Feb 3, 2026
Deep inside every cell, a protein quietly governs how fast you age. It sits on cell membranes in the kidneys and brain, regulating mineral balance, dampening inflammation, and defending neurons against oxidative damage. When levels are high, tissues stay resilient. When levels fall, the body accelerates toward dysfunction.
This protein is called Klotho, and its discovery fundamentally changed how scientists think about biological aging.
Named after Clotho, the Greek goddess who spins the thread of life, Klotho was first identified when researchers noticed that mice lacking the gene aged at a terrifying pace. Their arteries calcified. Their bones thinned. Their skin atrophied, and their lifespans collapsed to a fraction of normal. But here is what made the scientific community take notice: mice engineered to produce extra Klotho lived 20 to 30 percent longer than their peers. Not just longer. Healthier.
That single observation launched an entire field of research. Scientists began mapping how Klotho works at the molecular level, tracing its influence through phosphate regulation, antioxidant defense, inflammatory signaling, and brain function. They found that Klotho levels decline predictably with age and even faster with chronic kidney disease, cardiovascular illness, and neurodegeneration. They also found that certain genetic variants of the Klotho gene grant some people naturally higher cognitive performance and longer healthspans. The implications were enormous. If declining Klotho drives aging, could restoring it slow the process down or even reverse it?
That question has driven researchers toward longevity peptides and gene therapies designed to raise Klotho levels in aging humans. From a 30-amino-acid peptide fragment called KP1 that blocks kidney fibrosis, to full-length Klotho gene therapy that extended mouse lifespan by nearly 20 percent in a single injection, the therapeutic landscape is evolving rapidly. Meanwhile, lifestyle interventions, specific nutrients, and even certain anti-aging peptides have demonstrated the ability to upregulate Klotho expression naturally. This guide covers all of it. Every mechanism. Every study. Every practical strategy for harnessing the longevity protein that your body is slowly losing.
What is klotho and why researchers call it the longevity protein
Klotho is a transmembrane protein encoded by the KL gene on chromosome 13. It was discovered almost by accident when a research team in Japan created a transgenic mouse line with an insertional mutation that disrupted the gene. Those mice developed a syndrome that looked remarkably like human aging, complete with growth retardation, skin atrophy, osteoporosis, vascular calcification, pulmonary emphysema, and dramatically shortened lifespan.
The researchers named the gene after the Greek Fate who spins the thread of life. It was a fitting choice.
Subsequent experiments revealed the opposite side of the coin. When scientists engineered mice to overexpress Klotho, the animals lived significantly longer, showed reduced age-related pathology, and maintained better organ function throughout their extended lives. The protein appeared to act as a master regulator of aging, touching nearly every system in the body. This placed Klotho alongside a small group of molecules, including NAD-related compounds and telomere-associated factors like Epitalon, that researchers consider central to the biology of aging.
Klotho is expressed most abundantly in three tissues. The kidney produces the highest concentrations, where it plays an essential role in mineral metabolism and phosphate handling. The brain, particularly the choroid plexus and hippocampus, expresses significant amounts as well, and this expression appears closely linked to cognitive function and memory. The parathyroid gland rounds out the primary expression sites, connecting Klotho to calcium regulation and hormonal balance.
What makes Klotho especially interesting from a therapeutic perspective is its dual nature. The protein exists in two functional forms, each with distinct biological roles. One form stays anchored in cell membranes. The other circulates freely through the bloodstream, acting more like a hormone than a structural protein. This circulating form is what researchers measure in blood tests, and its levels correlate powerfully with health outcomes across populations. Higher circulating Klotho associates with better kidney function, stronger cardiovascular health, sharper cognition, and lower all-cause mortality. Lower levels predict the opposite.
The decline is relentless. Circulating Klotho peaks in childhood and drops steadily through adulthood. By age 70, levels may fall to half or less of what they were at age 20. Chronic diseases accelerate this decline further. Patients with chronic kidney disease, diabetes, and heart failure show Klotho levels far below age-matched healthy controls. This pattern suggests that Klotho depletion is not merely a consequence of aging but may actively drive the degenerative processes that define it. Understanding how to measure, preserve, and restore Klotho has become one of the most active areas in anti-aging research.
The two forms of klotho and how they work
The biology of Klotho splits neatly into two stories. Each form of the protein operates through different mechanisms, targets different pathways, and produces different downstream effects. Understanding both is essential for grasping why Klotho matters so much to aging and disease.
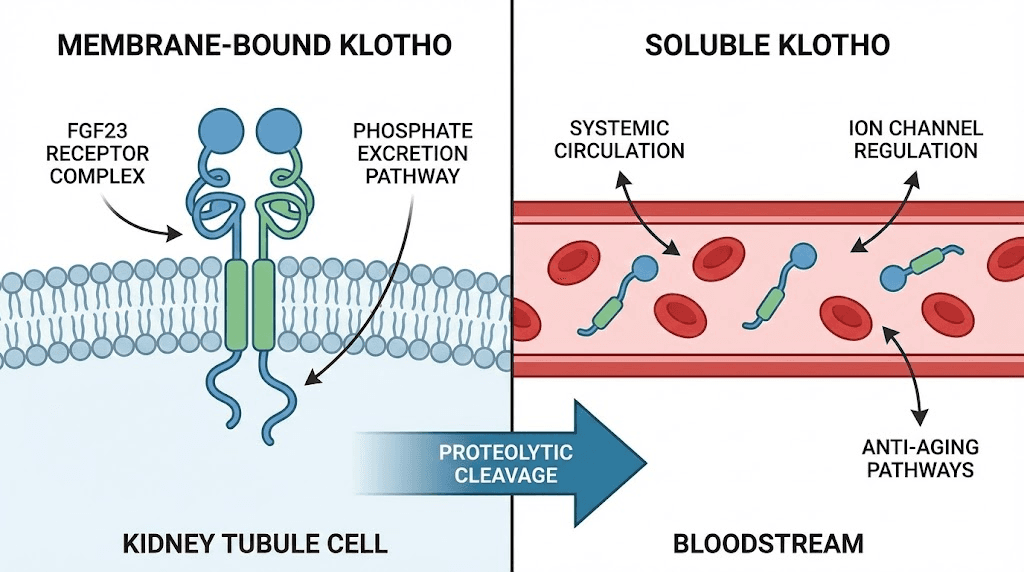
Membrane-bound klotho
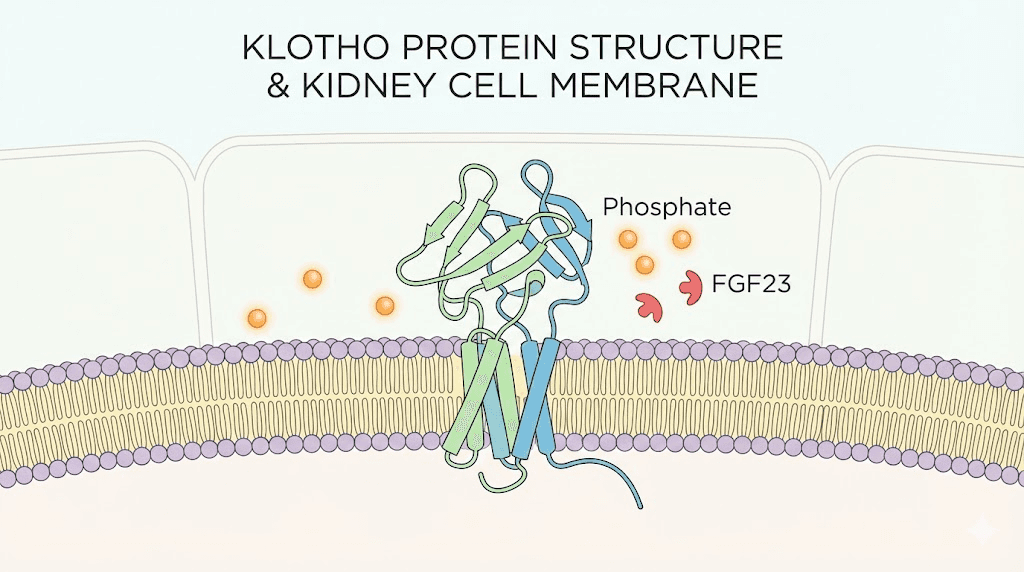
The full-length Klotho protein is a single-pass transmembrane protein with a large extracellular domain. In its membrane-bound form, it functions primarily as an obligate co-receptor for fibroblast growth factor 23, commonly known as FGF23. Without Klotho present on the cell surface, FGF23 cannot bind effectively to its receptor. The signaling simply does not occur.
This co-receptor function is critical for phosphate homeostasis. FGF23 is produced by bone cells and signals the kidneys to excrete excess phosphate. It also suppresses the enzyme that activates vitamin D. When membrane Klotho is present in sufficient quantities, FGF23 can perform these regulatory functions smoothly. When Klotho declines, as it does with aging and kidney disease, FGF23 signaling becomes impaired. Phosphate accumulates. Vitamin D metabolism goes haywire. The downstream consequences include bone mineral disorders, vascular calcification, and accelerated cardiovascular disease.
Membrane Klotho also participates in ion channel regulation, influencing calcium and potassium transport across cell membranes. This function connects it to cardiac rhythm stability and hormonal regulation in the parathyroid gland.
Soluble klotho
The extracellular domain of Klotho can be cleaved from the cell surface by enzymes called secretases, including ADAM10 and ADAM17. This cleavage releases soluble Klotho into the bloodstream, cerebrospinal fluid, and urine. Once free, soluble Klotho circulates throughout the body and acts as a hormone-like factor with remarkably diverse effects.
Soluble Klotho does not require FGF23 to function. It operates independently through several mechanisms. It inhibits insulin and insulin-like growth factor 1 signaling, which connects it to the well-established relationship between reduced IGF-1 activity and extended lifespan observed across multiple species. It suppresses Wnt signaling, a pathway that when overactive promotes cellular senescence and stem cell exhaustion. It activates antioxidant defenses by upregulating manganese superoxide dismutase and the FoxO family of transcription factors, which are critical for mitochondrial protection and cellular stress resistance.
Perhaps most remarkably, soluble Klotho inhibits NF-kB signaling, one of the master switches of inflammation. This anti-inflammatory action extends across tissues and organ systems, reducing the chronic low-grade inflammation, sometimes called inflammaging, that characterizes biological aging. It also blocks TGF-beta signaling, the primary driver of fibrosis in kidneys, lungs, heart, and liver. This anti-fibrotic property is what inspired the development of the Klotho-derived peptide KP1, which we will explore in detail later.
The dual nature of Klotho, acting locally as a co-receptor and systemically as a circulating hormone, makes it unique among aging-related proteins. Few molecules influence so many pathways simultaneously. Few decline so predictably with age. And few offer so many potential intervention points for researchers trying to slow biological aging.
The FGF23-klotho axis and mineral metabolism
The partnership between FGF23 and Klotho represents one of the most important endocrine axes discovered in recent decades. It governs phosphate balance, vitamin D activation, and calcium metabolism, three processes that directly affect bone density, cardiovascular health, and cellular energy production.
Here is how the axis works under normal conditions. Bone cells, specifically osteocytes, sense rising phosphate levels and secrete FGF23 into the bloodstream. FGF23 travels to the kidneys, where it binds to FGF receptor 1c. But this binding requires a partner. Membrane-bound Klotho must be present on the kidney cell surface to form a stable ternary complex with FGF23 and the receptor. Only then does signaling proceed.
Once activated, the FGF23-Klotho complex triggers two key actions. First, it downregulates sodium-phosphate co-transporters in the proximal tubule, increasing phosphate excretion in urine. Second, it suppresses the enzyme 1-alpha-hydroxylase, which converts inactive 25-hydroxyvitamin D into its active form, 1,25-dihydroxyvitamin D. This reduces intestinal phosphate absorption and helps maintain phosphate levels within a healthy range.
The system is elegant. It is also fragile.
When Klotho levels decline, whether from aging, kidney damage, or genetic factors, the axis breaks down. FGF23 can no longer signal effectively despite rising to extremely high concentrations in the blood. The kidneys lose their ability to excrete phosphate efficiently. Vitamin D metabolism becomes dysregulated. Phosphate accumulates in the bloodstream, driving a cascade of pathological events. Calcium-phosphate crystals deposit in blood vessel walls, causing vascular calcification. Bone mineralization becomes disordered, leading to both osteoporosis and paradoxical soft-tissue calcification simultaneously.
This scenario plays out most dramatically in chronic kidney disease, where Klotho loss is both early and severe. But subtler versions of the same disruption occur in normal aging. Researchers have found that even in otherwise healthy older adults, declining Klotho correlates with higher phosphate levels, stiffer arteries, and reduced bone mineral density. The FGF23-Klotho axis may be one of the fundamental drivers of age-related cardiovascular and skeletal deterioration. Maintaining this axis through Klotho preservation or restoration could have profound implications for healthy aging, particularly when combined with other bioregulator peptide approaches that target tissue-specific aging.
For researchers studying peptide protocols, the FGF23-Klotho axis adds an important layer of understanding. Mineral metabolism is not just about diet and supplements. It is about whether the signaling machinery is intact. Without adequate Klotho, no amount of phosphate restriction or vitamin D supplementation can fully compensate for the broken feedback loop.
How klotho protects the brain and enhances cognition
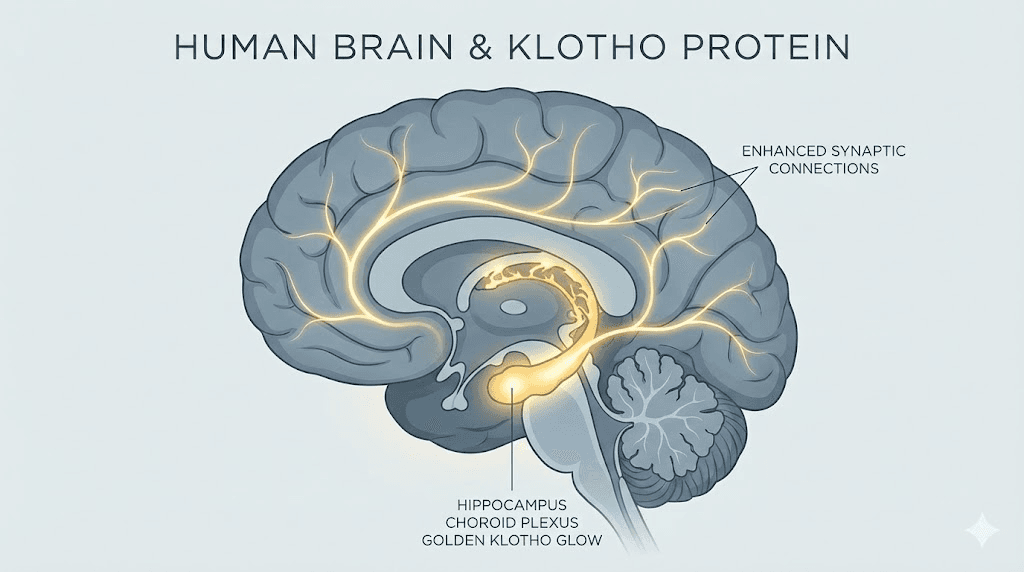
The brain is hungry for Klotho. The choroid plexus, the tissue that produces cerebrospinal fluid, expresses some of the highest Klotho levels in the body. The hippocampus, the brain region most associated with learning and memory formation, also produces significant amounts. This expression pattern is not accidental. Klotho plays a direct role in cognitive function, neuroprotection, and brain aging.
Cognitive enhancement and learning
Multiple large population studies have established a consistent association between higher circulating Klotho levels and better cognitive performance. This relationship holds across age groups, though it becomes especially pronounced in older adults. Higher Klotho correlates with faster processing speed, stronger executive function, better episodic memory, and larger volume in the prefrontal cortex, the brain region responsible for planning, decision-making, and complex thought.
Animal studies confirm these findings. Mice engineered to overexpress Klotho show enhanced spatial learning and improved long-term potentiation, the cellular mechanism underlying memory formation. Conversely, Klotho-deficient mice demonstrate severe cognitive impairment. The mechanism appears to involve modulation of NMDA receptor function, specifically enrichment of the GluN2B subunit at synapses. GluN2B-containing NMDA receptors are associated with synaptic plasticity and learning, and Klotho overexpression increases their abundance. This molecular detail connects Klotho to the same neurotransmitter systems targeted by many nootropic peptides and cognitive enhancement strategies.
What excites neuroscientists is that these cognitive benefits appear independent of age-related brain pathology. Klotho does not just protect against decline. It actively enhances cognitive capacity, even in young, healthy brains.
Neuroprotection against alzheimer pathology
The relationship between Klotho and Alzheimer disease has generated substantial research interest. Studies in both mice and humans suggest that higher Klotho levels protect against the accumulation of amyloid-beta plaques and neurofibrillary tau tangles, the two hallmark pathologies of Alzheimer disease.
The mechanism is multifaceted. Klotho reduces oxidative stress in neurons through upregulation of the Nrf2 antioxidant pathway and FoxO-mediated stress resistance genes. It suppresses neuroinflammation by inhibiting NF-kB activation in microglia, the brain resident immune cells that become chronically activated in Alzheimer disease. It promotes BDNF-related neurogenesis in the hippocampus, supporting the birth of new neurons even in aging brains. And critically, recent research suggests that Klotho directly reduces amyloid-dependent tau accumulation, potentially breaking the cascade that drives Alzheimer progression.
For individuals interested in brain health and cognitive longevity, Klotho represents a compelling target. It works through mechanisms distinct from but complementary to other neuroprotective agents like Cerebrolysin, Semax, and Selank. Where those peptides directly modulate neurotrophic factors and neurotransmitter systems, Klotho provides upstream protection by maintaining the cellular environment in which neurons thrive.
Protection against neurodegeneration
Beyond Alzheimer disease, Klotho shows protective effects in models of Parkinson disease, multiple sclerosis, and general age-related cognitive decline. Its anti-inflammatory properties reduce the microglial activation that damages neurons in all of these conditions. Its antioxidant effects protect mitochondria in neurons, cells with extraordinarily high energy demands and corresponding vulnerability to oxidative damage. Research into peptides for multiple sclerosis increasingly recognizes the role of factors like Klotho in modulating the neuroinflammatory environment.
Interestingly, Klotho also appears to support myelination, the process of wrapping nerve fibers in the insulating sheath that allows rapid signal transmission. Animal studies show that Klotho promotes oligodendrocyte maturation and myelin repair, findings with direct relevance to demyelinating diseases and age-related white matter decline. This adds yet another dimension to Klotho neuroprotection, suggesting that the protein supports not just neuronal survival but the integrity of the neural circuitry connecting brain regions.
The brain benefits of Klotho extend to mood and mental health as well. Lower Klotho levels have been associated with increased risk of depression and anxiety, conditions that often accompany aging and chronic disease. This connection may partly explain why some peptide strategies for anxiety produce benefits beyond their primary mechanisms, they may indirectly support Klotho-related pathways through reduced inflammation and improved metabolic health.
The KL-VS genetic variant and what it means for you
Not everyone starts with the same Klotho hand. A common genetic variant called KL-VS, found in approximately 20 to 25 percent of the population in heterozygous form, significantly influences Klotho function and cognitive outcomes. Understanding this variant provides a window into the genetics of aging and cognition.
KL-VS is a functional variant in the Klotho gene that contains two amino acid substitutions. People who carry one copy of this variant, heterozygotes, produce Klotho with altered properties. They tend to have higher levels of circulating soluble Klotho. And the cognitive benefits are measurable.
Large studies have shown that KL-VS heterozygotes consistently outperform non-carriers on tests of executive function, processing speed, and general cognitive ability. Brain imaging reveals that they also tend to have larger prefrontal cortex volume and greater overall brain volume, even after controlling for age and education. The effect size is meaningful, roughly equivalent to being several years cognitively younger than age-matched non-carriers. For context, this is a larger cognitive benefit than most pharmaceutical interventions have achieved in clinical trials.
There is a twist, though.
KL-VS homozygotes, the approximately 3 percent of the population carrying two copies of the variant, do not receive doubled benefits. They actually show worse outcomes than non-carriers. Their circulating Klotho levels tend to be lower, and their cognitive advantages disappear or reverse. This paradoxical dose-response, where one copy helps but two copies harm, is unusual in genetics and suggests a complex relationship between Klotho quantity, Klotho quality, and cellular signaling capacity.
The practical implications are straightforward but important. If you carry one copy of KL-VS, your biology already provides a cognitive and longevity advantage. Your goal should be preservation, maintaining the Klotho levels that your genetics support through lifestyle optimization. If you do not carry the variant, or if you carry two copies, strategies to upregulate Klotho expression become even more relevant. In either case, the same interventions that support Klotho, exercise, diet, specific supplements, and complementary peptides like Epitalon and GHK-Cu, apply regardless of genetic background.
Genetic testing services can identify KL-VS status, though the clinical utility of knowing your genotype remains debated. What is not debated is that Klotho levels matter for cognition, and that those levels are modifiable through lifestyle and potentially through peptide stacking strategies that target the pathways Klotho influences.
Klotho and kidney health
The kidney is ground zero for Klotho biology. It produces the most Klotho of any organ. It depends on Klotho for its most critical functions. And it is the first organ to suffer when Klotho declines.
In healthy kidneys, membrane-bound Klotho enables proper FGF23 signaling, maintaining phosphate balance and vitamin D metabolism as described earlier. But Klotho also provides direct protective effects on kidney tissue. It inhibits TGF-beta signaling, the primary driver of renal fibrosis, the progressive scarring that destroys kidney architecture and function. It reduces oxidative stress within kidney cells. It suppresses inflammatory signaling that would otherwise damage the tubular epithelium. And it modulates autophagy, the cellular housekeeping process that clears damaged proteins and organelles.
Chronic kidney disease presents one of the clearest examples of Klotho deficiency driving disease progression. Klotho expression in the kidney begins to decline in the earliest stages of CKD, often before clinical symptoms appear. As Klotho falls, the FGF23-Klotho axis destabilizes. FGF23 levels rise dramatically in an attempt to compensate, sometimes reaching hundreds of times normal concentrations. But without adequate Klotho, these elevated FGF23 levels become counterproductive, driving cardiac hypertrophy and further kidney damage through Klotho-independent signaling pathways.
The result is a vicious cycle. Kidney damage reduces Klotho. Reduced Klotho impairs FGF23 signaling. Impaired signaling accelerates mineral imbalance and fibrosis. Fibrosis causes more kidney damage. Breaking this cycle requires either restoring Klotho or mimicking its downstream effects, which is precisely what the Klotho-derived peptide KP1 aims to do.
For individuals focused on overall health maintenance, kidney function preservation deserves more attention than it typically receives. The kidneys influence blood pressure, electrolyte balance, red blood cell production, and metabolic waste clearance. Supporting kidney health through adequate hydration, blood pressure management, anti-inflammatory nutrition, and potentially through peptide protocols with established safety profiles represents a cornerstone of any longevity strategy.
Researchers investigating tissue repair peptides have noted that many regenerative compounds show enhanced effects in kidneys when Klotho levels are preserved. This makes intuitive sense. A kidney with adequate Klotho has better antioxidant defenses, less inflammation, and more functional repair capacity than a Klotho-depleted kidney. Maintaining Klotho may amplify the benefits of other regenerative interventions.
Cardiovascular protection through klotho pathways
Heart disease remains the leading cause of death globally, and Klotho research has revealed why some people resist cardiovascular aging better than others. Lower Klotho levels consistently predict higher cardiovascular mortality in population studies. The mechanisms linking Klotho to cardiovascular health are both direct and indirect, operating through vascular function, cardiac muscle biology, and metabolic regulation.
Vascular calcification and arterial stiffness
One of the most visible signs of cardiovascular aging is arterial stiffness, the loss of elasticity in blood vessel walls that drives systolic hypertension and increases cardiac workload. A major contributor to arterial stiffness is vascular calcification, the deposition of calcium-phosphate crystals in the smooth muscle layer of arteries. Klotho inhibits this process through multiple pathways.
By maintaining proper phosphate homeostasis through the FGF23 axis, Klotho prevents the hyperphosphatemia that promotes calcification. Soluble Klotho also directly suppresses the transformation of vascular smooth muscle cells into osteoblast-like cells, a pathological switch that drives active mineral deposition in vessel walls. Additionally, Klotho inhibits the Wnt-beta-catenin signaling that promotes this osteogenic transformation. The net effect is powerful protection against the arterial rigidity that characterizes cardiovascular aging.
This protection extends to the microvasculature as well. Klotho supports endothelial function, the ability of blood vessel linings to produce nitric oxide and regulate blood flow. Endothelial dysfunction is one of the earliest detectable changes in cardiovascular disease, preceding plaque formation by years or decades. By preserving endothelial health, Klotho helps maintain the vascular flexibility that supports healthy circulation to every organ, including the brain. Researchers exploring vascular bioregulator peptides like Vesugen recognize that these agents may work partly by supporting pathways that overlap with Klotho function.
Cardiac hypertrophy and heart failure
The heart itself is a direct target of Klotho protection. Animal studies demonstrate that Klotho deficiency promotes left ventricular hypertrophy, the thickening of heart muscle that increases the risk of heart failure. This occurs partly through uncontrolled FGF23 signaling in Klotho-depleted conditions and partly through the loss of Klotho direct cardioprotective effects.
Soluble Klotho inhibits the TRPC6 calcium channel in cardiac myocytes, reducing abnormal calcium signaling that drives pathological hypertrophy. It also suppresses inflammatory and fibrotic signaling within heart tissue, preventing the scarring that replaces functional heart muscle with stiff, noncompliant tissue. Cardiogen, a cardiac bioregulator peptide, may complement these pathways by providing tissue-specific support for heart muscle maintenance.
The cardiovascular benefits of Klotho converge with those of metabolic health more broadly. Insulin resistance, metabolic syndrome, and type 2 diabetes all associate with lower Klotho levels and higher cardiovascular risk. Whether Klotho decline drives metabolic dysfunction or vice versa remains actively debated, but the interconnection is clear. Strategies that improve metabolic health, including fat loss peptide protocols, weight management approaches, and exercise, also tend to support Klotho levels. The relationship is bidirectional and reinforcing.
Atherosclerosis and lipid metabolism
Klotho interacts with the pathways that drive atherosclerotic plaque formation. By reducing oxidative stress, suppressing inflammatory cytokine production, and modulating lipid handling in blood vessel walls, Klotho creates an environment less favorable to plaque development. Animal models show that Klotho overexpression reduces atherosclerotic lesion area, while Klotho deficiency accelerates plaque growth.
These findings have particular relevance for individuals managing cardiovascular risk through peptide-based strategies. Combining Klotho-supportive interventions with compounds that target inflammation, metabolic health, and vascular function creates opportunities for synergistic protection. The Ventfort peptide, for instance, specifically targets vascular tissue health and may work through complementary mechanisms to those of Klotho.
Anti-inflammatory and antioxidant mechanisms
Inflammation and oxidative stress are two sides of the same aging coin. They feed each other in a self-amplifying cycle that damages tissues, degrades cellular function, and accelerates biological aging. Klotho stands at a critical intersection in this cycle, suppressing both arms simultaneously.
NF-kB suppression and inflammaging
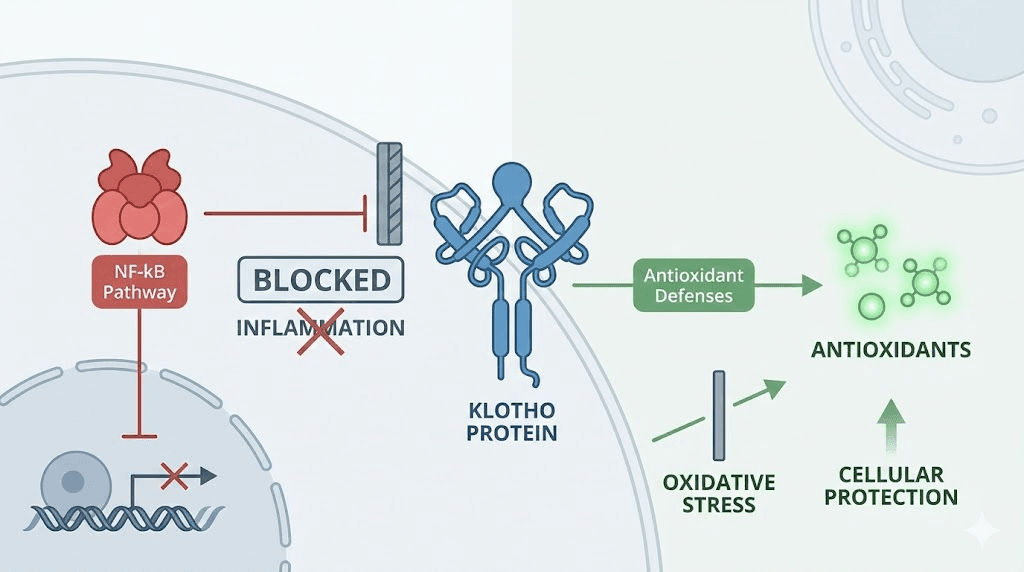
Nuclear factor kappa-B, NF-kB, is the master transcription factor controlling inflammatory gene expression. When activated, it drives production of inflammatory cytokines like interleukin-6, tumor necrosis factor alpha, and interleukin-1 beta. In aging, NF-kB becomes chronically activated even without infection or injury, producing the persistent low-grade inflammation that researchers call inflammaging. This chronic inflammation damages DNA, impairs stem cell function, disrupts tissue repair, and contributes to nearly every age-related disease.
Soluble Klotho directly inhibits NF-kB activation. It suppresses the phosphorylation of RelA, a critical subunit of the NF-kB complex, reducing its ability to enter the nucleus and activate inflammatory gene transcription. The result is a broad dampening of inflammatory signaling that extends across tissues and organ systems.
This anti-inflammatory action connects Klotho to the wider landscape of anti-inflammatory peptides. Where compounds like KPV target specific inflammatory mediators, Klotho operates upstream, reducing the overall inflammatory tone of the body. The two approaches are complementary rather than redundant. Combining upstream Klotho support with targeted anti-inflammatory peptides could provide layered protection against inflammaging.
Antioxidant pathway activation
Klotho enhances cellular antioxidant defenses through several mechanisms. It upregulates manganese superoxide dismutase, Mn-SOD, the enzyme that neutralizes superoxide radicals within mitochondria. It activates the Nrf2 pathway, the master regulator of cellular antioxidant and detoxification gene expression. And it supports FoxO transcription factors, which control a suite of stress resistance genes including catalase and other free radical scavengers.
The mitochondrial angle is particularly important. Mitochondria generate most of the cell energy supply but also produce most of the reactive oxygen species that drive oxidative damage. As mitochondria accumulate damage with age, they produce more reactive oxygen species and less energy, a downward spiral that Klotho helps counteract. The SS-31 peptide targets mitochondria directly by concentrating in the inner mitochondrial membrane, while Klotho supports mitochondrial health through broader antioxidant pathway activation. Together, they represent different but complementary approaches to preserving the cellular powerhouses that every tissue depends on.
Klotho antioxidant effects also extend to protecting DNA integrity. By reducing oxidative damage to both nuclear and mitochondrial DNA, Klotho helps maintain genomic stability, a hallmark of cellular youth. DNA damage accumulation drives cellular senescence, the permanent growth arrest that contributes to tissue dysfunction in aging. By preventing this damage, Klotho indirectly reduces the senescent cell burden that degrades tissue function over time.
Anti-fibrotic properties
Fibrosis, the excessive deposition of collagen and other extracellular matrix components, is a final common pathway for tissue damage in aging. It stiffens organs, disrupts their architecture, and replaces functional tissue with scar-like material. Fibrosis affects the kidneys, lungs, heart, liver, and skin, contributing to the progressive organ dysfunction that characterizes advanced aging.
Klotho is a potent anti-fibrotic factor. It inhibits TGF-beta signaling, the central pathway driving fibrotic responses in virtually all tissues. TGF-beta activates fibroblasts to produce excessive extracellular matrix, stimulates the transition of epithelial cells into matrix-producing mesenchymal cells, and suppresses the enzymes that degrade excess matrix. Klotho blocks these actions at multiple points, including direct binding to TGF-beta type 2 receptor and suppression of downstream Smad signaling.
This anti-fibrotic property is what makes the Klotho-derived peptide KP1 so therapeutically interesting. By concentrating the anti-fibrotic mechanism of Klotho into a small, deliverable peptide, researchers have created a potential treatment for fibrotic diseases that affect millions of people worldwide. We will examine KP1 in detail in the next section.
Klotho-derived peptide KP1 and therapeutic applications
The full Klotho protein is large, approximately 130 kilodaltons, making it challenging to produce, deliver, and stabilize as a therapeutic agent. Researchers at Yale University solved this problem by identifying the specific region of Klotho responsible for its anti-fibrotic activity and creating a small peptide that mimics this function. The result was KP1, a 30-amino-acid peptide derived from the KL1 domain of Klotho.
How KP1 works at the molecular level
KP1 binds directly to transforming growth factor beta receptor 2, TbR2, with an affinity measured at a dissociation constant of 1.4 micromolar. This binding disrupts the engagement between TGF-beta and its receptor, effectively blocking the initiation of fibrotic signaling. Downstream, KP1 inhibits both Smad-dependent and Smad-independent, specifically MAPK, TGF-beta signaling cascades. The result is comprehensive suppression of the fibrotic response.
What makes KP1 elegant as a therapeutic candidate is its specificity. Intravenous injection of KP1 in mouse models showed preferential accumulation in injured kidneys rather than healthy tissue. This targeting behavior suggests that KP1 may naturally concentrate where it is needed most, reducing the risk of off-target effects while maximizing therapeutic impact at sites of active fibrosis. Understanding how peptides work at the receptor level helps explain why this targeting occurs, injured tissues express different surface markers that may facilitate KP1 binding and uptake.
Preclinical results in kidney fibrosis
In animal models of kidney fibrosis, KP1 demonstrated remarkable efficacy. Treated mice showed significantly reduced collagen deposition, lower expression of fibrotic markers including alpha-smooth muscle actin and fibronectin, and better preserved kidney function compared to untreated controls. The peptide reduced tubular injury scores and suppressed the inflammatory infiltrate that accompanies fibrotic remodeling.
These results are significant because kidney fibrosis has no approved treatment. Current management of chronic kidney disease focuses on slowing progression through blood pressure control, glucose management in diabetics, and dietary modification. A direct anti-fibrotic agent could fundamentally change the therapeutic landscape for the millions of people worldwide living with progressive kidney disease.
The preclinical safety profile of KP1 has been encouraging. Studies in mice detected no significant adverse effects at therapeutic doses. The peptide did not appear to impair normal wound healing or tissue remodeling, suggesting that its anti-fibrotic effects target pathological fibrosis without disrupting physiological repair processes. This selectivity is critical for any anti-fibrotic therapy, as some degree of fibrotic response is necessary for normal wound healing and tissue maintenance.
Potential applications beyond kidneys
While kidney fibrosis has been the primary focus of KP1 research, the underlying mechanism, TGF-beta blockade, has implications for fibrotic diseases throughout the body. Pulmonary fibrosis, hepatic fibrosis, cardiac fibrosis, and skin fibrosis all share TGF-beta as a central driver. If KP1 can be delivered effectively to these tissues, its anti-fibrotic benefits could extend far beyond the kidneys.
Researchers investigating injury recovery peptides have noted that excessive fibrosis is a common barrier to optimal healing. Scar tissue that forms after musculoskeletal injuries, surgeries, or chronic inflammation often lacks the functional properties of the original tissue. An anti-fibrotic peptide that could modulate the healing response, reducing excessive scarring while allowing appropriate tissue repair, would represent a significant advance in regenerative medicine.
Current KP1 research products are available for laboratory investigation only. No human dosage protocol has been established, and the peptide has not entered clinical trials as of the time of writing. Researchers working with KP1 must follow all applicable regulations regarding research versus pharmaceutical peptide use. For those interested in the broader category of what peptides are and how they function as research tools, KP1 represents a fascinating example of translating basic science discoveries into potential therapeutic applications.
How to increase klotho levels naturally
While gene therapy and peptide therapeutics represent the future of Klotho restoration, several evidence-based lifestyle interventions can support Klotho levels right now. These approaches work through varied mechanisms, from direct gene expression changes to indirect support through reduced inflammation and improved metabolic health.
Exercise and physical activity
Exercise is the most robustly documented natural Klotho booster. Multiple studies across different populations confirm that regular physical activity associates with higher circulating Klotho levels, and intervention studies show that starting an exercise program can measurably increase Klotho within weeks to months.
The evidence supports both aerobic exercise and resistance training. Programs of 150 or more minutes per week of moderate-intensity aerobic activity, roughly 30 minutes five days per week, consistently show positive effects on Klotho. A controlled trial using a 16-week progressive exercise program demonstrated significant increases in circulating Klotho compared to sedentary controls. Resistance training programs of similar duration have shown comparable benefits.
The mechanisms likely involve multiple pathways. Exercise reduces chronic inflammation, improves insulin sensitivity, enhances blood flow to the kidneys where most Klotho is produced, and modulates the hormonal environment in ways that support Klotho gene expression. For individuals who already incorporate exercise into their routine for muscle growth or athletic performance, the Klotho benefits represent an additional return on their investment in physical activity. Combining exercise with peptide strategies targeting muscle preservation and recovery, such as growth hormone secretagogues or BPC-157 and TB-500 stacks, could create synergistic benefits for both performance and longevity.
Dietary patterns and specific nutrients
The Mediterranean dietary pattern shows the strongest association with higher Klotho levels among studied dietary approaches. Rich in vegetables, fruits, whole grains, nuts, olive oil, and fish, this pattern provides anti-inflammatory and antioxidant compounds that may support Klotho expression through reduced oxidative stress and inflammation.
Specific nutrients deserve individual mention.
Folate-rich foods demonstrate a dose-dependent positive relationship with Klotho. Research has shown that each additional unit of dietary folate intake associates with approximately 0.11 picograms per milliliter higher circulating Klotho. Dark leafy greens, legumes, fortified grains, and citrus fruits provide the richest folate sources.
Nuts have been positively associated with Klotho levels in middle-aged adults in cross-sectional analyses. While the specific mechanisms remain under investigation, nuts provide a combination of healthy fats, minerals like magnesium and selenium, and polyphenolic compounds that may collectively support Klotho expression.
Curcumin, the active compound in turmeric, has been shown to upregulate Klotho expression in human proximal tubule cells in laboratory studies. This finding is particularly interesting because the proximal tubule is the primary site of Klotho production in the kidney. While the translation from cell culture to clinical benefit requires caution, curcumin is a well-studied compound with established anti-inflammatory properties and a favorable safety profile.
Vitamin D has a complex bidirectional relationship with Klotho. Klotho regulates vitamin D activation through the FGF23 axis, and vitamin D may in turn influence Klotho expression. In individuals with low vitamin D levels, supplementation may support Klotho indirectly by reducing the inflammatory and metabolic stress associated with deficiency. However, the evidence for vitamin D supplementation as a direct Klotho booster is inconsistent, and excessive vitamin D can paradoxically worsen calcium-phosphate imbalances.
Intermittent fasting and caloric restriction
Caloric restriction, the most well-established longevity intervention across species, appears to support Klotho levels. Intermittent fasting protocols have been shown to elevate circulating Klotho in some studies, potentially through improved insulin sensitivity, reduced mTOR signaling, and enhanced autophagy. These same pathways are targeted by several metabolic peptides like MOTS-c that have demonstrated anti-aging effects in preclinical research.
The link between fasting and Klotho fits within the broader framework of nutrient sensing pathways in aging. When the body perceives reduced nutrient availability, it shifts from growth-oriented signaling toward maintenance and repair. Klotho upregulation may be part of this protective shift, as the protein inhibits insulin and IGF-1 signaling, two pathways whose suppression is associated with lifespan extension across species from worms to primates.
Probiotics and gut health
An emerging area of research connects the gut microbiome to Klotho expression. Studies in aging mice have shown that supplementation with specific probiotic strains, particularly combinations of Lactobacillus acidophilus and Lactococcus lactis, increased Klotho expression. The gut-kidney axis, through which microbiome metabolites influence kidney function, likely mediates this effect.
This connection aligns with growing recognition that gut health influences systemic aging processes. The gut health peptide category has expanded significantly as researchers identify more connections between intestinal function and distant organ health. BPC-157, for example, has demonstrated gastrointestinal protective effects that extend to systemic anti-inflammatory benefits, potentially supporting the kind of reduced inflammatory environment in which Klotho expression thrives.
Supplemental compounds with evidence
Several natural compounds have demonstrated Klotho-upregulating properties in laboratory and animal studies.
Astaxanthin, a carotenoid antioxidant found in salmon, shrimp, and microalgae, has shown Klotho-inducing effects in rodent kidney tissue. Its potent antioxidant properties may support Klotho expression by reducing the oxidative stress that suppresses the KL gene.
Resveratrol, the polyphenol found in red grapes and wine, upregulates Klotho in animal models through mechanisms involving SIRT1 activation. The overlap between resveratrol and sirtuin biology connects Klotho to the broader longevity research surrounding caloric restriction mimetics.
Cordyceps, a medicinal fungus with a long history in traditional medicine, has demonstrated Klotho-inducing properties in rodent kidney tissue. Whether these effects translate to meaningful changes in humans remains to be established.
Ginseng extracts have shown similar Klotho-supportive effects in animal studies, potentially through anti-inflammatory and antioxidant mechanisms. The adaptogenic properties attributed to ginseng may partly relate to its effects on stress response pathways that overlap with Klotho biology.
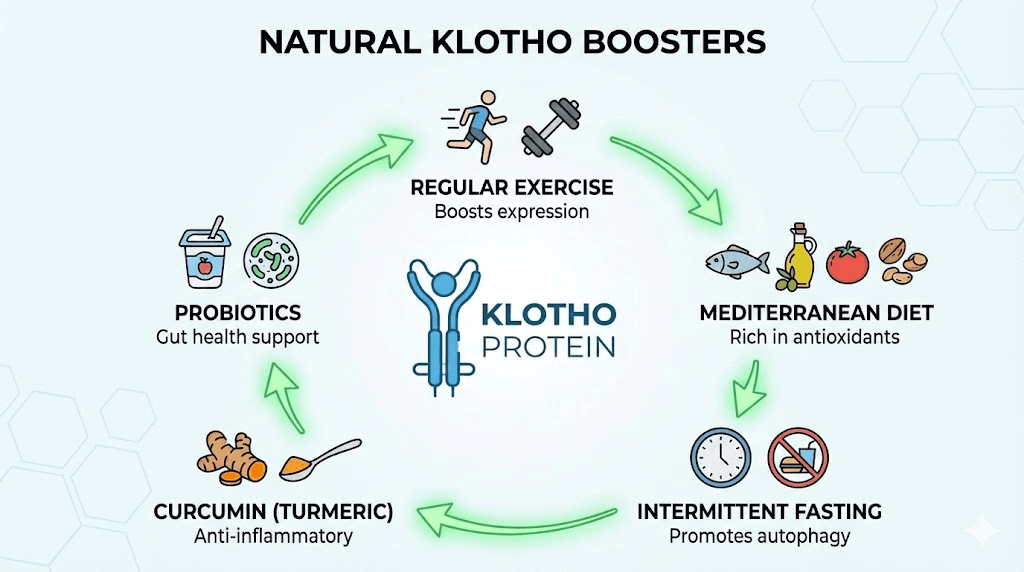
Peptides and compounds that upregulate klotho
Beyond lifestyle interventions, specific peptides and pharmacological agents have demonstrated the ability to increase Klotho expression. For researchers and biohackers already working within the peptide landscape, these findings create opportunities for targeted Klotho support.
GHK-Cu and klotho expression
GHK-Cu, the copper peptide tripeptide with established roles in skin repair and tissue remodeling, has been shown to upregulate Klotho gene expression. This finding adds an anti-aging dimension to a peptide already valued for its wound healing, collagen stimulation, and skin rejuvenation properties.
The mechanism by which GHK-Cu influences Klotho expression is not fully characterized but likely involves its known effects on gene expression patterns. GHK-Cu has been shown to modulate the expression of thousands of genes, generally shifting expression patterns toward a more youthful profile. Klotho upregulation fits within this broader gene expression resetting activity.
For those already using GHK-Cu injections for skin or tissue repair, the potential Klotho benefits represent an additional mechanism through which this peptide may support healthy aging. The peptide dosage chart can help researchers understand appropriate concentration ranges for various applications.
Epitalon and klotho
Epitalon, the tetrapeptide developed by Dr. Vladimir Khavinson and known for its telomerase-activating properties, has also been shown to upregulate Klotho expression. This creates a dual anti-aging mechanism, telomere maintenance through telomerase activation combined with Klotho-mediated antioxidant, anti-inflammatory, and metabolic benefits.
The Khavinson peptide research framework provides important context for understanding how short bioregulator peptides influence gene expression. Epitalon belongs to a class of peptides that interact with DNA and chromatin to modulate transcription of specific genes. Klotho upregulation may be one of several gene expression changes that collectively produce the anti-aging effects observed in Epitalon research.
Combining Epitalon with GHK-Cu in a planned peptide cycle could potentially provide Klotho upregulation through two independent mechanisms, an approach consistent with the multi-target strategies increasingly favored in longevity research. SeekPeptides provides resources for understanding how different peptides can be combined safely and effectively.
Pharmaceutical agents that boost klotho
Several approved medications have demonstrated Klotho-upregulating effects, though Klotho elevation is not their primary therapeutic purpose.
Angiotensin II receptor blockers, specifically losartan and valsartan, increase Klotho expression in kidney tissue. These blood pressure medications are commonly prescribed for hypertension and kidney protection, and their Klotho-boosting effects may partly explain why they provide cardiovascular and renal benefits beyond simple blood pressure reduction.
Fluvastatin, a cholesterol-lowering statin medication, has shown Klotho-upregulating effects. The mechanism may involve reduced oxidative stress and inflammation in kidney tissue, creating a more favorable environment for Klotho gene expression.
Rapamycin, the mTOR inhibitor that extends lifespan in multiple animal models, increases Klotho levels. This finding connects two major longevity pathways, mTOR suppression and Klotho elevation, and suggests they may be mechanistically linked. The overlap between rapamycin effects and Klotho biology reinforces the central role of nutrient sensing pathways in aging.
Metformin, the diabetes drug with emerging longevity applications, has been associated with higher Klotho levels in some studies. Like rapamycin, metformin modulates nutrient sensing pathways, specifically AMPK activation, and its Klotho effects may represent one mechanism through which it provides benefits beyond glucose control.
Pentoxifylline, a medication used for peripheral vascular disease, increases Klotho in chronic kidney disease patients. Its mechanism involves reduced TNF-alpha production, suggesting that anti-inflammatory effects indirectly support Klotho expression.
GLP-1 receptor agonists, the class of medications that includes semaglutide and has transformed obesity and diabetes treatment, have shown Klotho-elevating effects. For individuals already researching semaglutide dosing or exploring the semaglutide versus tirzepatide comparison, the potential Klotho benefits add another dimension to the risk-benefit analysis.
Building a klotho-supportive protocol
Combining multiple Klotho-boosting strategies creates the potential for additive or synergistic effects. A comprehensive approach might include regular exercise as the foundation, Mediterranean-style nutrition with emphasis on folate-rich foods and curcumin, intermittent fasting protocols, and targeted supplementation with compounds shown to upregulate Klotho in research settings.
For those incorporating peptides into their health optimization strategy, adding GHK-Cu or Epitalon to an existing peptide stack could provide Klotho support alongside the primary benefits these peptides offer. The peptide stack calculator can help researchers plan multi-peptide protocols that account for timing, dosing, and compatibility considerations. Understanding how many peptides you can take at once is important for anyone considering multi-compound approaches.
Clinical trials and the future of klotho therapy
The translation of Klotho research from animal models to human therapeutics has entered an exciting phase. Multiple companies and academic groups are pursuing different strategies for harnessing Klotho biology in clinical applications.
Gene therapy approaches
The most dramatic preclinical result in Klotho research came from a gene therapy study in mice. Researchers administered a single injection of an adeno-associated virus vector carrying the Klotho gene to middle-aged mice, equivalent to approximately 40-year-old humans. The treated mice lived 19.7 percent longer than controls, with improvements in bone density, muscle mass, brain aging markers, and overall functional capacity.
The breadth of improvements was remarkable. Treated mice showed restored muscle regeneration capacity, improved bone architecture, reversed astrocyte depletion in the brain, and better metabolic markers. This was from a single injection at middle age, not lifelong treatment. The results suggest that even partial restoration of Klotho levels in aging organisms can produce systemwide rejuvenation effects.
Minicircle, a biotechnology company, is developing a combination gene therapy approach that includes Klotho alongside Follistatin, another protein with anti-aging properties related to muscle preservation and metabolic function. Their approach targets healthy adults seeking preventive aging interventions, a relatively new concept in gene therapy that has traditionally focused on treating established diseases.
Recombinant protein and peptide therapeutics
Klotho Neurosciences is developing KLTO-202, a therapeutic designed to deliver Klotho protein for neurological conditions. Their initial target is amyotrophic lateral sclerosis, ALS, a devastating neurodegenerative disease with limited treatment options. Phase 1 clinical trials are expected to begin in the near future. If successful, the platform could expand to other neurodegenerative conditions including Alzheimer disease and Parkinson disease.
Jocasta Neuroscience is taking a different approach with JN-0413, a subcutaneous formulation designed for convenient repeated dosing to address cognitive decline. Their investigational new drug, IND, submission is planned for the near future. A subcutaneous formulation would make chronic Klotho therapy practical for outpatient use, similar to how many peptide injection protocols are already administered.
These clinical programs represent different strategies for the same underlying goal: getting functional Klotho into aging human bodies. Gene therapy aims for sustained endogenous production. Recombinant protein therapy provides exogenous Klotho directly. Small peptide mimetics like KP1 capture specific functional domains. Each approach has advantages and limitations.
Challenges in klotho therapeutics
Several significant challenges remain before Klotho-based therapies reach widespread clinical use.
The full-length Klotho protein is large and complex, making it difficult to manufacture at scale, maintain stability during storage and transport, and ensure consistent bioavailability after administration. These are the same challenges that face many protein therapeutics, but they are particularly acute for a protein that must reach diverse tissues throughout the body.
Delivery to the brain presents additional hurdles. The blood-brain barrier restricts passage of large molecules, and while Klotho does appear in cerebrospinal fluid naturally, achieving therapeutic concentrations through exogenous administration may require specialized delivery strategies. Research into nasal spray peptide delivery and other alternative routes reflects the broader challenge of delivering biologics to the central nervous system.
Dosing optimization presents another challenge. The KL-VS genetics illustrate that more is not always better with Klotho. Homozygous carriers, with presumably more variant Klotho, fare worse than heterozygotes. Finding the therapeutic window, enough to provide benefit without triggering compensatory suppression or adverse effects, will require careful dose-finding studies.
Long-term safety data in humans does not yet exist for exogenous Klotho administration. While the preclinical safety profile is encouraging and the protein is naturally present in the body, chronic supplementation at supraphysiological levels could have unforeseen consequences. Researchers approaching any novel therapeutic intervention should familiarize themselves with peptide safety principles and risk assessment frameworks.
The convergence with other longevity strategies
Klotho therapy will not exist in isolation. Its future clinical applications will almost certainly integrate with other longevity interventions to create comprehensive anti-aging protocols. The peptide research community is already exploring how different compounds can work together to target multiple aging pathways simultaneously.
Consider the potential synergies. MOTS-c targets mitochondrial function and metabolic health. Epitalon addresses telomere maintenance and potentially Klotho upregulation. SS-31 protects mitochondrial membranes directly. Thymalin supports immune system rejuvenation. Pinealon targets brain-specific aging pathways. Klotho addresses upstream regulatory mechanisms that influence all of these systems. A future in which these interventions are combined based on individual biomarker profiles represents the promise of personalized longevity medicine.
The regulatory landscape for peptide therapeutics continues to evolve, and researchers should stay informed about how new approvals and restrictions affect access to these compounds. Understanding peptide legality in different jurisdictions helps ensure that research activities comply with applicable laws.
Frequently asked questions
What does the klotho protein do in the body?
Klotho serves as both a membrane-bound co-receptor and a circulating hormone-like factor. In its membrane form, it enables FGF23 to regulate phosphate excretion and vitamin D metabolism in the kidneys. In its soluble form, it circulates through the bloodstream and suppresses inflammation via NF-kB inhibition, activates antioxidant defenses through FoxO and Nrf2 pathways, blocks fibrosis by inhibiting TGF-beta signaling, and modulates insulin and IGF-1 signaling. Higher circulating levels associate with better brain function, stronger cardiovascular health, and longer lifespan across populations.
Can you take klotho as a supplement?
Direct Klotho protein supplementation is not currently available as a consumer product. The protein is large and complex, presenting manufacturing and delivery challenges. However, several approaches can support Klotho levels indirectly. Exercise, Mediterranean diet, folate-rich foods, curcumin, and intermittent fasting all associate with higher Klotho. Certain longevity peptides including GHK-Cu and Epitalon have demonstrated Klotho-upregulating properties in research. The Klotho-derived peptide KP1 exists as a research compound but has no established human dosage protocol.
What is the KL-VS variant and should I get tested?
KL-VS is a common genetic variant in the Klotho gene carried by approximately 20 to 25 percent of the population in heterozygous form. One copy associates with higher circulating Klotho, better cognitive performance, larger prefrontal cortex volume, and potential longevity benefits. Two copies, found in about 3 percent of people, paradoxically associate with worse outcomes. Genetic testing can identify your KL-VS status, though the clinical actionability is limited since the same Klotho-supporting lifestyle interventions benefit everyone regardless of genotype.
How does klotho decline with age?
Circulating Klotho levels peak during childhood and decline steadily throughout adulthood. By the seventh decade of life, levels may fall to half or less of youthful values. This decline accelerates in the presence of chronic kidney disease, diabetes, cardiovascular disease, and chronic inflammation. The decline is considered a contributor to, not merely a consequence of, biological aging. Preserving Klotho through lifestyle interventions and potentially through peptide-based strategies represents a proactive approach to anti-aging.
Does exercise increase klotho levels?
Yes. Regular exercise is the most consistently documented natural Klotho booster. Both aerobic exercise at 150 or more minutes per week and resistance training programs of 16 weeks or longer have demonstrated significant increases in circulating Klotho compared to sedentary controls. The mechanisms involve reduced inflammation, improved insulin sensitivity, enhanced renal blood flow, and favorable hormonal changes. Exercise provides Klotho benefits alongside the well-documented effects on muscle health, cardiovascular fitness, and metabolic function.
What is the connection between klotho and kidney disease?
Klotho and kidney health are deeply intertwined. The kidney produces more Klotho than any other organ, and Klotho is essential for proper kidney function through FGF23-mediated phosphate regulation. In chronic kidney disease, Klotho expression declines early, often before symptoms appear, creating a vicious cycle where reduced Klotho impairs mineral metabolism, promotes fibrosis, and accelerates further kidney damage. Restoring Klotho, through either the KP1 peptide or other interventions, is a major research focus for breaking this destructive cycle.
Can klotho help with brain fog and cognitive decline?
Research strongly supports a connection between Klotho and cognitive function. Higher circulating Klotho associates with better performance on tests of executive function, processing speed, and memory. Klotho enhances NMDA receptor function by promoting GluN2B subunit expression, supports hippocampal neurogenesis, reduces neuroinflammation, and protects against amyloid and tau pathology associated with Alzheimer disease. While direct Klotho supplementation for cognitive enhancement is not yet available, supporting Klotho through exercise, nutrition, and Klotho-upregulating peptides may benefit brain health alongside dedicated nootropic peptide strategies.
What foods increase klotho naturally?
The Mediterranean dietary pattern shows the strongest overall association with higher Klotho. Specific foods and nutrients with evidence include folate-rich foods such as dark leafy greens, legumes, and citrus fruits, with each unit of additional folate intake associated with measurably higher Klotho. Nuts have shown positive associations in middle-aged adults. Curcumin from turmeric upregulates Klotho expression in kidney cells in laboratory studies. Omega-3 rich fish, extra virgin olive oil, and antioxidant-rich fruits and vegetables round out the dietary pattern most supportive of Klotho levels.
Is klotho peptide the same as full klotho protein?
No. The Klotho-derived peptide KP1 is a 30-amino-acid fragment derived from the KL1 domain of the full Klotho protein, which is approximately 130 kilodaltons and contains over 1,000 amino acids. KP1 specifically mimics the anti-fibrotic activity of Klotho by binding TGF-beta receptor 2 and blocking fibrotic signaling. It does not replicate all of Klotho many other functions, including FGF23 co-receptor activity, IGF-1 modulation, or Wnt signaling suppression. KP1 is a research compound without established human dosing protocols. Understanding the difference between different peptide structures is important for any researcher working with these compounds.
How is klotho related to other longevity peptides?
Klotho operates through mechanisms that both overlap with and complement other longevity peptides. Epitalon targets telomere maintenance and has been shown to upregulate Klotho expression, creating a synergistic anti-aging effect. MOTS-c addresses mitochondrial function and metabolic health, pathways that Klotho influences through its effects on insulin and IGF-1 signaling. SS-31 protects mitochondrial membranes, while Klotho upregulates mitochondrial antioxidant enzymes. The Khavinson bioregulator peptides target tissue-specific aging, while Klotho provides systemic anti-aging support. These compounds address aging from different angles and may produce additive benefits when combined thoughtfully.
External resources
NCBI Gene Database: KL (Klotho) gene overview, National Center for Biotechnology Information
Original Klotho discovery paper, Kuro-o et al., Nature (1997)
ClinicalTrials.gov: Active Klotho clinical trials
The science of Klotho is still being written. Every month brings new publications, new mechanisms, new potential applications. But the foundational picture is clear. This protein, quietly declining with every passing decade, touches nearly every system that determines how well and how long you live. Your kidneys need it for mineral balance. Your heart needs it to resist calcification and hypertrophy. Your brain needs it for sharp cognition and protection against neurodegeneration. Your entire body needs it to keep inflammation and oxidative stress in check.
The good news is that Klotho levels are not fixed by fate. Exercise raises them. Diet supports them. Specific compounds and peptides upregulate their expression. Gene therapy may soon restore them directly. The tools are emerging. The understanding is deepening. And the opportunity to act on this knowledge is available now, not in some distant future.
SeekPeptides members gain access to detailed guides on longevity peptides, including protocols for compounds like Epitalon and GHK-Cu that have demonstrated Klotho-upregulating properties. From dosage charts and reconstitution guides to in-depth articles on peptide stacking strategies and cycle planning, the SeekPeptides platform provides the knowledge base that serious researchers need to navigate the rapidly evolving world of longevity science. Whether you are investigating Klotho-specific interventions or building a comprehensive anti-aging protocol, having accurate, current information is the foundation of informed decision-making.
In case I do not see you, good afternoon, good evening, and good night. Join SeekPeptides.



