Feb 4, 2026
Deep inside prostate tissue, a cascade of cellular events determines whether a man spends his later decades in comfort or in a urologist waiting room. Peptides sit at the center of this cascade. They regulate inflammation, modulate hormone signaling, and direct the repair processes that keep prostatic tissue functioning properly. When those peptide signals break down, problems follow. Enlargement. Inflammation. Dysfunction. And for millions of men over forty, those problems arrive sooner than expected.
The numbers are sobering. Benign prostatic hyperplasia affects roughly 50% of men by age sixty, and that number climbs to nearly 90% by the ninth decade. Those are not distant statistics for someone else. They describe the biological reality facing every man who ages. Traditional approaches to hormone balance and prostate management rely on pharmaceuticals that often carry significant side effects, from sexual dysfunction to cardiovascular risks. This reality has driven a growing number of researchers toward peptide-based solutions that work with the body rather than against it.
But peptides for prostate health is not a simple topic. Different peptides target different mechanisms. Some reduce inflammation through NF-kB suppression. Others modulate testosterone and dihydrotestosterone at the receptor level. Still others work through bioregulatory pathways that restore protein synthesis in aging prostate cells. Understanding which peptide does what, and how they interact with existing prostate conditions, requires more than surface-level knowledge.
This guide covers every peptide with meaningful research connections to prostate health. From Khavinson bioregulator peptides like Libidon that target the prostate directly, to anti-inflammatory peptides that address the chronic inflammation driving BPH progression, to advanced immunotherapy peptides showing promise against prostate cancer itself. SeekPeptides has compiled the research, the protocols, and the practical considerations that matter for men serious about protecting their prostate health through peptide science.
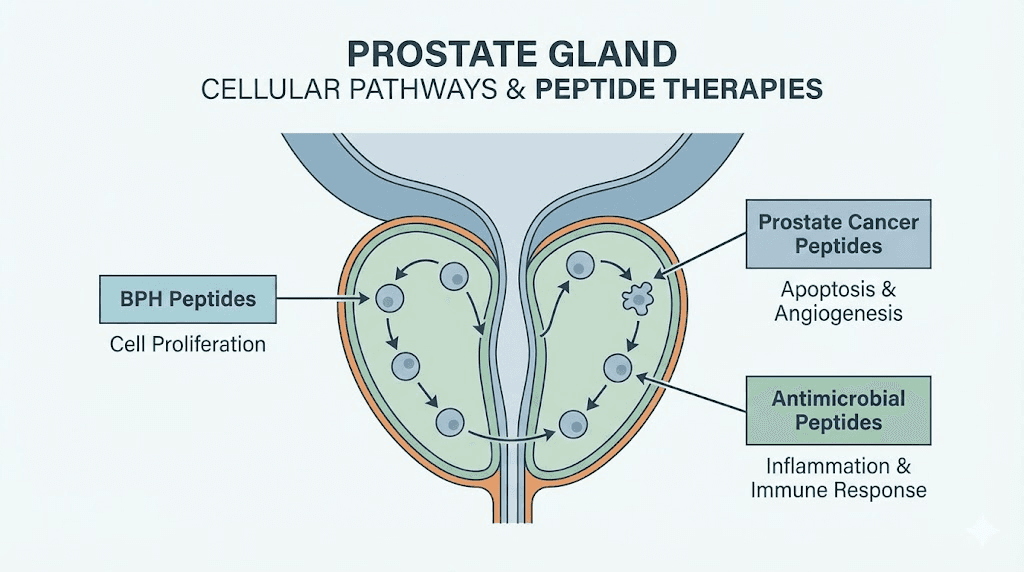
Understanding prostate health and why peptides matter
The prostate gland sits just below the bladder, wrapped around the urethra like a ring around a finger. It is small. About the size of a walnut in younger men. But it grows. And that growth drives the most common prostate condition men face as they age.
BPH, or benign prostatic hyperplasia, involves the proliferation of both stromal and epithelial cells in the prostate transition zone. This is the region that surrounds the urethra. As cells multiply, the tissue compresses the urethra and obstructs bladder outflow. The result is a collection of lower urinary tract symptoms that range from mildly annoying to significantly life-disrupting. Frequent urination. Weak stream. Incomplete emptying. Nighttime urgency that fragments sleep.
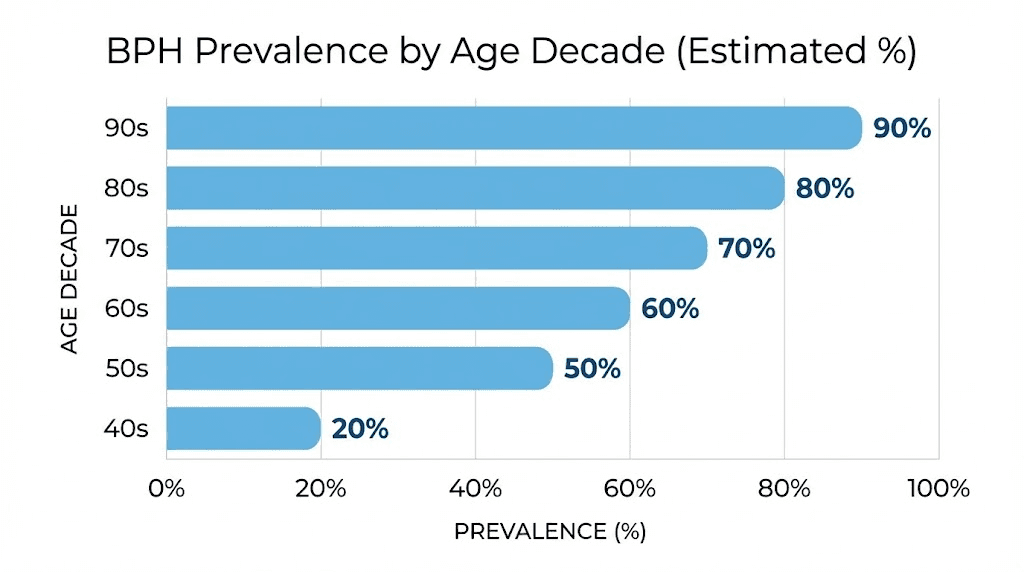
Histological evidence of BPH appears in about 8% of men between ages 31 and 40. By the sixth decade, that number reaches 50-60%. By age seventy and beyond, autopsy studies show BPH tissue in 80-90% of men. These are not numbers that suggest a rare condition. This is the default aging trajectory for the male prostate.
What drives this growth? Several interacting factors. Testosterone converts to dihydrotestosterone through 5-alpha reductase, and DHT is the primary hormonal driver of prostatic cell proliferation. But inflammation plays an equally critical role. Chronic prostatic inflammation activates NF-kB pathways, releases TNF-alpha and IL-6, and creates a microenvironment that promotes continuous cell growth. Research published in npj Aging identified that aging-related changes in GH-RH and LH-RH biology promote BPH through both hormonal and inflammatory processes simultaneously.
This dual mechanism explains why single-target pharmaceutical approaches often fall short. 5-alpha reductase inhibitors like finasteride address the DHT pathway but leave inflammation unchecked. Alpha-blockers relax smooth muscle but do nothing about the underlying growth. And both carry side effects, including erectile dysfunction, decreased libido, and cardiovascular concerns, that make long-term compliance difficult.
Peptides offer a fundamentally different approach. Rather than blocking a single pathway, they can modulate multiple mechanisms simultaneously. Anti-inflammatory peptides suppress NF-kB. Immune-modulating peptides restore proper immune surveillance. Bioregulator peptides restore protein synthesis in aging tissue. And hormone-modulating peptides can influence the testosterone-DHT axis without the blunt-force suppression of pharmaceutical options.
The inflammation connection most men miss
Chronic prostatitis, the inflammatory counterpart to BPH, affects an estimated 10-15% of men at some point in their lives. But subclinical prostatic inflammation, the kind that does not produce obvious symptoms, is far more common. Autopsy and biopsy studies consistently find inflammatory infiltrates in prostate tissue from men who never reported prostate symptoms.
This silent inflammation matters because it drives proliferation. Inflammatory cytokines like IL-6 and TNF-alpha stimulate prostatic stromal and epithelial cell growth. They activate growth factor pathways. They create oxidative stress that damages DNA and promotes abnormal cell division. Over years and decades, this chronic low-grade inflammation transforms normal prostate tissue into the enlarged, obstructing mass that sends men to the bathroom six times a night.
Understanding this inflammation connection is essential for understanding why anti-inflammatory peptides represent such a promising approach to prostate health. They target the root cause, not just the symptoms.
Risk factors that accelerate prostate problems
Several modifiable and non-modifiable factors influence prostate health trajectory. Family history of BPH or prostate cancer significantly increases individual risk. Obesity shows a consistently positive association with prostate volume, meaning greater body fat correlates with larger prostate size. Type 2 diabetes, insufficient physical activity, and hormonal imbalances all contribute to accelerated prostate growth.
Studies have increasingly observed that modifiable lifestyle factors substantially influence the natural history of BPH. This is encouraging. It means prostate health is not entirely predetermined by genetics. Strategic interventions, including peptide protocols, exercise, diet modification, and stress management, can meaningfully alter the trajectory.
Bioregulator peptides: targeting the prostate directly
Among all peptide approaches to prostate health, bioregulator peptides stand apart because they were specifically designed for prostate tissue. Developed through decades of research at the St. Petersburg Institute of Bioregulation and Gerontology under Professor Vladimir Khavinson, these peptides represent a fundamentally different paradigm from general anti-inflammatory or hormone-modulating approaches.
Libidon: the prostate-specific bioregulator
Libidon is a peptide complex containing low-molecular-weight peptide fractions isolated from the prostate gland of young, sexually mature calves. These peptides have molecular weights up to 5000 Daltons and are designed to interact directly with prostate cell DNA to restore normal protein synthesis.
The mechanism is elegant in its specificity. Khavinson research identified that each organ and tissue uses highly specific short-chain peptides as shortcuts to initiate protein synthesis. When those peptides become deficient due to aging or disease, protein synthesis in that tissue slows. Cellular repair declines. Function deteriorates. Libidon restores the peptide signals specific to prostate tissue, effectively resupplying what aging has depleted.
Clinical evidence supports this approach. A study conducted at the Medical Center of the St. Petersburg Institute of Bioregulation and Gerontology evaluated 48 patients aged 38-65 with diagnosed BPH and chronic prostatitis. The clinical study established that Libidon demonstrated efficiency in complex treatment of patients with both conditions, supporting restoration of sexual system function in men affected by disease, extreme environmental factors, nutritional deficiency, and aging.
The specific benefits documented include reduction of BPH symptoms, decreased prostatic inflammation, improvement in urinary function, support for erectile function affected by prostatic conditions, and enhanced overall prostate cellular health. Additional research published in the International Journal of Molecular Sciences showed that Khavinson peptides including Libidon can lower levels of TNF and IL-6, two major inflammatory cytokines involved in prostate pathology. The peptides also reduced monocyte adhesion to inflamed blood vessel cells, suggesting a vascular protective benefit relevant to cardiovascular health as well.
Typical protocol: 1-2 capsules, 1-2 times daily with meals, for 30 days. Repeat courses every 4-6 months. For men over 50, three courses per year provides more comprehensive support.
Safety data from decades of Russian clinical use shows remarkable tolerability. Natural peptide bioregulators have no known immunogenic or mutagenic properties. Their molecular weight is far below the threshold for prion contamination or foreign DNA content, making them exceptionally clean products from a safety standpoint.
Other prostate-relevant bioregulators
Libidon does not work in isolation. The bioregulator system includes complementary peptides that support related functions.
Testagen targets the testes and supports healthy testosterone production. Since testosterone metabolism directly influences prostate health through DHT conversion, maintaining optimal testosterone function matters. Testagen helps ensure the upstream hormonal signals remain balanced, reducing the likelihood of compensatory DHT overproduction that drives prostatic growth.
Cortagen supports the adrenal cortex. Adrenal androgens contribute to prostatic DHT levels, and chronic stress-driven adrenal dysfunction can alter the hormonal milieu affecting the prostate. Cortagen helps maintain normal adrenal peptide signaling.
Vesilute targets the bladder. Since BPH primarily manifests through lower urinary tract symptoms affecting bladder function, supporting bladder tissue alongside prostate tissue creates a more complete approach. Vesilute restores peptide-mediated protein synthesis in bladder wall cells, helping maintain detrusor muscle function that BPH-related obstruction can compromise over time.
For a comprehensive peptide stacking approach to male reproductive health, the combination of Libidon, Testagen, and Glandokort (adrenal bioregulator) has been used clinically in Russian gerontological practice. SeekPeptides members can access detailed stacking protocols and dosing schedules for these bioregulator combinations.
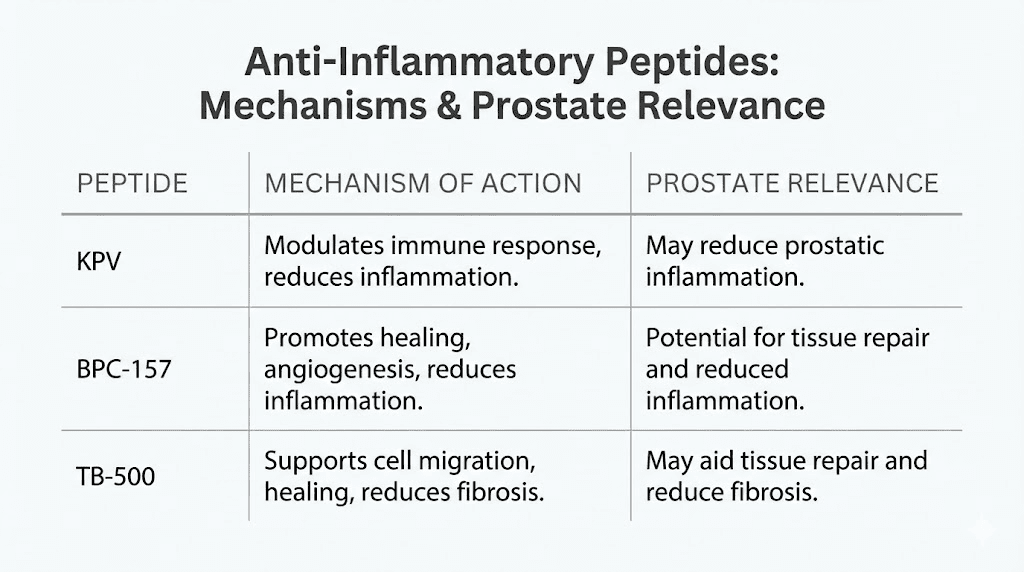
Anti-inflammatory peptides for prostate health
If bioregulators represent the targeted approach, anti-inflammatory peptides represent the foundational one. Chronic inflammation is not just a symptom of prostate problems. It is a cause. Addressing it may be the single most impactful intervention for long-term prostate health.
KPV: the inflammation master switch
KPV is a tripeptide fragment derived from alpha-melanocyte-stimulating hormone. Three amino acids: Lysine, Proline, Valine. Small in size. Enormous in anti-inflammatory impact.
KPV works primarily through MC1R and MC3R receptors on immune cells and inflamed tissues. Activation of these receptors reduces the release of TNF-alpha, IL-1-beta, and IL-6, the exact inflammatory cytokines implicated in prostatic inflammation and BPH progression. More importantly, KPV suppresses NF-kB activation, the master inflammatory transcription factor that drives expression of inflammatory genes and regulates the survival and differentiation of inflammatory T cells.
A 2012 study published in the International Journal of Physiology, Pathophysiology and Pharmacology confirmed this NF-kB suppression mechanism. And what makes KPV particularly interesting for systemic use is that it retains all the anti-inflammatory benefits of full-length alpha-MSH without stimulating melanocyte receptors. No pigmentation changes. No hormonal disruption. Just targeted inflammation control.
No published studies have directly investigated KPV for prostate inflammation specifically. That is an honest assessment. But the inflammatory pathways KPV modulates, specifically NF-kB, TNF-alpha, and IL-6, are the same pathways driving chronic prostatitis and BPH progression. The mechanistic rationale is strong even if prostate-specific clinical data does not yet exist.
For men using KPV as part of a prostate health protocol, the KPV dosage guide provides detailed information on administration routes and timing. The KPV for inflammation guide covers the broader anti-inflammatory applications.
BPC-157: tissue repair and vascular support
BPC-157, the Body Protection Compound, is a 15-amino-acid peptide derived from human gastric juice. It is perhaps the most widely researched peptide for tissue repair, and its mechanisms have relevant implications for prostate health.
BPC-157 activates VEGFR2 and nitric oxide synthesis through the Akt-eNOS axis, promoting angiogenesis and fibroblast activity. It engages ERK1/2 signaling. It facilitates endothelial repair. And it exerts anti-inflammatory effects across multiple tissue types. In safety studies, BPC-157 showed no histologic toxicity to prostate tissue across a wide range of doses from 6 micrograms per kilogram to 20 milligrams per kilogram. The prostate was specifically evaluated and showed no damage.
The direct evidence for BPC-157 in prostate conditions is preliminary. No peer-reviewed clinical trials have specifically investigated BPC-157 for BPH or prostatitis. However, its general anti-inflammatory and tissue-regenerative mechanisms are theoretically applicable to prostatic tissue. BPC-157 increases blood flow to damaged areas, promotes new blood vessel formation, encourages cell survival and growth, facilitates cell migration to injury sites, and balances inflammatory responses.
One consideration worth noting: the same growth-promoting pathways that make BPC-157 effective for tissue repair, particularly FAK and paxillin signaling, are also pathways that cancer cells can exploit. No clinical trial has evaluated whether BPC-157 could promote tumor growth or metastasis in prostate tissue, and no such outcomes have been reported. But for men with active prostate cancer or elevated PSA, this theoretical concern warrants discussion with a healthcare provider before use.
For detailed BPC-157 dosing protocols, reconstitution instructions, and stacking considerations, see the complete guide. The BPC-157 vs TB-500 comparison helps researchers understand which tissue repair peptide may be more appropriate for their specific situation.
TB-500: complementary tissue repair
TB-500, the synthetic analog of Thymosin Beta-4, complements BPC-157 through different but overlapping mechanisms. TB-500 regulates actin-cytoskeleton interactions, promotes cell migration, supports angiogenesis, and facilitates extracellular matrix remodeling.
For prostate health specifically, TB-500 anti-inflammatory properties operate through different pathways than BPC-157. A 2021 retrospective study compared knee injections using BPC-157 alone versus a BPC-157 and TB-500 combination, finding that the combination produced superior outcomes. This combinatorial benefit may translate to other tissue types where inflammation and repair both need addressing.
The tissue repair peptides guide covers how BPC-157 and TB-500 work together in greater detail.
Hormone-modulating peptides and prostate health
Hormones drive prostate growth. That is not debatable. Testosterone, its conversion to dihydrotestosterone through 5-alpha reductase, and the sensitivity of prostatic androgen receptors collectively determine how much the prostate grows and how fast. Peptides that influence this hormonal axis can meaningfully impact prostate health outcomes.
Triptorelin: the GnRH agonist
Triptorelin is a synthetic GnRH agonist with established clinical applications in prostate conditions. It is one of the few peptides in this discussion with extensive human clinical data for prostate-specific use.
The mechanism involves initial stimulation of the pituitary gland to release luteinizing hormone and follicle-stimulating hormone. With repeated administration, this stimulation desensitizes the pituitary, causing LH and FSH production to drop. The downstream effect is a significant reduction in testosterone and, consequently, dihydrotestosterone levels.
Triptorelin (marketed as Decapeptyl and Trelstar) is FDA-approved for advanced prostate cancer therapy. Phase 3 studies comparing LHRH agonists like triptorelin to surgical castration demonstrated no survival difference between the two approaches. Multiple Phase 3 studies have confirmed that all GnRH agonist preparations have similar efficacy in reducing testosterone to castrate levels.
For BPH specifically, triptorelin research is ongoing but promising. By reducing testosterone and DHT levels, triptorelin may help manage or even prevent BPH progression. However, the side effect profile of full testosterone suppression is significant: impotence, loss of libido, hot flashes, anemia, weight gain, hair loss, osteopenia, and emerging evidence linking androgen deprivation therapy to diabetes and cardiovascular disease.
This is not a peptide for casual prostate health optimization. Triptorelin is a medical intervention with serious physiological consequences. Its relevance here is primarily for men with diagnosed prostate cancer under medical supervision, not for general prostate health maintenance. The peptides for hormone balance guide discusses less aggressive approaches to hormonal optimization.
GH-RH antagonists: a newer approach
Growth hormone-releasing hormone antagonists represent a more nuanced approach to hormone modulation for prostate health. A comprehensive review published in npj Aging in 2025 highlighted the therapeutic promise of GH-RH antagonists for BPH.
In preclinical studies, GH-RH antagonists reduced prostate volume, improved lower urinary tract symptoms, and modulated inflammation mediated by NF-kB and IGF-I. The dual action on both inflammation and growth factor signaling makes this approach potentially more targeted than pure androgen suppression.
Clinical trials are needed to validate these findings in humans, but the preclinical data suggests GH-RH antagonism could become an important peptide-based strategy for BPH management. Unlike triptorelin and other GnRH agonists, GH-RH antagonists do not directly suppress testosterone production, potentially avoiding the severe hormonal side effects associated with androgen deprivation.
Kisspeptin: upstream hormone regulation
Kisspeptin plays a master regulatory role in the hypothalamic-pituitary-gonadal axis. It stimulates GnRH release, which in turn drives the entire downstream cascade of LH, FSH, testosterone, and DHT production. Research into kisspeptin modulation offers potential for fine-tuning hormonal output rather than the all-or-nothing approach of GnRH agonists.
For prostate health, kisspeptin research is still in early stages. But understanding its role helps contextualize how peptide-based hormonal interventions might evolve beyond simple suppression toward more sophisticated regulation. The peptides for testosterone guide and TRT peptide comparison provide additional context on peptide-based hormonal optimization approaches.
Enclomiphene: selective estrogen receptor modulation
Enclomiphene deserves mention in the prostate health context even though it is technically a small molecule rather than a peptide. It works by blocking estrogen feedback at the hypothalamus, increasing GnRH pulsatility, and boosting LH-driven testosterone production without shutting down the HPG axis. For men seeking to optimize testosterone while minimizing prostatic DHT conversion, enclomiphene combined with appropriate monitoring represents an option worth understanding.
Immune-modulating peptides and prostate cancer prevention
Prostate cancer is the second most common cancer in men worldwide. The progression from normal prostate tissue to benign hyperplasia to potential malignancy involves immune system failures at multiple levels. Peptides that restore proper immune surveillance may offer protective benefits that extend beyond simple symptom management.
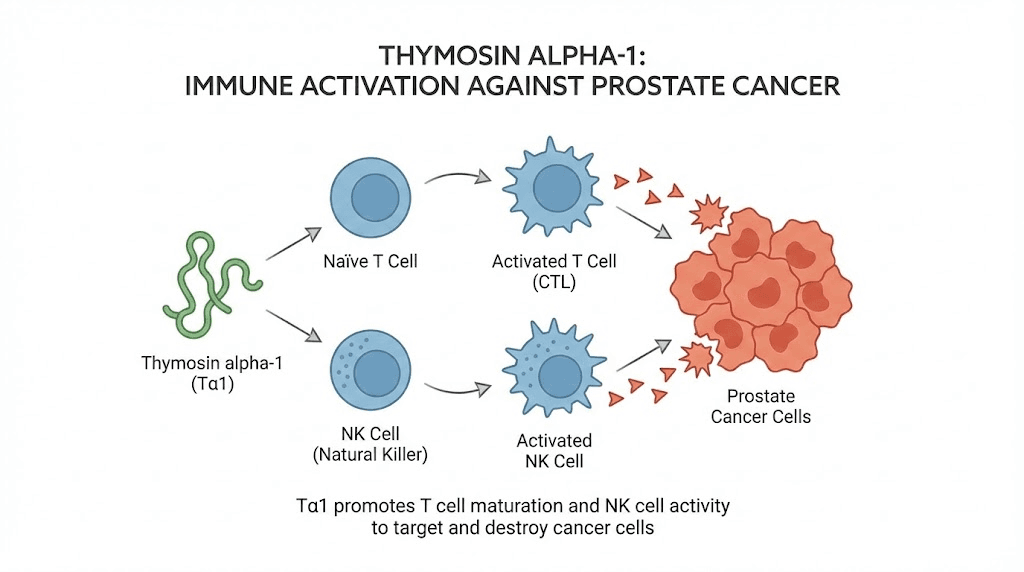
Thymosin alpha-1: restoring immune surveillance
Thymosin alpha-1 is a 28-amino-acid immunomodulating polypeptide originally isolated from thymic tissue. It has the most robust clinical evidence of any peptide discussed in this guide for cancer-related applications, including prostate cancer specifically.
The mechanism involves Toll-like receptor activation, specifically TLR2 and TLR9, in myeloid and plasmacytoid dendritic cells. This activation leads to differentiation of dendritic cells and T cells, initiation of interferon-gamma and interleukin-2 production, upregulation of CD4+ and CD8+ T cell production, and restoration of natural killer cell activity.
A particularly relevant study published on PubMed examined the combination of zoledronic acid and thymosin alpha-1 against prostate cancer. The findings were significant. The combination synergistically suppressed prostate cancer allograft tumor progression. It enhanced tumor inflammation and cytotoxic T-cell infiltration. It increased CD3+ and CD8+ T cell infiltration into prostate tumors. And it upregulated expression of cytotoxic effector molecules like granzyme B and perforin, the weapons immune cells use to destroy cancer cells.
Clinical data from the study showed quantifiable reduction in tumor area and serum PSA and PAP levels in prostate cancer patients receiving androgen deprivation therapy combined with zoledronic acid and thymosin alpha-1.
The synthetic form, thymalfasin (marketed as Zadaxin), received FDA orphan drug approval for treatment of malignant melanoma, chronic hepatitis B, DiGeorge anomaly, and hepatocellular carcinoma. Its well-established safety profile and demonstrated anticancer activity make it one of the most credible peptide candidates for prostate cancer adjunctive therapy.
For men concerned about longevity and cancer prevention, thymosin alpha-1 ability to restore age-declining immune function addresses a fundamental vulnerability. Glandular degeneration with aging leads to reduced thymic peptide output, resulting in an increased risk of cancer and age-related diseases. Supplementing with thymosin alpha-1 may help compensate for this natural decline in immune function.
Peptide-based immunotherapy: the research frontier
A major 2025 review published in Frontiers in Pharmacology explored peptide-based immunotherapy approaches for prostate cancer. The review identified several promising peptide strategies targeting the immunosuppressive tumor microenvironment that makes prostate cancer particularly resistant to standard immunotherapy.
Key approaches include stapled peptide Rh-2025u, which disrupts androgen receptor interactions with Filamin A, potentially overcoming androgen-driven cancer progression. Peptide 5a targets TGF-beta activation, inhibiting an immunosuppressive cytokine that helps prostate tumors evade immune detection. And M2pep-based therapies reprogram tumor-associated macrophages from cancer-supportive to cancer-fighting phenotypes.
These are not consumer-available peptides. They represent the cutting edge of research. But understanding where the science is heading helps contextualize why peptide approaches to prostate health are gaining momentum in the research community. The regulatory and legal landscape for peptides continues to evolve as more clinical evidence accumulates.
Epithalon: telomere and cellular aging
Epithalon is a tetrapeptide studied primarily for its anti-aging properties through telomerase activation and melatonin regulation. Its relevance to prostate health comes through its ability to enhance immune function and reduce oxidative damage at the cellular level.
Epithalon ability to regulate telomere length promotes cellular regeneration and longevity. For prostate cells specifically, this could potentially slow the aging process that makes prostatic tissue vulnerable to hyperplasia and malignant transformation. Combined with its documented immune-enhancing effects, Epithalon represents a systemic approach to the cellular aging that underlies prostate pathology.
GRP antagonists: a promising peptide frontier for BPH
Gastrin-releasing peptide is a potent growth factor in many proliferative conditions, and BPH is fundamentally a proliferative disorder. Research into GRP antagonists represents one of the most direct peptide-based approaches to reducing prostate size.
The peptide RC-3940-II showed particularly impressive preclinical results. It inhibited the proliferation of BPH-1 human prostate epithelial cells and WPMY-1 prostate stromal cells in a dose-dependent manner. It reduced prostatic cell volume in vitro. And in animal models, six weeks of treatment produced measurable prostate shrinkage: a 15.9% decline at 25 micrograms per day and an 18.4% reduction at 50 micrograms per day.
Those numbers may seem modest. They are not. A nearly 20% reduction in prostate volume translates to meaningful improvement in urinary symptoms for men with BPH. And the mechanism, direct inhibition of prostatic GRP receptors, is fundamentally different from hormonal approaches. GRP antagonists reduce prostate cell volume and lower prostate weight through direct antiproliferative effects, not through testosterone suppression.
Researchers concluded that GRP antagonists should be considered for further development as therapy for BPH. As these peptides advance through clinical development, they could offer a prostate-specific treatment option that avoids the systemic hormonal side effects of current pharmaceutical approaches.
GV1001: from cancer vaccine to BPH treatment
GV1001 is a cell-penetrating peptide originally developed as a cancer vaccine targeting human telomerase reverse transcriptase. Research has unexpectedly revealed its potential for BPH treatment through a completely different mechanism.
In laboratory studies, GV1001 effectively suppressed proliferation of prostatic stromal myofibroblasts and prostatic epithelial cells treated with dihydrotestosterone. In animal models of BPH, the characteristic hyper-proliferation, irregular glandular shapes, and inflammatory immune cell infiltration were all ameliorated by GV1001 treatment. The peptide reduced prostatic hypertrophy and inhibited cell proliferation, expression of Ki67 (a proliferation marker), PCNA (proliferating cell nuclear antigen), and prostate-specific antigen.
The finding that GV1001 interacts with the androgen receptor to inhibit prostate cell proliferation by regulating epithelial-mesenchymal transition represents a novel mechanism that could complement existing BPH treatments. This discovery illustrates how peptide research often reveals unexpected therapeutic applications.
Practical peptide protocols for prostate health
Research is valuable. Practical application is what changes outcomes. Here are evidence-informed protocol frameworks for different prostate health goals. These are research-based starting points that should be adapted based on individual response, existing conditions, and guidance from a qualified healthcare provider.
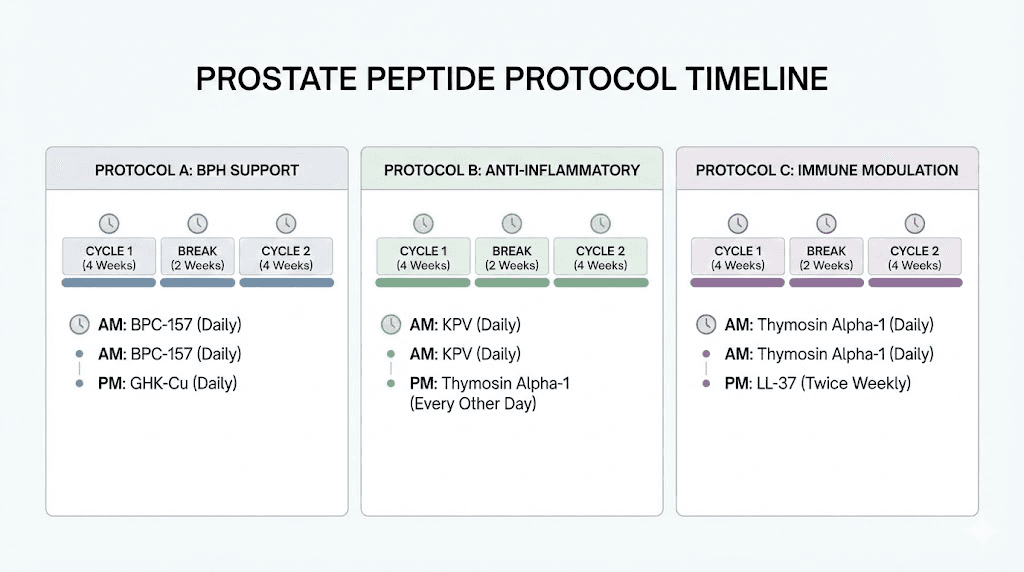
Protocol 1: prostate maintenance (men 40+, no symptoms)
Goal: Preventive support for prostate health and reduction of age-related decline.
Primary approach:
Libidon: 1-2 capsules daily with meals, 30-day course every 6 months
Testagen: 1-2 capsules daily, 30-day course bi-annually (supports healthy testosterone metabolism)
Anti-inflammatory support:
KPV: Per dosing guide recommendations for systemic inflammation management
Monitoring: Annual PSA testing, digital rectal exam per standard medical recommendations. Track peptide response timelines and adjust course frequency based on results.
Expected timeline: Bioregulator effects accumulate over multiple courses. Initial changes in urinary function may be noticed within the first 30-day course, with progressive improvements over 6-12 months of cyclical use.
Protocol 2: active BPH management (diagnosed BPH, symptomatic)
Goal: Reduce symptoms, support prostate tissue health, address underlying inflammation.
Primary approach:
Libidon: 2 capsules twice daily with meals, 30-day course. Repeat every 4 months (increased frequency for active condition)
Vesilute: 1-2 capsules daily, 30-day course (supports bladder function affected by BPH obstruction)
Anti-inflammatory stack:
KPV: For NF-kB suppression and cytokine reduction
BPC-157: For tissue repair and vascular support. Consult dosing guide for appropriate administration. Use the reconstitution calculator for accurate preparation
Hormonal support:
Testagen: For healthy testosterone metabolism (reducing compensatory DHT overproduction)
Important: This protocol is meant to complement, not replace, medical management. Men with diagnosed BPH should maintain their relationship with a urologist and continue any prescribed medications. Peptide safety considerations apply, especially regarding interactions with existing medications.
Protocol 3: immune optimization for cancer prevention
Goal: Enhance immune surveillance, reduce oxidative damage, support cellular health.
Primary approach:
Thymosin alpha-1: Research protocols typically involve subcutaneous administration. The synthetic form thymalfasin has the most clinical data
Epithalon: Per dosing guide for telomere and immune support
Foundational support:
Libidon: Prostate-specific cellular maintenance
KPV: Chronic inflammation reduction
Critical note: Men with active prostate cancer or elevated PSA must work with their oncologist before adding any peptide to their protocol. Immune-modulating peptides can theoretically interact with cancer treatments. The KPV cancer research guide discusses the complex relationship between anti-inflammatory peptides and cancer biology.
SeekPeptides members access detailed protocol builders, including weight-based calculators and expert-reviewed dosing guides that account for individual factors most general resources overlook. The peptide calculator helps determine exact dosing based on body weight and peptide concentration.
Understanding PSA: what peptide researchers need to know
Prostate-specific antigen is more than a number on a lab report. It is a serine protease enzyme with active roles in prostate biology that peptide researchers should understand.
PSA cleaves insulin-like growth factor binding proteins, releasing IGF-1, a growth factor involved in cell proliferation. It cleaves extracellular matrix components like fibronectin and laminin. It modifies latent TGF-beta-2. And research shows PSA itself has immunosuppressive properties, suppressing lymphocyte proliferation in a dose-dependent manner. This means elevated PSA is not just a marker of prostate problems. It may actively contribute to the immunosuppressive environment that allows prostate cancer to develop.
For peptide researchers, several PSA-related considerations matter.
First, inflammation confounds PSA readings. Prostatitis, urinary tract infections, and even vigorous exercise can transiently elevate PSA. When evaluating peptide protocol effectiveness through PSA monitoring, baseline readings should be established during periods of low inflammation, and changes should be interpreted in context.
Second, some peptides may influence PSA levels through their anti-inflammatory effects. A reduction in PSA after starting an anti-inflammatory peptide protocol might reflect reduced prostatic inflammation rather than actual changes in prostate size or cancer risk. This is clinically relevant and should be discussed with monitoring physicians.
Third, the GV1001 research showed direct reduction of PSA expression in prostate tissue, suggesting some peptides can influence PSA production through mechanisms beyond simple inflammation reduction. The understanding of peptide biomarkers continues to evolve.
The role of oxidative stress and how peptides address it
Oxidative stress accelerates every aspect of prostate pathology. It damages DNA, promoting mutations that can lead to cancer. It activates inflammatory pathways that drive BPH. It impairs the cellular repair mechanisms that keep prostate tissue healthy. And it increases with age, creating a progressively more hostile environment for prostate cells.
Several peptides address oxidative stress through different mechanisms.
Thymosin alpha-1 amplifies the activity of catalase, superoxide dismutase, and glutathione peroxidase, the body primary antioxidant enzymes. By enhancing endogenous antioxidant defenses rather than simply providing exogenous antioxidants, thymosin alpha-1 creates a more sustainable oxidative stress management approach.
SS-31 (Elamipretide) targets mitochondrial function directly. It concentrates in the inner mitochondrial membrane, stabilizing cardiolipin and optimizing electron transport chain function. Since mitochondrial dysfunction is a primary source of reactive oxygen species in aging tissues, including prostate tissue, SS-31 addresses oxidative stress at its source. The SS-31 guide covers its broader applications.
MOTS-c, a mitochondria-derived peptide, improves metabolic function and reduces oxidative stress through AMPK activation. Given the strong association between metabolic syndrome, obesity, and BPH, MOTS-c metabolic benefits may indirectly support prostate health. The MOTS-c dosing guide provides protocol details.
Epithalon reduces oxidative damage through telomerase activation and melatonin regulation. Melatonin itself is a potent antioxidant, and Epithalon capacity to normalize melatonin production supports nighttime antioxidant activity when cellular repair processes are most active.
For men building a comprehensive prostate health peptide stack, including an oxidative stress component alongside anti-inflammatory and bioregulatory peptides creates a multi-layered approach to prostate protection.
Peptides, DHT, and the 5-alpha reductase question
Dihydrotestosterone is the primary hormonal driver of prostate growth. Testosterone converts to DHT through the enzyme 5-alpha reductase, and DHT has roughly five times the binding affinity for androgen receptors compared to testosterone. This amplified signal drives the epithelial and stromal cell proliferation that characterizes BPH.
Pharmaceutical 5-alpha reductase inhibitors like finasteride and dutasteride effectively reduce DHT levels and prostate volume. But they come with costs. Sexual side effects affect a meaningful percentage of users. Post-finasteride syndrome, involving persistent sexual dysfunction even after discontinuation, remains controversial but reported. And these drugs reduce PSA levels by approximately 50%, complicating cancer screening interpretation.
Can peptides influence the testosterone-DHT axis without these drawbacks? The evidence is nuanced.
Bioregulator peptides like Testagen support normal testicular function, which may help maintain a healthy testosterone-to-DHT ratio by preventing the compensatory testosterone overproduction that can result from declining testicular function. When the testes produce appropriate testosterone levels, the drive toward excessive DHT conversion may be reduced.
GV1001 research demonstrated direct interaction with the androgen receptor, inhibiting prostate cell proliferation through regulation of epithelial-mesenchymal transition. This represents a peptide that modulates androgen signaling at the receptor level rather than by suppressing hormone production.
For men exploring peptides versus traditional testosterone approaches, understanding these hormonal interactions is critical. The testosterone peptide guide covers the broader topic of peptide-based hormonal optimization.
Lifestyle factors that amplify peptide effectiveness
Peptides do not work in a vacuum. Lifestyle factors significantly influence both prostate health trajectories and peptide protocol outcomes.
Exercise and prostate health
Physical activity reduces BPH risk through multiple mechanisms. It improves insulin sensitivity, reducing IGF-1 driven prostatic growth. It reduces systemic inflammation. It helps maintain healthy body composition, addressing the obesity-prostate volume link. And it supports the hormonal balance that peptide protocols aim to optimize.
Research consistently shows that moderate to vigorous physical activity reduces the risk of BPH symptom progression. For men on peptide protocols, regular exercise likely amplifies the anti-inflammatory and metabolic benefits of their peptide stack.
Dietary considerations
Lycopene, found primarily in tomatoes, shows specific prostate benefits. A study found that 15 mg daily for six months produced a statistically significant reduction in PSA levels compared to placebo, along with symptom improvement in BPH patients.
Zinc plays a critical role in prostate health. The prostate accumulates zinc at concentrations higher than nearly any other tissue in the body, and zinc deficiency has been linked to increased BPH risk. Adequate zinc intake supports the cellular processes that bioregulator peptides aim to restore.
Cruciferous vegetables provide sulforaphane, which activates Nrf2 antioxidant pathways. This complements the antioxidant effects of peptides like thymosin alpha-1 and SS-31 by supporting different but synergistic detoxification pathways.
Managing body composition through diet and exercise directly addresses the obesity-prostate connection. For men using fat-burning peptides alongside prostate-targeted protocols, the weight loss benefits compound with the prostate-specific benefits.
Stress management and sleep
Chronic stress elevates cortisol, which disrupts the HPG axis and alters adrenal androgen production. Both effects can influence prostatic DHT levels. Stress also elevates systemic inflammation through cortisol-mediated immune dysregulation.
Sleep quality directly affects testosterone production, growth hormone release, and immune function, all factors relevant to prostate health. Pineal peptides and DSIP can support sleep quality, but they work best when combined with good sleep hygiene practices.
Safety considerations for prostate-targeted peptide use
Safety deserves dedicated attention when discussing peptides for prostate health, particularly because prostate conditions require ongoing medical monitoring that peptide use should complement, never replace.
The cancer concern
Any substance that promotes cell growth or tissue repair raises theoretical questions about cancer risk. This applies to BPC-157, growth hormone secretagogues, and even bioregulator peptides that enhance protein synthesis. The concern is logical: if a peptide helps cells grow and repair, could it also help cancer cells grow?
The honest answer is that long-term cancer safety data for most peptides does not exist. BPC-157 activates FAK and paxillin pathways that cancer cells can exploit, though no clinical cases of peptide-induced cancer have been reported. Thymosin alpha-1, conversely, has documented anti-cancer activity through immune enhancement.
For men with active prostate cancer, elevated PSA, a family history of prostate cancer, or abnormal screening results, the precautionary approach is clear: consult with an oncologist before starting any peptide protocol. Immune-enhancing peptides like thymosin alpha-1 may be appropriate adjunctive therapy, but growth-promoting peptides warrant careful consideration.
For men with no cancer concerns and normal PSA, the risk profile is different. Bioregulator peptides like Libidon have decades of safety data from Russian clinical use. Anti-inflammatory peptides like KPV operate through pathways that reduce, rather than promote, the inflammatory conditions associated with cancer development.
Interactions with prostate medications
Men taking finasteride, dutasteride, tamsulosin, or other prostate medications should be aware of potential interactions. No formal drug-peptide interaction studies exist for most of these combinations. General considerations include the following.
Anti-inflammatory peptides that reduce PSA levels through inflammation reduction could mask PSA changes that urologists use for monitoring. Men on KPV or BPC-157 should inform their urologist about their peptide use so PSA results can be interpreted accurately.
Hormone-modulating peptides could theoretically interact with 5-alpha reductase inhibitors or GnRH agonists. Adding testosterone-supporting peptides while on androgen-suppressive medications could create conflicting signals. The peptide safety guide discusses general interaction considerations.
Saw palmetto, which many men take for BPH, acts similarly to 5-alpha reductase inhibitors. Adding it to a peptide protocol that also includes hormonal peptides creates additional complexity. The cycling guide discusses timing strategies that can help manage potential interactions.
Reconstitution and storage
For injectable peptides in prostate protocols (BPC-157, TB-500, thymosin alpha-1), proper handling is essential for both safety and efficacy. The reconstitution guide covers step-by-step preparation. The bacteriostatic water guide explains the appropriate mixing solution. And the storage guide ensures potency is maintained throughout the protocol.
Using the peptide reconstitution calculator eliminates dosing errors that could compromise results or safety. For men managing multiple peptides in a prostate health stack, the peptide stack calculator helps organize timing and dosing across compounds.
What the research says about combination approaches
The most compelling evidence in peptide-prostate research often involves combinations rather than single agents.
The thymosin alpha-1 plus zoledronic acid study demonstrated synergistic anti-cancer effects greater than either agent alone. The BPC-157 plus TB-500 research showed enhanced tissue repair outcomes from the combination. And the bioregulator system was designed from the outset as a multi-peptide approach, with Libidon, Testagen, and complementary bioregulators intended to work as a coordinated system.
This pattern makes biological sense. Prostate health involves multiple interacting systems, hormonal, inflammatory, immune, metabolic, and oxidative. A single peptide targeting one system may produce modest benefits. A carefully designed combination addressing multiple systems creates the potential for compounding effects.
However, complexity introduces risk. More peptides mean more variables, more potential interactions, and more difficulty attributing observed changes to specific compounds. For researchers building prostate health peptide stacks, the recommended approach is sequential introduction: start with one peptide, establish baseline response over a full cycle, then add a second compound. This methodical approach allows for identifying both benefits and any adverse effects before increasing protocol complexity.
SeekPeptides members get access to detailed combination protocols, interaction databases, and community experiences from researchers who have navigated these exact questions. For those serious about evidence-based prostate health optimization, the guide to combining multiple peptides provides essential guidance.
Comparing peptide approaches: which is right for you
Peptide | Primary mechanism | Prostate evidence level | Administration | Best for |
|---|---|---|---|---|
Bioregulation, protein synthesis restoration | Clinical studies (48 patients) | Oral capsules | General prostate maintenance, BPH support | |
Thymosin alpha-1 | Immune modulation, T-cell/NK cell activation | Prostate cancer clinical data | Subcutaneous injection | Cancer prevention, immune support |
NF-kB suppression, anti-inflammatory | Strong mechanistic rationale | Multiple routes | Chronic prostatitis, BPH inflammation | |
Tissue repair, angiogenesis, anti-inflammatory | Safety data includes prostate | Subcutaneous injection | Tissue repair support, pelvic health | |
Cell migration, matrix remodeling | General tissue repair evidence | Subcutaneous injection | Complementary tissue repair | |
Telomerase activation, antioxidant | Indirect prostate relevance | Subcutaneous injection | Cellular aging, oxidative stress | |
Metabolic optimization, AMPK activation | Indirect (metabolic-prostate link) | Subcutaneous injection | Metabolic syndrome, obesity-related BPH | |
Testicular bioregulation | Clinical use (bioregulator system) | Oral capsules | Testosterone balance, hormonal health | |
Bladder bioregulation | Clinical use (bioregulator system) | Oral capsules | Urinary symptoms from BPH | |
Triptorelin | GnRH agonism, testosterone suppression | FDA-approved for prostate cancer | Injection (medical) | Advanced prostate cancer (medical supervision only) |
This comparison makes one thing clear: there is no single "best" peptide for prostate health. The right choice depends on the specific condition being addressed, existing medications, cancer risk profile, and individual health goals. The peptide dosing guide and getting started guide help researchers navigate these decisions systematically.
What researchers are watching: emerging prostate peptide science
The peptide-prostate research pipeline contains several developments worth tracking.
PSMA-targeting peptides are being developed for both prostate cancer imaging and targeted drug delivery. At the 2025 PSMA and Beyond annual meeting, researchers emphasized the need to look beyond PSMA to other peptide targets like GRPR (gastrin-releasing peptide receptor) for more comprehensive prostate cancer management. These diagnostic and therapeutic peptides could fundamentally change how prostate cancer is detected and treated.
Peptide-drug conjugates (PDCs) represent a promising strategy for prostate cancer therapy. A 2025 review in the Journal of Peptide Science highlighted PDCs that deliver anticancer drugs specifically to prostate tumor cells, minimizing off-target toxicity. The conjugate GSG showed particular promise as both more potent and potentially safer than unconjugated drugs.
PSA-cleavable peptide prodrugs exploit prostate-specific antigen enzymatic activity to selectively release anticancer agents within prostate tumors. This approach uses the prostate own biology as a drug activation mechanism, an elegant strategy that multiple research groups are actively developing.
A $1.4 million Department of Defense grant awarded to Dr. Benyi Li at the University of Kansas Cancer Center supports development of short peptide-based therapy targeting the androgen receptor protein. Preliminary studies identified a small peptide capable of triggering androgen receptor breakdown in prostate cancer cells. This could offer a more targeted alternative to broad androgen deprivation therapy.
These developments confirm that peptide approaches to prostate health are not fringe science. They are a significant and growing area of funded research with the potential to transform prostate cancer treatment and BPH management. The peptide regulation news tracks the evolving legal and regulatory landscape.
Common questions and misconceptions
Does testosterone cause prostate problems?
This is one of the most persistent misconceptions in men health. The relationship between testosterone and prostate health is far more nuanced than the simplistic "testosterone feeds prostate growth" narrative suggests.
While DHT (derived from testosterone) does drive prostatic cell proliferation, the relationship is not linear. Men with higher testosterone levels do not consistently develop worse BPH or higher prostate cancer rates. In fact, some research suggests that extremely low testosterone may increase certain prostate cancer risks through loss of normal cellular differentiation signals.
The critical factor is DHT conversion rate and androgen receptor sensitivity, not raw testosterone levels. This is why peptides that support healthy testosterone production (Testagen) may actually support prostate health by maintaining normal hormonal patterns rather than the dysregulated patterns associated with aging. The complete guide to peptides for men covers hormonal optimization in broader context.
Can peptides replace my prostate medication?
No. Peptides should be considered complementary to, not replacements for, prescribed prostate medications. Men taking finasteride, tamsulosin, or other prostate drugs should not discontinue them in favor of peptides without explicit guidance from their prescribing physician.
That said, some men find that peptide protocols reduce their symptom burden to the point where their physician agrees to modify medication doses. This is a conversation to have with a urologist, not a decision to make independently.
Are bioregulator peptides just supplements?
Bioregulator peptides like Libidon are categorized as dietary supplements rather than pharmaceutical drugs. However, the "just supplements" framing understates their mechanism. These are specific peptide sequences that interact with cellular DNA to modulate gene expression and protein synthesis. The clinical research supporting them, while primarily from Russian institutions, follows legitimate scientific methodology.
The distinction matters for realistic expectations. Bioregulator peptides work gradually, through cumulative effects over multiple courses, rather than providing immediate symptomatic relief like pharmaceutical drugs. They address underlying cellular function rather than blocking specific pathways.
Frequently asked questions
What is the best peptide for prostate health?
Libidon has the most direct prostate-specific research, with clinical data from 48 patients showing benefits for both BPH and chronic prostatitis. For immune-focused prostate protection, thymosin alpha-1 has the strongest evidence, including specific prostate cancer research. The best choice depends on your specific goals, whether that is maintenance, symptom management, or immune optimization.
Can peptides shrink an enlarged prostate?
GRP antagonist research showed measurable prostate shrinkage (15-19%) in preclinical models. GV1001 demonstrated reduced prostatic hypertrophy in animal studies. Bioregulator peptides support normal prostate cellular function that may limit further enlargement. However, no peptide has been proven to shrink an enlarged prostate in human clinical trials with the same level of evidence as pharmaceutical 5-alpha reductase inhibitors.
Are peptides safe to use with prostate cancer?
This depends entirely on the specific peptide. Thymosin alpha-1 has demonstrated anti-cancer activity in prostate cancer studies and may be beneficial as adjunctive therapy under medical supervision. Growth-promoting peptides like BPC-157 carry theoretical concerns about promoting cell growth that should be discussed with an oncologist. Always inform your cancer treatment team about any peptide use. Review the peptide safety guide for general risk considerations.
How long do prostate peptide protocols take to show results?
Bioregulator peptides work gradually. Initial changes may be noticed during the first 30-day course, with progressive improvements over 6-12 months of cyclical use. Anti-inflammatory peptides like KPV may show effects sooner since inflammation reduction can produce noticeable symptom relief within weeks. The peptide timeline guide provides detailed expectations for various compounds.
What age should men start considering peptides for prostate health?
Given that histological BPH appears in 8% of men between ages 31 and 40, and clinical symptoms increase substantially after age 50, proactive peptide protocols starting around age 40 align with the biology. Earlier intervention through longevity peptides and anti-inflammatory support may help prevent the inflammatory cascade that accelerates prostate aging. The Khavinson bioregulator protocol recommends starting regular courses at age 40-45.
Can I combine prostate peptides with saw palmetto?
Saw palmetto acts similarly to 5-alpha reductase inhibitors, reducing DHT conversion. Combining it with bioregulator peptides and anti-inflammatory peptides should not create direct interactions, but the combined effect on hormonal pathways warrants monitoring. Start one intervention at a time and assess response before adding more. The cycle planning guide helps organize multi-compound protocols.
Do peptides affect PSA test results?
Anti-inflammatory peptides that reduce prostatic inflammation may lower PSA levels. This is not a false reduction, as inflammation genuinely contributes to PSA elevation. However, it could complicate cancer screening interpretation. Inform your urologist about any peptide use so they can factor it into their assessment. Understanding peptide research limitations helps set realistic expectations for monitoring.
What is the safest peptide for prostate health?
Libidon has the longest safety record, with decades of clinical use and no reported serious adverse effects. Khavinson bioregulator peptides in general have demonstrated remarkable tolerability across extensive Russian clinical experience. They have no known immunogenic or mutagenic properties, making them among the safest options available.
External resources
National Institute of Diabetes and Digestive and Kidney Diseases - Enlarged Prostate (BPH)
PMC - Peptides in Prostate Cancer Immunotherapy (2025)
For researchers serious about optimizing their prostate health through peptide protocols, SeekPeptides offers the most comprehensive resource available, with evidence-based guides, proven protocols, and a community of thousands who have navigated these exact questions.
In case I do not see you, good afternoon, good evening, and good night. May your prostate stay healthy, your protocols stay evidence-based, and your PSA stay in the normal range.



