Feb 4, 2026
The numbness started in your toes. Then it crept into your feet. Now it is in your calves, and the burning keeps you awake at three in the morning. You have tried gabapentin. You have tried pregabalin. You have tried duloxetine. Each one blunted the pain slightly while adding its own collection of side effects, from dizziness to weight gain to that unsettling mental fog that makes you feel like you are thinking through wet concrete. And none of them, not a single one, addressed the actual nerve damage underneath. They managed symptoms. They did not repair anything.
That distinction matters more than most people realize.
Neuropathy affects roughly 20 million Americans, and that number only counts the diagnosed cases. The actual figure is likely much higher. Peripheral nerves do possess the capacity to regenerate, unlike central nervous system neurons, but the process is agonizingly slow, roughly one millimeter per day under optimal conditions, and existing medications do nothing to accelerate it. This is where peptide research has opened an entirely different conversation. Instead of blocking pain signals downstream, certain peptides appear to promote actual nerve fiber regrowth, reduce the neuroinflammation driving ongoing damage, and restore function rather than merely masking dysfunction. The research ranges from compelling animal models to actual human clinical trials showing measurable nerve fiber regeneration in as little as 28 days. SeekPeptides built this guide to cover every peptide with meaningful neuropathy research behind it, from mechanisms and dosage protocols to the honest limitations of current evidence. Whether you are dealing with chronic pain, numbness, burning sensations, or progressive nerve degeneration, this resource covers what actually works, what shows promise, and what remains speculative.
Understanding neuropathy and why conventional treatments fall short
Neuropathy is not a single disease. It is an umbrella term covering damage to peripheral nerves, the vast network of fibers that carry signals between your brain, spinal cord, and the rest of your body. When these nerves are damaged, the signals become garbled. Pain receptors fire when nothing is wrong. Touch receptors go silent when something is. Motor fibers weaken. Autonomic fibers that control blood pressure, digestion, and heart rate malfunction in ways that seem disconnected from the original nerve injury.
The causes are numerous. Diabetes accounts for roughly 30% of all neuropathy cases, with chronic hyperglycemia damaging small blood vessels that supply peripheral nerves. Chemotherapy-induced peripheral neuropathy affects 30 to 40% of cancer patients receiving neurotoxic agents like taxanes, platinum compounds, and vinca alkaloids. Autoimmune conditions including Guillain-Barre syndrome and chronic inflammatory demyelinating polyneuropathy attack nerve myelin directly. Alcohol abuse, vitamin B12 deficiency, kidney disease, infections, and physical trauma each contribute their own share. And in roughly 25% of cases, the cause remains completely unknown, classified as idiopathic neuropathy.
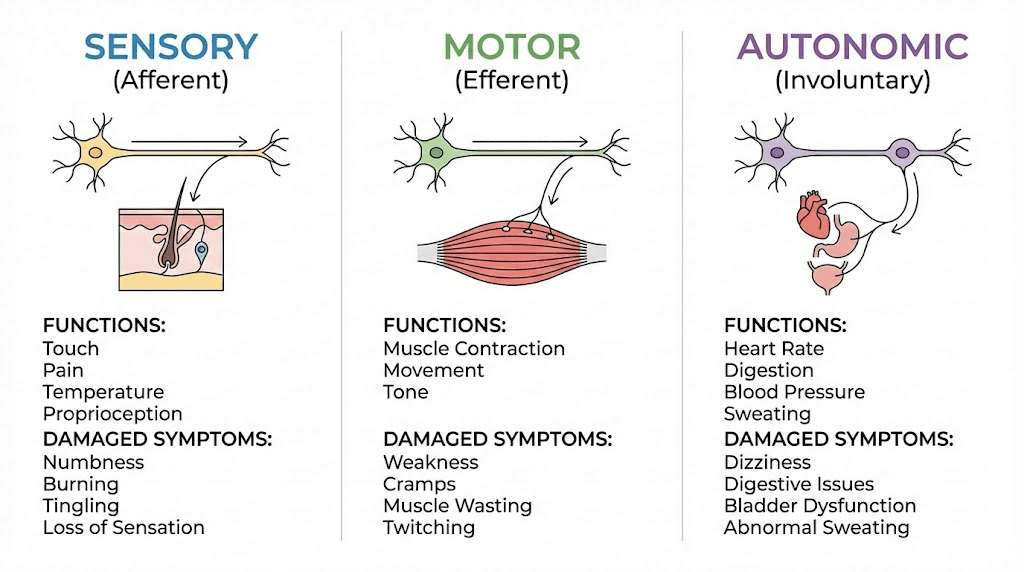
Understanding which nerves are damaged determines what symptoms appear.
Sensory nerve damage produces the symptoms most people associate with neuropathy: numbness, tingling, burning pain, pins-and-needles sensations, and hypersensitivity to touch. Small fiber neuropathy, which affects the thinnest unmyelinated nerve fibers, creates burning and stinging pain often concentrated in the feet and hands. Large fiber neuropathy, affecting myelinated fibers, produces numbness, loss of balance, and difficulty sensing joint position.
Motor nerve damage causes muscle weakness, cramping, atrophy, and difficulty with coordination. When motor nerves in the feet are affected, gait changes and foot deformities can develop over time.
Autonomic nerve damage disrupts involuntary body functions. This manifests as blood pressure instability, digestive problems, bladder dysfunction, abnormal sweating, and sexual dysfunction. The connection between autonomic neuropathy and conditions addressed by gut health peptides is increasingly recognized by researchers.
Conventional neuropathy treatments fall into two categories, and neither addresses the underlying nerve damage. The first category includes pain management medications: gabapentin, pregabalin, duloxetine, tricyclic antidepressants, and topical lidocaine or capsaicin. These modify pain signaling but leave the damaged nerves exactly as damaged as they were before. The second category includes disease management, controlling blood sugar in diabetic neuropathy, adjusting chemotherapy regimens, or treating underlying infections. These slow further damage but do not reverse existing injury.
There is no FDA-approved medication that regenerates peripheral nerves. None. Zero.
That gap in treatment options is precisely why peptide research for neuropathy has generated such intense interest among researchers. Several peptides demonstrate mechanisms that go beyond symptom management, actually promoting nerve fiber regrowth, reducing neuroinflammation, and supporting the biological environment needed for nerve healing. The evidence ranges from preclinical animal studies to human clinical trials, and the mechanisms are diverse enough that different peptides address different aspects of nerve damage. Researchers exploring tissue repair peptides consistently find that nerve regeneration is among the most promising applications.
How peptides promote nerve repair at the cellular level
Peripheral nerve regeneration is a complex biological process involving multiple cell types, signaling molecules, and structural changes. Understanding these mechanisms explains why certain peptides show such compelling neuropathy research and why a multi-peptide approach often makes more sense than targeting a single pathway.
When a peripheral nerve fiber is damaged, the portion of the axon beyond the injury point degenerates through a process called Wallerian degeneration. Schwann cells, the support cells that form myelin sheaths around nerve fibers, shift from their normal myelinating role to a repair phenotype. They clear debris, form guidance tubes called bands of Bungner, and release neurotrophic factors that signal the surviving neuron to regenerate.
This is where peptides enter the picture.
Neurotrophic factors are the molecular signals that tell neurons to grow, survive, and repair. The most important include Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and neurotrophin-3 (NT-3). Each supports different neuron populations. NGF primarily supports small-fiber sensory neurons, the exact neurons damaged in the most common forms of neuropathy. BDNF supports a broader range of sensory and motor neurons. Several peptides discussed in this guide directly increase production of these neurotrophic factors, effectively amplifying the body natural repair signaling. The connection between BDNF and neural health is explored in depth in dedicated guides.
Neuroinflammation is the second major target. After nerve injury, inflammatory cells flood the damage site. Initially, this inflammation serves a purpose, clearing debris and recruiting repair cells. But when inflammation becomes chronic, as it does in diabetic neuropathy and many other forms, it actively prevents regeneration. Pro-inflammatory cytokines like TNF-alpha, IL-1beta, and IL-6 damage surviving nerve fibers, interfere with Schwann cell repair functions, and create a hostile environment for axonal regrowth. Peptides that reduce this chronic neuroinflammation remove a major barrier to nerve healing. This anti-inflammatory approach connects with broader research on inflammation-targeting peptides.
Angiogenesis, the formation of new blood vessels, represents the third critical mechanism. Peripheral nerves depend on tiny blood vessels called vasa nervorum for their oxygen and nutrient supply. In diabetic neuropathy, microangiopathy damages these vessels, starving nerves of the resources needed for survival and repair. Peptides that promote angiogenesis restore blood flow to damaged nerves, creating the metabolic conditions necessary for regeneration.
The fourth mechanism involves direct neuroprotection, preventing further nerve cell death while repair processes are underway. Some peptides stabilize neuronal membranes, reduce oxidative stress, or modulate excitotoxic signaling that kills vulnerable neurons during the recovery period.
Each peptide discussed in the following sections addresses one or more of these mechanisms. The most compelling candidates address multiple mechanisms simultaneously, which is why they show stronger results than compounds targeting a single pathway. Researchers using the peptide stack calculator can explore how combining peptides with complementary mechanisms might address neuropathy from multiple angles.
BPC-157 for neuropathy and nerve regeneration
BPC-157 stands as arguably the most researched peptide for peripheral nerve regeneration, with a body of preclinical evidence that spans multiple nerve injury models, administration routes, and outcome measures. This 15-amino-acid peptide, derived from a protective protein in human gastric juice, has demonstrated effects on nerve healing that go well beyond what its gastroprotective origins might suggest.
The sciatic nerve transection study
The landmark study establishing BPC-157 nerve regeneration capacity comes from Gjurasin and colleagues, who studied rats with completely transected sciatic nerves, one of the most severe nerve injury models available. This is not a compression injury or a partial damage model. The nerve was cut completely through.
Rats receiving BPC-157 at doses of 10 micrograms or 10 nanograms per kilogram showed improvement across every measured outcome: clinical recovery, microscopic nerve architecture, morphometric analysis, electromyography (EMG), and functional walking recovery measured by the sciatic functional index. The BPC-157 research profile continues to expand as more nerve-related studies emerge.
Specifically, BPC-157 treated rats exhibited faster axonal regeneration, improved presentation of neural fascicles, a homogeneous regeneration pattern (indicating organized rather than chaotic regrowth), increased density and size of regenerative fibers, and evidence of both epineural and perineural regeneration. The regeneration was not just faster. It was structurally better.
Perhaps most significant for neuropathy patients was the finding on autotomy behavior. Autotomy, where animals mutilate a denervated limb, is considered a behavioral marker of neuropathic pain. BPC-157 completely eliminated autotomy behavior, suggesting direct effects on neuropathic pain reduction alongside structural nerve repair.
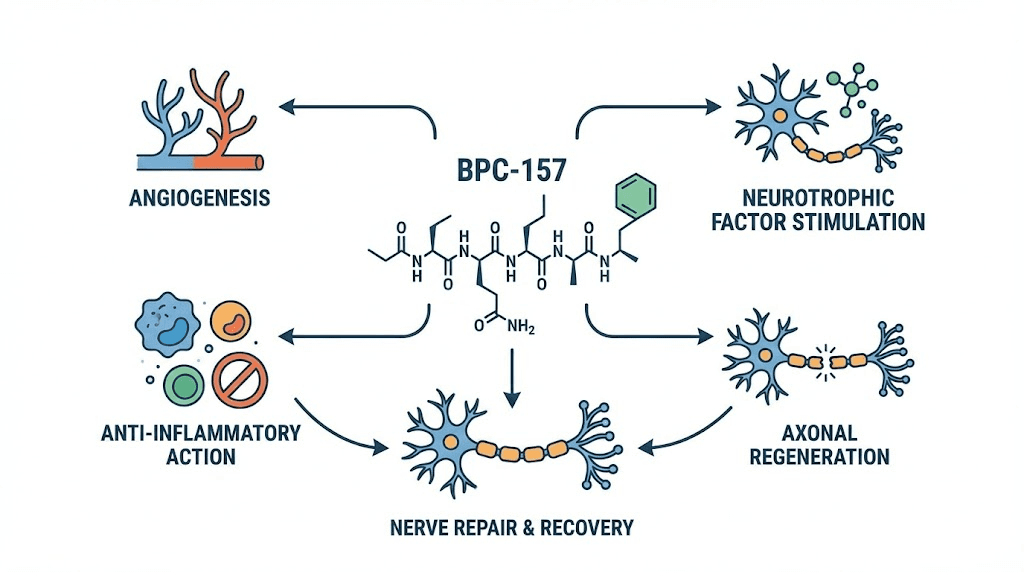
How BPC-157 promotes nerve healing
BPC-157 activates several overlapping pathways relevant to nerve regeneration. The VEGFR2 pathway and nitric oxide synthesis via the Akt-eNOS axis promote angiogenesis, restoring blood supply to damaged nerve tissue. This addresses the vascular component of nerve damage that is particularly relevant in diabetic neuropathy where microangiopathy starves nerves of oxygen and nutrients.
The peptide also modulates serotonergic and dopaminergic systems, which has implications beyond mood. Serotonin receptors are present on peripheral nerve fibers and play roles in pain modulation and nerve fiber maintenance. Dopaminergic modulation influences neuronal survival signaling. These neurotransmitter interactions, detailed in the peptide solutions guide, contribute to both the analgesic and regenerative effects observed in neuropathy models.
BPC-157 protects somatosensory neurons, promotes peripheral nerve regeneration after transection, counteracts the otherwise progressing course after traumatic brain injury, and in rat spinal cord compression models with tail paralysis, addresses axonal and neuronal necrosis, demyelination, and cyst formation while rescuing tail function. The breadth of neurological applications suggests that BPC-157 acts on fundamental nerve repair mechanisms rather than pathway-specific targets.
BPC-157 dosage protocols for neuropathy research
Research protocols for BPC-157 in neuropathy contexts typically use subcutaneous administration at 250 to 500 mcg once or twice daily for 4 to 8 weeks. The BPC-157 dosage calculator helps researchers determine precise amounts based on concentration and body weight. For nerve-specific applications, subcutaneous injection near the affected area (when accessible) may offer advantages over distant-site injection, though systemic administration also shows efficacy in the published research.
The animal studies used both local application at the injury site and systemic administration (intraperitoneal and intragastric), with both routes showing significant effects. This versatility in administration route is practically important because neuropathy often affects diffuse nerve territories rather than a single discrete injury point. Researchers working with BPC-157 should review the reconstitution guide and the reconstitution calculator to ensure accurate preparation.
Proper storage is critical for maintaining peptide integrity. The peptide storage guide covers optimal conditions, and the guide on how long peptides last after reconstitution provides specific stability data. For BPC-157 specifically, reconstituted peptide should be refrigerated at 2 to 8 degrees Celsius and used within a reasonable timeframe to maintain biological activity.
Limitations of BPC-157 evidence
Intellectual honesty requires noting that virtually all BPC-157 nerve regeneration evidence comes from animal models. Only three pilot human studies have examined BPC-157, focusing on knee pain, interstitial cystitis, and intravenous safety rather than neuropathy specifically. The preclinical evidence is robust, consistent, and mechanistically plausible, but the gap between rat sciatic nerve transection models and human diabetic neuropathy is significant. Until well-designed human clinical trials specifically examine BPC-157 for neuropathy, the compound remains investigational for this application. The broader context of peptide legality and regulatory status is important for researchers to understand.
ARA-290 (cibinetide) for neuropathy: the clinical trial peptide
If BPC-157 represents the strongest preclinical evidence for peptide-based nerve repair, ARA-290 represents the strongest clinical evidence. This synthetic 11-amino-acid peptide, also known as cibinetide, has completed multiple human clinical trials specifically targeting neuropathy, with results that have attracted FDA Fast Track designation and orphan drug status.
What makes ARA-290 different
ARA-290 was engineered from the tissue-protective domain of erythropoietin (EPO). EPO is well known for stimulating red blood cell production, but it also has powerful tissue-protective and neuroprotective effects. The problem with using EPO for neuropathy is that its blood-stimulating effects create dangerous side effects, increasing blood viscosity and thrombosis risk. ARA-290 was specifically designed to retain the neuroprotective and tissue-repair properties of EPO while completely eliminating its erythropoietic activity. It does this by activating the innate repair receptor (IRR), a heteromer of the EPO receptor and the beta-common receptor (CD131), which simultaneously triggers anti-inflammatory and tissue repair pathways. The ARA-290 benefits guide covers the full scope of this peptide research profile.
What sets ARA-290 apart mechanistically is that it does not simply mask pain. It reduces the underlying inflammation driving nerve damage and stimulates actual nerve fiber regrowth from damaged axons. This was not just observed in animal models. It was measured in human clinical trials using corneal confocal microscopy, a technique that directly visualizes and quantifies small nerve fiber density.
Clinical trial results
Sarcoidosis-associated small fiber neuropathy: In a blinded, placebo-controlled trial, 28 days of daily subcutaneous ARA-290 administration in patients with documented small nerve fiber loss produced significant improvements. Neuropathic and autonomic symptoms improved. Quality of life scores improved. Exercise capacity, measured by the 6-minute walk test, showed that 50% of ARA-290 patients could walk at least 25 meters more compared with only 12% of placebo patients. Most remarkably, ARA-290 initiated measurable regrowth of small nerve fibers in the cornea, providing direct evidence of nerve regeneration in humans.
Type 2 diabetes with neuropathy: Forty-eight patients with moderate to severe small fiber neuropathy-induced chronic pain received daily subcutaneous injections of ARA-290 at 4 mg or placebo for 28 days. The ARA-290 group showed improvement in hemoglobin A1c and lipid profiles throughout the 56-day observation period. Neuropathic symptoms improved significantly. Patients with reduced corneal nerve fiber density showed significant increases in nerve fiber density compared with no change in the placebo group.
The principal investigator, Dr. Daniel Culver of the Cleveland Clinic, stated that the magnitude of effect in only 28 days was remarkable and suggested cibinetide has the potential to become a transformative disease-modifying therapy. No existing neuropathy treatment has demonstrated actual nerve fiber regeneration in human clinical trials at this scale.
Current status and access
Cibinetide has been granted US and EU Orphan Drug Designation for sarcoidosis treatment and received US Orphan Drug and Fast Track designations for neuropathic pain in sarcoidosis patients. However, it remains investigational and is not yet approved by the FDA for routine therapeutic use. Access is currently limited to clinical trials and expanded access programs. For researchers following neuropathy peptide developments, ARA-290 represents the most advanced compound in the clinical pipeline.
GHK-Cu for nerve regeneration and neuroprotection
GHK-Cu (glycyl-L-histidyl-L-lysine copper complex) is best known for its remarkable effects on skin healing and collagen production. But its nerve-related research, while less publicized, reveals a peptide with significant potential for neuropathy applications through mechanisms that are fundamentally different from BPC-157 or ARA-290.
Nerve growth factor stimulation
When severed nerves in rats are placed in a collagen tube impregnated with GHK-Cu, nerve outgrowth increases significantly. GHK-Cu increased production of nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), sped up regeneration of nerve fibers from nerve stubs, and increased axon count and proliferation of Schwann cells compared to controls. The GHK-Cu comprehensive guide covers the full range of this peptide biological activities.
The Schwann cell proliferation finding deserves particular attention for neuropathy researchers. Schwann cells are the support cells responsible for myelinating peripheral nerve fibers, and their shift to a repair phenotype after nerve injury is essential for regeneration. By increasing Schwann cell proliferation, GHK-Cu enhances the cellular infrastructure needed for nerve regrowth.
Multiple independent laboratories have confirmed that GHK stimulates nerve outgrowth. Monique Sensenbrenner at a French lab and Gertrude Lindler at a German lab both demonstrated this effect in cell cultures, providing cross-laboratory validation of the nerve growth-promoting properties.
Gene expression and the nervous system
Broad Institute Connectivity Map data reveals that GHK influences the expression of a staggering number of human genes, with 50% or greater change in expression in roughly 31% of all human genes examined. When researchers specifically searched for GHK effects on neuron-related gene ontology, they found 408 genes upregulated and 230 genes downregulated. This massive genomic footprint on the nervous system suggests that GHK-Cu acts as an epigenetic modifier, resetting pathological gene expression patterns toward healthier states. The GHK-Cu injection dosage guide covers administration specifics for researchers.
The neuroprotective effects have been demonstrated in animal models of brain injury. Rats pretreated with GHK showed significantly improved neurological deficits and reduced brain water content after intracerebral hemorrhage. While brain injury and peripheral neuropathy are different conditions, the neuroprotective mechanisms, including reduced oxidative stress and inhibited neuronal death, are relevant to both. For a detailed overview of brain repair peptides, the dedicated guide provides broader context.
Copper regulation and nerve health
GHK-Cu role as a copper-binding and copper-regulating molecule adds another dimension to its neuropathy relevance. Copper is essential for nerve function, playing roles in neurotransmitter synthesis, myelin formation, and antioxidant defense (through copper-zinc superoxide dismutase). Both copper excess and copper deficiency can cause neuropathy. GHK-Cu ability to regulate copper homeostasis rather than simply adding copper makes it relevant for maintaining the precise copper balance nerves require.
The peptide natural decline with age is noteworthy. Blood plasma levels drop from roughly 200 nanograms per milliliter in young adults to about 80 nanograms per milliliter by age 60. This age-related decline coincides with increased susceptibility to both neuropathy and neurodegenerative conditions, suggesting a possible protective role for endogenous GHK that diminishes with aging. Researchers interested in the longevity implications of peptides often include GHK-Cu in their protocols for this reason.
TB-500 for nerve repair and neuroinflammation
TB-500, the synthetic form of thymosin beta-4, is a 43-amino-acid peptide widely studied for tissue repair across multiple organ systems. Its relevance to neuropathy centers on three mechanisms: cell migration promotion, neuroinflammation reduction, and direct effects on nervous system repair cells.
Neural repair mechanisms
TB-500 enhances the repair of oligodendrocytes and astrocytes, the glial cells that support nerve fiber function and maintenance. In the peripheral nervous system, analogous support cells, specifically Schwann cells, play critical roles in nerve regeneration. TB-500 reduces glial scarring, which represents one of the major physical barriers to nerve regeneration after injury. When scar tissue forms at a nerve injury site, it blocks regenerating axons from crossing the gap and reaching their targets. By reducing this scarring, TB-500 removes a fundamental obstacle to nerve repair. The TB-500 benefits profile covers the full spectrum of tissue repair applications.
Anti-inflammatory effects compound the nerve repair potential. TB-500 reduces production of pro-inflammatory cytokines at injury sites, creating a more permissive environment for nerve regeneration. Since chronic neuroinflammation is a driving force in diabetic neuropathy, chemotherapy-induced neuropathy, and many other forms, this anti-inflammatory mechanism addresses a root cause rather than a downstream symptom.
Angiogenesis promotion adds the third mechanism. TB-500 stimulates the formation of new blood vessels, which is critically important for nerve repair. Regenerating nerve fibers have high metabolic demands, requiring abundant oxygen and nutrient delivery. By promoting blood vessel growth at repair sites, TB-500 ensures the vascular infrastructure needed to support nerve regeneration.
Dosage protocols for TB-500
TB-500 is typically dosed at 2 to 2.5 mg twice weekly for 4 to 6 weeks in research protocols targeting tissue repair, followed by lower maintenance doses. The TB-500 dosage calculator helps researchers determine precise dosing based on their protocol requirements. Subcutaneous injection is the standard administration route. For neuropathy applications, some researchers consider injection sites near affected nerve territories, though systemic effects from distant-site injection are also documented.
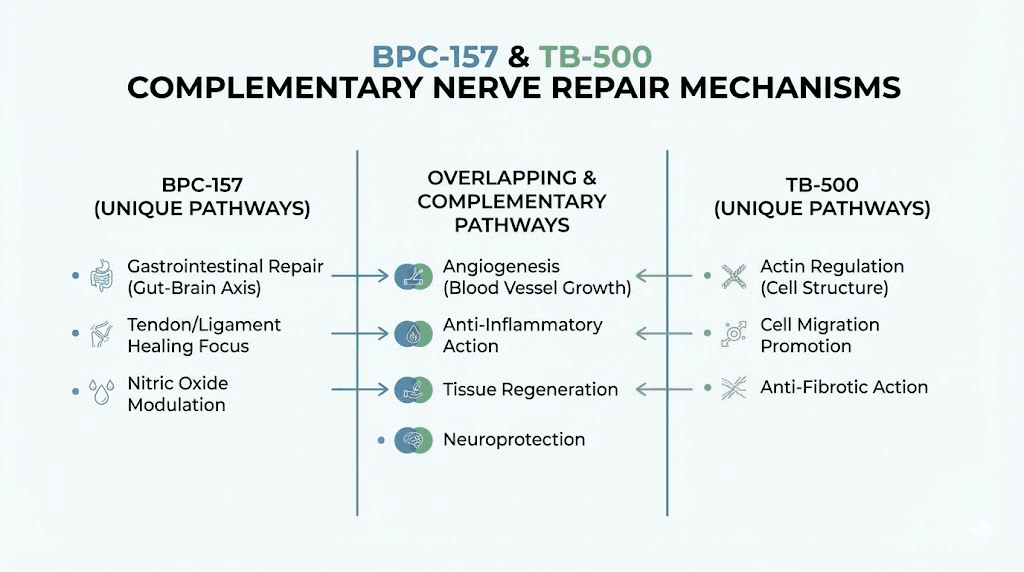
Stacking BPC-157 and TB-500 for neuropathy
The combination of BPC-157 and TB-500 represents one of the most commonly discussed peptide stacks for tissue repair, and their complementary mechanisms make particular sense for neuropathy. BPC-157 promotes axonal regeneration and neurotrophic factor production while TB-500 reduces glial scarring and supports repair cell function.
BPC-157 activates the nitric oxide pathway while TB-500 promotes angiogenesis through different mechanisms. Together, they address nerve regeneration, scar reduction, blood supply restoration, and inflammation modulation. The BPC-157 and TB-500 stacking guide covers combination protocols in detail. Researchers can compare the approaches head to head using the BPC-157 versus TB-500 comparison.
Cerebrolysin for neuropathy and neurological recovery
Cerebrolysin occupies a unique position among neuropathy-relevant peptides. It is not a single synthetic peptide but rather a complex mixture of low molecular weight neuropeptides and free amino acids derived from porcine brain tissue. Its composition includes active fragments of nerve growth factor, BDNF, ciliary neurotrophic factor, and other neurotrophic molecules. This complexity mirrors the natural neurotrophic environment more closely than any single synthetic peptide can.
Diabetic peripheral neuropathy evidence
A mouse model study specifically examining Cerebrolysin effects on diabetic peripheral neuropathy produced dose-dependent results. Mice with established type 2 diabetes received Cerebrolysin via intraperitoneal injection for 10 consecutive days. After treatment, the number, diameter, and area of myelinated nerve fibers increased in the sciatic nerves of treated mice. The effects were clearly dose-dependent, with higher doses producing better therapeutic outcomes. The Cerebrolysin benefits guide covers the full range of neurological applications.
This finding is particularly relevant because it demonstrates actual structural nerve improvement in a metabolic neuropathy model, not just a traumatic injury model. Diabetic neuropathy involves ongoing metabolic insult combined with microvascular damage, and the fact that Cerebrolysin improved nerve fiber parameters in this challenging context suggests robust neurotrophic effects.
Human clinical evidence
Cerebrolysin has been used clinically in some countries for decades, primarily for stroke recovery, dementia, and traumatic brain injury. Published clinical data suggest that Cerebrolysin is associated with more rapid neurological recovery after various peripheral nerve lesions compared to conventional therapies including steroids and supportive treatments such as vitamins and antioxidants.
Clinical dosing typically ranges from 5 to 30 milliliters daily for 10 to 20 day courses, administered by injection. The clinical use track record in Eastern Europe and Asia provides a level of real-world safety and efficacy data that purely preclinical peptides lack, though large-scale randomized controlled trials specifically for peripheral neuropathy are still needed. Researchers interested in broader brain function optimization often encounter Cerebrolysin as a well-established option in the neuropeptide space.
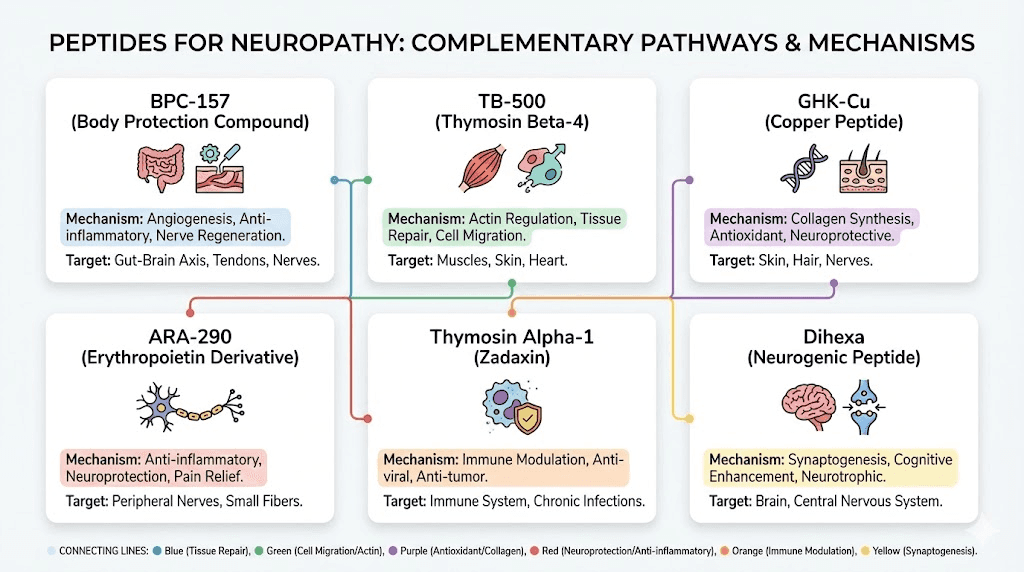
Additional peptides with neuropathy-relevant mechanisms
Beyond the primary peptides discussed above, several additional compounds demonstrate mechanisms relevant to neuropathy through distinct biological pathways. While their evidence bases for neuropathy specifically may be less developed, their mechanisms warrant consideration by researchers building comprehensive nerve repair protocols.
Semax for neurotrophic support
Semax, a synthetic heptapeptide derived from ACTH(4-10), upregulates BDNF expression in brain regions relevant to neurological function. While studied primarily for cognitive enhancement and stroke recovery, its BDNF-boosting properties have direct relevance to peripheral nerve maintenance and repair. BDNF supports the survival and function of sensory and motor neurons, and its deficiency contributes to nerve degeneration. The Semax dosage guide covers administration protocols for researchers interested in neurotrophic applications.
Semax also modulates neuroinflammation through multiple pathways, reducing the chronic inflammatory signaling that drives ongoing nerve damage in many neuropathy subtypes. Intranasal administration delivers the peptide efficiently to central nervous system targets, though peripheral effects on neurotrophic factor production may also benefit peripheral nerve health indirectly.
Selank for neuroinflammation
Selank, a synthetic analog of the immunomodulatory peptide tuftsin, demonstrates anti-inflammatory and neuroprotective properties that are relevant to neuropathy through the inflammation-reduction pathway. By modulating immune cell activity and reducing neuroinflammatory signaling, Selank may create a more favorable environment for nerve repair. Its anxiolytic effects are also practically relevant, since chronic neuropathy pain frequently coexists with anxiety and sleep disruption, both of which impair healing. Detailed protocol information is available in the Selank dosage guide.
KPV for inflammation-driven neuropathy
KPV, a tripeptide (Lys-Pro-Val), blocks NF-kB activation, the master regulator of inflammatory gene expression. This mechanism is relevant to neuropathy because NF-kB-driven inflammation is a central mediator of nerve damage in diabetic neuropathy, autoimmune neuropathies, and chemotherapy-induced peripheral neuropathy. By suppressing pro-inflammatory cytokine production at the transcriptional level, KPV addresses a root cause of inflammatory nerve damage. The KPV benefits profile explores the full anti-inflammatory mechanism, and its gut-healing effects are additionally relevant for neuropathy patients with concurrent gastrointestinal symptoms.
SS-31 for mitochondrial neuropathy
SS-31 (Elamipretide) targets the inner mitochondrial membrane, stabilizing cardiolipin and improving electron transport chain efficiency. Mitochondrial dysfunction is increasingly recognized as a contributor to neuropathy, particularly in diabetic and chemotherapy-induced forms where mitochondrial damage in dorsal root ganglion neurons precedes and contributes to axonal degeneration. By restoring mitochondrial function at the cellular level, SS-31 addresses one of the upstream causes of nerve damage that other peptides do not directly target. The SS-31 benefits guide covers the mitochondrial support mechanism in detail, while the broader role of energy-supporting peptides provides additional context.
MOTS-c for metabolic neuropathy support
MOTS-c, a mitochondrial-derived peptide, influences cellular energy production and metabolic function. Its relevance to neuropathy is most direct in the metabolic neuropathies, particularly diabetic peripheral neuropathy, where impaired glucose metabolism, insulin resistance, and mitochondrial dysfunction all contribute to nerve damage. By improving metabolic function at the cellular level, MOTS-c may support the high-energy demands of nerve repair and maintenance. The MOTS-c benefits guide covers the metabolic mechanisms, and the MOTS-c dosage chart provides protocol specifics.
Pinealon for neuroprotection
Pinealon (Glu-Asp-Arg) is a tripeptide bioregulator targeting the pineal gland and central nervous system. Its relevance to neuropathy comes through serotonin synthesis promotion and neuroprotective gene expression modulation. Serotonin receptors are present on peripheral sensory neurons, and serotonergic signaling plays roles in pain modulation and nerve fiber maintenance. The Pinealon benefits guide explores the broader neuroprotective profile, and the bioregulator peptides guide covers the Khavinson research framework that produced Pinealon and related compounds.
Peptide stacking strategies for neuropathy
Neuropathy is a multi-mechanism disorder. Nerve damage involves inflammation, vascular compromise, neurotrophic factor deficiency, oxidative stress, and mitochondrial dysfunction. No single peptide addresses all of these simultaneously. This is why strategic stacking, combining peptides with complementary mechanisms, offers a logical approach to comprehensive nerve support.
Stack 1: structural nerve repair (BPC-157 + TB-500)
This is the foundational neuropathy stack, combining the two peptides with the most direct evidence for nerve tissue regeneration. BPC-157 promotes axonal regrowth, neurotrophic factor production, and angiogenesis through the nitric oxide pathway. TB-500 reduces glial scarring, promotes repair cell migration, and supports angiogenesis through complementary mechanisms. Together, they address the structural requirements for nerve repair from multiple angles.
Standard protocols use BPC-157 at 250 to 500 mcg daily (subcutaneous) alongside TB-500 at 2 to 2.5 mg twice weekly. Some researchers begin with BPC-157 alone for the first two weeks to establish a baseline response before adding TB-500. The peptide stacking guide covers general principles for multi-peptide protocols, and the cycle planning guide addresses timing and cycling considerations.
Stack 2: neurotrophic + anti-inflammatory (Semax + Selank)
This intranasal stack targets the neurochemical environment supporting nerve repair. Semax upregulates BDNF production and modulates neurotransmitter balance. Selank reduces neuroinflammation and provides neuroprotective effects. Both peptides are administered intranasally, simplifying the protocol for researchers already managing subcutaneous injections of other compounds. The nasal spray peptide guide covers intranasal administration techniques.
Stack 3: metabolic neuropathy support (BPC-157 + SS-31 or MOTS-c)
For neuropathies with a strong metabolic component, particularly diabetic peripheral neuropathy, combining a direct nerve repair peptide with a mitochondrial support peptide addresses both the damage and its upstream metabolic cause. BPC-157 repairs nerve tissue while SS-31 or MOTS-c restores the mitochondrial function needed to sustain nerve cell health and support the energy-intensive process of regeneration.
Cycling considerations for neuropathy protocols
Long-term neuropathy protocols require thoughtful cycling to prevent receptor desensitization and maintain efficacy. BPC-157 cycling typically follows patterns of four to six weeks on, two to four weeks off. TB-500 loading phases of four to six weeks are often followed by maintenance dosing at reduced frequency. The peptide cycling guide provides compound-specific cycling recommendations, and the timeline guide helps set realistic expectations for when nerve-related improvements might become noticeable.
Comparison table: peptides for neuropathy
Peptide | Primary mechanism | Evidence level | Route | Best for |
|---|---|---|---|---|
BPC-157 | Axonal regeneration, angiogenesis, neurotransmitter modulation | Strong preclinical | Subcutaneous, oral | Traumatic nerve injury, general nerve repair |
ARA-290 | IRR activation, anti-inflammatory, nerve fiber regrowth | Human clinical trials | Subcutaneous | Small fiber neuropathy, diabetic neuropathy |
GHK-Cu | NGF/NT-3/NT-4 production, Schwann cell proliferation, gene modulation | Moderate preclinical | Subcutaneous, topical | Age-related neuropathy, neuroprotection |
TB-500 | Glial scar reduction, cell migration, anti-inflammatory | Moderate preclinical | Subcutaneous | Injury-related neuropathy, nerve scar tissue |
Cerebrolysin | Multi-neurotrophic factor support, myelination | Clinical use data | Injection | Diabetic neuropathy, broad neurological repair |
Semax | BDNF upregulation, monoamine tuning | Moderate clinical | Intranasal | Neurotrophic support, cognitive-nerve overlap |
SS-31 | Mitochondrial stabilization | Early clinical | Subcutaneous | Metabolic and mitochondrial neuropathies |
KPV | NF-kB inhibition, cytokine reduction | Moderate preclinical | Oral, subcutaneous | Inflammation-driven neuropathy |
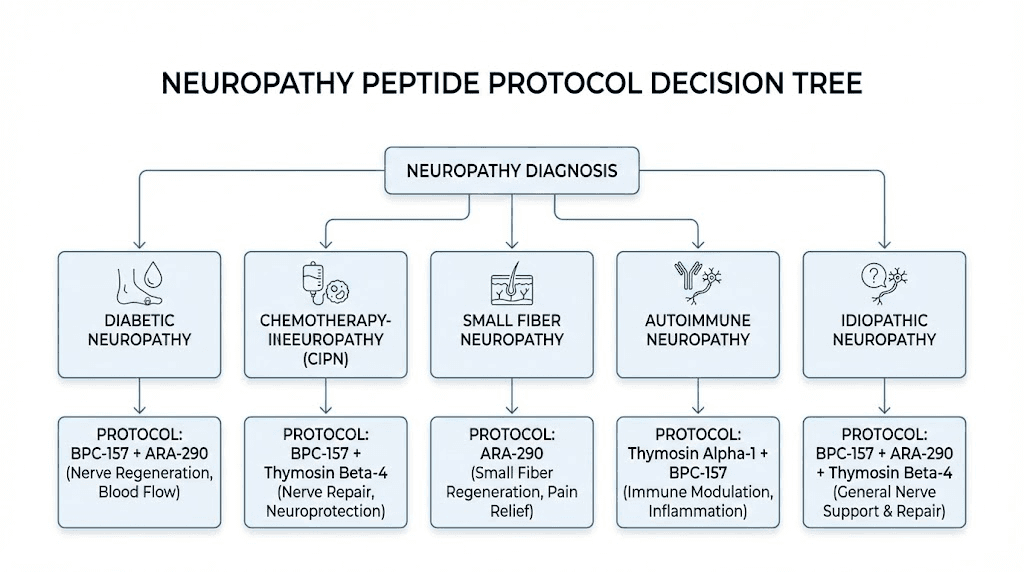
Neuropathy type-specific peptide recommendations
Different neuropathy subtypes involve different pathological mechanisms, which means the optimal peptide approach varies by diagnosis. The following recommendations match peptide mechanisms to specific neuropathy causes.
Diabetic peripheral neuropathy
Diabetic neuropathy involves chronic hyperglycemia damaging small blood vessels that feed peripheral nerves (microangiopathy), along with metabolic disruption, oxidative stress, and advanced glycation end-products damaging nerve proteins. The ideal peptide approach addresses both the vascular compromise and the nerve damage itself.
BPC-157 addresses the angiogenic component by promoting new blood vessel formation through the VEGFR2/Akt-eNOS pathway, while simultaneously promoting axonal regeneration. Cerebrolysin has specific evidence in a diabetic neuropathy mouse model showing dose-dependent nerve fiber improvements. SS-31 or MOTS-c addresses the mitochondrial dysfunction that underlies metabolic nerve damage. KPV reduces the inflammatory signaling from chronic hyperglycemia that drives ongoing nerve injury.
Blood sugar management remains the foundation of diabetic neuropathy treatment. Peptides work alongside, not instead of, metabolic control. Researchers interested in the metabolic aspects should also review the weight management peptide research, as body composition improvements can meaningfully impact glycemic control and neuropathy progression.
Chemotherapy-induced peripheral neuropathy
CIPN involves direct neurotoxic damage to dorsal root ganglion neurons, primarily through mitochondrial dysfunction, disrupted axonal transport, and neuroinflammation. The damage typically affects the longest nerves first, producing the classic stocking-and-glove distribution of numbness and pain in feet and hands.
BPC-157 neuroprotective properties, demonstrated in multiple neurotoxicity models, suggest potential for both prevention and treatment of CIPN. TB-500 anti-inflammatory and tissue repair effects address the glial response to chemotherapy-induced nerve damage. SS-31 directly targets the mitochondrial dysfunction that is a primary mechanism of taxane and platinum-based chemotherapy neurotoxicity.
Timing matters in CIPN. Early intervention, ideally concurrent with chemotherapy rather than after nerve damage has become established, may provide better outcomes than delayed treatment. However, researchers must consider potential interactions between peptides and chemotherapy drugs, a topic that requires oncologist involvement. The autoimmune and immune-modulating peptide guide covers considerations for peptide use in the context of cancer treatment.
Small fiber neuropathy
Small fiber neuropathy (SFN) affects unmyelinated C-fibers and thinly myelinated A-delta fibers, producing burning pain, tingling, and autonomic symptoms. It is diagnosed through skin biopsy showing reduced intraepidermal nerve fiber density (IENFD) or corneal confocal microscopy showing reduced corneal nerve fiber density.
ARA-290 has the strongest specific evidence for SFN, with human clinical trials demonstrating measurable nerve fiber regrowth in the cornea of SFN patients. GHK-Cu ability to stimulate NGF production is relevant because NGF is the primary neurotrophic factor supporting the small sensory neurons most affected in SFN. BPC-157 broad nerve regeneration effects apply to small fibers as well as larger myelinated nerves.
Autoimmune neuropathies
Guillain-Barre syndrome, CIDP, and other autoimmune neuropathies involve immune-mediated damage to nerve myelin or axons. The primary treatment is immunomodulation (IVIG, plasma exchange, or immunosuppressants). Peptides may serve adjunctive roles by reducing inflammation (KPV, Selank), supporting nerve repair during and after immune-mediated damage (BPC-157, TB-500), and providing neurotrophic support (Semax, GHK-Cu). The immune system peptide guide covers the broader context of peptide immunomodulation.
Idiopathic neuropathy
When the cause of neuropathy is unknown, which affects roughly 25% of patients, a broad-spectrum approach targeting multiple mechanisms makes the most sense. The BPC-157 plus TB-500 foundation addresses structural repair. Adding GHK-Cu provides neurotrophic factor support and neuroprotection. KPV addresses any inflammatory component that may be contributing even without an identified inflammatory cause. Semax provides BDNF support for neuron survival and maintenance.
Practical considerations for neuropathy peptide research
Understanding the science behind neuropathy peptides is necessary but not sufficient. Practical implementation involves decisions about administration, sourcing, monitoring, and professional guidance that determine whether a research protocol produces useful data or wasted time and money.
Administration routes and considerations
Subcutaneous injection is the standard route for most neuropathy-relevant peptides including BPC-157, TB-500, ARA-290, and GHK-Cu. For neuropathy applications, some researchers consider injection sites near affected nerve territories. The logic is that local administration creates higher peptide concentrations at the relevant tissue. However, systemic effects from distant-site injection are also documented, and for diffuse neuropathies affecting multiple nerve territories, systemic administration may provide more uniform coverage. The injectable peptides guide covers subcutaneous technique, and the peptide injection pen guide discusses tools for more comfortable administration.
Intranasal administration is preferred for Semax and Selank, delivering these peptides efficiently to the central nervous system where they influence BDNF production and neuroinflammation. Intranasal delivery partially bypasses the blood-brain barrier, which is relevant for the central mechanisms of neuropathic pain processing. The nasal spray guide covers device selection and administration technique.
Oral administration works for select peptides. BPC-157 has oral bioavailability, though it is lower than subcutaneous, and higher doses may be needed. Small bioregulator peptides like Pinealon can be taken orally. KPV can be administered orally for its gut anti-inflammatory effects. The injectable versus oral comparison helps researchers evaluate route tradeoffs. Peptide capsules offer convenience for oral administration protocols.
Reconstitution and storage
Most peptides arrive as lyophilized (freeze-dried) powder requiring reconstitution with bacteriostatic water before use. The reconstitution guide walks through the process step by step, and the reconstitution calculator eliminates math errors. For neuropathy protocols that may run for extended periods, understanding how long peptides last in powder form and after reconstitution is essential for maintaining potency throughout the protocol. Proper post-reconstitution storage at 2 to 8 degrees Celsius preserves biological activity.
Source quality and testing
Peptide quality directly affects both safety and efficacy. For neuropathy research where consistent dosing over extended periods is the norm, source reliability is non-negotiable. Third-party testing for identity, purity, and absence of endotoxin contamination should be verified for any peptide intended for research use. The peptide testing labs guide identifies reputable testing services. The peptide vial research guide covers what to evaluate when assessing sources, and understanding the grey market peptide landscape helps researchers navigate sourcing challenges.
Monitoring nerve function
Measuring progress in neuropathy requires objective tools, not just subjective symptom reporting. Nerve conduction studies (NCS) and electromyography (EMG) quantify large fiber function. Quantitative sensory testing (QST) measures sensory thresholds. Skin biopsy with IENFD counting provides the gold standard for small fiber neuropathy assessment. Corneal confocal microscopy offers a noninvasive alternative for tracking small fiber changes over time.
For home monitoring, standardized questionnaires such as the Michigan Neuropathy Screening Instrument (MNSI) and the Neuropathy Total Symptom Score (NTSS-6) provide structured self-assessment tools. Tracking symptom severity, distribution, and functional impact on a consistent schedule reveals trends that day-to-day subjective impressions would miss.
Working with healthcare providers
Neuropathy requires proper medical diagnosis. The differential diagnosis is broad, from diabetes to B12 deficiency to autoimmune conditions to malignancy, and treatment varies dramatically by cause. Starting a peptide protocol without a proper diagnosis risks missing a treatable underlying condition. A neurologist can perform nerve conduction studies, order appropriate blood work, and potentially perform a skin biopsy to establish an objective baseline against which to measure changes.
Never discontinue prescribed neuropathy medications to start a peptide protocol. Gabapentin and pregabalin in particular should not be stopped abruptly due to withdrawal risks. Any medication changes should be gradual, medically supervised, and separate from peptide protocol changes to avoid confounding variables. The online peptide therapy guide covers how to find practitioners familiar with peptide research.
Cost and duration expectations
Neuropathy peptide protocols are typically longer-term commitments compared to acute injury healing. Peripheral nerves regenerate at approximately one millimeter per day under optimal conditions. For someone with neuropathy affecting nerves in the feet, where the nerve distance from the spinal cord can be over a meter, even dramatically accelerated regeneration takes months to produce measurable clinical improvement.
The peptide therapy cost guide helps researchers budget for extended protocols, and the peptide cost calculator provides per-dose cost estimates.
Setting realistic timelines prevents premature discouragement. Most researchers report initial symptom changes (reduced pain intensity, less burning, improved sensation) within 4 to 8 weeks. Measurable nerve function improvements on objective testing typically require 3 to 6 months of consistent protocol adherence. Complete nerve regeneration in severely affected individuals may take longer. The dosing guide emphasizes the importance of consistent, long-term adherence for nerve-related applications.
What the evidence actually shows versus what it does not
Responsible reporting of peptide research for neuropathy requires separating what is well-established from what is promising but preliminary. The enthusiasm around peptides for nerve repair is justified by the science, but overstating the evidence helps no one.
What is well-established:
Peripheral nerves can regenerate. Unlike central nervous system neurons, peripheral nerve fibers have inherent regenerative capacity. The challenge is speed, completeness, and supporting the biological environment for repair.
Neurotrophic factors (NGF, BDNF, NT-3) are essential for nerve survival, maintenance, and regeneration. Increasing their levels supports nerve health.
Chronic neuroinflammation actively impedes nerve repair. Reducing it creates conditions more favorable for regeneration.
Microangiopathy (small blood vessel damage) starves nerves of oxygen and nutrients. Restoring blood supply supports nerve health.
What has strong preclinical evidence:
BPC-157 promotes peripheral nerve regeneration in animal models of sciatic nerve transection, with improved functional, morphometric, and electrophysiological outcomes.
TB-500 reduces glial scarring and promotes tissue repair cell migration in animal models.
GHK-Cu stimulates NGF, NT-3, and NT-4 production and increases Schwann cell proliferation and nerve fiber regeneration in collagen tube models.
Cerebrolysin dose-dependently improves myelinated nerve fiber parameters in diabetic neuropathy mouse models.
What has human clinical evidence:
ARA-290 produced measurable nerve fiber regrowth in humans with small fiber neuropathy in placebo-controlled trials over 28 days. It has FDA Fast Track designation.
Cerebrolysin has decades of clinical use for neurological recovery, with published data suggesting faster nerve recovery than conventional therapies.
What remains speculative or early-stage:
Specific peptide stacking protocols for neuropathy have not been tested in controlled clinical trials.
Optimal dosing for neuropathy applications is extrapolated from general tissue repair protocols rather than neuropathy-specific dose-finding studies for most peptides.
Long-term safety data for multi-peptide neuropathy protocols does not exist.
Whether preclinical nerve regeneration results translate to equivalent human outcomes is uncertain for all peptides except ARA-290, which has direct human data.
Managing expectations appropriately serves researchers better than overpromising. Peptides for neuropathy represent a genuine frontier in nerve repair research, with mechanisms that conventional treatments simply do not address. But they remain investigational compounds, and combining them with proper medical care, lifestyle optimization, and realistic timelines produces the most honest and sustainable approach.
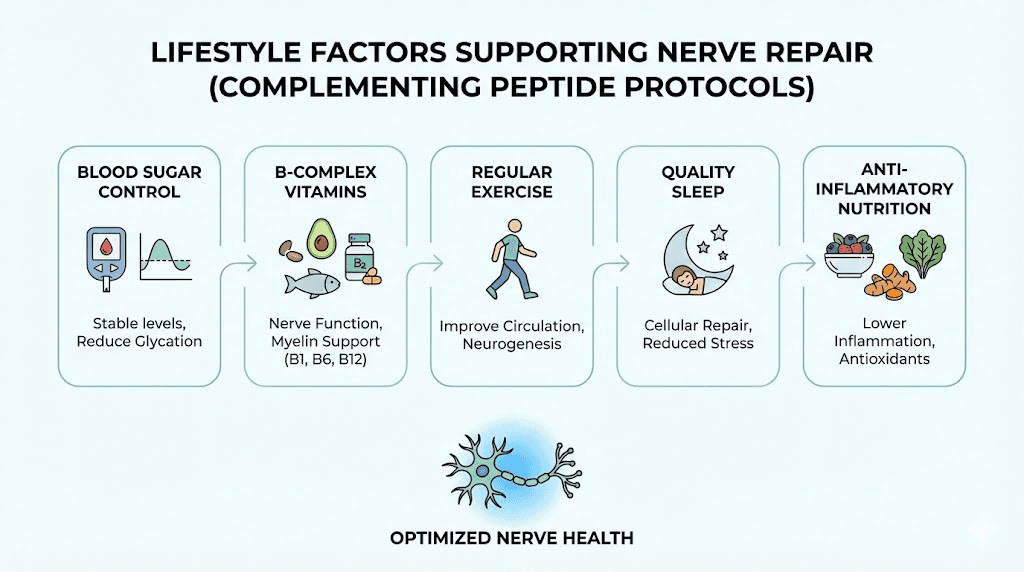
Lifestyle factors that support nerve repair alongside peptide protocols
Peptides work within a biological context. That context, determined by nutrition, activity, sleep, and metabolic health, either supports or undermines the nerve repair processes peptides are trying to promote. Ignoring these foundational factors while investing in peptide protocols is like fertilizing a garden without watering it.
Blood sugar control remains the single most important modifiable factor for diabetic neuropathy. No peptide can outpace the damage caused by chronic hyperglycemia. Keeping HbA1c below 7% slows progression, and tighter control may allow repair processes, including peptide-supported repair, to operate effectively.
B vitamin status directly affects nerve health. B12 deficiency is a common and often overlooked cause of neuropathy. B6 is required for neurotransmitter synthesis. B1 (thiamine) deficiency, particularly common in alcohol users, damages peripheral nerves. Ensuring adequate B vitamin levels removes nutritional barriers to nerve repair.
Exercise improves nerve function through multiple mechanisms: increased blood flow to peripheral nerves, reduced inflammation, improved insulin sensitivity, and upregulation of endogenous neurotrophic factor production. Walking, swimming, and cycling are generally well-tolerated by neuropathy patients. The exercise-induced increase in BDNF production complements the neurotrophic effects of peptides like Semax.
Sleep quality matters because growth hormone release during deep sleep supports tissue repair processes. Neuropathic pain often disrupts sleep, creating a vicious cycle where poor sleep impairs healing while nerve damage disrupts sleep. Addressing sleep directly, whether through sleep hygiene, sleep-supporting peptides like DSIP, or medical sleep interventions, removes a significant barrier to recovery.
Alpha-lipoic acid, an antioxidant with specific evidence in diabetic neuropathy, may complement peptide protocols by reducing oxidative stress in peripheral nerves. Clinical trials have demonstrated improvement in neuropathic symptoms at doses of 600 mg daily.
Omega-3 fatty acids reduce systemic inflammation and support nerve cell membrane integrity. Anti-inflammatory dietary patterns in general create a metabolic environment more conducive to the repair processes peptides are promoting.
Frequently asked questions
What is the best peptide for neuropathy?
It depends on the type and cause of neuropathy. ARA-290 has the strongest human clinical evidence for small fiber neuropathy, showing measurable nerve fiber regrowth in 28 days. BPC-157 has the most extensive preclinical evidence for peripheral nerve regeneration after traumatic injury. Cerebrolysin has specific evidence in diabetic neuropathy models. The best peptides for pain guide provides additional context on peptides for neuropathic pain relief.
Can peptides actually regrow nerves?
Preclinical evidence shows that several peptides promote peripheral nerve fiber regrowth in animal models. ARA-290 has demonstrated actual small nerve fiber regrowth in human clinical trials, measured by corneal confocal microscopy. Peripheral nerves do possess regenerative capacity, and peptides appear to accelerate and enhance this natural process rather than creating regeneration from nothing.
How long do peptides take to work for neuropathy?
Initial symptom improvements, such as reduced pain and burning, may appear within 4 to 8 weeks. Measurable improvements on nerve function testing typically require 3 to 6 months. Peripheral nerves regenerate at roughly 1 mm per day under optimal conditions, so protocols for neuropathy tend to be longer than those for acute injuries. The peptide timeline guide provides compound-specific expectations.
Are peptides for neuropathy safe?
Safety profiles vary by peptide and are generally favorable in available research. BPC-157 shows a strong safety profile across extensive animal studies and limited human data. ARA-290 was well-tolerated in clinical trials with no serious adverse events. Cerebrolysin has decades of clinical safety data. However, long-term safety data for multi-peptide neuropathy protocols does not exist, and individual responses vary. Professional medical supervision is important. The peptide legality guide covers regulatory considerations.
Can I use peptides alongside gabapentin or pregabalin?
This question requires medical guidance specific to your situation. There are no published studies examining interactions between neuropathy peptides and gabapentinoids. In general, peptides work through different mechanisms than gabapentin or pregabalin, which modulate calcium channel subunits. However, any combination of compounds should be discussed with a healthcare provider who understands both your neuropathy treatment and the peptides you are considering.
What is BPC-157 dosage for nerve damage?
Research protocols typically use 250 to 500 mcg administered subcutaneously once or twice daily for 4 to 8 weeks. The BPC-157 calculator helps determine precise dosing based on concentration and vial size. Some researchers administer the injection near affected nerve territories, while others use standard subcutaneous sites. Both approaches show efficacy in published research.
Is ARA-290 available for neuropathy treatment?
ARA-290 (cibinetide) is currently investigational and not approved by the FDA for routine therapeutic use. It has received Orphan Drug and Fast Track designations, indicating regulatory recognition of its potential. Access is limited to clinical trials and expanded access programs. For the latest trial information, check ClinicalTrials.gov.
Do peptides work for diabetic neuropathy specifically?
Several peptides have evidence relevant to diabetic neuropathy. Cerebrolysin improved nerve fiber parameters in a diabetic neuropathy mouse model. ARA-290 improved neuropathic symptoms and metabolic markers in type 2 diabetes patients. BPC-157 angiogenic properties address the microvascular damage central to diabetic nerve damage. Blood sugar control remains the foundation of diabetic neuropathy management, with peptides serving as potential adjuncts rather than replacements.
External resources
PubMed - National Library of Medicine - Search for peer-reviewed peptide and neuropathy research studies
National Institute of Neurological Disorders and Stroke - Peripheral Neuropathy - Comprehensive overview of neuropathy types and treatments
The Foundation for Peripheral Neuropathy - Patient education and research updates
ClinicalTrials.gov - Search for active neuropathy and peptide clinical trials
Cleveland Clinic - Peripheral Neuropathy - Evidence-based medical information on neuropathy diagnosis and treatment
For researchers serious about understanding and optimizing peptide protocols for nerve repair, SeekPeptides offers the most comprehensive resource available. Members access evidence-based protocol guides, detailed dosing information, and a community of experienced researchers who have navigated these exact challenges. When neuropathy disrupts your quality of life and conventional treatments only manage symptoms, having access to structured guidance on regenerative approaches makes the difference between hoping and knowing.
In case I do not see you, good afternoon, good evening, and good night. Join us here.



