Feb 2, 2026
What is multiple sclerosis and why do current treatments fall short?
Multiple sclerosis strips the protective myelin coating from nerve fibers. Slowly. Relentlessly. The immune system, confused by signals it cannot properly interpret, attacks the very tissue that allows electrical impulses to travel from brain to body. Over time, this process creates lesions, scars that interrupt communication along the central nervous system and produce symptoms ranging from numbness and fatigue to vision loss and difficulty walking.
More than 2.8 million people worldwide live with MS. Most receive their diagnosis between the ages of 20 and 40, right in the prime of their careers, relationships, and physical lives. Women are affected roughly three times more often than men, making MS-relevant peptide research especially important for women exploring peptide therapy.
The disease follows different patterns in different people, with relapsing-remitting MS being the most common form, accounting for roughly 85% of initial diagnoses. But even in remission, the underlying damage often continues silently, a process neurologists call "smoldering inflammation." For women over 40 and those approaching menopause, hormonal changes can complicate MS progression, adding another layer of complexity to disease management.
Current disease-modifying therapies have improved outcomes considerably. Drugs like ocrelizumab, natalizumab, and fingolimod can reduce relapse rates by 50-70%. That is significant progress. But these medications share a fundamental limitation: they suppress the immune system broadly rather than targeting the specific autoimmune pathways driving myelin destruction. The result is increased vulnerability to infections, potential liver toxicity, and a long list of side effects that many patients find difficult to tolerate over years or decades of treatment.
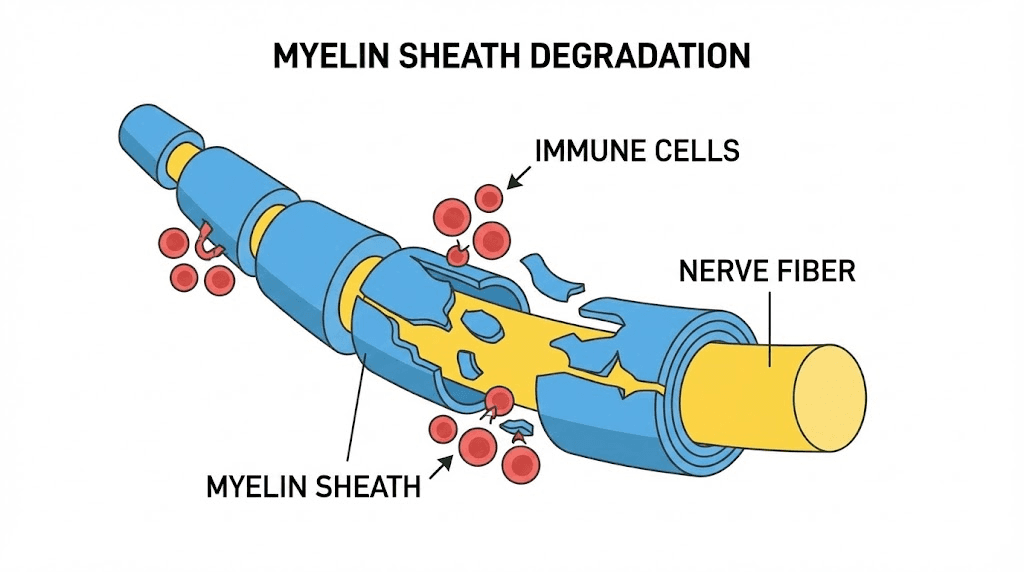
Perhaps more importantly, none of the currently approved MS drugs can reliably repair damaged myelin. They slow the attack.
They do not fix what has already been broken. This gap between managing autoimmune conditions and actually restoring lost function is where peptide-based research enters the picture, and where the science gets genuinely exciting.
Peptides offer something fundamentally different from conventional immunosuppressants. These small chains of amino acids, each with a specific molecular structure, can modulate specific immune pathways without shutting down the entire system. Some promote tissue repair directly. Others reduce neuroinflammation through targeted mechanisms.
A few even show potential to stimulate remyelination, the regrowth of the protective nerve coating that MS destroys. The research is still early for many of these compounds. No peptide has received FDA approval specifically for MS treatment. But the preclinical evidence is substantial enough that researchers at major institutions, including those investigating longevity-oriented peptide therapies and immune-focused compounds, are actively pursuing these molecules as the next frontier in MS therapy.
This guide examines every peptide that current research connects to multiple sclerosis management, from compounds with direct evidence in MS animal models to those whose mechanisms of action make them theoretically relevant. You will find specific study findings, proposed mechanisms, dosing information where available, and an honest assessment of where the science stands for each one. SeekPeptides exists precisely for situations like this, where the research landscape is complex and the stakes are high, and where having accurate, comprehensive information can make the difference between informed decisions and dangerous guesswork.
How the immune system attacks myelin in MS
Understanding which peptides might help requires understanding what goes wrong in the first place. MS is not a simple disease. Multiple overlapping mechanisms drive myelin destruction, and different peptides target different parts of this cascade.
It starts with T cells.
In healthy immune systems, T cells learn to distinguish between self and non-self during development in the thymus gland. Autoreactive T cells, those that would attack the body own tissues, are normally eliminated or suppressed. In MS, this process fails. Myelin-reactive CD4+ T helper cells escape into the bloodstream, cross the blood-brain barrier, and encounter myelin antigens presented by local immune cells within the central nervous system.
Once activated inside the brain and spinal cord, these T cells release pro-inflammatory cytokines, particularly interleukin-17 (IL-17), interferon-gamma (IFN-gamma), and tumor necrosis factor-alpha (TNF-alpha). These chemical signals recruit additional immune cells, including macrophages and B cells, creating an inflammatory environment that directly damages oligodendrocytes, the cells responsible for producing and maintaining myelin.
The damage extends beyond myelin itself. Activated microglia, the resident immune cells of the brain, amplify the inflammatory response by producing reactive oxygen species and additional cytokines. Astrocytes become reactive, forming scar tissue (gliosis) that further impedes remyelination. Meanwhile, complement activation and antibody-mediated damage add additional layers of destruction.
And then there is the gut.
Recent research has revealed a critical connection between gut health and MS. People with MS consistently show altered gut microbiomes compared to healthy controls, with elevated levels of certain bacteria that correlate with plasma concentrations of IL-17A, a key driver of MS pathology. Some researchers now believe that molecular mimicry, where bacterial peptides in the gut resemble myelin proteins closely enough to confuse the immune system, may be one of the initial triggers for MS in genetically susceptible individuals. This gut-brain axis connection has profound implications for peptide therapy, since several peptides that reduce gut inflammation also appear to modulate neuroinflammation.
The disease creates a vicious cycle. Inflammation damages myelin and oligodendrocytes. Damaged tissue releases more antigens, which activate more immune cells. Oxidative stress compounds the damage. And the blood-brain barrier, weakened by inflammation, allows even more immune cells to enter the CNS.
Breaking this cycle requires interventions at multiple points: suppressing the aberrant immune response, protecting oligodendrocytes from ongoing damage, promoting remyelination of denuded axons, and reducing the oxidative stress and neuroinflammation that perpetuate the process. Different peptides address different parts of this cascade, which is why researchers increasingly believe that combination approaches may prove most effective.
The four types of MS each present different challenges for peptide-based interventions. Relapsing-remitting MS (RRMS) involves clear episodes of inflammation followed by partial or complete recovery, making it potentially responsive to immunomodulatory peptides that could prevent relapses. Secondary progressive MS (SPMS) develops in many RRMS patients over time, characterized by gradual worsening independent of relapses. Primary progressive MS (PPMS) involves steady deterioration from onset without clear relapses, representing the form most resistant to current therapies. Progressive-relapsing MS combines steady worsening with occasional flares. For SPMS and PPMS, where neurodegeneration and failure to remyelinate drive disability accumulation, peptides that promote myelin repair and provide neuroprotection may be especially relevant because current drugs offer the least benefit in these progressive forms.
The fatigue, cognitive dysfunction, and mood disturbances that accompany MS are not just symptoms. They reflect the widespread neuroinflammation and neurodegeneration affecting brain regions beyond the obvious white matter lesions visible on MRI. Gray matter atrophy, cortical lesions, and diffuse microglial activation all contribute to the cognitive and psychological burden of MS. Peptides that address neuroinflammation broadly, rather than targeting only focal demyelination, may help with these often-overlooked aspects of the disease that significantly impact daily functioning and quality of life.
Thymosin beta-4: the strongest evidence for remyelination
If one peptide stands above the rest for MS-relevant research, it is thymosin beta-4 (TB4). This 43-amino-acid peptide, produced naturally by the thymus gland, has been studied extensively in two separate animal models of MS and has demonstrated something that almost no currently approved MS drug can claim: genuine remyelination of damaged nerve fibers.
The research is compelling. In the experimental autoimmune encephalomyelitis (EAE) model, which mimics the autoimmune aspects of MS, prophylactic treatment with TB4 significantly delayed disease onset and produced measurable neurological functional recovery. Animals treated with TB4 showed improved motor function compared to untreated controls, with reduced clinical disability scores across the study period.
But the cuprizone model results are even more striking.
Cuprizone is a chemical that specifically kills oligodendrocytes, producing demyelination without immune involvement. When researchers administered TB4 to cuprizone-treated animals, they observed a significant increase in oligodendrocyte progenitor cell (OPC) differentiation, the process by which immature precursor cells mature into functional myelin-producing oligodendrocytes. The treatment also increased remyelination in the corpus callosum, one of the brain regions most commonly affected in MS patients.
The mechanism appears to involve the epidermal growth factor receptor (EGFR) signaling pathway. TB4 treatment increased both the expression and activation of EGFR in demyelinating brain regions, suggesting that the peptide does not merely protect existing myelin but actively promotes the generation of new myelin-producing cells. This is a critical distinction. Most neuroprotective approaches focus on preventing further damage. TB4 appears to actually reverse it.
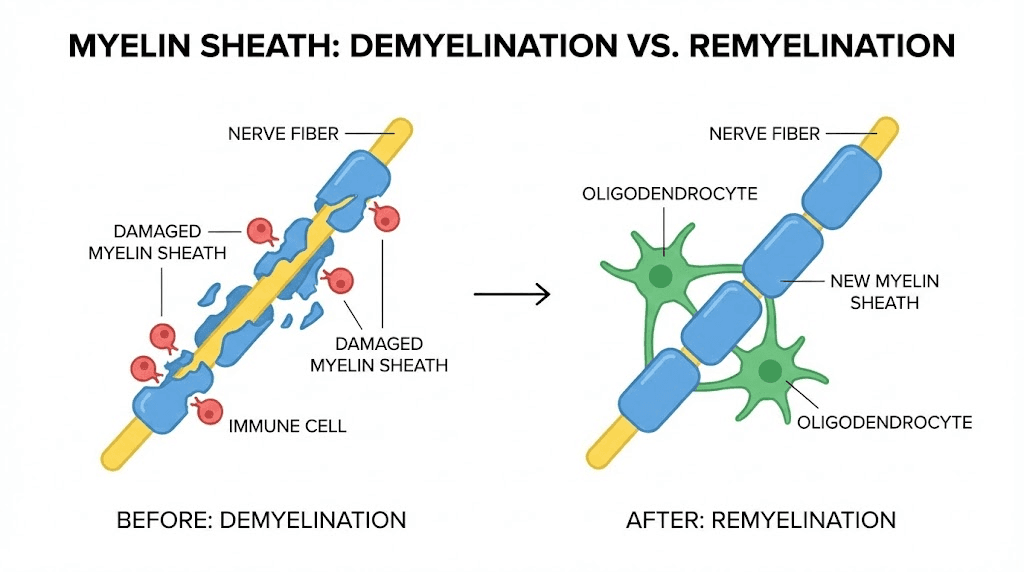
Additional mechanisms contribute to TB4's relevance for MS. The peptide suppresses nuclear factor-kappa B (NF-kB) activation, a master regulator of inflammatory gene expression.
By inhibiting NF-kB, TB4 may protect oligodendrocytes from inflammatory damage while simultaneously promoting their regeneration. The peptide also demonstrates immunosuppressive properties that help modulate the overactive immune response characteristic of MS.
Safety data supports the therapeutic potential. RegeneRx Biopharmaceuticals conducted randomized, double-blind, placebo-controlled Phase 1A and 1B studies of intravenous TB4 administration in healthy volunteers, finding the drug safe and well tolerated. This safety profile stands in contrast to the concerns surrounding some other research peptides, where regulatory questions and limited human data create uncertainty. The company has three drug candidates in clinical development for ophthalmic, cardiac, and dermal indications, with RGN-259 receiving orphan drug designation for neurotrophic keratopathy.
What does this mean for MS patients? TB4 is not approved for MS treatment, and human clinical trials specifically for MS remain limited. But the preclinical evidence, spanning multiple animal models and multiple research groups, consistently points to the same conclusion: this peptide promotes oligodendrogenesis and remyelination through mechanisms directly relevant to MS pathology. For anyone tracking the bioregulator peptide research landscape, TB4 represents perhaps the most promising remyelination candidate currently under investigation.
TB-500: the synthetic fragment
TB-500 is not the same molecule as full-length thymosin beta-4. It is the N-acetylated 17-23 fragment (Ac-LKKTETQ), corresponding to TB4's actin-binding region. This distinction matters because while TB-500 shares some properties with its parent peptide, particularly regarding actin dynamics, cell migration, and angiogenesis, the remyelination research was conducted with full-length TB4, not the TB-500 fragment.
That said, TB-500 is far more widely available in the research peptide market than full-length TB4. Its mechanisms, regulating actin dynamics and driving cell migration, are theoretically relevant to OPC migration toward demyelinated lesions. Many researchers investigating BPC-157 and TB-500 combinations are interested in the potential for synergistic tissue repair effects that might extend to neural tissue.
The honest assessment: TB-500 may share some of full-length TB4's properties relevant to MS, but extrapolating the MS-specific research conducted with full-length TB4 to the TB-500 fragment requires caution. The remyelination and oligodendrogenesis data specifically apply to TB4. Whether TB-500 can achieve similar effects in the CNS remains an open question that requires dedicated research. Anyone using our TB-500 dosage calculator should understand this distinction clearly.
Thymosin alpha-1: immune modulation for MS
While thymosin beta-4 targets remyelination, its thymic cousin thymosin alpha-1 (TA1) addresses the other side of the MS equation: the dysfunctional immune response driving myelin destruction in the first place.
TA1 is a 28-amino-acid peptide that has been studied more extensively in clinical settings than almost any other peptide discussed in this guide. It is approved in over 30 countries for hepatitis B and C treatment and as an immune adjuvant. Its mechanism centers on modulating the immune system rather than broadly suppressing it, a distinction that makes it particularly interesting for autoimmune conditions where the goal is to restore biological balance rather than eliminate function. As one of the most established alpha peptides in clinical use, TA1 has a safety track record that few research peptides can match.
The MS-specific research reveals several relevant mechanisms.
First, TA1 regulates the defective phenotype of monocytes in MS patients, impacting T cell activation. In MS, monocytes (a type of white blood cell) display abnormal behavior that promotes excessive T cell activation against myelin proteins. TA1 appears to normalize this monocyte function, reducing the inappropriate immune activation that drives disease progression.
Second, the peptide promotes differentiation of regulatory T cells (Tregs), the immune system natural braking mechanism. MS patients consistently show deficiencies in Treg number and function. By boosting Treg activity, TA1 may help restore the immune tolerance that MS disrupts, potentially reducing relapse frequency without the broad immunosuppression caused by conventional DMTs.
Third, TA1 displays what researchers call "pleiotropic" activity, meaning it promotes anti-inflammatory and regulatory conditions across multiple immune pathways simultaneously. This multi-pathway approach is significant because MS involves numerous immune mechanisms, and single-target therapies often fail to address the full scope of immune dysregulation.
Early clinical observations suggest that TA1 may reduce relapse frequency in MS patients by stabilizing immune responses. These findings are preliminary and need confirmation through larger trials. But they align with the robust preclinical data showing TA1's ability to modulate, rather than suppress, immune system function.
There is also evidence of a thymus-pituitary-adrenal axis modulated by TA1. Injection of thymosin fraction V and TA1 into the lateral ventricles of the brain produces a significant increase in circulating corticosterone, indicating a neuroendocrine site of action. This connection between thymic peptides and the stress response system has implications for MS, since cortisol dysregulation is common in MS patients and corticosteroids remain a frontline treatment for acute relapses.
Researchers reviewing the combined evidence for thymosin alpha-1 and thymosin beta-4 in MS have proposed that their complementary mechanisms, immune modulation from TA1 and remyelination from TB4, make them strong candidates for combination therapy. This approach would simultaneously address the autoimmune attack (TA1) and promote repair of existing damage (TB4), potentially offering more comprehensive disease management than either peptide alone.
BPC-157: neuroprotection from the gut
BPC-157, the body protection compound derived from human gastric juice, has generated some of the most intriguing preclinical data relevant to MS. This 15-amino-acid peptide, widely studied for tissue repair applications, has been tested directly in the cuprizone model of MS demyelination, with results that caught the attention of the neuroscience community.
The cuprizone study demonstrated that BPC-157-treated animals had consistently less nerve damage across all affected brain areas compared to untreated controls. Specifically, BPC-157 reduced damage in the corpus callosum, laterodorsal thalamus, nucleus reuniens, and anterior horn motor neurons, all regions commonly affected in MS. Perhaps most importantly from a functional standpoint, the peptide counteracted cerebellar ataxia and impaired forelimb function, suggesting that neuroprotection translated into preserved motor ability.
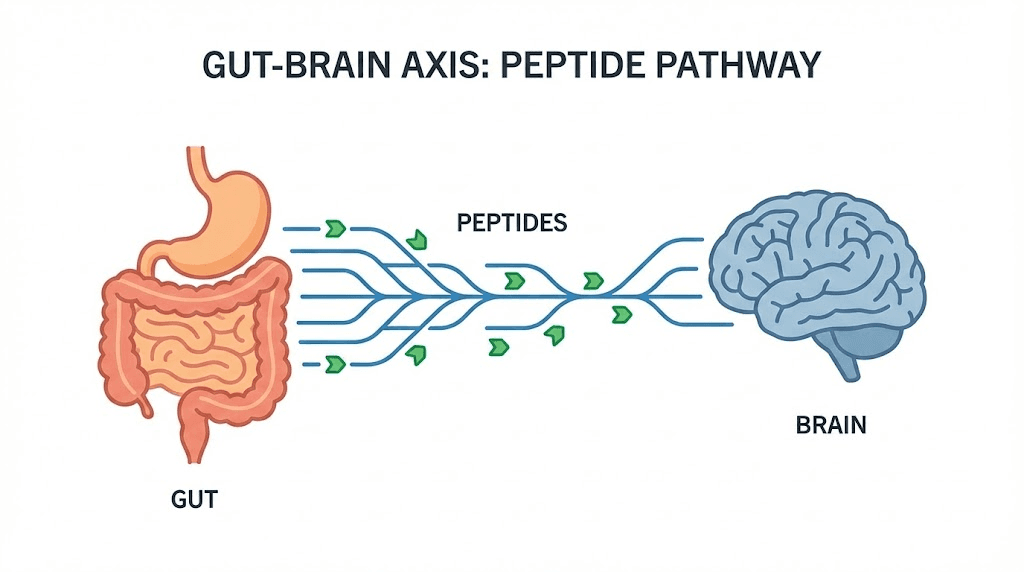
What makes BPC-157 particularly interesting for MS is the gut-brain connection.
The researchers who conducted the cuprizone study specifically proposed that BPC-157 links inflammatory bowel disease and multiple sclerosis, two conditions that share epidemiological and mechanistic connections. MS patients have higher rates of inflammatory bowel disease than the general population. Both conditions involve dysregulated inflammation, disrupted barrier function (the blood-brain barrier in MS, the intestinal barrier in IBD), and altered immune responses to self-antigens.
BPC-157 was shown to equally counteract models of both diseases, suggesting that its mechanism addresses shared pathological pathways. Oral ingestion at an estimated 10 micrograms per kilogram was similarly effective as subcutaneous injections, which aligns with the peptide established oral bioactivity for gut-related conditions.
The broader neuroprotective profile of BPC-157 further supports its relevance. The peptide markedly improves healing of traumatic nerve and brain injuries, ameliorates encephalopathies in severely intoxicated animals, and modulates serotonergic and dopaminergic systems. In spinal cord injury models, BPC-157 counteracted axonal and neuronal necrosis, demyelination, and cyst formation, with functional improvements maintained for up to 360 days after a single treatment. These findings, while not MS-specific, demonstrate tissue repair capabilities directly relevant to the type of neural damage MS causes. The peptide effects on healing pathways and injury recovery extend across multiple tissue types, reflecting a broad cytoprotective mechanism that may apply to CNS tissues as well.
A US patent presents the use of BPC-157 in the treatment of MS in combination with corticosteroids. The inventors argued that in addition to the anti-inflammatory effect, BPC-157 could benefit the depression, muscle weakness, and other common symptoms of MS.
They also suggested the peptide could prevent relapse or treat MS in remission.
Important caveats apply. In late 2023, the FDA flagged BPC-157 as a Category 2 substance, citing insufficient information to determine safety when administered to humans. Human clinical data for BPC-157 remains extremely limited, with only three published human studies to date. The MS-specific evidence comes exclusively from animal models. Anyone considering BPC-157 should do so only under qualified medical supervision and with a clear understanding that the evidence, while promising, is preclinical. Use our BPC-157 dosage calculator to understand reconstitution and dosing fundamentals, but recognize that MS-specific protocols have not been established in human trials.
KPV: the anti-inflammatory tripeptide
KPV is a deceptively simple molecule. Just three amino acids, lysine-proline-valine, derived from the C-terminal end of alpha-melanocyte stimulating hormone (alpha-MSH). But this tripeptide carries most of the anti-inflammatory power of its much larger parent molecule, and its mechanism targets pathways directly relevant to MS neuroinflammation.
The anti-inflammatory effects of alpha-MSH have been confirmed across multiple animal models of inflammation, including brain inflammation specifically. Most of these effects can be attributed to the KPV fragment. The peptide works by downregulating nuclear factor-kappa B (NF-kB) signaling, the same master inflammatory pathway that TB4 also targets. It reduces production of pro-inflammatory cytokines including IL-1-beta, IL-6, and TNF-alpha, all of which play central roles in MS pathology.
KPV also reduces reactive oxygen species (ROS) production, offering cellular protection against the oxidative stress that compounds myelin damage in MS. This dual mechanism, suppressing inflammatory signaling while reducing oxidative damage, addresses two of the key pathological processes that perpetuate MS progression.
The peptide relevance to MS extends through the gut-inflammation connection. KPV has demonstrated efficacy in models of inflammatory bowel disease, where it reduces intestinal inflammation and supports barrier integrity. Given the established link between gut dysbiosis and MS, and the evidence that gut-derived inflammatory signals can cross-react with myelin proteins through molecular mimicry, reducing gut inflammation may indirectly benefit neuroinflammation.
One advantage of KPV over many other peptides in this guide is its relatively simple structure and favorable pharmacokinetic properties. As a tripeptide, it is smaller and potentially more bioavailable than larger peptide molecules. Researchers have explored various delivery routes including oral, topical, and injectable administration. The peptide limited spectrum of biologic activities, focused primarily on anti-inflammatory rather than broadly immunosuppressive effects, makes it an interesting candidate for immune-mediated inflammatory diseases where targeted intervention is preferred over broad suppression.
The broader KPV research also shows antimicrobial activity against pathogens like Staphylococcus aureus and Candida albicans, which is relevant because MS patients on immunosuppressive therapies are vulnerable to opportunistic infections. A peptide that reduces inflammation without compromising antimicrobial defense would represent a meaningful advantage over conventional approaches.
No clinical trials have tested KPV specifically for MS. The evidence supporting its potential comes from its established anti-inflammatory mechanism, its efficacy in neuroinflammation models, and its effects on the gut-immune-brain axis. More research is needed, but the mechanistic rationale is sound.
For anyone researching KPV alongside other anti-inflammatory peptides, understanding the KPV dosing landscape provides important context. The peptide favorable safety profile in preclinical studies, combined with its targeted anti-inflammatory mechanism, positions it as one of the more conservative peptide options for researchers interested in neuroinflammation reduction without broad immunosuppression.
Epitalon: the telomere peptide with immune implications
Epitalon, a synthetic tetrapeptide (Ala-Glu-Asp-Gly) developed by Vladimir Khavinson, is primarily known for its telomerase-activating properties. But its relevance to MS extends beyond anti-aging applications. The peptide has demonstrated immunomodulatory effects that touch on pathways relevant to autoimmune neuroinflammation.
The connection works through the pineal gland. Epitalon stimulates melatonin production by the pineal gland, and melatonin itself has documented neuroprotective and immunomodulatory properties. In MS research, melatonin has been shown to reduce EAE severity, decrease pro-inflammatory cytokine production, and promote regulatory T cell differentiation. By restoring pineal function and melatonin secretion, epitalon may indirectly support these same pathways.
More directly, epitalon belongs to the Khavinson bioregulator peptide family, a group of short peptides designed to regulate specific organ functions. Several bioregulators in this family have documented effects on immune cell function. The standard research protocols for epitalon involve cyclic administration, typically 10 days on followed by extended breaks, which may allow the immune system to recalibrate during off periods.
The evidence for epitalon in MS specifically is indirect. No studies have tested it in EAE or cuprizone models. Its relevance is inferred from its effects on melatonin, telomere maintenance (relevant to the accelerated cellular aging seen in MS), and general immunomodulation. It represents one of the more speculative entries in the MS peptide landscape, but the biological rationale is not unreasonable.
Bioregulator peptides: organ-specific immune support
The bioregulator peptide concept, pioneered by Khavinson and colleagues in Russia, involves short peptides (typically 2-4 amino acids) that target specific organs and tissues. Several of these peptides have theoretical relevance to MS through their effects on the immune system, brain tissue, and blood vessels.
Thymalin, a thymus bioregulator peptide, directly supports the organ that produces T cells. Since MS involves T cell dysfunction, supporting thymic health could theoretically improve T cell education and reduce the escape of autoreactive T cells. Thymalin has demonstrated analgesic activity in inflammatory conditions and in both severe and mild progressive forms of EAE, the animal model of MS. When bound to polybutylcyanoacrylate (PBCA) nanoparticles to enhance bioavailability, both free and nanoparticle-bound thymalin were evaluated in relapsing and remitting EAE, providing direct MS-relevant preclinical data.
Cortagen, targeting the brain cortex, and pinealon, targeting the pineal gland, are other bioregulators that may support CNS function in contexts of neuroinflammation. Vesugen, a vascular bioregulator, could theoretically support blood-brain barrier integrity, one of the structures compromised early in MS pathogenesis. And Chonluten, targeting bronchial and lung tissue, has immunomodulatory properties that extend beyond its primary organ target.
The bioregulator approach to MS would involve supporting multiple organ systems simultaneously: the thymus for T cell regulation, the brain for neuroprotection, the pineal gland for melatonin-mediated immune balance, and the vasculature for BBB integrity. This multi-organ strategy aligns with the understanding that MS is a systemic disease affecting far more than just myelin sheaths. For researchers interested in bioregulator protocols, SeekPeptides offers comprehensive guides covering the full Khavinson bioregulator family, including cycling schedules and combination strategies.
Evidence for individual bioregulator peptides in MS remains limited. The strongest preclinical support exists for thymalin, with its direct EAE testing. The others are included based on mechanistic rationale rather than MS-specific data.
Selank and Semax: nootropic peptides with immune-modulating properties
Selank and Semax were not developed with MS in mind. Both peptides emerged from Russian pharmaceutical research as cognitive enhancers and anxiolytics. But their mechanisms of action, involving both neuroprotection and immune modulation, have drawn attention from researchers studying neuroinflammatory conditions.
Selank
Selank is a synthetic heptapeptide analogue of the human immune peptide tuftsin. It modulates several pathways relevant to MS pathology. The peptide suppresses pro-inflammatory cytokines including IL-6 and TNF-alpha, two of the primary drivers of neuroinflammation in MS. It affects the balance of T helper cell cytokines, potentially shifting the immune response away from the Th17-dominant profile that characterizes active MS.
The connection to neuroinflammation is direct. Chronic inflammation in the brain contributes to multiple neurodegenerative diseases, and Selank has demonstrated the ability to modulate the immune response in the brain specifically by reducing production of inflammatory compounds. Its interaction with interleukin-1 (IL-1) plays a role in dampening the inflammatory response in the central nervous system, which is pivotal in mitigating neuroinflammation. The peptide modulation of transforming growth factor-beta 1 (TGF-beta-1) contributes to maintenance of neuronal health and promotes neurogenesis.
Critically, unlike traditional anti-inflammatory agents, Selank does not suppress immune function broadly. This is an important distinction for MS patients, who need targeted immune modulation rather than general immunosuppression. The peptide anxiolytic properties, mediated through GABA and serotonin pathways, are also relevant since anxiety and mood disorders are common comorbidities in MS that significantly impact quality of life.
For more on Selank dosing protocols, refer to our detailed guide. Standard research protocols involve nasal spray administration, which provides direct CNS access.
Semax
Semax is a synthetic analogue of ACTH(4-10), the fragment of adrenocorticotropic hormone. It is approved as a prescription drug in Russia for stroke, cognitive disorders, and immune support, though it has not been evaluated or approved in most other countries.
The immune and neuroprotective properties of Semax are well-documented. In conditions of focal brain ischemia, Semax altered the expression of genes that modulate immune cell quantity and mobility, enhanced expression of genes encoding chemokines and immunoglobulins, and influenced genes that promote formation and functioning of the vascular system. The immunomodulating effect and vascular impact during ischemia are considered key mechanisms underlying the peptide neuroprotective effects.
A recent study published in the British Journal of Pharmacology demonstrated that Semax improved functional recovery in spinal cord injury by targeting mu-opioid receptors, which regulated USP18 and subsequently promoted deubiquitination pathways. The peptide decreased oxidative stress and inhibited pyroptosis-related neuroinflammation. These mechanisms are directly relevant to MS, where oxidative stress and neuroinflammation drive progressive disability.
Semax also stimulates brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), two molecules critical for neuronal survival and myelin maintenance.
The BDNF pathway is of particular interest in MS because BDNF levels are reduced in MS patients during relapses, and restoring neurotrophic support may help protect neurons from ongoing damage.
The connection to MS is reinforced by the fact that ACTH, the parent hormone from which Semax derives, has established clinical utility in treating MS relapses, and enkephalins (whose degradation Semax inhibits) are implicated in multiple sclerosis pathophysiology.
Both Selank and Semax inhibit enzymes that degrade enkephalins and other endogenous regulatory peptides, suggesting overlapping mechanisms relevant to opioid growth factor pathways in MS. This connection to endogenous opioid peptides links these nootropics to the same biological system that low-dose naltrexone targets in MS patients.
The opioid growth factor connection: LDN and endogenous peptides
Low-dose naltrexone (LDN) is not a peptide itself, but its mechanism of action revolves entirely around endogenous peptide systems. Understanding this connection illuminates why certain peptides may benefit MS and provides context for several peptide-based approaches.
The key molecule is opioid growth factor (OGF), chemically termed [Met5]-enkephalin. This endogenous peptide is produced in an autocrine fashion throughout the body and exerts potent inhibitory effects on cell growth through interaction with the opioid growth factor receptor (OGFr). In autoimmune disorders including MS, the OGF-OGFr axis becomes dysregulated.
LDN works by briefly blocking opioid receptors at very low doses (typically 3-4.5mg taken once daily), causing a rebound upregulation of both endogenous opioid peptides and their receptors. This intermittent blockade paradoxically increases the body production of regulatory peptides, restoring the balance that MS disrupts.
The evidence from EAE models is striking. After two weeks of LDN treatment, saline-treated mice showed approximately 22% demyelination compared to less than 5% for LDN-treated animals. Neuropathological studies revealed no demyelination at day 60 in mice treated with OGF or LDN that had not displayed disease symptoms. The treatment halted disease progression, reversed neurological deficits, and prevented onset of neurological dysfunction across a considerable time span.
Mechanistically, LDN reduces nitric oxide synthase activity, decreasing peroxynitrite formation, which prevents inhibition of glutamate transporters. It also inhibits microglial activation in the brain and spinal cord, reducing production of reactive oxygen species and potentially neuroexcitatory chemicals. OGF treatment specifically impaired CNS infiltration by CD4+ T lymphocytes, directly addressing one of the primary drivers of MS pathology.
Clinical data supports these preclinical findings. A placebo-controlled crossover study showed that LDN at 4.5mg nightly significantly improved mental health quality of life indices in MS patients, including measures of anxiety and depression. A long-term observational study found that patients taking LDN maintained stable health over an average of approximately 1,095 days (about three years). These findings are especially relevant given that mental health comorbidities affect the majority of MS patients and can significantly worsen overall disability.
The relevance to peptide therapy is this: the endogenous opioid peptides that LDN upregulates, particularly OGF/met-enkephalin, are the actual therapeutic agents. LDN is merely the tool that increases their production. This same peptide system is modulated by Selank and Semax through their inhibition of enkephalin-degrading enzymes. Understanding these overlapping pathways helps explain why multiple peptide approaches may benefit MS through convergent mechanisms.
Experimental peptides in the MS research pipeline
Beyond the well-studied peptides discussed above, several experimental compounds show enough promise to warrant attention from anyone following MS research closely.
TDP6: the synthetic myelin repair peptide
Researchers at the University of Melbourne developed TDP6, a synthetic peptide designed to function as a smaller, more directed version of brain-derived neurotrophic factor (BDNF). This approach mirrors the broader trend of engineering biomimetic peptides that replicate the beneficial effects of larger proteins while improving stability and selectivity. The team identified that the TrkB receptor, expressed by myelin-producing cells, responds to BDNF. They engineered TDP6 to selectively target only this receptor, making it more stable and more selective than natural BDNF.
The results in mouse models were impressive. TDP6 boosted myelin regeneration significantly better than BDNF itself by increasing the number of myelin-producing cells. The newly formed myelin layers were thicker than restored myelin typically is, an important finding since thin remyelination is a common limitation of natural repair processes. Intact TDP6 was still detected in treated mice after seven days, suggesting favorable stability.
TDP6 represents a next-generation approach to peptide-based remyelination, designed from the ground up to target the specific receptor that drives oligodendrocyte maturation and myelin production.
Cyclotides: plant-derived peptides
MedUni Vienna demonstrated in animal models that a specially synthesized plant peptide from the cyclotide family prevented further progression of MS symptoms. The one-off oral administration brought about a substantial improvement in symptoms with no further disease attacks observed.
Cyclotides are macrocyclic peptides found across major plant families, representing a large group of natural substances. Their cyclic structure provides exceptional stability, and the ability to administer them orally is a significant practical advantage. Oral administration for a neurological condition is notable because it eliminates the need for injections, which is particularly relevant for peptide delivery in chronic conditions requiring long-term treatment.
Myelin peptide patches
One of the most clinically advanced antigen-specific approaches involves transdermal delivery of myelin peptides. In a controlled clinical trial, patients receiving a skin patch containing 1mg each of three myelin peptides (MBP85-99, MOG35-55, and PLP139-155) showed a 66.5% reduction in cumulative active brain lesions compared to placebo over 12 months. The annual relapse rate dropped from 1.4 to 0.43, with no serious adverse events reported.
This approach works through immune tolerance induction. By presenting myelin antigens through the skin in a non-inflammatory context, the immune system learns to tolerate these proteins rather than attack them. It is fundamentally different from immunosuppression because it targets only the specific immune response against myelin, leaving the rest of the immune system intact.
FTX-101: targeting the remyelination pathway
FTX-101 is a first-in-class therapeutic peptide that modulates the Plexin A1/Neuropilin 1 (NRP1) transmembrane receptor complex in the brain to promote remyelination. This receptor complex normally mediates the inhibitory effects of semaphorin 3A, a guidance molecule that impedes OPC migration and differentiation.
By blocking this inhibitory signal, FTX-101 essentially removes a natural brake on remyelination, allowing the brain existing repair mechanisms to function more effectively.
ISP: the scar tissue breakthrough
Intercellular Sigma Peptide (ISP) targets a different obstacle to MS recovery: scar tissue. In MS, reactive astrocytes form glial scars around lesion sites that physically block remyelination by preventing OPCs from reaching damaged areas. ISP inhibits proteins found in this scar tissue, enabling tissue remodeling. Mouse models of MS and spinal cord injury have shown reversals in CNS plaquing and scarring following ISP treatment, a finding that addresses one of the most stubborn barriers to MS repair. This scar-breaking mechanism complements the tissue repair capabilities of other peptides and could theoretically work synergistically with remyelination-promoting compounds like TB4.
GLP-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) receptor agonists, best known for diabetes and weight management applications, have shown unexpected benefits in MS models. The long-acting GLP-1 receptor agonist NLY01 demonstrated anti-inflammatory and neuroprotective effects in EAE models. A research program funded by the Congressionally Directed Medical Research Programs is specifically investigating GLP-1R agonists as a new therapeutic for MS, recognizing that the benefits of calorie restriction on MS may be mediated partly through GLP-1 pathways.
The overlap between metabolic peptides and neuroinflammation is an emerging research area. Semaglutide and similar GLP-1 receptor agonists may have neuroimmune effects beyond their metabolic applications, though MS-specific clinical trials are still in early stages. The connection between metabolic peptides and neurological health represents one of the more surprising developments in recent MS research, suggesting that metabolic optimization may contribute to neuroinflammation management through mechanisms involving insulin sensitivity, appetite-regulating hormones, and direct anti-inflammatory effects on microglia. For MS patients dealing with weight changes related to reduced mobility or corticosteroid treatment, the potential dual benefit of GLP-1 peptides, addressing both metabolic and neurological health, is worth monitoring as research progresses.
The gut-brain axis: why gut peptides matter for MS
The connection between gut health and MS is no longer theoretical. It is supported by a substantial and rapidly growing body of evidence that has changed how neurologists think about the disease.
People with MS consistently show an altered gut microbiome compared to healthy controls. The gut microbiota in MS varies with disease activity, with specific bacterial populations correlating with relapse status and lesion formation. Multiple studies have identified elevated levels of disease-associated bacteria that correlate with plasma concentrations of IL-17A, a cytokine central to MS pathology. Both bacterial populations and cytokine levels correlate with active brain lesions, supporting a gut bacteria-cytokine-brain axis in MS.
The mechanisms linking gut and brain in MS include several pathways where peptides play roles.
Molecular mimicry stands out as perhaps the most consequential. Bacterial peptides produced by gut microorganisms can structurally resemble myelin proteins closely enough that immune cells trained to attack these bacterial peptides also attack myelin. For T cells to attack the myelin sheath inside the CNS, they must first be activated peripherally, possibly by a bacterial peptide. In the brains of MS patients, bacterial peptidoglycan has been found in antigen-presenting cells, suggesting that bacterial products directly participate in MS pathophysiology.
Intestinal barrier integrity is another critical factor. MS patients show increased intestinal permeability, the so-called "leaky gut" that allows bacterial products and immune signals to enter systemic circulation and ultimately reach the CNS. Peptides like BPC-157 and KPV that strengthen intestinal barrier function and reduce gut inflammation may benefit MS by addressing this upstream trigger.
Oxidative stress provides yet another connection. Both pathogenic and commensal gut bacteria produce reactive oxygen species directly or indirectly through metabolites like short-chain fatty acids and formyl-peptides. Excessive ROS generation from gut dysbiosis can worsen intracellular signaling pathways and amplify inflammation in MS patients. Peptides with antioxidant properties, including BPC-157 and KPV, may help break this cycle.
The therapeutic implications are significant. Modification of the microbiome through probiotics, fecal microbiota transplantation, bile acid supplementation, and intestinal barrier enhancement are all being investigated as MS interventions. Peptides that address gut inflammation, barrier integrity, and the microbiome-immune interface, including PDA peptide and glutamine peptides which support intestinal health, represent a complementary approach that targets the disease at one of its potential points of origin. The growing peptide solutions landscape for gut conditions may prove unexpectedly relevant to neurological disease management.
For anyone managing MS, the gut-brain connection suggests that gut health optimization is not merely a wellness practice. It may be a meaningful component of disease management that current pharmaceutical approaches largely ignore.
Peptide-based targeted drug delivery for MS
Even if a molecule has perfect therapeutic properties, it is useless if it cannot reach the brain. The blood-brain barrier (BBB), which normally protects the central nervous system from circulating pathogens and toxins, also blocks most therapeutic molecules from entering the brain. This is a fundamental challenge for MS treatment, and peptide-based drug delivery systems offer a solution.
Researchers are developing peptide targeting ligands that enable therapeutic cargos to cross the BBB and accumulate specifically at MS lesion sites. These targeting peptides recognize molecular signatures unique to MS lesions, including upregulated adhesion molecules on inflamed blood vessel walls and exposed myelin antigens in active plaques.
The approach involves conjugating therapeutic agents (which could include other peptides, small molecules, or antibodies) to BBB-penetrating peptides. This targeted accumulation enhances local drug concentration at the pathological area, increasing therapeutic efficacy while decreasing systemic side effects. Nanoparticle delivery systems incorporating peptide targeting ligands further improve stability, enable co-delivery of multiple therapeutic agents, and allow sustained release at the lesion site.
This technology has implications beyond just delivering existing drugs more effectively. It opens the possibility of delivering remyelination-promoting peptides like TB4 or neuroprotective peptides like BPC-157 directly to MS lesions at concentrations that might not be achievable through systemic administration. Lipid nanoparticle platforms, similar to those used in mRNA vaccines, can even permit in situ expression of therapeutic peptides or immunomodulatory molecules directly within the CNS.
The field is still primarily preclinical, but the concept of peptide-guided drug delivery to MS lesions represents a genuine paradigm shift in how treatment might work. Rather than flooding the entire body with immunosuppressants and hoping enough reaches the brain, targeted delivery could put the right molecules exactly where they are needed.
Comparing peptide approaches for MS
With so many peptides potentially relevant to MS, understanding how they differ is essential for anyone researching this space. The following comparison addresses the key distinctions in mechanism, evidence level, and potential application.
Peptide | Primary mechanism | MS evidence level | Administration | Best suited for |
|---|---|---|---|---|
Thymosin beta-4 | Remyelination via OPC differentiation | Strong preclinical (EAE + cuprizone) | Injectable | Myelin repair, functional recovery |
TB-500 | Actin dynamics, cell migration | Inferred from TB4 data | Injectable | General tissue repair |
Thymosin alpha-1 | Immune modulation, Treg promotion | Moderate preclinical + early clinical | Injectable | Reducing relapse frequency |
BPC-157 | Neuroprotection, gut-brain axis | Moderate preclinical (cuprizone) | Injectable or oral | Neuroprotection, gut-MS connection |
KPV | Anti-inflammatory (NF-kB), antioxidant | Mechanistic (no direct MS studies) | Injectable, oral, topical | Neuroinflammation reduction |
Selank | Cytokine modulation, anxiolytic | Mechanistic | Nasal spray, injectable | Neuroinflammation, mood support |
Semax | BDNF stimulation, neuroprotection | Moderate preclinical (SCI, ischemia) | Nasal spray, injectable | Neuroprotection, cognitive support |
TDP6 | TrkB receptor-targeted remyelination | Preclinical (mouse models) | Injectable | Myelin regeneration |
Cyclotides | Immune tolerance, disease arrest | Preclinical (animal model) | Oral | Disease progression arrest |
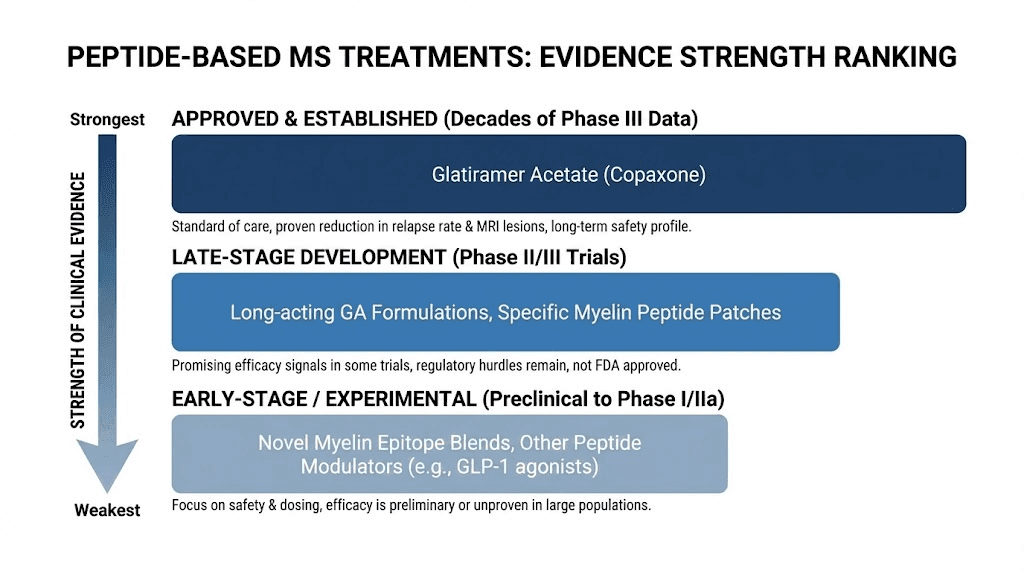
The key insight from this comparison is that different peptides address different aspects of MS pathology. No single peptide addresses everything.
Thymosin beta-4 excels at promoting remyelination but does not address the underlying autoimmune attack. Thymosin alpha-1 modulates the immune system but does not promote myelin repair. BPC-157 offers neuroprotection and addresses the gut-brain connection but has limited direct remyelination evidence. This is why researchers increasingly advocate for combination approaches, similar to how peptide stacking in other contexts targets multiple goals simultaneously.
The administration route matters too. Some peptides in this table work best as nasal sprays for direct CNS access, while others require injection or can be taken orally. For anyone exploring these compounds, understanding the differences between injectable and oral delivery helps in evaluating practical feasibility. The general peptide dosing principles apply across these compounds, though MS-specific protocols remain to be established through clinical research.
Safety considerations and the reality of peptide therapy for MS
Honesty matters more than hope when discussing unproven therapies for serious diseases. Here is the reality of peptide therapy for MS in its current state.
No peptide discussed in this guide has received FDA approval for treating multiple sclerosis. The evidence, while often compelling, comes primarily from animal models. Animal models of MS, particularly EAE and the cuprizone model, have poor track records of predicting human outcomes. Many treatments that dramatically improve EAE fail in human clinical trials. This is not unique to peptides. It is a fundamental challenge in MS drug development.
The translation gap is partly biological. EAE is an induced disease in genetically identical animals, while human MS develops over years or decades in genetically diverse individuals exposed to different environmental triggers. Cuprizone produces demyelination without immune involvement, while MS involves both immune attack and demyelination. These differences mean that even strong preclinical results should be interpreted cautiously.
Specific safety concerns vary by peptide. BPC-157 has been flagged by the FDA as a Category 2 substance with insufficient safety data for human use. TB-500 and full-length thymosin beta-4 have different safety profiles despite being related molecules. Thymosin alpha-1 has the most extensive human safety data, being approved in over 30 countries for other indications, but has not been specifically tested in large MS trials. Selank and Semax are approved in Russia but have not been evaluated by the FDA or EMA.
Drug interactions represent another concern. MS patients typically take disease-modifying therapies that alter immune function. Adding peptides with immunomodulatory properties creates the potential for unpredictable interactions. No controlled studies have evaluated the safety of combining research peptides with approved MS medications like ocrelizumab, natalizumab, or fingolimod.
Quality and purity issues are significant. Research-grade peptides vary widely in quality. Without pharmaceutical-grade manufacturing standards, contamination, degradation, and incorrect potency are real risks. Proper reconstitution, storage, and handling are critical. Using our peptide reconstitution calculator and following proper bacteriostatic water protocols helps ensure accurate preparation, but does not address fundamental purity concerns.
Third-party testing from reputable labs should be considered non-negotiable for anyone using research peptides, especially for a condition as serious as MS. Certificates of analysis should confirm identity, purity (ideally greater than 98%), and the absence of endotoxins and heavy metals.
The bottom line: peptide therapy for MS shows genuine scientific promise. The mechanisms are sound. The preclinical data is encouraging. But anyone considering peptides for MS management should do so in partnership with their neurologist, not as a replacement for proven therapies, and with realistic expectations about the current state of the evidence. Reviewing the complete landscape of available peptides and understanding how many peptides can be used simultaneously provides important context for anyone evaluating their options. The differences between peptides and other research compounds also matter, since the specificity and generally favorable safety profiles of peptides distinguish them from broader-acting molecules.
Practical considerations for MS researchers exploring peptides
For those who have discussed peptide research with their healthcare providers and want to understand practical aspects, several considerations deserve attention.
Starting with the strongest evidence
If MS-relevant peptide research interests you, prioritizing compounds with the strongest evidence base makes sense. Thymosin alpha-1 has the most extensive human safety data and the most direct MS research. Thymosin beta-4 has the strongest remyelination evidence. BPC-157 has the most data on the gut-brain connection relevant to MS. These three represent the most evidence-supported starting points for conversations with healthcare providers. SeekPeptides provides detailed protocol information for each of these compounds, along with dosing calculators and community discussion that can help inform those conversations.
Understanding peptide quality
Peptide quality is not negotiable for research involving serious health conditions. Key considerations include:
Purity matters enormously. A peptide advertised at 95% purity means 5% unknown impurities. For compounds being administered to people with compromised immune systems, those impurities could include endotoxins, heavy metals, or degradation products. Seek peptides with 98% or higher purity, verified by independent third-party testing.
Storage and handling affect potency. Most lyophilized peptides are relatively stable when stored properly, but reconstituted peptides degrade much faster. Understanding how long reconstituted peptides last and maintaining proper refrigeration is essential for maintaining potency throughout the research period.
The monitoring question
MS disease activity can be tracked through MRI scans, relapse frequency, disability progression scales (EDSS), and various blood biomarkers including neurofilament light chain (NfL) levels. Anyone adding peptide research to an existing MS management program should ensure that regular monitoring continues. Changes in disease activity, whether positive or negative, need documentation to make informed decisions about continuing, adjusting, or discontinuing any approach.
Combining approaches thoughtfully
The multi-mechanism nature of MS suggests that combination approaches addressing different disease pathways might be more effective than single agents. The thymosin alpha-1 (immune modulation) plus thymosin beta-4 (remyelination) combination has been specifically proposed in the research literature. Adding BPC-157 for gut-brain axis support creates a three-pronged strategy addressing immune dysregulation, myelin repair, and the gut-inflammation connection.
However, combining multiple research compounds without clinical trial data on interactions introduces uncertainty. Cycling different peptides rather than combining them simultaneously is a more conservative approach. Use the peptide stack calculator to understand dosing fundamentals, but recognize that MS-specific combination protocols have not been validated in human trials.
Administration routes and CNS access
Reaching the central nervous system is a challenge for any therapeutic molecule. Different administration routes offer different advantages for MS-relevant peptides.
Nasal spray delivery provides relatively direct CNS access through the olfactory and trigeminal nerve pathways. Selank and Semax are commonly administered this way, and the route bypasses the blood-brain barrier partially. For peptides targeting neuroinflammation specifically, intranasal delivery may achieve higher brain concentrations than systemic injection.
Subcutaneous injection remains the most common route for peptides like BPC-157 and thymosin variants. While this route requires the peptide to cross the blood-brain barrier to reach CNS targets, the BBB is actually compromised in active MS lesions, which paradoxically may improve drug penetration at the sites where treatment is most needed. Proper injection technique and sterile vial handling become especially important for immunocompromised individuals.
Oral administration works for select peptides. BPC-157 has demonstrated oral bioactivity in animal studies, with oral doses achieving neuroprotective effects comparable to injections. Cyclotide peptides are specifically designed for oral delivery. For MS patients already managing multiple injectable medications, oral peptide options reduce treatment burden considerably. Understanding the differences between injectable and oral peptides helps inform route selection.
Reconstitution and handling for MS research
Proper peptide preparation is critical for anyone, but doubly so for individuals with autoimmune conditions. MS patients on immunosuppressive therapy face heightened infection risk, making sterile technique essential.
Start with bacteriostatic water from a sealed vial. Never use plain sterile water for multi-use preparations because bacteriostatic water contains 0.9% benzyl alcohol that inhibits microbial growth between uses. Use our peptide calculator to determine the correct volume of diluent, and follow the mixing guide precisely. Direct the stream of water down the side of the vial rather than directly onto the peptide cake, and swirl gently rather than shaking.
Once reconstituted, peptides have a limited shelf life. The general guideline is 28-30 days refrigerated for most reconstituted peptides, though stability varies by compound. Lyophilized powder lasts much longer, often years when stored properly at -20C. For anyone managing a multi-peptide protocol, understanding these timelines prevents using degraded compounds that may be ineffective or produce unexpected byproducts.
Cost considerations
MS is already an expensive disease to manage. Adding peptide research compounds introduces additional costs that need realistic assessment. Peptide pricing varies dramatically depending on the compound, purity level, quantity, and vendor. Thymosin alpha-1 tends to be among the more expensive research peptides due to its longer amino acid chain. BPC-157 and KPV are generally more affordable.
Use the peptide cost calculator to estimate monthly research expenses based on specific compounds and dosing schedules. Factor in the cost of bacteriostatic water, syringes, alcohol swabs, and third-party testing. For a chronic condition like MS where peptide research might continue for months or years, total costs add up significantly and should be part of any informed decision-making process.
What does the future hold for peptides and MS?
The MS treatment landscape is shifting. After decades focused almost exclusively on immunosuppression, the field is increasingly recognizing that effective MS management requires repair and neuroprotection alongside immune modulation. Peptides are uniquely positioned to address this expanded therapeutic vision.
Several trends point toward a growing role for peptides in MS.
First, the tolerance revolution. Antigen-specific peptide therapies that induce immune tolerance to myelin proteins represent perhaps the most elegant approach to MS treatment. Rather than suppressing the immune system broadly, these therapies teach it to stop attacking myelin specifically. The clinical trial showing a 66.5% reduction in brain lesions with a myelin peptide skin patch demonstrates that this concept works in humans. Advances in delivery technology, including nanoparticles, lipid nanoparticles, and hydrogels, are improving peptide stability and enabling targeted antigen presentation that could make these approaches more practical and effective.
Second, the remyelination imperative. Current MS drugs can slow the immune attack. They cannot repair the damage already done. The remyelination evidence for thymosin beta-4 and TDP6, while preclinical, addresses what many neurologists consider the most critical unmet need in MS treatment. As these compounds move toward clinical trials, they could fundamentally change what "treating MS" means, shifting from damage limitation to active repair.
Third, the gut-brain paradigm. The rapidly accumulating evidence linking gut dysbiosis, microbial peptides, and MS pathogenesis is opening entirely new therapeutic avenues. Peptides that address gut inflammation and barrier integrity, particularly BPC-157 and KPV, may prove relevant to MS management in ways that were inconceivable a decade ago.
Fourth, precision delivery. Peptide-based targeted drug delivery systems that can guide therapeutic cargos across the blood-brain barrier and to specific MS lesion sites could solve one of the biggest practical challenges in MS treatment, getting the right drug to the right place in the right concentration.
The honest assessment is that most peptide approaches for MS remain years away from potential clinical availability. The preclinical evidence is often strong but the path from animal models to approved human therapies is long, expensive, and uncertain. However, for a disease where current treatments remain unable to fully prevent progression or restore function, the peptide research pipeline offers genuine reasons for cautious optimism. The evolving regulatory landscape for peptides will also shape how quickly these compounds move from research to clinical application. Staying informed about the distinction between research and pharmaceutical grade peptides helps set realistic expectations about availability and quality standards.
SeekPeptides members access the most current research updates, detailed protocol information, and a community of researchers navigating these complex decisions with evidence-based guidance. For a condition as serious as MS, having access to comprehensive, regularly updated resources is not a luxury. It is a necessity.
Frequently asked questions
Can peptides cure multiple sclerosis?
No peptide has been shown to cure MS in humans. The research shows potential for peptides to modulate the immune response driving MS, promote tissue repair including remyelination, and reduce neuroinflammation. However, all MS-specific peptide evidence is preclinical or in early clinical stages. Peptides should be considered potential adjuncts to, not replacements for, established disease management approaches.
Which peptide has the strongest evidence for MS specifically?
Thymosin beta-4 has the strongest preclinical evidence for the remyelination aspect of MS, with demonstrated ability to promote oligodendrocyte progenitor cell differentiation in both EAE and cuprizone models. For immune modulation, thymosin alpha-1 has the most clinical experience, being approved in over 30 countries for other immune-related conditions. BPC-157 has the most data connecting gut health to MS neuroprotection.
Is BPC-157 safe for people with MS?
The FDA has classified BPC-157 as a Category 2 substance, citing insufficient safety data for human use. Only three published human studies exist, and none specifically involved MS patients. The preclinical safety profile appears favorable, with the peptide showing no toxicity in animal studies, but this does not guarantee safety in immunocompromised humans. Anyone considering BPC-157 should discuss it with their neurologist. Understanding proper reconstitution and storage is essential for minimizing contamination risks.
Can I take peptides alongside my current MS medication?
No clinical studies have evaluated the safety of combining research peptides with approved MS medications. Combining immunomodulatory peptides with drugs like ocrelizumab or natalizumab that also affect the immune system creates the potential for unpredictable interactions. This is a conversation that must happen with your neurologist before any experimentation. Thymosin alpha-1 has the most data on combination use with other drugs, though not specifically with MS medications.
How do gut health peptides relate to MS?
MS patients consistently show altered gut microbiomes, increased intestinal permeability, and changes in bile acid metabolism. Gut bacteria produce peptides that can trigger molecular mimicry with myelin proteins, potentially activating myelin-reactive T cells. Peptides like BPC-157 and KPV that reduce gut inflammation and strengthen intestinal barrier function may address this upstream trigger of MS pathology.
What is the difference between TB-500 and thymosin beta-4?
TB-500 is the N-acetylated 17-23 fragment of thymosin beta-4, not the full-length protein. The remyelination research in MS models was conducted with full-length thymosin beta-4. While TB-500 shares some properties with its parent peptide, including effects on cell migration and angiogenesis, the MS-specific findings cannot be directly extrapolated to the fragment without dedicated research.
Are any peptide treatments for MS in clinical trials?
Yes. Antigen-specific peptide approaches, including myelin peptide skin patches and tolerance-inducing peptide therapies delivered via nanoparticles, are in various stages of clinical investigation. Thymosin beta-4 has passed Phase 1 safety trials for other indications. A clinical trial safety and efficacy study for thymosin beta-4 (NCT05485818) is registered. The GLP-1 receptor agonist NLY01 is being investigated specifically for MS through a funded research program.
How long would peptide therapy take to show results for MS?
This question cannot be answered with current data because no standardized peptide protocol for MS exists. In animal models, thymosin beta-4 showed measurable remyelination within the study periods (typically weeks to months). BPC-157 showed neuroprotective effects within days to weeks in cuprizone models. In the myelin peptide patch trial, significant differences between treatment and placebo groups emerged over the 12-month study period. Any human timeline would depend on the specific peptide, dosing, administration route, disease stage, and individual factors. Understanding how long peptides generally take to work provides some baseline context, though MS applications fall outside the typical use cases.
Do peptides need to be refrigerated for MS research protocols?
Yes. Most reconstituted peptides require refrigeration at 2-8C and should be used within 28-30 days. Unreconstituted lyophilized peptides are more stable but still benefit from proper cold storage. For long-term storage, -20C freezing is recommended for powder form. Given that MS research protocols may extend over months, proper peptide storage directly impacts whether you are getting the intended compound or a degraded version. Never use peptides that have been left at room temperature for extended periods.
Can peptides help with MS-related fatigue?
Fatigue is the most commonly reported symptom in MS, affecting up to 80% of patients. Several peptides discussed in this guide have mechanisms relevant to fatigue. Semax stimulates BDNF and supports cognitive function. Thymosin alpha-1 modulates the immune dysfunction that contributes to inflammation-related fatigue. Peptides targeting energy and vitality may address the mitochondrial dysfunction increasingly recognized as a contributor to MS fatigue. The SS-31 peptide, which targets mitochondrial function specifically, represents another compound of theoretical interest for MS-related energy deficits, though no MS-specific studies exist.
Are there peptides specifically for MS-related pain?
Neuropathic pain affects a significant percentage of MS patients and is often poorly managed by conventional treatments. BPC-157 has demonstrated analgesic effects in inflammatory and neuropathic pain models. Thymalin shows analgesic activity in inflammatory conditions including EAE. The opioid growth factor pathway, modulated by LDN and influenced by Selank and Semax, plays a role in pain processing. For general information on peptides for pain management, see our dedicated guide, though keep in mind that MS neuropathic pain involves different mechanisms than musculoskeletal pain.
Should I tell my neurologist about peptide research interest?
Absolutely, without exception. MS is a serious disease that requires ongoing medical management. Peptides are unproven therapies that can interact with disease-modifying treatments. Your neurologist needs complete information about everything you take or plan to take to make informed treatment decisions and monitor for complications. Many neurologists are familiar with the peptide research landscape and can provide informed guidance. Those who are not will appreciate the transparency and can research specific compounds to advise you appropriately. Whether the topic is peptide legality, drug interactions, or monitoring needs, your neurologist is an essential partner in this process.
External resources
Thymosins in multiple sclerosis and its experimental models - PMC/NCBI
Peptide-Based Molecules as Potential Drug Candidates for MS - PMC/NCBI
For researchers committed to understanding the full scope of peptide potential for multiple sclerosis, SeekPeptides provides the most comprehensive, regularly updated resource available. Members access detailed protocol databases, evidence-based guides covering every peptide discussed in this article, and a community of experienced researchers navigating the complex intersection of peptide science and autoimmune disease management.
In case I do not see you, good afternoon, good evening, and good night. May your myelin stay protected, your inflammation stay managed, and your research stay informed.



