Jan 31, 2026
Your immune system is supposed to protect you.
It fights off infections, neutralizes toxins, and repairs damaged tissue every single day without you thinking about it. But what happens when that same system turns on the very body it was designed to defend?
That is the fundamental crisis behind every autoimmune disease, from rheumatoid arthritis grinding down your joints to lupus attacking your kidneys, from multiple sclerosis stripping the protective coating off your nerves to Crohn's disease tearing apart your gut lining. The immune system stops distinguishing self from threat. And once that distinction breaks down, the consequences cascade through every organ, every system, every day of your life.
Conventional treatments for autoimmune diseases share a common problem. They suppress the immune system broadly. Corticosteroids, methotrexate, biologics like infliximab and adalimumab, they all work by dampening immune activity across the board. This approach reduces symptoms. It can slow disease progression. But it also leaves you vulnerable to infections, impairs wound healing, and comes with side effects that sometimes rival the disease itself. The question researchers have been asking for decades is simple but profound: can we modulate the immune system without suppressing it? Can we restore balance instead of forcing silence?
Peptides offer one of the most promising answers to that question. These short chains of amino acids, smaller than proteins but more targeted than most conventional drugs, interact with immune signaling pathways in ways that are remarkably precise. Some promote regulatory T cell expansion. Others reduce inflammatory cytokines without shutting down immune surveillance entirely. A few even help repair the tissue damage that autoimmune diseases leave behind. The research spans decades and multiple disease models, from animal studies showing dramatic reductions in disease markers to early clinical trials in humans yielding cautious optimism.
This guide covers every major peptide being studied for autoimmune applications, the science behind how they work, specific protocols researchers use, and the honest limitations of what we know so far. SeekPeptides has compiled this resource because understanding these mechanisms is not optional for anyone serious about peptide research, it is foundational.
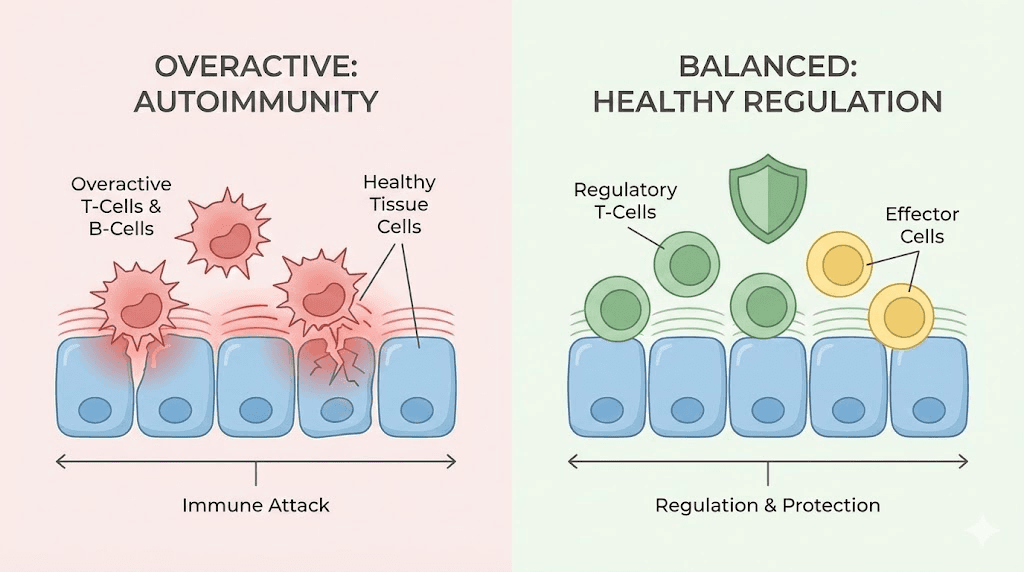
How autoimmune diseases actually work at the cellular level
Before understanding how peptides might help, you need to understand what goes wrong. Autoimmune disease is not just "your immune system attacking you." That description, while accurate on the surface, misses the precise mechanisms that researchers target with peptide therapies.
The thymus gland sits behind your breastbone. It is where T cells mature and learn the difference between self and foreign. During development, T cells undergo two critical selection processes. Positive selection keeps T cells that can recognize molecules presented on your own cells. Negative selection eliminates T cells that react too strongly to your own tissues. And a third pathway, one that has become central to autoimmune research, generates regulatory T cells, also called Tregs. These Tregs function as the immune system's referees. They prevent other immune cells from overreacting.
When this system fails, the results are devastating.
Self-reactive T cells escape the thymus and enter circulation. Without enough Tregs to keep them in check, these rogue cells begin attacking healthy tissue. The specific tissue they target determines which autoimmune disease develops. Attack the joint synovium and you get rheumatoid arthritis. Attack the myelin sheath around nerves and you get multiple sclerosis. Attack the intestinal lining and you get inflammatory bowel disease. The mechanism is remarkably similar across diseases. The target tissue is what differs.
A key gene called AIRE (autoimmune regulator) plays a critical role in thymic selection. It ensures that thymic epithelial cells express proteins from tissues throughout the body, allowing the thymus to test developing T cells against a comprehensive library of self-antigens. Mutations in the AIRE gene cause autoimmune polyendocrinopathy, a condition where multiple organs come under immune attack simultaneously. Similarly, mutations in the Foxp3 gene, which controls Treg development, cause IPEX syndrome, a devastating autoimmune condition affecting multiple organ systems.
This is where peptides enter the picture. Several classes of peptides interact directly with these immune mechanisms. Some support thymic function and Treg generation. Others reduce the inflammatory signaling that drives tissue destruction. A few do both simultaneously. Understanding which peptide does what, and why, is the foundation for evaluating their potential in autoimmune research.
The cytokine storm in autoimmune flares
Cytokines are the chemical messengers of the immune system. In autoimmune disease, certain pro-inflammatory cytokines become chronically elevated. TNF-alpha, interleukin-6 (IL-6), interleukin-1-beta (IL-1B), and interferon-gamma (IFN-gamma) drive persistent inflammation that damages tissue over time. This is not a short-term immune response that resolves. It is a sustained, unregulated assault.
Many conventional autoimmune drugs target these cytokines directly. TNF inhibitors like infliximab block TNF-alpha. IL-6 receptor antagonists like tocilizumab block IL-6 signaling. These drugs are effective, but they leave significant gaps in immune defense. Peptide researchers are exploring compounds that reduce these inflammatory cytokines while simultaneously supporting anti-inflammatory pathways, creating a more balanced immune environment rather than simply blocking one side of the equation.
The gut-immune connection
Approximately 70% of your immune system resides in the gut. This is not a coincidence. The intestinal lining serves as the boundary between the outside world and your internal environment, and the immune cells stationed there must constantly distinguish between harmless food particles, beneficial bacteria, and genuine threats. When this barrier breaks down, a condition researchers call intestinal permeability or "leaky gut," antigens that should stay in the gut escape into the bloodstream. The immune system encounters these foreign molecules in places they should never be, and the resulting immune activation can trigger or worsen autoimmune responses throughout the body.
Several peptides being studied for autoimmune applications work specifically at this gut-immune interface. BPC-157 promotes gut mucosal healing. KPV reduces intestinal inflammation through NF-kB pathway modulation. These gut-focused mechanisms are particularly relevant for autoimmune diseases with gastrointestinal involvement, but researchers increasingly believe that gut barrier integrity influences autoimmune activity far beyond the digestive tract.
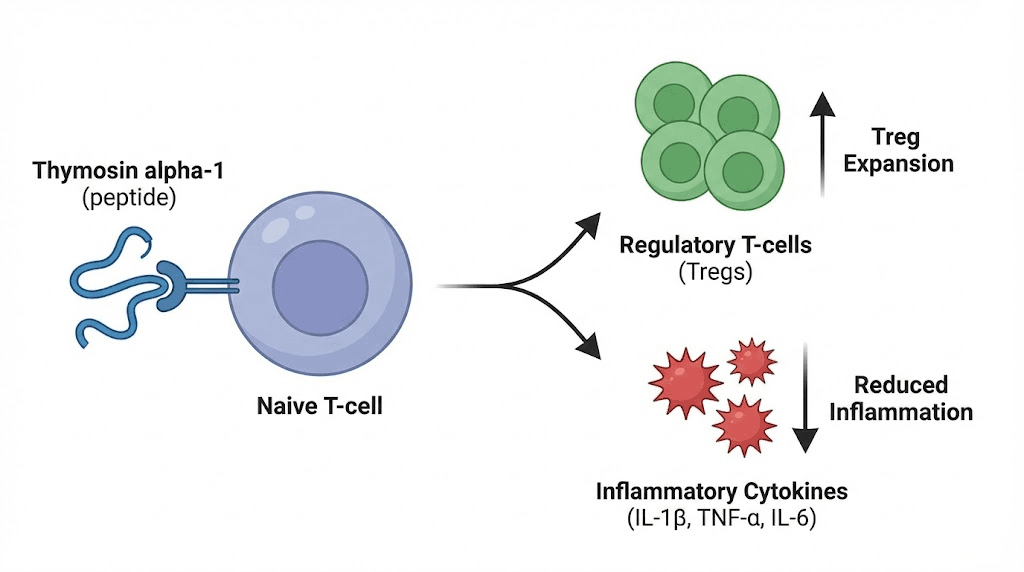
Thymosin alpha-1: the immune modulator with the longest track record
If any peptide has earned its reputation in autoimmune research, it is thymosin alpha-1. This 28-amino-acid peptide was originally isolated from the thymus gland, the very organ responsible for T cell maturation and immune tolerance. It has been studied for over four decades, used clinically in more than 30 countries, and investigated across conditions ranging from chronic hepatitis to cancer to autoimmune disease.
Thymosin alpha-1, also known as thymalfasin and marketed as Zadaxin in some countries, works through a mechanism that is genuinely unusual among immune-affecting compounds. It is bidirectional. When the immune system is underactive, thymosin alpha-1 enhances immune function. When it is overactive, as in autoimmune disease, it promotes regulatory pathways that calm the response. This bidirectional activity is what makes it particularly interesting for autoimmune applications, where the goal is not suppression but rebalancing.
How thymosin alpha-1 modulates autoimmune activity
The mechanisms are multi-layered. First, thymosin alpha-1 promotes the expansion of regulatory T cells, the very cells whose deficiency allows autoimmune disease to develop. By enhancing Treg populations, the peptide helps restore the immune balance between effector cells (which attack) and regulatory cells (which prevent inappropriate attacks).
Second, it modulates cytokine production. Research shows thymosin alpha-1 increases beneficial cytokines like IL-2 and IL-10 while suppressing excessive production of pro-inflammatory markers like IL-6 and TNF-alpha. IL-10 is particularly important because it is one of the primary anti-inflammatory cytokines that Tregs use to suppress autoimmune responses.
Third, thymosin alpha-1 influences dendritic cell maturation. Dendritic cells are the immune system's scouts. They capture antigens and present them to T cells, essentially telling the immune system what to attack. In autoimmune disease, dendritic cells often present self-antigens in ways that trigger immune attacks against the body's own tissue. Thymosin alpha-1 appears to promote a more tolerogenic dendritic cell phenotype, meaning these cells are more likely to induce tolerance rather than activation when they encounter self-antigens.
Research in specific autoimmune diseases
A study published in the journal Annals of the New York Academy of Sciences examined serum thymosin alpha-1 levels in patients with autoimmune diseases including psoriatic arthritis, rheumatoid arthritis, and systemic lupus erythematosus. The findings were striking: patients with these conditions had significantly lower serum thymosin alpha-1 levels compared to healthy controls (18.38 versus 53.08, P less than 0.0001). This suggests that autoimmune patients may have a natural deficiency in this regulatory peptide.
For multiple sclerosis specifically, research by Giacomini and colleagues found that thymosin alpha-1 expands deficient IL-10-producing regulatory B cell subsets in patients with relapsing-remitting MS. The researchers first documented that RRMS patients had low serum levels of thymosin alpha-1, then demonstrated that supplementation promoted the expansion of regulatory B cells known to dampen autoimmune inflammation.
In lupus, the peptide's immune-modulating activity helps lower autoantibody levels and supports tissue recovery. While large-scale clinical trials specifically for autoimmune indications remain limited, the existing research demonstrates a consistent pattern: thymosin alpha-1 supports immune regulation through multiple complementary pathways.
Thymosin alpha-1 protocol considerations
The most well-established dosing protocol comes from clinical use of Zadaxin. The standard dose is 1.6 mg administered subcutaneously twice per week for 6 to 12 months. In clinical trials involving over 2,000 patients across various conditions, no clinically significant adverse reactions were attributed to thymosin alpha-1 administration. Side effects were infrequent and mild, consisting primarily of local discomfort at the injection site.
Some practitioners use alternative protocols. The 1.6 mg bi-weekly dose can be split into smaller daily doses (approximately 0.45 mg daily) due to the peptide's short half-life. This approach aims for more consistent serum levels. Others escalate to higher weekly totals of 3.2 to 6.4 mg for more aggressive immune modulation, though this exceeds the standard protocol. Use the SeekPeptides peptide calculator to determine exact dosing based on your reconstitution method.
One important caveat: thymosin alpha-1 should be used cautiously in individuals who are deliberately immunosuppressed, such as organ transplant recipients. The peptide's immune-enhancing properties could theoretically interfere with immunosuppressive regimens. Always consult a qualified healthcare professional before considering any peptide protocol, especially when managing autoimmune conditions.
BPC-157: gut healing and tissue repair in autoimmune conditions
BPC-157 (Body Protection Compound-157) is a 15-amino-acid peptide originally isolated from human gastric juice. Its primary reputation is as a tissue repair peptide, and its relevance to autoimmune disease centers on two key capabilities: gut mucosal healing and broad anti-inflammatory activity.
Why does gut healing matter for autoimmune disease? Because intestinal permeability acts as both a trigger and an amplifier of autoimmune activity. When the gut barrier breaks down, bacterial endotoxins and food-derived antigens enter the bloodstream. The immune system responds to these misplaced molecules with inflammation. In genetically susceptible individuals, this chronic immune activation can initiate or worsen autoimmune responses against distant tissues. Restoring gut barrier integrity addresses one of the root drivers of autoimmune inflammation, not just the symptoms.
BPC-157 in inflammatory bowel disease research
The most direct autoimmune application for BPC-157 involves inflammatory bowel diseases, specifically Crohn's disease and ulcerative colitis. These conditions represent autoimmune attacks against the intestinal lining, making them a natural fit for a peptide derived from gastric juice that promotes mucosal healing.
Animal studies paint a compelling picture. In mouse models of Crohn's disease, BPC-157 administration led to significant decreases in the size of intestinal lesions. Treated mice demonstrated greater weight gain and overall health compared to controls. In colitis models simulating ulcerative colitis, BPC-157 reduced inflammation and promoted colon healing. The peptide was given the designation PL-10 and PL14736 during early clinical trial development by Pliva, a Croatian pharmaceutical company, specifically for inflammatory bowel disease.
The mechanisms behind these effects involve multiple pathways. BPC-157 enhances growth hormone receptor expression, modulates VEGF and FGF signaling to promote angiogenesis and tissue repair, reduces pro-inflammatory cytokines TNF-alpha, IL-6, and IFN-gamma, and interacts with the nitric oxide system to provide endothelial protection. It promotes tissue repair, reduces inflammation, and supports the formation of new blood vessels needed to deliver nutrients to healing tissue.
One case study documented dramatic improvement in a patient with therapy-resistant ulcerative colitis who had not responded to conventional treatments including biologics. While this is a single case and cannot establish efficacy on its own, it adds to the body of evidence suggesting BPC-157's potential in autoimmune gastrointestinal conditions.
BPC-157 for autoimmune conditions beyond the gut
The anti-inflammatory properties of BPC-157 extend beyond the gastrointestinal tract. Research demonstrates that the peptide reduces inflammation in joint tissues relevant to rheumatoid arthritis. Its pro-angiogenic effects support tissue repair wherever autoimmune-mediated damage occurs. And its interaction with the gut-brain axis may improve both intestinal and neurological symptoms, which is relevant given that many autoimmune patients also experience anxiety and depression alongside their primary condition.
The peptide's stability is another advantage for autoimmune research. Unlike many peptides that degrade rapidly in acidic environments, BPC-157 is resistant to stomach acid and stable at room temperature. This opens the possibility of oral administration, which is particularly relevant for gastrointestinal autoimmune conditions where delivering the peptide directly to the affected tissue makes biological sense.
BPC-157 protocol considerations for autoimmune research
Standard research protocols for BPC-157 use 200 to 300 mcg administered subcutaneously once or twice daily. For gastrointestinal applications, oral administration is also studied, with the same dose range delivered in capsule form. Improvements in animal models are typically observed within 3 to 7 days, with fuller healing occurring over 2 to 4 weeks depending on severity.
Important contraindications exist. Because BPC-157 promotes angiogenesis, it should be avoided by individuals with certain cancers where new blood vessel formation could feed tumor growth. It may also interact with blood thinners like warfarin. Safety considerations are essential when exploring any peptide for autoimmune research, and BPC-157 is no exception.
Store reconstituted BPC-157 according to proper peptide storage guidelines. Refrigeration at 2 to 8 degrees Celsius is standard for reconstituted solutions, which typically remain stable for up to several weeks in the fridge. Lyophilized powder is more stable and can be stored longer according to powder form storage protocols.
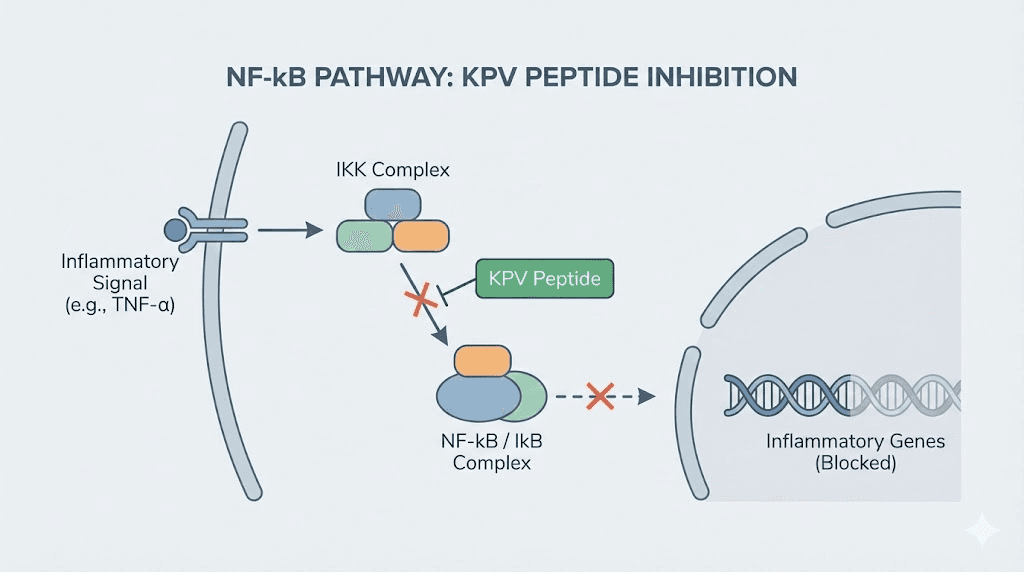
KPV: the anti-inflammatory tripeptide for immune modulation
KPV is a tripeptide consisting of just three amino acids: lysine, proline, and valine. It is derived from the C-terminal end of alpha-melanocyte-stimulating hormone (alpha-MSH), a larger peptide hormone that plays central roles in immune regulation and inflammation control. What makes KPV remarkable is that most of alpha-MSH's anti-inflammatory activity can be attributed to this tiny three-amino-acid fragment.
Small size, massive impact.
KPV's primary mechanism involves inhibiting NF-kB activation. NF-kB is not just any inflammatory pathway. It is considered the master regulator of inflammation, controlling the expression of hundreds of genes involved in immune activation, cytokine production, and cell survival. In autoimmune disease, NF-kB is chronically activated, driving the persistent inflammation that characterizes these conditions. By blocking this pathway, KPV addresses one of the root signaling cascades rather than a single downstream effect.
KPV mechanisms in autoimmune inflammation
Beyond NF-kB inhibition, KPV reduces the production of multiple pro-inflammatory cytokines. It decreases TNF-alpha, IL-1-beta, and IL-6 through melanocortin receptor-mediated pathways. It also reduces neutrophil infiltration, which is critical in both acute and chronic inflammatory conditions associated with autoimmune flares.
A key research finding involves KPV's interaction with the PepT1 transporter. PepT1 is a di/tripeptide transporter normally expressed in the small intestine and induced in the colon during inflammatory bowel disease. Research published in Gastroenterology demonstrated that nanomolar concentrations of KPV inhibit NF-kB and MAP kinase inflammatory signaling pathways through PepT1-mediated transport, and reduce pro-inflammatory cytokine secretion. Furthermore, oral administration of KPV reduced the incidence of colitis in both DSS and TNBS mouse models.
This PepT1 connection is particularly elegant. During IBD, the colon upregulates PepT1 expression, essentially creating more cellular doorways for KPV to enter. The inflamed tissue becomes more receptive to the very peptide that can calm it. The sicker the tissue, the more effectively it can absorb the treatment.
KPV for specific autoimmune conditions
KPV is most studied for autoimmune conditions involving the gut, skin, and mucosal surfaces. For inflammatory bowel disease, the evidence from animal models is substantial: KPV reduces intestinal inflammation, promotes epithelial stability, and reduces disease severity markers. For skin-related autoimmune conditions, KPV's ability to reduce inflammatory cytokines makes it relevant to inflammatory skin conditions including psoriasis-related autoimmune inflammation.
KPV also shows synergistic effects when combined with other peptides. Researchers have paired it with BPC-157 for comprehensive gut repair, addressing both the inflammatory signaling (KPV) and the physical tissue damage (BPC-157) simultaneously. This combination approach targets two different aspects of the same problem, potentially yielding better outcomes than either peptide alone.
KPV protocol considerations
KPV is studied in both oral and subcutaneous forms. For KPV dosing, oral administration makes particular sense for gastrointestinal autoimmune conditions because the PepT1 transporter directly facilitates absorption in inflamed intestinal tissue. Standard research protocols use 200 to 500 mcg daily, either orally or subcutaneously. Some protocols suggest taking KPV in the morning for optimal absorption, while others divide the dose between morning and evening.
One receptor-binding nuance deserves attention: whether KPV binds to melanocortin receptors and uses identical signaling pathways as its parent peptide alpha-MSH is not yet fully clear. Several binding studies suggest KPV does not directly bind MC-1R and does not increase cAMP levels. However, it does block IL-1-beta surface binding to T cells and inhibits hyperalgesic effects of IL-1-beta in vivo, suggesting alternative signaling mechanisms that researchers continue to investigate.
Thymosin beta-4: tissue repair meets immune modulation
Thymosin beta-4 (TB-4 or TB-500) is a 43-amino-acid peptide that exists naturally in nearly all human cell types. While it is primarily known for its tissue repair capabilities, its relevance to autoimmune disease extends into two specific areas: remyelination in multiple sclerosis and general tissue repair in autoimmune-damaged organs.
TB-500 promotes cell migration, angiogenesis, and anti-inflammatory activity through mechanisms distinct from other peptides on this list. It works by sequestering actin monomers, which affects cellular processes including migration, proliferation, and differentiation. For autoimmune applications, these wound-healing properties address the damage side of the equation rather than the immune dysregulation itself.
Thymosin beta-4 in multiple sclerosis research
Multiple sclerosis involves the autoimmune destruction of myelin, the protective sheath surrounding nerve fibers. Loss of myelin impairs nerve signal transmission, causing the progressive neurological symptoms characteristic of MS. Remyelination, the process of rebuilding this myelin sheath, is one of the most sought-after therapeutic goals in MS research.
Thymosin beta-4 has been hypothesized as an effective remyelination treatment based on its ability to promote differentiation of oligodendrocyte progenitor cells (OPCs). Oligodendrocytes are the cells responsible for producing myelin in the central nervous system, and OPCs are their precursors. Research using two demyelination animal models, including experimental autoimmune encephalomyelitis (EAE), which is the standard animal model for MS, demonstrated that thymosin beta-4 promoted OPC differentiation and supported remyelination.
This is not immune modulation in the traditional sense. TB-500 does not correct the underlying autoimmune attack that destroys myelin. But by promoting the repair of damaged myelin, it addresses one of the most devastating consequences of the disease. In combination with immune-modulating peptides like thymosin alpha-1, TB-500 could theoretically address both the cause and the consequence of autoimmune nerve damage.
TB-500 for autoimmune tissue damage
Beyond MS, the tissue repair properties of TB-500 apply to any autoimmune condition where chronic inflammation causes structural damage. Tendon and joint damage from rheumatoid arthritis, gut mucosal erosion from IBD, skin lesions from psoriasis, and organ damage from lupus all involve tissue that needs repair. TB-500's ability to promote new blood vessel formation, reduce inflammation, and stimulate cellular migration to damaged areas makes it a candidate for supporting recovery across autoimmune conditions.
Research protocols for TB-500 typically involve stacking it with BPC-157 for enhanced tissue repair. The combination addresses different but complementary repair pathways: BPC-157 promotes growth factor signaling and angiogenesis through the VEGF pathway, while TB-500 facilitates cellular migration and differentiation through actin modulation. For autoimmune conditions involving significant tissue damage, this combination represents a multi-mechanism approach to repair. Compare these two peptides in our BPC-157 vs TB-500 guide.
LL-37: antimicrobial defense and immune regulation
LL-37 is a cathelicidin-derived antimicrobial peptide that serves a dual role in the immune system. It directly kills pathogens by disrupting their cell membranes, and it modulates immune responses by influencing cytokine production, immune cell migration, and tissue healing. This dual role makes it relevant to autoimmune conditions where microbial dysbiosis contributes to immune activation.
The connection between infections, microbial imbalance, and autoimmune disease is well established. Molecular mimicry, where microbial proteins resemble self-proteins, can trigger autoimmune responses. Chronic infections in the gut can maintain immune activation that spills over into autoimmune pathology. And dysbiosis itself alters the balance of immune-promoting versus immune-regulating signals from the microbiome.
How LL-37 supports autoimmune management
LL-37 addresses these issues from the microbial side. By directly killing pathogenic bacteria, fungi, and some viruses, it helps restore a healthier microbial balance. By promoting immune cell migration to sites of genuine infection, it redirects immune activity away from self-tissue and toward actual threats. And by supporting tissue healing and angiogenesis, it helps repair damage caused by both infection and autoimmune activity.
LL-37 also stabilizes macrophages and microglia, reducing what researchers describe as the "fight everything" immune posture that fuels autoimmune progression. This stabilization effect is particularly relevant in neurological autoimmune conditions where overactive microglia contribute to nerve damage.
However, LL-37 is more immune-activating than KPV. While KPV is characterized by its calming, anti-inflammatory effects, LL-37 actively stimulates certain immune pathways. This makes it better suited for situations where microbial infection or dysbiosis is contributing to autoimmune activation, rather than situations where pure immune calming is needed. For systemic autoimmune inflammation without a significant infectious component, KPV may be the more appropriate choice.
LL-37 protocol considerations
LL-37 is typically administered subcutaneously. Due to its immune-activating properties, it may not be suitable for chronic daily use in autoimmune patients. Researchers often use it in short courses targeted at specific infectious or dysbiotic contributions to autoimmune activation, rather than as a long-term immune-modulating agent. It combines well with KPV and thymosin alpha-1, with LL-37 addressing microbial triggers while the other peptides handle immune regulation.
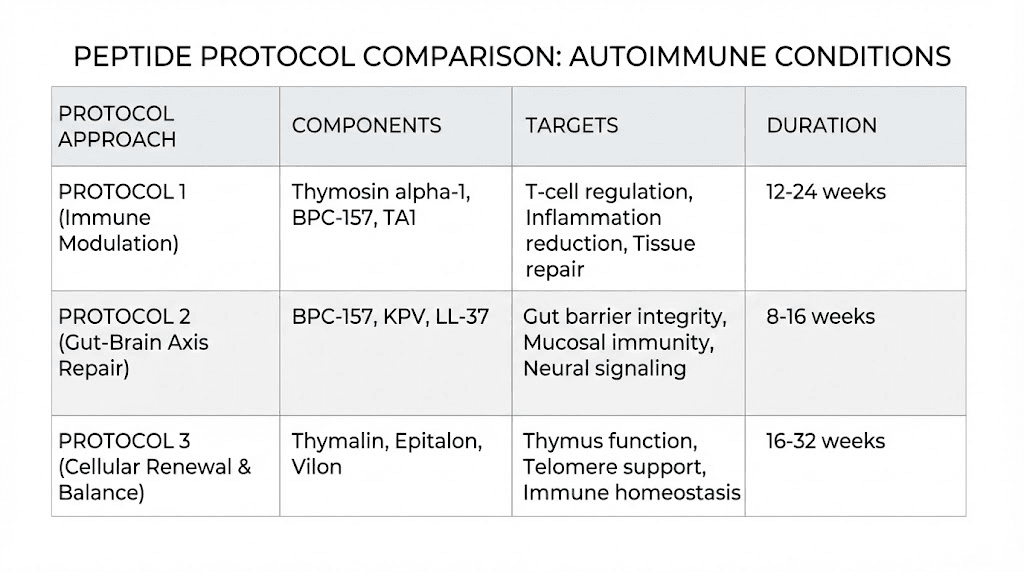
Peptide combinations for autoimmune research protocols
Autoimmune disease involves multiple dysfunctional mechanisms simultaneously. The immune system overreacts. Regulatory cells are deficient. Inflammatory cytokines run unchecked. Tissue damage accumulates. Gut barrier integrity is compromised. No single peptide addresses all of these problems. This is why combination protocols are central to peptide research for autoimmune conditions.
Protocol approach 1: immune rebalancing (thymosin alpha-1 plus KPV)
Goal: Restore regulatory immune function while reducing inflammatory signaling
Components:
Thymosin alpha-1: 1.6 mg subcutaneously twice per week (promotes Treg expansion, modulates dendritic cells)
KPV: 200-500 mcg daily, orally or subcutaneously (inhibits NF-kB, reduces TNF-alpha and IL-6)
Rationale: Thymosin alpha-1 works at the cellular level to rebuild the regulatory arm of the immune system, while KPV addresses the inflammatory signaling that drives acute symptoms. Together they target both the structural immune deficit and its downstream consequences.
Duration: Research protocols often run 8 to 12 weeks minimum, with some extending to 6 months for thymosin alpha-1 to allow full Treg population recovery.
Protocol approach 2: gut-focused autoimmune support (BPC-157 plus KPV)
Goal: Restore gut barrier integrity and reduce intestinal inflammation
Components:
BPC-157: 200-300 mcg twice daily, orally or subcutaneously near the abdomen (promotes mucosal healing, angiogenesis)
KPV: 200-500 mcg daily, orally (NF-kB inhibition, cytokine reduction, PepT1-mediated gut absorption)
Rationale: For autoimmune conditions with significant gastrointestinal involvement, including IBD, celiac-associated inflammation, or any autoimmune condition complicated by leaky gut, this combination addresses both the physical barrier damage (BPC-157) and the inflammatory signaling (KPV). Oral administration delivers both peptides directly to the affected tissue.
Duration: Gut healing protocols typically run 4 to 8 weeks, with reassessment at the 4-week mark. Use the peptide calculator for precise dosing.
Protocol approach 3: comprehensive repair (thymosin alpha-1 plus BPC-157 plus TB-500)
Goal: Immune rebalancing combined with aggressive tissue repair
Components:
Thymosin alpha-1: 1.6 mg subcutaneously twice per week
BPC-157: 250 mcg subcutaneously twice daily near affected tissue
TB-500: 2-5 mg subcutaneously twice per week for loading, then weekly for maintenance
Rationale: This three-peptide approach targets immune regulation (TA1), mucosal and tissue healing (BPC-157), and cellular migration plus repair (TB-500). It is the most comprehensive protocol but also the most complex. It is typically reserved for autoimmune conditions with significant accumulated tissue damage.
Duration: Loading phase of 4 weeks followed by maintenance for 8 to 12 additional weeks. Researchers typically cycle peptide protocols with breaks to prevent receptor desensitization.
Understanding how to properly reconstitute these peptides is essential before beginning any protocol. Use bacteriostatic water for all reconstitutions and follow sterile injection techniques rigorously.
Specific autoimmune diseases and peptide applications
The general principles above apply across autoimmune conditions, but each disease has specific characteristics that affect which peptides are most relevant and how protocols might be structured. Here is a disease-by-disease breakdown based on current research.
Rheumatoid arthritis
Rheumatoid arthritis involves autoimmune attack against the synovial membrane lining the joints. Chronic inflammation leads to cartilage destruction, bone erosion, and progressive joint deformity. The primary inflammatory drivers are TNF-alpha, IL-6, and IL-1-beta, all of which are targeted by multiple peptides on this list.
Most relevant peptides:
Thymosin alpha-1 for immune rebalancing and Treg promotion
KPV for TNF-alpha and IL-6 reduction
BPC-157 for joint tissue repair and anti-inflammatory effects
TB-500 for tendon and connective tissue repair
Research shows that BPC-157 reduces inflammation in joint tissues relevant to RA. Combined with the systemic immune modulation of thymosin alpha-1 and the cytokine reduction of KPV, a multi-peptide approach addresses both the root immune dysfunction and the resulting joint damage. TB-500 adds cellular repair capacity for damaged cartilage and surrounding structures. For managing pain associated with RA, these repair peptides may help address the structural damage that drives chronic pain.
Systemic lupus erythematosus (SLE)
Lupus is one of the most complex autoimmune diseases because it can attack virtually any organ system. The immune system produces autoantibodies against nuclear components (DNA, histones, ribosomes), and immune complex deposition causes inflammation in kidneys, skin, joints, brain, and blood vessels. Lupus flares are driven by a combination of B cell hyperactivity, T cell dysregulation, and interferon pathway activation.
Most relevant peptides:
Thymosin alpha-1 for restoring T cell regulation and reducing autoantibody production
KPV for systemic NF-kB inhibition and cytokine reduction
An important lupus-specific peptide in clinical development is P140/Lupuzor, a 21-amino-acid phosphopeptide that has completed Phase IIb clinical trials and entered Phase III trials for SLE. Data indicate that P140 depletes hyper-activated autoreactive T and B cells and restores normal immune homeostasis. It belongs to a new family of non-immunosuppressive immunoregulators. While P140 is not available outside clinical trials, it demonstrates that peptide-based approaches can achieve antigen-specific tolerance in lupus, precisely targeting the dysfunctional immune cells while leaving normal immune function intact.
The finding that lupus patients have significantly lower serum thymosin alpha-1 levels (the Pica et al. study mentioned earlier) provides a direct rationale for thymosin alpha-1 supplementation in SLE. Restoring deficient regulatory peptide levels may help rebalance the immune dysfunction underlying the disease.
Multiple sclerosis
MS involves autoimmune destruction of myelin in the central nervous system, leading to progressive neurological disability. The disease pattern alternates between relapses (acute autoimmune attacks) and remissions (partial recovery periods), at least in the relapsing-remitting form that accounts for about 85% of initial MS diagnoses.
Most relevant peptides:
Thymosin alpha-1 for expanding regulatory B cell subsets and reducing relapse frequency
Thymosin beta-4/TB-500 for promoting remyelination through OPC differentiation
BPC-157 for neuroprotective anti-inflammatory effects
The Giacomini et al. research specifically demonstrated thymosin alpha-1's ability to expand IL-10-producing regulatory B cells in RRMS patients, a finding that directly addresses one of the documented immune deficits in MS. Combined with TB-500's remyelination potential, this represents a dual approach: reduce the autoimmune attack (TA1) while repairing its primary damage (TB-500). Research on peptides for brain and neurological function continues to expand as more is understood about these mechanisms.
Inflammatory bowel disease (Crohn's and ulcerative colitis)
IBD represents one of the most directly applicable areas for peptide research in autoimmune disease. The gut is both the site of autoimmune attack and the location where several key peptides have their strongest documented effects.
Most relevant peptides:
BPC-157 for mucosal healing, angiogenesis, and anti-inflammatory effects in gut tissue
KPV for PepT1-mediated intestinal anti-inflammatory activity and NF-kB inhibition
Thymosin alpha-1 for systemic immune rebalancing
This is the one autoimmune application where the research evidence is strongest for peptide interventions. BPC-157 was specifically developed for clinical trials in IBD (designated PL14736). KPV has published research showing colitis reduction through a well-characterized mechanism (PepT1 transport). And the gut barrier restoration achieved by both peptides addresses a fundamental driver of autoimmune activity throughout the body, not just in the intestines. Our guides on peptides for gut health and KPV for inflammation provide additional detail on these applications.
Hashimoto's thyroiditis and autoimmune thyroid conditions
Hashimoto's thyroiditis is the most common autoimmune disease, involving immune attack against the thyroid gland that progressively destroys its ability to produce thyroid hormones. The condition is characterized by elevated anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin antibodies, lymphocytic infiltration of thyroid tissue, and gradual development of hypothyroidism.
Most relevant peptides:
Thymosin alpha-1 for immune regulation and autoantibody reduction
KPV for reducing the inflammatory environment that drives thyroid tissue destruction
While direct research on peptides for Hashimoto's is limited compared to lupus or MS, the mechanisms are consistent. Thymosin alpha-1's ability to promote Tregs and reduce autoantibody production applies directly to the anti-TPO and anti-thyroglobulin antibodies that characterize Hashimoto's. KPV's systemic anti-inflammatory effects could reduce the chronic inflammation that drives progressive thyroid destruction. Additionally, addressing gut health is relevant for Hashimoto's because of the well-documented gut-thyroid axis connection.
Psoriasis and psoriatic arthritis
Psoriasis involves immune-mediated inflammation of the skin, while psoriatic arthritis adds joint involvement. Both are driven by TNF-alpha, IL-17, and IL-23 overproduction. The skin repair properties of certain peptides make them particularly relevant here.
Most relevant peptides:
KPV for skin and mucosal anti-inflammatory effects
Thymosin alpha-1 for immune regulation (notably, the Pica study specifically included psoriatic arthritis patients)
BPC-157 for tissue repair in joint involvement
KPV is particularly relevant for psoriasis because of its calming effects on skin inflammation. Its ability to reduce IL-6, TNF-alpha, and NF-kB activation addresses the core inflammatory drivers of psoriatic plaques. Thymosin alpha-1 addresses the broader immune dysregulation, and BPC-157 supports repair of damaged skin tissue and arthritic joints.
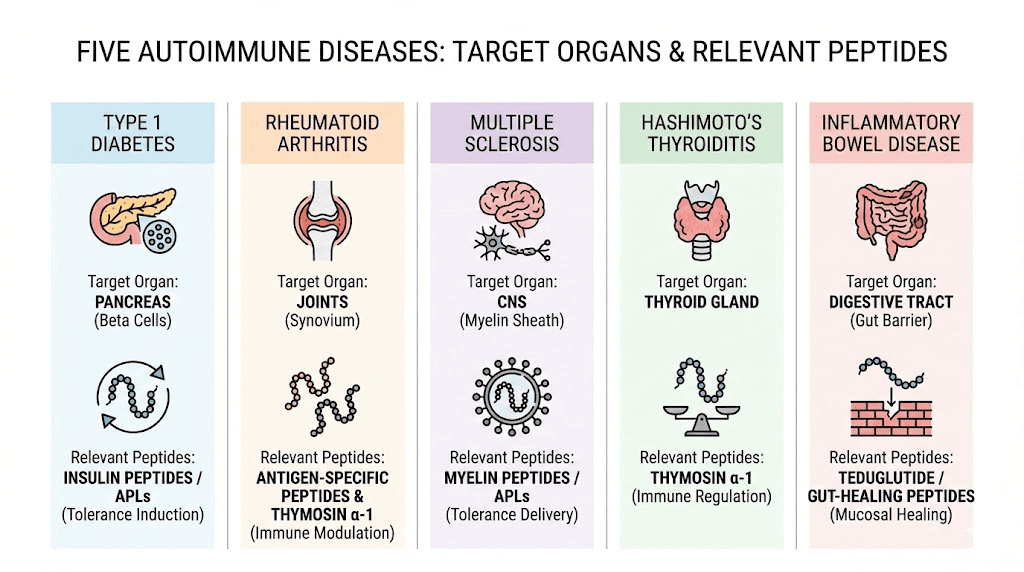
Emerging peptides in autoimmune research
Beyond the established peptides above, several newer compounds are generating research interest for autoimmune applications. These are earlier in the research pipeline but represent the direction the field is heading.
Bioregulator peptides from the Khavinson school
The Khavinson bioregulator peptides are short peptides (2-4 amino acids) developed by Russian researcher Vladimir Khavinson. Several of these bioregulators are relevant to autoimmune disease through organ-specific mechanisms:
Thymalin is a thymus-derived peptide that supports thymic function and T cell maturation. In the context of autoimmune disease, Thymalin's ability to support proper thymic selection, the very process whose failure leads to autoimmune disease, makes it a theoretical upstream intervention. By supporting the organ that generates immune tolerance, Thymalin could help prevent the production of self-reactive T cells that drive autoimmune attacks.
Vilon is a dipeptide (Lys-Glu) that also acts on the thymus, promoting thymic regeneration and immune function in aging organisms. As thymic involution with age is associated with increased autoimmune disease incidence, supporting thymic function may have preventive value.
Bronchogen targets bronchial tissue and may be relevant for autoimmune conditions affecting the lungs.
Cortagen acts on the brain and nervous system, with potential relevance to neuroimmune conditions like MS.
The bioregulator peptide approach is fundamentally different from the larger peptides discussed earlier. These tiny molecules are thought to interact with DNA expression, potentially influencing gene regulation in specific tissues. The evidence base is predominantly from Russian research institutions, and Western clinical validation remains limited. But the concept of tissue-specific regulatory peptides that restore normal function rather than suppressing disease processes aligns with the broader goal of immune modulation in autoimmune disease.
SS-31 (elamipretide) for mitochondrial autoimmune mechanisms
SS-31 is a mitochondria-targeted peptide that has primarily been studied for aging and cellular energy. Its relevance to autoimmune disease involves a less obvious but increasingly recognized mechanism: mitochondrial dysfunction drives inflammatory signaling in immune cells. Damaged mitochondria release molecules that activate the NLRP3 inflammasome, a protein complex that amplifies inflammatory cytokine production. By protecting mitochondria from oxidative damage, SS-31 may reduce one of the upstream triggers of autoimmune-associated inflammation.
This mitochondrial connection is particularly relevant for lupus, where mitochondrial dysfunction in immune cells has been documented, and for fibromyalgia and chronic fatigue that frequently accompany autoimmune conditions.
Epitalon and pineal-immune connections
Epitalon is a tetrapeptide that acts on the pineal gland to promote melatonin production. Melatonin, in turn, has documented immunomodulatory effects. It reduces inflammatory cytokines, promotes Treg function, and exhibits antioxidant effects that protect tissues from inflammatory damage. The longevity and immune-supportive effects of epitalon make it a peripheral but potentially valuable addition to autoimmune protocols, particularly for patients whose autoimmune conditions are exacerbated by sleep disturbances or circadian rhythm disruption.
Selank for neuroimmune modulation
Selank is a synthetic analogue of the immunomodulatory peptide tuftsin. It was originally developed for anxiety and cognitive enhancement, but its immune-modulating properties make it relevant to autoimmune conditions with neuropsychiatric components. Selank affects the balance of T helper cell subsets and influences cytokine expression, including reductions in IL-6. For autoimmune patients who experience significant anxiety or cognitive symptoms alongside their physical disease, Selank addresses both the neuropsychiatric and immune components simultaneously.
The science of immune tolerance and why it matters for peptides
The ultimate goal of autoimmune therapy is not just to suppress inflammation or repair damage. It is to restore immune tolerance, the state where the immune system correctly identifies self-tissue as friendly and stops attacking it. This is fundamentally different from immune suppression, which simply reduces all immune activity regardless of its target.
Central tolerance versus peripheral tolerance
Central tolerance occurs in the thymus, where self-reactive T cells are eliminated before they enter circulation. Peripheral tolerance operates throughout the body, using Tregs and other regulatory mechanisms to suppress any self-reactive cells that escape thymic selection. Autoimmune disease represents a failure of both systems.
Peptide-based approaches can target both types of tolerance. Thymus-supporting peptides like thymosin alpha-1, Thymalin, and Vilon enhance central tolerance by supporting the organ responsible for T cell selection. Immune-modulating peptides like KPV and thymosin alpha-1 enhance peripheral tolerance by promoting Treg expansion and function. The ability to support tolerance at multiple levels simultaneously is what makes peptide combinations particularly interesting for autoimmune research.
Tolerogenic peptide vaccines: the frontier
The most advanced application of peptides in autoimmune disease is the development of tolerogenic vaccines. These are specific peptide sequences derived from the self-antigens that the immune system inappropriately attacks, administered in ways that induce tolerance rather than immunity.
The concept works like this: by presenting the body's own peptide epitopes, the fragments of self-proteins that T cells recognize, in a tolerogenic context, researchers can selectively delete or suppress the specific T cell clones responsible for autoimmune attack. This is antigen-specific therapy. It does not affect the immune system's ability to fight infections or cancer. It only suppresses the exact immune responses causing autoimmune damage.
P140/Lupuzor for lupus is the most advanced example. In type 1 diabetes, tolerogenic vaccines containing multiple islet-derived peptides combined with cyclosporine A have induced regulatory T cells and prevented disease onset in mouse models. In MS research, tolerogenic peptide approaches targeting myelin basic protein have shown early promise. Advances in delivery technologies, including nanoparticles, lipid nanoparticles, and hydrogels, are improving peptide stability and enabling targeted presentation to the immune system.
These approaches represent the future of autoimmune treatment: not blanket suppression, but precision correction of the specific immune error driving each patient's disease.
Practical considerations for peptide research in autoimmune conditions
Anyone exploring peptides for autoimmune applications needs to understand several practical realities that affect safety, efficacy, and protocol design.
Interaction with conventional autoimmune medications
Many autoimmune patients are already taking immunosuppressive medications. Corticosteroids, methotrexate, mycophenolate, biologics (anti-TNF agents, anti-IL-6 agents, anti-CD20 agents), and JAK inhibitors each have specific mechanisms that could interact with peptide effects.
Thymosin alpha-1's immune-enhancing properties could theoretically counteract immunosuppressive medications. If you are on drugs designed to suppress immune function, adding a peptide that enhances immune activity requires careful medical oversight. This is not something to experiment with independently.
BPC-157 and TB-500, as tissue repair peptides, are less likely to directly interfere with immunosuppressive medications. However, BPC-157's angiogenic properties could theoretically affect the pharmacokinetics of injected biologics by altering tissue blood flow at injection sites.
KPV's mechanism of action (NF-kB inhibition) overlaps with some conventional autoimmune drugs, particularly corticosteroids and JAK inhibitors, which also suppress NF-kB-mediated inflammation. Combining these could potentially produce additive anti-inflammatory effects, which might be beneficial or could result in excessive immune suppression depending on the context.
The bottom line: peptide protocols for autoimmune conditions should never be designed or implemented without the involvement of a qualified healthcare provider who understands both the conventional treatment regimen and the peptide mechanisms involved. Peptide safety is always a priority, but it becomes critical when autoimmune medications are already in play.
Timing around autoimmune flares
Autoimmune diseases characteristically cycle between flares (active disease) and remission (quiescent disease). The optimal timing for peptide protocols relative to these cycles is an important consideration that lacks definitive research guidance.
Some researchers suggest that immune-modulating peptides like thymosin alpha-1 are best started during remission, when the immune system is more stable and regulatory mechanisms have a better chance of re-establishing control. Starting during an active flare might be less effective because the inflammatory environment overwhelms regulatory mechanisms.
Tissue repair peptides like BPC-157 and TB-500, by contrast, may be most valuable during or immediately after flares, when tissue damage is greatest and repair mechanisms are most needed. The timeline for peptide effects varies by compound, with anti-inflammatory effects often appearing within days while immune rebalancing may take weeks to months.
Monitoring and assessment
Objective monitoring is essential when using peptides for autoimmune conditions. Relevant blood markers include:
Inflammatory markers: C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), TNF-alpha levels, IL-6 levels
Immune cell counts: CD4/CD8 ratios, Treg percentages (CD4+CD25+FoxP3+), B cell subsets
Disease-specific markers: Anti-CCP and RF for RA, ANA and anti-dsDNA for lupus, anti-TPO for Hashimoto's
Organ function: Kidney function (creatinine, BUN) for lupus, liver function for drug monitoring, thyroid hormones for Hashimoto's
Baseline bloodwork before starting any peptide protocol is non-negotiable. Follow-up testing at 4-week intervals allows tracking of both efficacy and safety. Documenting these results systematically using tools available through SeekPeptides helps researchers track progress and adjust protocols based on objective data rather than subjective symptoms alone.
Reconstitution and administration
Proper peptide handling is always important, but it takes on additional significance for immunocompromised autoimmune patients. Contaminated injections pose a greater infection risk for individuals whose immune systems are already dysfunctional or suppressed by medication.
Follow proper reconstitution protocols rigorously. Use bacteriostatic water rather than sterile water to prevent microbial growth in multi-use vials. Maintain proper cold-chain storage at all times. Alcohol-swab injection sites. Use fresh syringes for each injection. These are standard practices for all peptide users, but the consequences of contamination are more severe for autoimmune patients.
The peptide reconstitution calculator ensures accurate mixing. Getting the concentration right matters not just for efficacy but for avoiding unnecessarily concentrated solutions that could cause injection site reactions, an issue that autoimmune patients with skin sensitivity may be more prone to.
What the research says and does not say
Honest evaluation of the evidence is essential. The peptide research landscape for autoimmune disease spans a wide range of evidence quality, and understanding where each compound stands prevents both false hope and premature dismissal.
Strong evidence
Thymosin alpha-1 has the strongest overall evidence base among peptides discussed here. It has been studied in clinical trials involving over 2,000 individuals, is approved for use in over 30 countries (primarily for hepatitis and cancer immunotherapy), and has specific published research in autoimmune populations including RA, SLE, and MS patients. The Pica et al. study documenting deficient thymosin alpha-1 levels in autoimmune patients provides a direct biological rationale for supplementation. The safety profile is well-characterized and favorable.
KPV has strong mechanistic evidence from published research in Gastroenterology and other peer-reviewed journals, with well-characterized pathways (NF-kB inhibition, PepT1 transport) and positive results in established animal models of colitis. The evidence for KPV in non-gut autoimmune conditions is less robust but mechanistically plausible.
Moderate evidence
BPC-157 has extensive animal model data showing impressive gut healing and anti-inflammatory effects, including specific IBD models. It was advanced to clinical trial designations (PL-10, PL14736) for IBD. However, published human clinical trial data remains extremely limited, with only three published human studies and no large-scale randomized controlled trials. The gap between the promising animal data and the limited human data is the most significant limitation.
TB-500 has published research demonstrating remyelination in MS animal models and well-characterized tissue repair mechanisms. However, its application specifically for autoimmune conditions (rather than general tissue repair) is largely extrapolated from its known mechanisms rather than directly studied.
Early/limited evidence
LL-37, Selank, SS-31, Epitalon, and the Khavinson bioregulators have varying levels of evidence for immune modulation, but their specific application to autoimmune disease involves significant extrapolation from general immune effects to specific autoimmune mechanisms. They are mentioned here for completeness and because their mechanisms are relevant, not because they have robust autoimmune-specific clinical data.
Researchers should calibrate their expectations accordingly. Thymosin alpha-1 and KPV have the most direct evidence for autoimmune applications. BPC-157 has strong evidence for the gut-specific aspects of autoimmune disease. The others have theoretical relevance supported by varying amounts of indirect evidence.
Peptides versus conventional autoimmune treatments
How do peptide approaches compare to the drugs currently used for autoimmune disease? This comparison reveals both the potential advantages and the significant limitations of peptide-based strategies.
Factor | Conventional immunosuppressants | Biologic drugs (TNF inhibitors, etc.) | Peptide approaches |
|---|---|---|---|
Mechanism | Broad immune suppression | Targeted cytokine blockade | Immune modulation and rebalancing |
Specificity | Low (affects all immune cells) | Medium (targets one cytokine) | Variable (some highly targeted) |
Infection risk | High | Moderate to high | Low (for modulatory peptides) |
Side effects | Extensive and common | Moderate | Generally mild |
Evidence level | Extensive RCTs, FDA-approved | Extensive RCTs, FDA-approved | Varies widely by peptide |
Tissue repair | None (may impair healing) | None | Several peptides promote repair |
Cost | Low to moderate | Very high ($20,000+/year) | Low to moderate |
FDA approval for autoimmune | Yes | Yes | Not in the US (TA1 approved elsewhere) |
The critical distinction: conventional treatments have extensive clinical trial evidence and FDA approval for autoimmune indications. Peptides do not (with the partial exception of thymosin alpha-1 in non-US markets).
This means peptides should not be viewed as replacements for proven treatments. They should be viewed as complementary approaches that may enhance outcomes, reduce medication side effects, or address aspects of disease (like tissue repair) that conventional treatments do not.
For anyone currently on conventional autoimmune medications, the decision to explore peptides should involve their treating physician. Abruptly stopping proven medications in favor of peptides with less clinical evidence is not a sound approach. Gradual integration under medical supervision, with objective monitoring of disease markers, is the responsible path. Understanding how peptide research is conducted helps set realistic expectations about what the evidence can and cannot support.
The role of lifestyle factors in autoimmune peptide protocols
Peptides do not operate in isolation. The inflammatory environment of the body significantly affects how well immune-modulating peptides can work. Several lifestyle factors either support or undermine peptide-based autoimmune protocols.
Diet and gut health
Given the gut-immune connection discussed earlier, dietary choices directly influence the baseline level of inflammation that peptides must overcome. Processed foods, refined sugars, industrial seed oils, and alcohol all increase intestinal permeability and systemic inflammation. Eliminating these reduces the inflammatory burden and gives immune-modulating peptides a better starting point.
Anti-inflammatory diets like the autoimmune protocol (AIP), Mediterranean diet, or elimination-based approaches reduce dietary triggers of immune activation. Fermented foods support a diverse microbiome, which in turn supports regulatory immune pathways. Adequate fiber feeds beneficial bacteria that produce short-chain fatty acids, natural anti-inflammatory compounds that support gut barrier integrity.
When using gut-healing peptides like BPC-157 and KPV, dietary factors can significantly amplify or negate their effects. Healing the gut lining with BPC-157 while continuing to consume foods that damage it is like mopping the floor while the faucet is still running.
Sleep and circadian rhythm
Sleep deprivation increases inflammatory cytokines and impairs Treg function. Studies show that even partial sleep restriction for one week increases CRP levels and shifts immune cell populations toward a more inflammatory profile. For autoimmune patients using peptides to promote immune regulation, inadequate sleep actively works against their protocols.
Seven to nine hours of quality sleep in a dark, cool environment is foundational. For those with sleep disturbances, peptides like DSIP (delta sleep-inducing peptide) or Pinealon may help restore sleep quality, indirectly supporting immune regulation.
Stress management
Chronic psychological stress activates the hypothalamic-pituitary-adrenal (HPA) axis, producing cortisol that initially suppresses inflammation but eventually leads to cortisol resistance and a paradoxical increase in inflammatory signaling. Many autoimmune patients report that flares are triggered or worsened by stressful periods. Managing stress through evidence-based approaches (regular exercise, meditation, social connection, time in nature) reduces the baseline inflammatory burden that peptide protocols must overcome.
Exercise
Moderate exercise has documented anti-inflammatory effects and promotes Treg expansion. However, excessive intense exercise can transiently increase inflammation and stress the immune system. For autoimmune patients, moderate activity (walking, swimming, yoga, light resistance training) provides immune benefits without triggering flares. Heavy training sessions, particularly during active disease, may be counterproductive.
The combination of appropriate lifestyle factors with targeted peptide protocols creates a synergistic environment for immune rebalancing. Peptides work better when the body's baseline inflammatory state is not working against them.
Common questions and misconceptions about peptides for autoimmune disease
The intersection of peptides and autoimmune disease generates many questions, some based on legitimate concerns and others on widespread misconceptions. Addressing these directly is more useful than letting them persist.
Will peptides cure my autoimmune disease?
No. No peptide, no combination of peptides, and no current therapy of any kind cures autoimmune disease. Autoimmune conditions can go into remission, sometimes sustained remission, but the underlying genetic susceptibility persists. Peptides may help modulate immune function, reduce inflammation, promote tissue repair, and potentially extend or deepen remission periods. Framing them as curative does a disservice to anyone managing a chronic autoimmune condition.
Can I stop my medications if I start peptides?
This decision must be made by a qualified healthcare provider based on objective disease markers, not by the patient alone and certainly not based on general information in a blog post. Some patients may eventually reduce or eliminate certain medications if peptide-supported remission is sustained, but this process requires careful monitoring, gradual tapering, and clinical oversight. Abrupt discontinuation of immunosuppressive medications can trigger severe flares.
Are immune-enhancing peptides safe for autoimmune patients?
This is the most nuanced question. Thymosin alpha-1 is sometimes described as "immune-enhancing," which understandably worries people whose disease involves an overactive immune system. The key distinction is that thymosin alpha-1 is not simply an immune stimulant. It is an immune modulator that enhances regulatory pathways (Tregs, tolerogenic dendritic cells) more than effector pathways. The net effect in autoimmune patients is typically toward balance rather than further activation. However, individual responses vary, and this underscores the importance of monitoring.
How long until I see results?
This varies dramatically by peptide and by the aspect of disease being targeted:
Anti-inflammatory effects (KPV, BPC-157): Often noticeable within days to 2 weeks
Gut healing (BPC-157, KPV): Initial improvements in 1-2 weeks, more substantial healing over 4-8 weeks
Immune rebalancing (thymosin alpha-1): Treg expansion takes weeks to months. Meaningful immune rebalancing typically requires 3-6 months
Tissue repair (TB-500, BPC-157): Varies by tissue type and damage extent, typically 4-12 weeks for meaningful structural improvement
Set realistic expectations. Autoimmune conditions took months or years to develop. Restoring immune balance is not an overnight process.
Frequently asked questions
What is the best peptide for autoimmune disease?
There is no single "best" peptide because autoimmune diseases vary widely in their mechanisms and affected tissues. Thymosin alpha-1 has the broadest immune-modulating evidence and the longest clinical track record. KPV is strongest for reducing inflammation. BPC-157 is best for gut-related autoimmune conditions. Most researchers consider combinations more effective than individual peptides for complex autoimmune conditions.
Can peptides replace biologics for autoimmune treatment?
Not based on current evidence. Biologics have extensive clinical trial data and FDA approval for autoimmune indications. Peptides have promising research but lack the large-scale randomized controlled trials needed to establish them as replacements. They are better viewed as potential complements to conventional treatment, not substitutes. Any changes to established medication regimens should be discussed with a physician.
Are peptides safe to use with methotrexate or prednisone?
Safety data on specific peptide-drug combinations in autoimmune patients is limited. Thymosin alpha-1's immune-enhancing properties could theoretically reduce the effectiveness of immunosuppressive drugs. KPV's anti-inflammatory effects may have additive interactions with corticosteroids. BPC-157 and TB-500 are primarily tissue repair peptides with less likelihood of direct drug interactions. However, always consult a healthcare provider before combining peptides with any existing medication regimen.
How do I know which peptides to use for my specific autoimmune condition?
Start with the disease-specific sections in this guide to understand which peptides have the most relevant mechanisms for your condition. Then consult with a healthcare provider experienced in both autoimmune disease management and peptide research. SeekPeptides members access detailed protocol guidance and community support for navigating these decisions with the help of experienced researchers and practitioners.
Do peptides help with autoimmune fatigue?
Fatigue is one of the most common and debilitating symptoms across autoimmune diseases. Thymosin alpha-1 has been shown to help reduce fatigue and body aches in autoimmune patients. Energy-supporting peptides like SS-31 address mitochondrial dysfunction that contributes to autoimmune fatigue. Reducing systemic inflammation with KPV can also alleviate the fatigue driven by chronic cytokine elevation. Addressing the underlying immune dysfunction often improves energy as a secondary benefit.
Can peptides help prevent autoimmune disease if I have a family history?
Prevention-focused peptide research is in early stages. Thymus-supporting peptides like thymosin alpha-1 and Thymalin theoretically support the immune tolerance mechanisms whose failure leads to autoimmune disease. Supporting gut barrier integrity with BPC-157 could reduce one known trigger of autoimmune activation. However, no peptide has been proven to prevent autoimmune disease in humans, and genetic susceptibility involves multiple factors beyond what any single intervention can address.
What is the difference between immune suppression and immune modulation?
Immune suppression reduces all immune activity broadly, leaving you vulnerable to infections and cancers. Immune modulation adjusts the balance between different immune pathways, ideally suppressing the overactive autoimmune responses while preserving or even enhancing protective immunity. Peptides like thymosin alpha-1 and KPV are classified as immune modulators because they promote regulatory pathways rather than simply suppressing all immune function. This distinction is what makes them theoretically better suited for long-term autoimmune management than broad immunosuppressants.
How do I stack peptides for autoimmune conditions?
Peptide stacking for autoimmune disease should address multiple aspects of the condition: immune regulation, inflammation reduction, and tissue repair. A common research approach combines thymosin alpha-1 (immune rebalancing) with KPV (anti-inflammatory) and BPC-157 (tissue repair). Review our peptide stacking guide for general principles, and consult our peptide stack calculator for protocol planning. Always start peptides one at a time to assess individual responses before combining.
For researchers committed to understanding and optimizing their autoimmune peptide protocols, SeekPeptides provides the most comprehensive resource available, with evidence-based guides, proven protocols, detailed dosing information, and a community of thousands who have navigated these exact questions. The intersection of peptide science and autoimmune management is complex, and having access to structured, current information makes the difference between guessing and researching systematically.
External resources
PubMed Central: Thymosin Alpha-1 comprehensive literature review
MDPI: Peptide-based therapeutics in autoimmune diseases (January 2026)
PubMed: PepT1-mediated KPV uptake reduces intestinal inflammation
In case I do not see you, good afternoon, good evening, and good night. May your immune system find its balance, your inflammation stay quiet, and your protocols stay grounded in evidence.



