Feb 4, 2026
After analyzing hundreds of recovery protocols across tendons, muscles, joints, and nerves, one pattern keeps emerging. The researchers who recover fastest are not simply choosing the right peptide. They are choosing the right combination of regenerative peptides, timing them correctly, and targeting specific molecular pathways that drive cellular repair. That distinction matters more than most guides admit.
Regenerative peptides represent a category of short-chain amino acid sequences that directly influence how the body repairs damaged tissue. They are not generic supplements. They are signaling molecules. Each one triggers specific cascades within cells, from angiogenesis and collagen synthesis to stem cell activation and inflammation resolution. And when combined strategically, they produce effects that exceed what any single peptide achieves alone.
The complexity here is real. BPC-157 activates different pathways than TB-500. GHK-Cu upregulates over 4,000 genes that neither of those touch. MGF initiates satellite cell proliferation in muscle tissue through mechanisms distinct from all three. Understanding which peptide does what, and how they interact, separates effective protocols from wasted time and money. This guide covers every major regenerative peptide, their mechanisms, the research behind them, practical protocols, stacking strategies, and the specific tissue types each one targets.
SeekPeptides has compiled the most comprehensive breakdown available, drawing from published research, documented protocols, and real-world application data that experienced researchers rely on daily.
What makes a peptide regenerative
Not every peptide qualifies as regenerative. The term gets applied loosely across the industry, but true regenerative peptides share specific characteristics that set them apart from performance peptides or fat loss peptides.
A regenerative peptide must directly promote tissue repair. That means stimulating cellular processes like angiogenesis (new blood vessel formation), collagen synthesis, fibroblast proliferation, or stem cell activation. Peptides that only reduce inflammation without triggering repair do not qualify. Peptides that boost growth hormone without local tissue effects do not qualify either.
The distinction matters for protocol design. When someone needs to heal a torn tendon, reduce scar tissue after surgery, or accelerate nerve recovery, they need peptides that act at the tissue level. General wellness peptides will not get the job done. Regenerative peptides work through several overlapping molecular pathways. The most important ones include PI3K/Akt for cell survival and proliferation, MAPK for fibroblast activity and collagen production, TGF-beta for extracellular matrix remodeling, VEGFR2 for angiogenesis, and NF-kB inhibition for resolving chronic inflammation that blocks healing. Understanding these pathways helps explain why certain peptide stacks work better than individual compounds, and why timing and sequencing affect outcomes so dramatically.
The four pillars of tissue regeneration
Every successful tissue repair process requires four things to happen in sequence. First, inflammation must be controlled. Not eliminated, controlled. Acute inflammation is necessary for signaling repair cells to the injury site. Chronic inflammation prevents those cells from doing their work.
Second, new blood vessels must form. Without angiogenesis, repair cells cannot reach the damage site and nutrients cannot feed new tissue growth. This is where many healing protocols fail silently.
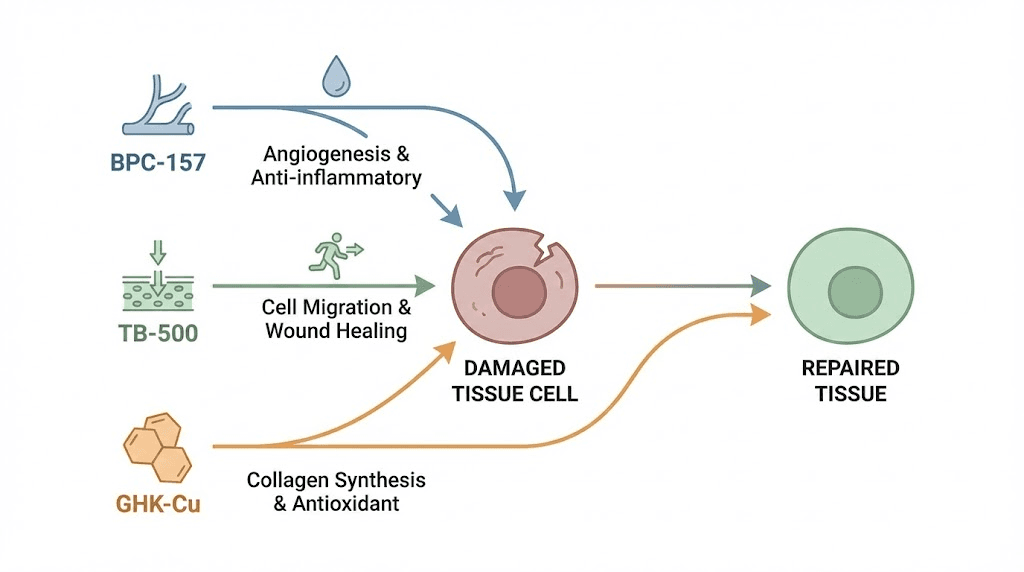
Third, structural proteins must be synthesized. Collagen, elastin, and other extracellular matrix components form the scaffolding that new tissue grows on. Without adequate structural protein production, repairs are weak and re-injury becomes likely. Fourth, progenitor and stem cells must be recruited and activated. These cells differentiate into the specific tissue types needed, whether that is tendon, muscle, cartilage, or nerve. The most effective regenerative peptides address multiple pillars simultaneously. BPC-157 handles inflammation control and angiogenesis. TB-500 excels at cell migration and anti-fibrosis. GHK-Cu drives gene expression changes and structural protein synthesis. This is why the BPC-157 and TB-500 combination has become the foundation of most regenerative protocols.
BPC-157: the foundational regenerative peptide
BPC-157 stands as the most studied and widely used regenerative peptide in research settings. This 15-amino-acid sequence, originally isolated from human gastric juice, demonstrates remarkable stability and broad tissue repair activity across nearly every tissue type studied.
What makes BPC-157 exceptional is its multi-pathway activation. Most peptides influence one or two signaling cascades. BPC-157 activates at least five distinct mechanisms simultaneously, which explains its effectiveness across such diverse tissue types.
Mechanism of action
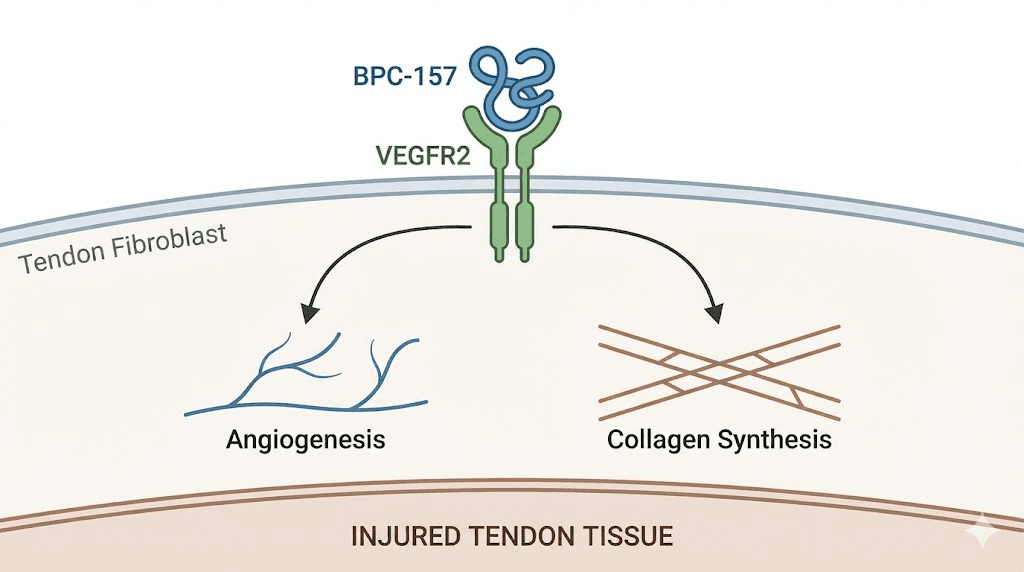
At the molecular level, BPC-157 triggers a cascade that begins with VEGFR2 activation. This receptor, once engaged, initiates the Akt-eNOS signaling pathway. The result is increased nitric oxide production, which dilates blood vessels and promotes the formation of entirely new vascular networks at injury sites. Research shows BPC-157 enhances ERK1/2 phosphorylation in a dose-dependent manner. Higher concentrations produce stronger activation, leading to increased cellular proliferation, enhanced migration of repair cells, and accelerated vascular tube formation. These are the foundational processes of tissue repair.
Beyond vascular effects, BPC-157 upregulates cytoprotective factors including heme oxygenase-1 and heat shock proteins. These protect mitochondrial integrity under stress conditions, reducing oxidative damage that would otherwise slow or prevent healing. The peptide also prevents activation of the transcription factor erg-1, interrupting the release of proinflammatory and prothrombic mediators that sustain chronic inflammation at injury sites.
One finding that consistently surprises researchers involves growth hormone. In tendon tissue studies, BPC-157 produced a sevenfold increase in local growth hormone levels by day three of administration. This localized growth hormone surge accelerates tissue remodeling without the systemic effects associated with exogenous growth hormone administration. For anyone using the BPC-157 dosage calculator, understanding these mechanisms helps explain why proximity of injection to the injury site matters so much.
Tissue-specific applications
The research on BPC-157 spans nearly every connective tissue in the body. For tendons and ligaments, studies demonstrate accelerated reticulin and collagen formation alongside enhanced angiogenesis. Macrophage and fibroblast infiltration increase significantly, with measurable improvements in tendon-to-bone healing within 10 to 14 days in animal models.
Muscle tissue responds strongly as well. BPC-157 restores complete function of injured muscles by modulating immune cell activity at the damage site while simultaneously promoting structural repair. For researchers dealing with muscle-related protocols, this dual action, controlling inflammation while building new tissue, explains the compound rapid reputation among athletic recovery applications.
Gut tissue repair represents perhaps the most well-established application. Given that BPC-157 originates from gastric juice, its affinity for gastrointestinal tissue makes biological sense. The peptide demonstrates protective and healing effects on the intestinal lining, making it relevant for gut health protocols and conditions involving intestinal permeability.
Neural tissue is another area where BPC-157 shows promise. The peptide promotes nerve growth factor expression and accelerates peripheral nerve healing in animal models. For those researching peptides for brain and nerve repair, BPC-157 often forms the foundation of neuroregeneration stacks.
Vascular and cardiovascular repair
BPC-157 has been closely implicated in the recovery of blood flow to ischemic muscle tissue. By stimulating angiogenesis through peptide-induced expression of VEGFR2 and subsequent activation of the VEGFR2-Akt-eNOS signaling pathway, BPC-157 restores perfusion to tissues that have been deprived of adequate blood supply. This mechanism has direct relevance for post-surgical recovery, crush injuries, and any condition where vascular compromise threatens tissue viability.
The cardiovascular applications extend further. BPC-157 has demonstrated protective effects on endothelial cells, the cells lining blood vessels. It inhibits the formation of thrombi and counteracts vascular dysfunction caused by various toxins and pharmacological agents in preclinical models. For researchers interested in the intersection of cardiovascular health and regenerative protocols, BPC-157 vascular protective profile adds a dimension that most tissue repair peptides lack.
Current human evidence
Three pilot studies have examined BPC-157 in humans. One investigated intraarticular knee pain, delivering the peptide directly into the joint space. Another focused on interstitial cystitis, a chronic bladder condition with limited treatment options. A third assessed intravenous safety and pharmacokinetics, establishing basic tolerability data in human subjects.
While the preclinical evidence is extensive, spanning hundreds of published studies across multiple tissue types and injury models, human data remain limited. This is important context for anyone building protocols around this compound. The preclinical results are robust and the mechanistic rationale is strong, but caution is warranted until larger, controlled human trials are completed. Several clinical investigations are reportedly in progress, which should provide more definitive human data in the coming years.
TB-500: the cell migration specialist
TB-500, the synthetic form of Thymosin Beta-4, brings a fundamentally different mechanism to regenerative protocols. While BPC-157 excels at creating the vascular infrastructure for repair, TB-500 specializes in getting repair cells to where they need to be.
Thymosin Beta-4 is a 43-amino-acid peptide present in nearly all human cells and body fluids. Concentrations run highest in platelets and white blood cells at wound sites, which makes biological sense. The body naturally upregulates this peptide in response to tissue injury. TB-500 amplifies that natural response.
How TB-500 promotes regeneration
The primary mechanism involves actin polymerization. TB-500 enables key repair cells, including fibroblasts, keratinocytes, and endothelial cells, to reorganize their internal cytoskeleton and migrate effectively toward injury sites. Without adequate cell migration, all the angiogenesis and growth factor signaling in the world produces nothing. Cells must physically arrive at the damage site to begin repairs.
TB-500 also modulates transcriptional pathways involved in angiogenesis, cytokine production, and tissue remodeling. The PI3K/Akt and NF-kB signaling cascades respond to TB-500 administration, producing downstream effects on both inflammation and structural repair. Suppression of IL-6 and TNF-alpha, two key inflammatory cytokines, helps create conditions favorable for tissue rebuilding rather than ongoing damage. The complete profile of TB-500 benefits extends well beyond what most summaries cover.
Anti-fibrotic properties
One of TB-500 lesser known but most valuable effects is scar tissue reduction. The peptide limits myofibroblast activity and downregulates fibrosis-associated pathways. In preclinical studies, wounds treated with TB-500 exhibited more organized collagen alignment and lower levels of inflammatory cytokines compared to untreated controls.
This matters enormously for functional recovery. Scar tissue is structurally inferior to native tissue. It lacks the elasticity, strength, and organized fiber patterns of the original structure. By reducing fibrosis during the healing process, TB-500 helps ensure that repaired tissue more closely resembles what was there before the injury.
For tendon and ligament injuries specifically, this property is critical. Tendons repaired with excessive scar tissue remain prone to re-injury.
The organized collagen alignment promoted by TB-500 produces more functional repairs, which is why the compound features prominently in protocols for tendon repair and joint recovery.
Cardiac and multi-tissue applications
TB-500 demonstrates efficacy across cardiac, muscular, ocular, and dermal injury models. The cardiac applications are particularly interesting. Following heart muscle damage, TB-500 promotes regeneration of cardiomyocytes while reducing the fibrotic scarring that typically replaces damaged heart tissue. The combination of cell migration support, anti-fibrotic effects, and anti-inflammatory cytokine modulation makes TB-500 uniquely suited for cardiac repair applications where scar tissue formation traditionally limits functional recovery.
In dermal wound models, TB-500 accelerates closure rates and produces wounds with better cosmetic outcomes due to organized collagen deposition. Ocular injury models show improvements in corneal repair and reduced scarring after chemical or mechanical damage. These diverse applications confirm that TB-500 mechanisms are not tissue-specific but rather fundamental repair processes that operate across virtually all tissue types. While these applications remain preclinical, they illustrate the broad regenerative potential of this peptide and explain why TB-500 features in so many multi-tissue recovery protocols.
For practical protocol purposes, TB-500 pairs exceptionally well with BPC-157. The BPC-157 versus TB-500 comparison reveals complementary rather than overlapping mechanisms. BPC-157 builds the vascular infrastructure and controls inflammation. TB-500 mobilizes repair cells and prevents fibrosis. Together, they cover more regenerative pillars than either compound addresses alone. Use the TB-500 dosage calculator to determine appropriate amounts based on body weight and protocol goals.
GHK-Cu: the genetic reprogramming peptide
GHK-Cu operates on a completely different level than BPC-157 or TB-500. While those peptides work primarily through receptor activation and pathway signaling, GHK-Cu literally reprograms gene expression at scale.
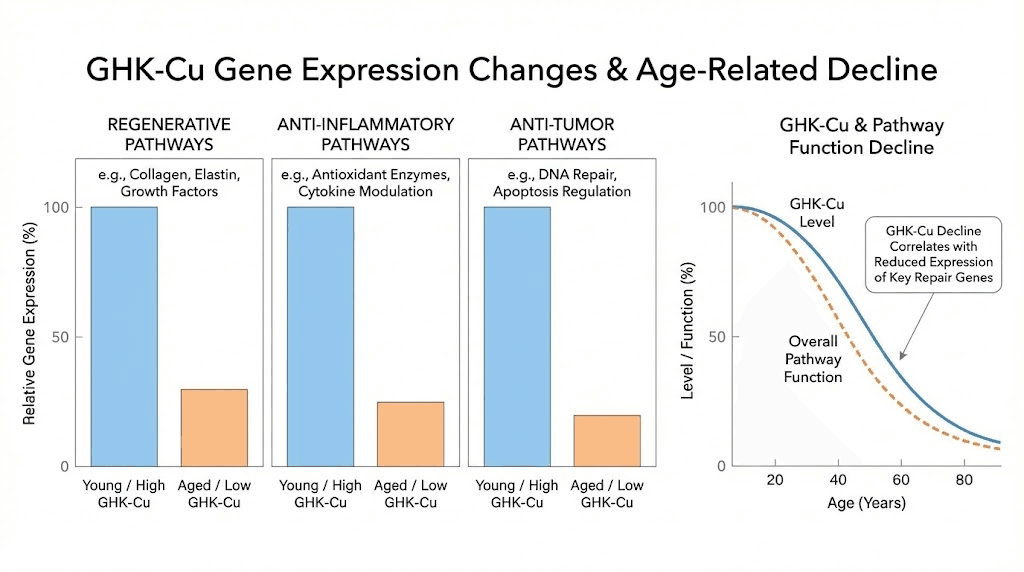
This naturally occurring tripeptide binds copper ions and is found in plasma, saliva, and urine. Serum levels sit around 200 nanograms per milliliter at age 20 but decline to approximately 80 nanograms per milliliter by age 60. That decline in GHK-Cu correlates directly with the age-related decrease in regenerative capacity that everyone experiences.
Gene expression on a massive scale
Supplementing GHK-Cu has been shown to upregulate over 4,000 regenerative genes while simultaneously suppressing pro-inflammatory and pro-tumor pathways. The scale of this genetic reprogramming is unlike anything else in the peptide space. No other single compound influences gene expression across so many systems simultaneously. The complete GHK-Cu guide covers the full scope of these gene expression changes.
Among the pathways affected, GHK-Cu contributes to redox homeostasis and extracellular matrix balance. It stimulates metalloproteinase activity, the enzymes responsible for breaking down and remodeling damaged tissue, while simultaneously increasing anti-protease expression to prevent excessive tissue degradation. This balance between breakdown and rebuilding is essential for clean, functional tissue repair.
Stem cell activation
GHK-Cu promotes the survival of basal stem cells in skin tissue and enhances cellular stemness. It increases the secretion of trophic factors by mesenchymal stem cells, the multipotent cells capable of differentiating into bone, cartilage, muscle, fat, and connective tissue. This stem cell activation makes GHK-Cu relevant for conditions ranging from bone healing to skin regeneration to hair follicle recovery.
Copper-dependent antioxidant enzyme regulation adds another layer. GHK-Cu participates in managing oxidative stress at injury sites, preventing the cellular damage that reactive oxygen species cause during the inflammatory phase of healing. This protective effect helps preserve viable tissue around injury margins, giving repair processes more healthy tissue to work with.
Topical versus injectable applications
GHK-Cu has an established track record in topical formulations for skin protection and post-procedure wound care. The GHK-Cu injection dosage guide covers systemic administration, while injection technique details address practical application. Intra-articular injections have shown improved graft healing post-surgery in animal models, and incorporating GHK-Cu into collagen matrices enhances wound contraction in diabetic models.
For researchers building regenerative stacks, GHK-Cu adds the genetic reprogramming layer that BPC-157 and TB-500 lack. It reinforces and stabilizes the repairs initiated by those peptides, making regenerated tissue more resilient over time. The GLOW peptide blend, which combines GHK-Cu with BPC-157 and TB-500, was specifically designed around this synergistic principle.
Additional regenerative peptides worth knowing
BPC-157, TB-500, and GHK-Cu form the core of most regenerative protocols. But several other peptides contribute meaningful regenerative effects through distinct mechanisms. Understanding these expands the toolkit available for complex or resistant injuries.
MGF (mechano growth factor)
MGF is a splice variant of IGF-1, but its sequence and function differ significantly from the systemic IGF-1 produced by the liver. Where liver-derived IGF-1 has broad systemic effects, MGF acts locally at the site of mechanical tissue damage.
The peptide initiates hypertrophy and repair of damaged muscle by activating satellite cells. These are the muscle-specific stem cells that donate nuclei to damaged muscle fibers, enabling both repair and growth. MGF is expressed as a pulse following muscle damage, making it a first-responder in the muscle regeneration sequence. For muscle growth protocols, MGF addresses a specific regenerative bottleneck that other peptides do not directly target. The satellite cell activation it provides is a prerequisite for meaningful muscle fiber repair and hypertrophy following training-induced damage.
SS-31 (elamipretide)
While most regenerative peptides act on extracellular signaling or gene expression, SS-31 works inside the cell at the mitochondrial level. It targets cardiolipin in the inner mitochondrial membrane, restoring electron transport chain function and reducing the production of reactive oxygen species.
Mitochondrial dysfunction underlies many chronic conditions where tissue regeneration stalls. When mitochondria cannot produce adequate ATP, cells lack the energy needed for repair processes. SS-31 addresses this fundamental bottleneck. The SS-31 benefits profile extends across cardiac, muscular, renal, and neurological tissues. For aging-related regenerative decline, SS-31 may be one of the most directly relevant compounds because mitochondrial function deteriorates progressively with age. Athletes interested in recovery applications can review the SS-31 bodybuilding applications for context on physical performance.
KPV: the anti-inflammatory tripeptide
KPV is a three-amino-acid peptide derived from alpha-melanocyte-stimulating hormone. Its primary regenerative contribution is powerful anti-inflammatory activity that creates conditions favorable for tissue repair.
While KPV does not directly stimulate tissue growth in the way BPC-157 or GHK-Cu do, its ability to suppress NF-kB signaling and reduce inflammatory cytokine production makes it a valuable addition to regenerative stacks. Chronic inflammation is the most common barrier to successful tissue healing. KPV benefits include gut healing, skin repair, and systemic inflammation control. The KPV dosage guide covers practical application for various protocols. KPV is a key component of the KLOW peptide blend, which adds it to the BPC-157, TB-500, and GHK-Cu combination for enhanced anti-inflammatory coverage.
Epitalon
Epitalon approaches regeneration from the telomere level. This synthetic tetrapeptide activates telomerase, the enzyme that maintains telomere length at the ends of chromosomes. Telomere shortening limits how many times a cell can divide before becoming senescent. By preserving telomere length, Epitalon potentially extends the replicative capacity of cells involved in tissue repair.
The Epitalon benefits and dosage protocols are covered in dedicated guides. For regenerative applications, Epitalon supports the longevity of the repair process itself, ensuring that progenitor cells maintain their ability to divide and differentiate over extended healing periods. This peptide is part of the broader bioregulator peptide category developed through decades of Russian research.
MOTS-c
MOTS-c is a mitochondria-derived peptide that activates AMPK signaling, influencing metabolism, exercise capacity, and cellular energy production. While primarily known for metabolic effects, MOTS-c contributes to regeneration by optimizing the metabolic environment within which tissue repair occurs.
Cells repairing tissue have enormous energy demands. MOTS-c helps ensure adequate metabolic capacity to meet those demands. The MOTS-c benefits guide covers its full profile, while the dosage chart provides practical implementation details. Be aware of the documented MOTS-c side effects when incorporating this compound into stacking protocols.
Thymosin Alpha-1
Thymosin Alpha-1 modulates immune function in ways that support tissue regeneration indirectly. By optimizing immune cell activity, it ensures that the inflammatory phase of healing resolves appropriately and that immune surveillance supports rather than hinders the repair process. The thymalin peptide guide covers the broader thymic peptide family and their roles in immune-mediated tissue repair.
Cerebrolysin
For neural regeneration specifically, Cerebrolysin provides a mixture of neurotrophic peptides that promote neuroplasticity and neural repair. The Cerebrolysin benefits are particularly relevant for brain injury recovery and neurodegenerative conditions where neural tissue needs active rebuilding. Combined with other brain-targeted peptides, Cerebrolysin addresses a regenerative niche that general tissue repair peptides do not fully cover.
BDNF-related peptides
Brain-derived neurotrophic factor peptides support neural plasticity and nerve cell survival. The BDNF peptide guide explains how these compounds promote neuronal growth, differentiation, and survival. For researchers working on neuropathy protocols or brain repair stacks, BDNF peptides add a regenerative dimension that complements the vascular and anti-inflammatory effects of BPC-157.
Regenerative peptides by tissue type
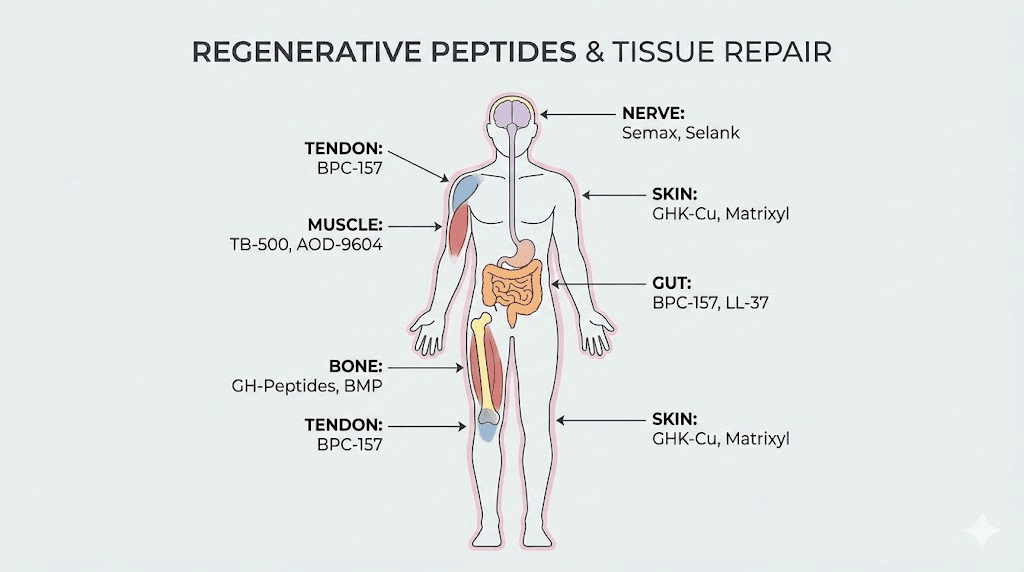
Different tissues require different regenerative approaches. A tendon heals differently than muscle, which heals differently than nerve tissue. Matching the right peptides to the target tissue dramatically improves outcomes.
Tendons and ligaments
Tendon injuries are among the most common and most frustrating to heal. The limited blood supply to tendon tissue creates a natural bottleneck for nutrient delivery and repair cell access. This is precisely why angiogenesis-promoting peptides like BPC-157 are so effective here.
The primary protocol for tendon regeneration centers on BPC-157 for vascular support and local growth hormone upregulation, combined with TB-500 for cell migration and anti-fibrotic effects. GHK-Cu adds collagen remodeling and gene expression changes that improve the quality of repaired tissue. Researchers working on tendon-specific protocols often include all three compounds. For specific joint applications, guides on shoulder pain, back pain, and general joint support provide targeted protocol details.
Muscle tissue
Muscle regeneration follows a well-characterized sequence. Satellite cells activate, proliferate into myoblasts, and then differentiate into new myofibers. MGF initiates this sequence by activating satellite cells. BPC-157 provides the vascular support needed for nutrient delivery. TB-500 ensures repair cells reach the damage site efficiently.
For muscle growth and recovery, the combination addresses the full regenerative pathway. Muscle-specific peptide protocols often include IGF-1 variants alongside the core regenerative compounds. The IGF peptide guide and IGF-1 LR3 guide cover these options in detail. Athletic performance protocols frequently combine regenerative peptides with performance compounds for simultaneous recovery and adaptation.
Bone and cartilage
Bone regeneration requires peptides that promote osteoblast differentiation and mineralization. BPC-157 demonstrates bone healing properties, but the addition of GHK-Cu, which activates mesenchymal stem cells capable of differentiating into bone tissue, significantly enhances the process.
Cartilage presents unique challenges because it lacks direct blood supply and has limited self-repair capacity. Peptide-based approaches for cartilage regeneration promote chondrogenesis, enhance cell viability, and induce differentiation of chondrocytes from mesenchymal stem cells. The bone healing guide covers protocols for fracture recovery and bone density support. For degenerative joint conditions, the osteoporosis peptide guide addresses long-term bone health maintenance. Herniated disc protocols combine cartilage and nerve repair peptides for these complex injuries.
Neural tissue
Neural regeneration represents the frontier of peptide-based tissue repair. The central nervous system has historically been considered incapable of significant regeneration, but recent peptide research challenges that assumption.
BPC-157 promotes nerve growth factor expression. Cerebrolysin provides neurotrophic peptide support. BDNF peptides enhance neural plasticity. And emerging research on cell-permeable peptides shows they can dissolve stress granules formed by the G3BP1 protein, which inhibit the protein synthesis necessary for axon repair. This approach has shown effectiveness in both peripheral and central nervous system neurons.
For peripheral nerve injuries, self-assembling peptide hydrogels used as scaffolds within nerve conduits have achieved axonal regeneration comparable to autografts in animal models. These biomaterial approaches combine the signaling properties of regenerative peptides with physical scaffolding that guides nerve growth along the correct pathways. The neuropathy guide covers practical protocols, while brain repair applications address central nervous system targets.
Skin and wound healing
Skin regeneration draws on all three core peptides effectively. BPC-157 accelerates wound closure through angiogenesis and collagen formation. TB-500 reduces scarring by limiting myofibroblast activity. GHK-Cu promotes organized collagen deposition and stem cell-mediated skin renewal.
The complete skin peptide guide covers applications from wound healing to anti-aging. For specific concerns, dedicated resources address skin tightening, natural skin peptides, acne, and eczema. Collagen peptides for cellulite and Snap-8 for expression lines represent more targeted cosmetic applications of regenerative peptide technology.
Gut and gastrointestinal tissue
BPC-157 originates from gastric juice, and its regenerative effects on gut tissue are among the best documented. The peptide protects and heals intestinal lining, reduces inflammation in the GI tract, and promotes mucosal repair.
For gut-specific protocols, the gut health category page provides an overview, while inflammation management guides address the underlying inflammatory processes. KPV adds significant value to gut regeneration protocols through its NF-kB suppression and direct gut anti-inflammatory effects. The combination of BPC-157 and KPV for gut repair has become one of the most popular peptide stacking approaches for gastrointestinal conditions.
Stacking regenerative peptides for synergistic effects
Single-peptide protocols produce results. Multi-peptide stacks produce dramatically better results. The reason is straightforward. Tissue regeneration involves multiple simultaneous processes. One peptide cannot optimally drive all of them.
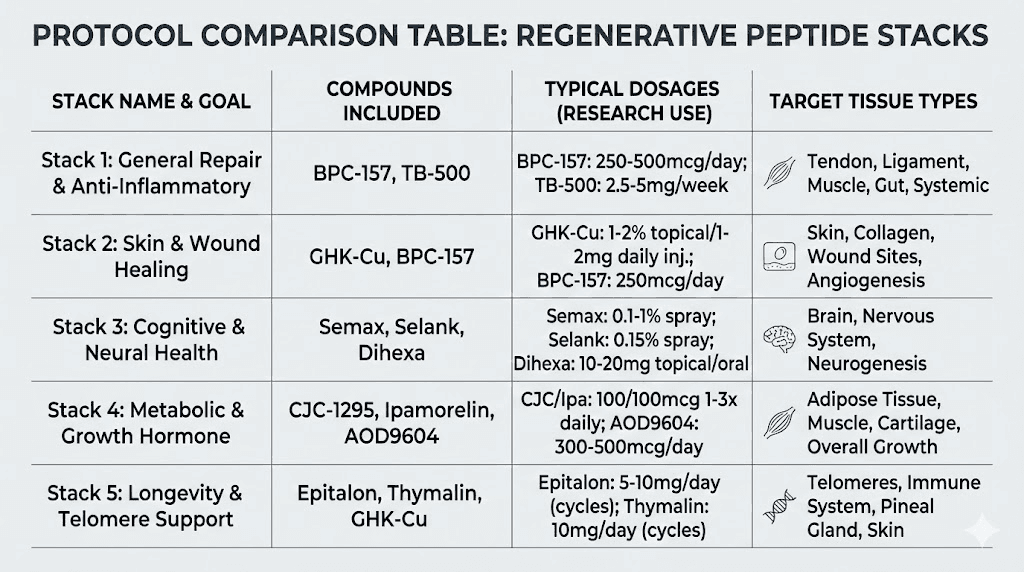
The foundational stack: BPC-157 plus TB-500
This is the most widely used regenerative combination in the research community. BPC-157 provides angiogenesis, inflammation control, and local growth factor upregulation. TB-500 provides cell migration, anti-fibrotic effects, and cytokine modulation. Together, they address four of the five pillars of tissue regeneration.
The complete BPC-157 and TB-500 stacking guide covers protocol design in detail. Dosing typically follows weight-based calculations, and the peptide stack calculator helps determine appropriate amounts for combined protocols. Understanding the differences between BPC-157 and TB-500 is essential for protocol optimization.
The GLOW stack: BPC-157 plus TB-500 plus GHK-Cu
Adding GHK-Cu to the foundational stack introduces gene expression reprogramming, enhanced stem cell activation, and superior collagen remodeling. This three-peptide combination addresses all five regenerative pillars.
The GLOW blend and GLOW stack guide provide detailed protocols. GHK-Cu doses are covered in the injection dosage guide. The GLOW peptide overview explains how the three compounds complement each other at the molecular level.
The KLOW stack: BPC-157 plus TB-500 plus GHK-Cu plus KPV
For situations involving significant inflammatory burden, adding KPV to the GLOW combination creates the KLOW stack. This four-peptide protocol adds dedicated NF-kB suppression and anti-inflammatory cytokine modulation on top of the tissue repair, cell migration, and gene expression effects.
The KLOW peptide guide covers this combination in detail. Researchers dealing with autoimmune-related tissue damage, chronic inflammatory conditions, or gut-related repair often prefer this expanded stack.
The Wolverine stack
Named for obvious reasons, the Wolverine stack represents a more aggressive regenerative approach. The Wolverine peptide concept combines multiple regenerative compounds at higher relative doses for accelerated healing timelines. This approach is typically reserved for significant injuries or post-surgical recovery where aggressive tissue repair is the priority.
Stacking principles
Effective regenerative stacking follows several key principles. First, combine peptides that target different mechanisms rather than doubling up on the same pathway. BPC-157 and TB-500 work well together precisely because their mechanisms are complementary, not redundant.
Second, consider the tissue type and match peptide selection accordingly. Neural injuries benefit from adding Cerebrolysin or BDNF peptides. Muscle injuries benefit from adding MGF. Bone injuries benefit from emphasizing GHK-Cu for its stem cell differentiation effects.
Third, timing matters. Some researchers stagger their compounds rather than administering everything simultaneously. The peptide cycle planning guide addresses timing and sequencing strategies. Understanding how many peptides can be taken simultaneously and how to cycle different peptides helps prevent protocol complexity from becoming counterproductive.
Practical protocols for regenerative peptides
Theory without application is useless. This section provides practical framework protocols for common regenerative goals. These are starting points based on commonly reported approaches in the research community, not medical prescriptions.
Protocol 1: general tissue repair (moderate injury)
Goal: Accelerate healing of moderate soft tissue injuries such as muscle strains, ligament sprains, or tendinopathy.
Core compounds:
BPC-157: 250 to 300 mcg, administered twice daily (morning and evening)
TB-500: 2 to 2.5 mg, administered twice weekly
Duration: 4 to 6 weeks, with reassessment at week 4.
Administration: Subcutaneous injection, ideally within 1 to 2 inches of the injury site for BPC-157. TB-500 can be administered subcutaneously at any site due to its systemic distribution properties.
Expected timeline: Initial improvements around days 7 to 10. Significant healing progress by weeks 3 to 4. Protocol completion at week 6 with reassessment.
Use the BPC-157 calculator and TB-500 calculator for weight-based dosing. The peptide dosing guide covers general principles for all protocols.
Protocol 2: aggressive regeneration (severe injury or post-surgical)
Goal: Maximum tissue repair for severe injuries, post-surgical recovery, or chronic conditions that have not responded to simpler protocols.
Core compounds:
BPC-157: 300 to 500 mcg, twice daily
TB-500: 2.5 mg, twice weekly for first 4 weeks, then once weekly
GHK-Cu: 200 to 500 mcg, once daily
Duration: 6 to 8 weeks minimum, with reassessment intervals every 2 weeks.
Expected timeline: Measurable improvements by week 2. Significant tissue remodeling by weeks 4 to 6. Continued improvement through week 8 and beyond as collagen maturation occurs.
This protocol combines the GLOW stack principles with more aggressive dosing. The peptide stack calculator helps balance multiple compounds. Review the stacking guide for interaction considerations.
Protocol 3: neural regeneration
Goal: Support peripheral nerve healing, neuropathy recovery, or post-concussion neuroplasticity.
Core compounds:
BPC-157: 250 to 500 mcg, twice daily
Cerebrolysin: Protocol-specific dosing (see Cerebrolysin guide)
Semax: 200 to 600 mcg intranasally, once or twice daily (see Semax dosage guide)
Optional additions: Selank for anxiety and stress modulation during recovery (Selank dosage guide), PE-22-28 for additional neurotrophic support (PE-22-28 guide).
Duration: 8 to 12 weeks for peripheral nerve injuries. Longer protocols may be appropriate for central nervous system applications.
Protocol 4: gut regeneration
Goal: Heal intestinal lining, reduce gut inflammation, and restore gastrointestinal barrier function.
Core compounds:
BPC-157: 250 to 500 mcg, twice daily (oral or subcutaneous)
KPV: Protocol-specific dosing for gut anti-inflammatory effects
Notes: BPC-157 can be administered orally for gut-specific applications, a unique advantage among regenerative peptides. The injectable versus oral comparison explains the differences in bioavailability and tissue targeting for each administration route. Peptide capsules and nasal spray options offer additional administration alternatives.
Protocol 5: anti-aging and longevity regeneration
Goal: Support overall tissue quality, counter age-related regenerative decline, and maintain cellular repair capacity.
Core compounds:
GHK-Cu: 200 to 500 mcg, daily
Epitalon: Protocol-specific dosing for telomere maintenance
SS-31: Protocol-specific dosing for mitochondrial support
MOTS-c: Protocol-specific dosing for metabolic optimization
Duration: Cyclical protocols with periods of active use and rest. The cycle planning guide addresses long-term protocol structure. Review the longevity peptides guide and anti-aging category page for comprehensive context on age-related peptide protocols.
Preparation, storage, and administration
The most carefully designed regenerative protocol fails if the peptides are improperly prepared or stored. Degraded peptides produce degraded results. This section covers the practical essentials.
Reconstitution
Most regenerative peptides arrive as lyophilized (freeze-dried) powder that requires reconstitution before use. The complete reconstitution guide covers the step-by-step process. Key considerations include using bacteriostatic water for multi-use vials, proper injection technique, and maintaining sterility throughout the process.
The reconstitution calculator determines exact water volumes for target concentrations. Understanding how much bacteriostatic water to add and how to properly mix peptides prevents the dosing errors that compromise protocol outcomes. For those new to the process, the difference between lyophilized and liquid peptides is worth understanding.
Storage requirements
Unreconstituted peptide powder stores well in freezer conditions, remaining stable for extended periods. The powder storage duration guide covers specific timelines. Once reconstituted, peptides require refrigeration and should be used within defined timeframes.
The comprehensive storage guide addresses all scenarios. Key resources include information on refrigerator storage duration, room temperature stability, post-reconstitution storage, and peptide expiration. Temperature excursions can degrade peptides rapidly, and once degraded, no amount of correct dosing will recover their effectiveness.
Administration methods
Subcutaneous injection remains the primary administration route for most regenerative peptides. The peptide injection overview covers fundamentals, while the injection pen guide addresses more convenient administration tools. The complete list of injectable peptides provides a comprehensive reference.
Alternative routes include oral administration (primarily for BPC-157 and gut-targeted protocols), nasal spray delivery (useful for neurological peptides like Semax), and topical application (primarily for GHK-Cu skin applications). Nasal spray peptide delivery offers advantages for compounds targeting the brain, while injectable versus oral comparisons help determine the best route for each specific compound and application. SeekPeptides provides detailed protocol guides covering every administration method with specific instructions for each peptide.
Quality, testing, and sourcing considerations
Peptide quality varies enormously across the market. Regenerative peptides are only as effective as their purity and structural integrity allow. This is not an area where cutting costs makes sense.
Purity testing
Third-party testing via HPLC (high-performance liquid chromatography) and mass spectrometry confirms both identity and purity. Reputable sources provide certificates of analysis for each batch. The peptide testing labs guide covers what to look for in quality documentation and which testing methods provide reliable results.
Peptide vial research addresses how to evaluate what arrives in the mail. Contaminants, degradation products, and incorrect peptide sequences all reduce effectiveness and potentially introduce risks. Researching research versus pharmaceutical grade peptides helps set realistic expectations for different quality tiers.
Legal and regulatory landscape
The legal status of peptides varies by jurisdiction. The peptide legality guide covers the current regulatory framework, while regulation news tracks ongoing changes. Understanding drug testing implications matters for athletes and professionals subject to testing requirements.
The FDA has not approved most regenerative peptides for medical treatment. BPC-157, TB-500, GHK-Cu, and related compounds are available for research purposes. Clinical trials are ongoing for several of these compounds, and the regulatory landscape continues evolving. Staying informed through reliable sources is essential for responsible research practices.
Cost considerations
Regenerative peptide protocols involve ongoing costs that vary based on compound selection, dosing, and protocol duration. The peptide cost calculator helps estimate expenses for different protocols. Comprehensive cost breakdowns appear in the peptide therapy cost guide and general cost overview.
The most common mistake researchers make is choosing cheaper sources to save money. Lower purity means lower effectiveness, which means longer protocols and more compound needed to achieve the same results. In many cases, quality peptides at proper doses end up costing less than cheap peptides at compensatory higher doses.
The science of regenerative peptide combinations
Understanding why combinations outperform individual peptides requires examining what happens at the molecular level when multiple regenerative signals converge simultaneously.
Synergistic pathway activation
BPC-157 activates the VEGFR2-Akt-eNOS axis. TB-500 activates PI3K/Akt through different upstream receptors. GHK-Cu drives gene expression changes through copper-dependent transcription factor interactions. When all three are present simultaneously, the cell receives converging pro-regenerative signals through multiple pathways.
This convergent signaling produces amplified responses. A fibroblast receiving VEGF stimulation (from BPC-157), actin polymerization support (from TB-500), and genetic upregulation of collagen synthesis (from GHK-Cu) simultaneously produces more collagen faster than it would in response to any single signal. The math is not simply additive. It is multiplicative.
Temporal coverage
Different peptides have different pharmacokinetic profiles. BPC-157 acts quickly and is administered twice daily for consistent tissue levels. TB-500 has a longer half-life and distributes systemically, providing sustained background activity between the twice-weekly doses. GHK-Cu drives gene expression changes that persist beyond the immediate presence of the peptide itself.
This temporal layering means that regenerative signaling never drops to zero between doses. Some compound is always active, some pathway is always engaged, and the repair process never fully stalls between administrations.
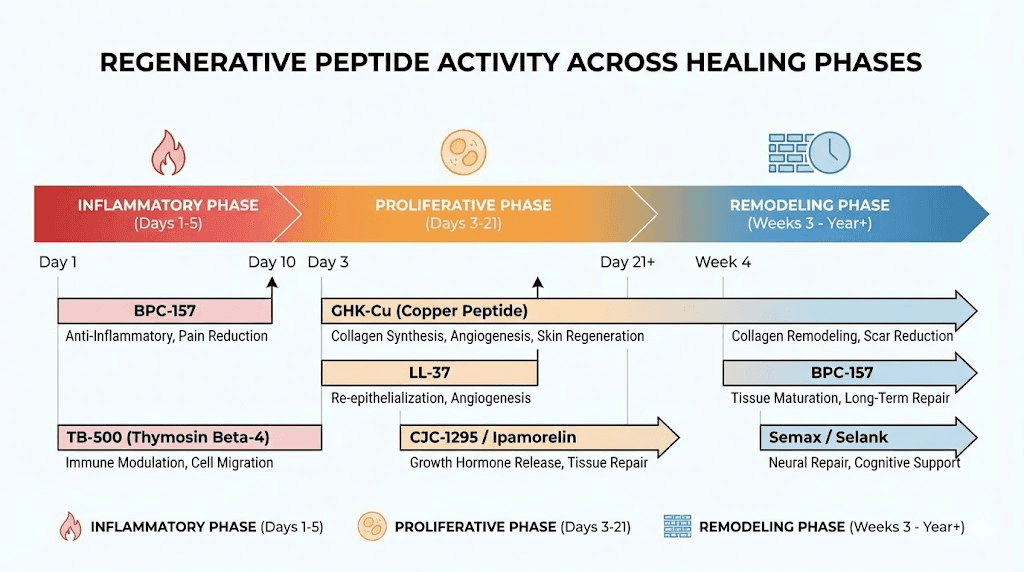
Phase-specific support
Tissue healing progresses through distinct phases. The inflammatory phase (days 0 to 3) requires inflammation control without suppression. The proliferative phase (days 3 to 21) requires angiogenesis, cell migration, and collagen synthesis. The remodeling phase (weeks 3 to 12) requires organized tissue restructuring and maturation.
Different peptides contribute maximally during different phases. KPV provides the strongest support during the inflammatory phase. BPC-157 and TB-500 drive the proliferative phase. GHK-Cu and Epitalon support the remodeling phase. A well-designed regenerative stack provides phase-appropriate support throughout the entire healing timeline. This is why understanding cycle planning matters as much as compound selection.
Common mistakes in regenerative peptide protocols
Knowing what works is half the equation. Knowing what fails, and why, prevents wasted effort and resources.
Mistake 1: insufficient duration
Tissue regeneration operates on biological timelines, not human patience. Tendon collagen requires 6 to 12 weeks to mature. Nerve regeneration proceeds at roughly 1 millimeter per day. Cartilage has virtually no direct blood supply and repairs slowly even under optimal conditions.
The most common protocol error is stopping too early. Researchers who run 2-week protocols for tendon injuries and conclude peptides do not work have not given the biology enough time. The timeline guide sets realistic expectations. View before and after documentation from longer protocols to understand what adequate duration produces.
Mistake 2: poor injection site selection
BPC-157 demonstrates enhanced local effects when administered near the injury site. Injecting it into the abdomen for a knee injury sacrifices this localized advantage. While systemic distribution still provides some benefit, proximity injection capitalizes on the local growth hormone upregulation and concentrated signaling that make BPC-157 so effective for targeted tissue repair.
The sevenfold growth hormone increase documented in tendon tissue studies occurs locally, at the injection site. That localized surge drives accelerated healing in the surrounding tissue. Researchers who consistently inject near the injury report faster and more complete outcomes than those using distant injection sites. For multi-site injuries, some protocols involve rotating injection locations to deliver concentrated BPC-157 activity to each affected area throughout the treatment cycle. The fast injury healing guide covers injection site optimization strategies for various injury types and locations.
Mistake 3: ignoring the inflammatory phase
Some researchers aggressively suppress inflammation from day one. This actually slows healing. The initial inflammatory response recruits immune cells that clear debris and release signaling molecules that initiate the repair cascade. Suppressing this too early removes essential first steps in the regenerative process.
The correct approach is modulation, not suppression. BPC-157 and KPV modulate inflammation without eliminating it. They prevent chronic inflammation from establishing while allowing the acute inflammatory phase to complete its essential functions.
Mistake 4: single-peptide protocols for complex injuries
A complex injury involving multiple tissue types, such as a shoulder injury affecting the rotator cuff tendon, surrounding muscle, and potentially the labral cartilage, requires a multi-peptide approach. Running BPC-157 alone addresses vascular repair and inflammation but misses the cell migration support (TB-500), gene expression changes (GHK-Cu), and tissue-specific growth factors that a comprehensive protocol provides.
The research community has moved decisively toward combination protocols for good reason. Each regenerative peptide addresses different bottlenecks in the healing process. A protocol limited to one compound leaves those other bottlenecks unaddressed, which limits the overall speed and quality of recovery regardless of how well that single compound performs its specific function. The stacking guide explains how to combine compounds without creating unnecessary complexity or interaction concerns. The minimum effective combination for most injuries is BPC-157 plus TB-500. Adding GHK-Cu elevates results further. Adding tissue-specific compounds like Cerebrolysin for neural injury or MGF for muscle damage adds precision targeting on top of the broad-spectrum regenerative foundation.
Mistake 5: poor peptide quality
Under-dosed, contaminated, or degraded peptides produce nothing. Testing lab reports, certificate of analysis verification, and sourcing from reputable providers are non-negotiable prerequisites. The testing labs guide and peptide solutions overview address quality assurance in detail.
Regenerative peptides versus other recovery approaches
Regenerative peptides exist within a broader landscape of recovery and repair strategies. Understanding where they fit, and where other approaches might be more appropriate, leads to better decision-making.
Peptides versus SARMs
SARMs (Selective Androgen Receptor Modulators) promote muscle growth through androgenic signaling. They build tissue but do not specifically promote repair of damaged tissue. The peptides versus SARMs comparison explains the fundamental differences. For injury recovery, regenerative peptides are more targeted and appropriate. For muscle growth without injury, the conversation becomes more nuanced.
Peptides versus TRT
Testosterone replacement therapy supports muscle maintenance and recovery capacity but operates through hormonal pathways rather than direct tissue repair mechanisms. The peptides versus TRT comparison covers the differences. Some researchers combine both approaches, using TRT for hormonal optimization and regenerative peptides for targeted tissue repair.
Peptides versus PRP and stem cell therapies
Platelet-rich plasma (PRP) and stem cell injections are established regenerative medicine approaches with growing clinical evidence. A major systematic review evaluated the clinical efficacy of multiple regenerative therapies. Meta-regression analysis identified mesenchymal stem cell therapy as the most effective intervention for pain reduction (with a statistically significant effect size), while PRP and peptide-based therapies showed moderate improvements across musculoskeletal conditions.
The advantage of peptide protocols is accessibility, cost-effectiveness, and the ability to run sustained protocols outside clinical settings. PRP treatments require blood draws and centrifugation at each session. Stem cell treatments involve either bone marrow aspiration or adipose tissue harvesting. Both require clinical visits with trained practitioners and involve higher per-session costs than self-administered peptide injections.
However, the comparison is not strictly either-or. Some researchers combine approaches, using clinical PRP or stem cell treatments for the initial concentrated regenerative stimulus alongside sustained home peptide protocols for ongoing repair support. The PRP provides a concentrated burst of growth factors at the injury site, while peptides like BPC-157 and TB-500 maintain elevated regenerative signaling between clinical sessions. This layered approach addresses different timescales of the repair process simultaneously.
For conditions where clinical regenerative treatments have shown strong evidence, such as knee osteoarthritis or chronic tendinopathy, incorporating peptide support may enhance outcomes. For conditions where clinical options are limited or impractical, peptide-only protocols provide an accessible alternative. The online peptide therapy guide covers how researchers access these compounds for their protocols.
The integrated approach
Regenerative peptides work best within a comprehensive recovery framework that includes proper nutrition, adequate sleep, appropriate physical therapy, and stress management. Peptides amplify the body natural repair processes. They do not replace the fundamental inputs those processes require.
Protein intake matters significantly during regenerative protocols. The body needs adequate amino acid supply to build the new tissue that peptides are signaling it to create. Collagen synthesis requires specific amino acids including glycine, proline, and hydroxyproline. Adequate vitamin C supports collagen cross-linking. Zinc and copper are essential cofactors for many repair enzymes. Without these nutritional foundations, even the best peptide protocol operates at reduced capacity.
Sleep quality directly affects growth hormone release, immune function, and cellular repair rates. The majority of tissue repair occurs during deep sleep phases. Researchers who optimize their sleep hygiene alongside peptide protocols consistently report better outcomes than those who focus on compounds alone. Physical therapy and appropriate loading patterns help remodeling tissue align along functional stress lines, producing stronger repairs. Hormone balance, energy optimization, and stress management all contribute to the environment in which regenerative peptides operate. Chronic stress elevates cortisol, which directly opposes tissue repair processes. Managing stress through whatever methods work for the individual, whether exercise, meditation, or simply better work-life boundaries, removes a significant barrier to regeneration.
Emerging research and future directions
The field of regenerative peptides is advancing rapidly. Several areas of active research promise to expand the toolkit and improve outcomes significantly.
Self-assembling peptide scaffolds
Self-assembling peptides (SAPs) that form hydrogel scaffolds represent a convergence of biomaterial science and peptide therapeutics. These materials provide both physical structure and biochemical signaling, guiding tissue growth along correct anatomical pathways while delivering regenerative signals directly to cells within the scaffold.
In nerve repair applications, SAP-filled nerve conduits have achieved axonal regeneration comparable to autografts. The RADA/KLT scaffold designs show particular promise for long-gap peripheral nerve repair. For bone and cartilage, peptide-functionalized scaffolds incorporating 3D bioprinting technology are creating increasingly sophisticated tissue engineering solutions. The biomimetic peptides guide covers these emerging technologies.
Gene-targeted peptide delivery
Research on cell-permeable peptides that dissolve stress granules (G3BP1 protein aggregates) opens new possibilities for central nervous system regeneration. These peptides overcome a fundamental barrier to CNS repair by removing molecular structures that block protein synthesis needed for axon regrowth. The stress granule dissolution approach represents a paradigm shift in neural regeneration thinking.
Traditional approaches focused on providing growth factors and trophic support to injured neurons. This new approach removes an internal molecular brake that prevents neurons from synthesizing the proteins they need for axon extension. The cell-permeable peptide derived from G3BP1 has demonstrated effectiveness in mouse, rat, and human neurons, boosting axon regeneration in both the peripheral and central nervous systems. If these results translate to clinical applications, they could transform the treatment of spinal cord injuries, traumatic brain injuries, and neurodegenerative conditions that have long been considered irreversible.
Precision regenerative protocols
As genomic and proteomic data become more accessible, personalized regenerative peptide protocols are becoming feasible. The concept involves analyzing individual gene expression profiles to determine which regenerative pathways are most deficient and selecting peptides accordingly. Rather than using standard stacks, this approach tailors compound selection to individual biological needs.
For example, an individual with documented GHK-Cu deficiency (common in those over 50) might prioritize GHK-Cu supplementation alongside standard regenerative compounds. Someone with identified mitochondrial dysfunction markers might add SS-31 to their core stack. An individual with elevated inflammatory markers might emphasize KPV and Thymosin Alpha-1 alongside tissue repair compounds. The peptide formula guide explores how custom formulations are designed for specific applications. As testing becomes more accessible and affordable, precision approaches will likely replace the current one-size-fits-most protocol paradigm.
Bioregulator peptides
The Khavinson bioregulator peptides represent a distinct approach to tissue-specific regeneration. These short peptides (typically 2 to 4 amino acids) are designed to interact with specific DNA sequences and modulate gene expression in targeted tissues. Organ-specific bioregulators include Cartalax for cartilage, Vesugen for blood vessels, Ventfort for vascular tissue, Chonluten for respiratory tissue, Bronchogen for lungs, Cortagen for the brain, Livagen for liver, and Pancragen for the pancreas.
This organ-targeted approach allows regenerative protocols to be customized at an unprecedented level of tissue specificity. Combined with the broad-spectrum effects of BPC-157, TB-500, and GHK-Cu, bioregulator peptides add precision to an already powerful regenerative framework.
Special populations and considerations
Not everyone approaches regenerative peptides from the same starting point. Age, sex, underlying conditions, and concurrent treatments all influence protocol design.
Age-related considerations
Regenerative capacity declines with age. GHK-Cu levels drop by 60 percent between ages 20 and 60. Telomere length shortens. Mitochondrial function deteriorates. Stem cell populations decrease. For older researchers, regenerative protocols may need to be more comprehensive and longer in duration to compensate for these age-related deficits.
The anti-aging peptide guide and longevity peptide overview address age-specific protocol considerations. Protocols for women over 40 and the Klotho peptide guide cover emerging approaches to age-related regenerative decline.
Women-specific considerations
Hormonal fluctuations throughout the menstrual cycle and during perimenopause and menopause influence regenerative capacity and peptide response. The peptides for women guide, menopause peptide protocols, and perimenopause guide address these considerations specifically. Hormone balance optimization often precedes or accompanies regenerative protocols for better outcomes.
Autoimmune and inflammatory conditions
Autoimmune conditions create unique challenges for regenerative peptide protocols because the immune system actively attacks the tissues being repaired. The autoimmune peptide guide covers protocol modifications. Specific condition guides address psoriasis, lupus, multiple sclerosis, eczema, and allergies. The immune modulation provided by Thymosin Alpha-1 and KPV becomes particularly important in these contexts.
Pain management during regeneration
Pain is a common companion to the injuries that drive interest in regenerative peptides. The pain management peptide guide covers approaches to managing discomfort while supporting repair. Fibromyalgia and neuropathy represent chronic pain conditions where regenerative peptides may address the underlying cause rather than just masking symptoms.
Building your regenerative peptide protocol
Every effective protocol begins with clear identification of the target tissue, the specific repair goal, and the timeline available. Here is a framework for designing a personalized regenerative approach.
Step 1: identify the target
What tissue needs repair? Tendon, muscle, bone, cartilage, nerve, skin, gut, or a combination? The answer determines which peptides form the core of the protocol. Review the tissue-specific sections above and the relevant condition guides to narrow the compound selection.
Many injuries involve multiple tissue types simultaneously. A shoulder injury might affect the rotator cuff tendon, surrounding muscle, and the labral cartilage. A herniated disc involves both cartilage and nerve compression. In these cases, the protocol needs to address each tissue type, which is another reason why multi-peptide stacks outperform single compounds. Map every tissue involved in the injury before selecting your compounds.
Step 2: assess the severity
A mild muscle strain requires a different approach than a torn ACL. Moderate injuries respond well to the foundational BPC-157 plus TB-500 stack. Severe injuries may require the full GLOW or KLOW combinations with tissue-specific additions.
Step 3: select compounds
Start with the core regenerative peptides (BPC-157 and TB-500 for most injuries) and add tissue-specific compounds based on the target. The complete peptide list provides a comprehensive reference, and the stacking guide addresses combination principles.
Step 4: calculate dosing
Use the peptide calculator for individual compound dosing and the stack calculator for combinations. Weight-based dosing produces more consistent results than flat-dose approaches. The dosing guide covers principles that apply across all protocols.
Step 5: prepare supplies
Before starting, ensure proper supplies are on hand. Bacteriostatic water, appropriate syringes, alcohol swabs, and proper storage containers are all non-negotiable. The water guide covers reconstitution fluid selection. The reconstitution guide walks through preparation step by step.
Step 6: execute and monitor
Consistency matters more than perfection. Maintaining the dosing schedule, proper storage, and sterile technique throughout the protocol produces the best outcomes. Document progress to enable protocol adjustments at reassessment intervals. SeekPeptides members access detailed tracking tools, protocol adjustment guidance, and community support from experienced researchers who have navigated these exact decisions.
Understanding the research landscape
Responsible regenerative peptide use requires honest assessment of where the evidence stands. The preclinical data on these compounds is extensive and promising. Human data remains limited for most of them.
What the research supports
Animal model studies consistently demonstrate that BPC-157, TB-500, and GHK-Cu promote tissue regeneration across multiple tissue types. The mechanisms are well-characterized through molecular biology research. Safety profiles in animal studies are favorable across multiple species, dosing ranges, and administration routes. The molecular pathways involved, PI3K/Akt, VEGFR2, MAPK, NF-kB, and TGF-beta, are well-established pathways in cellular biology with extensive independent validation.
BPC-157 alone has been the subject of hundreds of published preclinical studies spanning gastrointestinal healing, tendon repair, muscle regeneration, bone healing, nerve regeneration, and cardiovascular protection. GHK-Cu gene expression research identifies over 4,000 genes affected by this peptide, with the results replicated across multiple research groups. TB-500 studies in cardiac, muscular, and wound healing models show consistent anti-fibrotic and pro-migratory effects. The breadth and consistency of preclinical evidence across independent laboratories provides strong mechanistic support for the therapeutic potential of these compounds.
What the research does not yet prove
Large-scale human clinical trials are lacking for most regenerative peptides. The three pilot human studies on BPC-157 are a start but do not constitute the robust evidence base needed for definitive clinical claims. Translating animal model results to human outcomes always involves uncertainty.
The responsible approach
Researchers who understand both the promise and the limitations make better decisions. The preclinical evidence supports cautious optimism. The mechanistic data explains why these compounds work as observed. But honest assessment requires acknowledging the evidence gaps that remain. The peptides and proteins report sheet guide helps researchers document their own observations systematically.
For researchers committed to evidence-based peptide protocols, SeekPeptides offers the most comprehensive resource available, with detailed guides, calculators for precise dosing, community support from experienced researchers, and regularly updated content that tracks the evolving evidence base as new studies are published.
Frequently asked questions
What are the most effective regenerative peptides?
BPC-157, TB-500, and GHK-Cu form the core of most regenerative protocols. BPC-157 drives tissue repair through angiogenesis and growth factor upregulation. TB-500 promotes cell migration and reduces fibrosis. GHK-Cu reprograms gene expression across thousands of regenerative pathways. The combination addresses all major pillars of tissue regeneration simultaneously.
Can regenerative peptides help with old injuries?
Chronic injuries respond to regenerative peptides, though typically requiring longer protocol durations than acute injuries. The key challenge with old injuries is established scar tissue and remodeled tissue architecture. TB-500 anti-fibrotic properties help break down existing scar tissue while BPC-157 promotes vascularization of previously under-supplied areas. Results with chronic injuries often become apparent after 4 to 6 weeks of consistent protocol adherence.
How long do regenerative peptide protocols typically last?
Protocol duration depends on the tissue type and injury severity. Muscle injuries often show significant improvement in 4 to 6 weeks. Tendon and ligament injuries require 6 to 12 weeks. Nerve injuries may need 8 to 12 weeks or longer. Cartilage repair follows the slowest timeline due to limited blood supply. The peptide timeline guide provides tissue-specific expectations.
Is it safe to stack multiple regenerative peptides?
Combining peptides that act through different mechanisms is the foundation of effective regenerative protocols. BPC-157, TB-500, and GHK-Cu have complementary rather than redundant mechanisms, which is why they are commonly combined. The multiple peptide guide addresses safety considerations and practical limitations. Starting with fewer compounds and adding based on response is a prudent approach.
Do regenerative peptides work for nerve damage?
BPC-157 promotes nerve growth factor expression and accelerates peripheral nerve healing. Cerebrolysin provides neurotrophic peptide support for both peripheral and central nervous system repair. Emerging research on self-assembling peptide scaffolds shows results comparable to autografts for nerve conduit applications. The neuropathy guide and brain repair guide cover neural regeneration protocols in detail.
What is the difference between regenerative peptides and growth hormone peptides?
Growth hormone secretagogues like Sermorelin, Ipamorelin, and CJC-1295 increase systemic growth hormone output. This provides general anabolic support but lacks the targeted tissue repair mechanisms of regenerative peptides. BPC-157, TB-500, and GHK-Cu act directly at injury sites through specific molecular pathways. Some protocols combine both categories for simultaneous systemic optimization and local tissue repair. The Ipamorelin versus CJC-1295 comparison covers the growth hormone peptide options.
How should regenerative peptides be stored?
Unreconstituted peptide powder should be stored in a freezer for maximum longevity. After reconstitution with bacteriostatic water, peptides require refrigeration at 2 to 8 degrees Celsius and should be used within the timeframes specified in the storage guide. Temperature excursions degrade peptides and reduce effectiveness.
Are regenerative peptides legal?
Regenerative peptides are available for research purposes in most jurisdictions. The FDA has not approved them for medical treatment. The legality guide and regulation updates provide current information on the evolving regulatory framework. Researchers should understand the legal status in their specific jurisdiction before proceeding.
External resources
Local and systemic peptide therapies for soft tissue regeneration: a narrative review (PMC)
Therapeutic peptides in orthopaedics: applications, challenges, and future directions (PMC)
Peptide-based biomaterials for bone and cartilage regeneration (Biomedicines)
In case I do not see you, good afternoon, good evening, and good night. May your tissues stay resilient, your recovery stay swift, and your protocols stay precise.



