Jan 22, 2026
Your cells are dying faster than they are being replaced. This process started in your twenties. By forty, it accelerates. By sixty, the imbalance becomes visible in every mirror, felt in every joint, measured in every blood test. The question is not whether aging is happening.
The question is whether you can slow it down.
This is where longevity peptides enter the conversation. These are not supplements in the traditional sense. They are signaling molecules, some encoded in your own mitochondrial DNA, others synthesized to replicate compounds your body produced abundantly in youth but now barely manufactures at all.
The science here is not speculative. Researchers have documented telomere extension in human clinical studies. They have measured metabolic improvements in aged mice that rival what exercise produces. They have observed mortality reductions in long-term human trials spanning more than a decade. These are not fringe claims. They are findings published in peer-reviewed journals, replicated across laboratories, and increasingly recognized by longevity medicine practitioners worldwide.
But the peptide landscape is confusing. Which compounds actually work? What dosages have research support? How do you combine them without creating counterproductive interactions? And perhaps most importantly, how do you distinguish evidence-based protocols from marketing hype?
This guide answers those questions with specificity. You will learn which peptides target the fundamental mechanisms of aging, from mitochondrial dysfunction to cellular senescence to telomere shortening. You will understand the research behind each compound, the practical protocols researchers use, and the considerations that determine whether a particular peptide makes sense for your goals. SeekPeptides has compiled this information from scientific literature, clinical data, and the experience of thousands of researchers who have used these compounds.
Longevity is not about living forever. It is about maintaining function, vitality, and quality of life for as long as possible. The peptides covered here offer tools for that objective. Tools that work at the cellular level, where aging actually happens.
Understanding the biology of aging and how peptides intervene
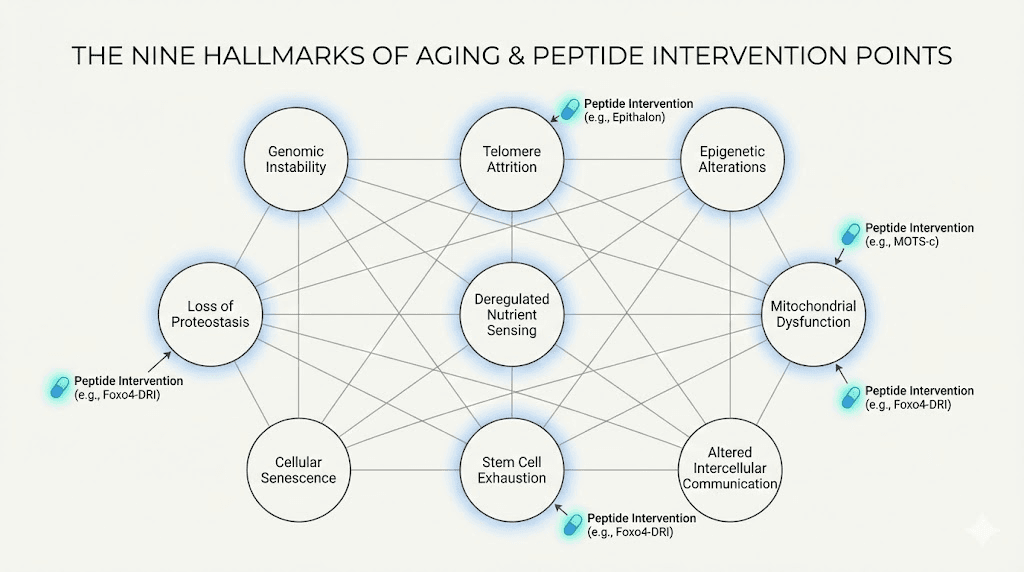
Aging is not a single process. It is a cascade of interconnected failures that compound over time. To understand how longevity peptides work, you first need to understand what is actually breaking down.
The hallmarks of aging, as defined by researchers, include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Every one of these processes can be measured. Every one of them accelerates with age. And remarkably, specific peptides have demonstrated effects on multiple hallmarks simultaneously.
Consider mitochondrial dysfunction. Your mitochondria produce the energy that powers every cellular process. They also produce reactive oxygen species as a byproduct, which damage cellular structures including the mitochondria themselves. With age, this damage accumulates. Energy production declines. Cells function less efficiently. Organs deteriorate.
Peptides like MOTS-c and SS-31 target this process directly. MOTS-c is actually encoded in mitochondrial DNA, making it one of the few peptides your body naturally produces specifically to regulate mitochondrial function. When researchers give MOTS-c to aged mice, they observe improvements in exercise capacity, metabolic health, and insulin sensitivity that mirror what younger animals exhibit.
Telomere attrition presents another target. Telomeres are the protective caps on your chromosomes. Each time a cell divides, these caps shorten. When they become too short, the cell either dies or enters a senescent state where it stops dividing but continues to produce inflammatory signals. This is why telomere length correlates so strongly with biological age and disease risk.
Epitalon, a synthetic version of a pineal gland peptide, has been shown to activate telomerase, the enzyme that rebuilds telomeres. In human clinical studies, elderly patients treated with Epitalon showed measurable increases in telomere length. This is not theoretical. It is documented in published research.
Cellular senescence represents yet another intervention point.
Senescent cells accumulate with age, releasing inflammatory molecules that damage surrounding tissues. This senescence-associated secretory phenotype contributes to chronic inflammation, often called inflammaging, which underlies most age-related diseases.
Peptides like Thymosin alpha-1 help modulate immune function, potentially reducing the burden of senescent cells and the inflammation they produce.
Others, like GHK-Cu, influence gene expression patterns associated with aging, essentially resetting cellular programming toward a more youthful state.
The point is that aging is not inevitable in the way most people assume. It is a biological process with identifiable mechanisms. And those mechanisms have molecular targets. Peptides provide tools to engage those targets with specificity that broad interventions like diet and exercise cannot match. This does not mean peptides replace lifestyle factors. It means they add precision to the intervention toolkit.
Epitalon: the telomere peptide with four decades of research
No longevity peptide has a longer research history than Epitalon. This tetrapeptide, consisting of just four amino acids, was developed by Russian gerontologist Vladimir Khavinson beginning in the 1970s. The original compound was epithalamin, an extract from bovine pineal glands. Epitalon is the synthetic version, standardized and purifiable.
The mechanism is straightforward. Epitalon activates telomerase, the enzyme responsible for adding nucleotide sequences to telomere ends. Without telomerase activity, telomeres shorten with each cell division until they reach a critical length that triggers cellular senescence or apoptosis. By reactivating telomerase, Epitalon potentially extends the replicative capacity of cells.
The research supporting this mechanism is substantial. In one study, human fibroblast cultures treated with Epitalon maintained proliferative capacity significantly longer than untreated controls. Control cells lost the ability to divide after 34 passages. Epitalon-treated cells continued dividing past 44 passages. The difference in cellular lifespan was dramatic and reproducible.
Human clinical data exists as well. In patients aged 60-80, treatment with Epitalon or the original epithalamin preparation significantly increased telomere lengths in blood cells. These were not short-term studies. Some trials tracked participants for six to twelve years, documenting sustained effects on mortality rates and biomarkers of aging.
One particularly notable study followed 266 elderly patients over eight years. Half received peptide bioregulator treatment including epithalamin. The treated group showed significantly lower mortality rates, reduced cardiovascular disease incidence, and improvements in multiple organ function markers. The results were published in peer-reviewed journals and have been cited extensively in longevity research.
The typical research protocol involves subcutaneous injection of 5 milligrams daily for 10-20 consecutive days, repeated one to two times per year. Some researchers use 10 milligrams daily for 10 days instead, achieving the same total dose over a shorter period. Both protocols have research support.
Beyond telomeres, Epitalon affects melatonin production. The pineal gland, where the natural form of this peptide originates, is responsible for melatonin synthesis. Melatonin declines significantly with age, which affects sleep quality, circadian rhythm regulation, and antioxidant capacity. Epitalon treatment has been shown to restore melatonin production toward youthful levels.
The circadian implications extend beyond sleep. Melatonin influences gene expression throughout the body. It modulates immune function. It affects metabolic processes. By restoring pineal function, Epitalon may produce downstream effects on multiple aging pathways simultaneously.
Researchers interested in Epitalon should understand the regulatory landscape. The FDA included Epitalon among peptides flagged for potential safety concerns in compounding, which affects availability through traditional channels. However, the safety data from decades of human use in clinical settings has not shown significant adverse effects. The primary concern is theoretical, related to telomerase activation in cells with malignant potential, though no evidence suggests Epitalon promotes cancer development.
SeekPeptides members access detailed protocols for Epitalon administration, including cycling schedules, combination strategies with other longevity peptides, and monitoring approaches to track results.
MOTS-c: the exercise mimetic encoded in mitochondrial DNA
MOTS-c stands apart from other longevity peptides because of its origin. This 16-amino acid peptide is encoded not in nuclear DNA but in mitochondrial DNA, specifically within the 12S rRNA gene. This makes MOTS-c a mitochondrial-derived peptide, or MDP, part of a recently discovered class of signaling molecules that regulate cellular metabolism and stress responses.
The significance of mitochondrial encoding cannot be overstated. Mitochondria are the powerhouses of cells, producing the ATP that fuels virtually every biological process. They also play central roles in aging, from energy production decline to oxidative stress accumulation to triggering apoptosis in damaged cells. A peptide that originates within mitochondria and regulates their function represents a fundamentally different intervention strategy than externally synthesized compounds.
Exercise causes a dramatic increase in MOTS-c expression. In one study of healthy young men, an acute bout of stationary cycling increased skeletal muscle MOTS-c levels by nearly 12-fold. Circulating levels rose by 1.6-fold and remained elevated for hours after exercise ended. This suggests MOTS-c may be one of the molecular mediators of exercise benefits, helping explain why physical activity improves metabolic health and extends lifespan.
The practical implication is that MOTS-c supplementation may function as an exercise mimetic. When researchers administer MOTS-c to mice, they observe improvements in physical capacity, metabolic parameters, and disease resistance that parallel what exercise produces. Old mice treated with MOTS-c can run nearly twice as far on treadmills as untreated controls. Their insulin sensitivity improves. Their body composition shifts toward less fat and more lean mass.
The metabolic effects operate through AMPK activation. AMPK, or AMP-activated protein kinase, is a master regulator of cellular energy homeostasis. When cellular energy is low, AMPK activates pathways that increase energy production and reduce energy consumption. Caloric restriction, exercise, and many longevity-promoting interventions work at least partly through AMPK. MOTS-c provides a direct molecular activator of this pathway.
Research dosing in animal studies typically uses 5-15 milligrams per kilogram of body weight, administered intraperitoneally. Translating this to human dosing requires careful consideration of pharmacokinetic differences between species. The peptide has a relatively short half-life, returning to baseline within about four hours after exercise-induced elevation. This suggests that maintaining elevated levels may require frequent administration or sustained-release formulations.
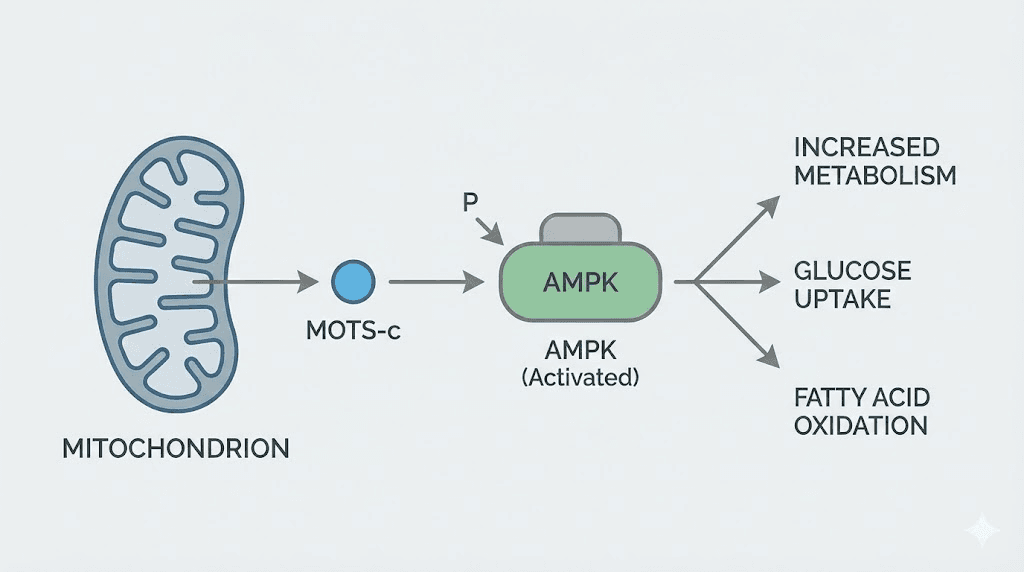
One challenge with MOTS-c is delivery. Like many peptides, it has limited bioavailability when taken orally and tends to persist at injection sites rather than distributing systemically. Current research explores various formulations to improve therapeutic utility.
Despite these challenges, the preclinical results are compelling enough that MOTS-c remains a peptide of significant interest for athletic performance and longevity applications.
The connection between MOTS-c and aging extends beyond metabolism. Levels of the peptide decline with age in multiple species. Mice experience a roughly 40 percent drop in circulating MOTS-c by 18 months of age. If MOTS-c is indeed mediating some of the protective effects of exercise, then age-related decline in this peptide may contribute to the increased disease susceptibility and metabolic dysfunction that characterize older organisms.
MOTS-c represents the cutting edge of peptide longevity research. It is not yet as well characterized as Epitalon or GHK-Cu. But its mitochondrial origin, its connection to exercise benefits, and its demonstrated effects on aging parameters make it one of the most scientifically intriguing compounds in the longevity peptide space. Researchers tracking this field should monitor ongoing MOTS-c studies closely.
GHK-Cu: the copper peptide that resets gene expression
GHK-Cu occupies a unique position among longevity peptides. Unlike compounds that target single pathways, GHK-Cu influences the expression of thousands of genes simultaneously, essentially reprogramming cells toward more youthful patterns of activity. This broad effect explains why a single tripeptide can produce benefits across such diverse areas as skin aging, wound healing, hair growth, inflammation, and tissue regeneration.
The peptide was discovered in 1973 when Loren Pickart isolated it from human plasma albumin. GHK, glycyl-L-histidyl-L-lysine, binds copper with very high affinity to form the complex GHK-Cu. This copper-peptide complex occurs naturally in your body, but levels decline substantially with age. Plasma concentrations drop from about 200 nanograms per milliliter at age 20 to approximately 80 nanograms per milliliter by age 60.
This decline coincides with observable reductions in regenerative capacity. Wounds heal more slowly. Skin loses elasticity and thickness. Hair follicles miniaturize. Inflammatory responses become dysregulated. The correlation between declining GHK-Cu and declining regenerative function suggests a causal relationship, and supplementation studies support this hypothesis.
Gene expression analysis reveals the scope of GHK-Cu activity. The peptide has been documented to up-regulate or down-regulate at least 4,000 genes in the human genome. Many of these genes relate to tissue remodeling, inflammation regulation, antioxidant defense, and DNA repair. The pattern of gene expression changes moves cells toward states characteristic of younger tissues.
For skin applications, GHK-Cu has particularly strong evidence. Clinical studies show improvements in skin thickness, elasticity, and collagen content. One trial found that facial cream containing GHK-Cu outperformed both vitamin C and retinoic acid for collagen synthesis in photoaged skin. Another 12-week study documented significant improvements in skin laxity, fine lines, wrinkle depth, and overall skin density compared to placebo.
The wound healing effects are equally well documented. GHK-Cu accelerates the formation of granulation tissue, promotes angiogenesis, and increases collagen production. It improves the success rate of skin grafts and reduces scarring. These effects make it valuable not just for cosmetic applications but for functional tissue repair.
What makes GHK-Cu particularly interesting for longevity is its anti-inflammatory activity. Chronic low-grade inflammation, or inflammaging, underlies most age-related diseases. GHK-Cu modulates inflammatory gene expression, reducing the production of pro-inflammatory cytokines while supporting resolution of inflammatory responses. This immunomodulatory effect may contribute to systemic benefits beyond local tissue applications.
Recent research has explored cognitive effects. In aged mouse models, GHK administration improved cognitive function by targeting anti-inflammatory and epigenetic pathways. The peptide can cross the blood-brain barrier, making it potentially relevant for neurological aging. While human cognitive data is limited, the mechanism suggests possible applications in age-related cognitive decline.
Dosing depends on the route of administration. Topical products typically contain 2-10 percent GHK-Cu, with 4 percent being standard for daily facial application. Results generally become visible after 4-8 weeks of consistent use, with more substantial improvements by three months. Injectable protocols in research settings use doses around 10 milligrams per kilogram body weight, though human translation of these doses requires medical supervision.
Because GHK-Cu increases copper availability, balancing with zinc becomes important, particularly with systemic use. Copper and zinc share transport pathways, and elevated copper without adequate zinc can create functional deficiencies. Researchers often recommend concurrent zinc supplementation when using injectable GHK-Cu.
GHK-Cu dosage protocols vary considerably based on the application. SeekPeptides provides comprehensive guidance on topical, oral, and injectable formulations, along with combination strategies that leverage GHK-Cu synergies with other longevity peptides.
Humanin: the mitochondrial peptide linked to centenarians
Humanin was discovered in 2001 by Japanese researchers investigating why some neurons resist Alzheimer disease pathology while others succumb. What they found was remarkable: a small peptide, just 24 amino acids long, encoded in the mitochondrial genome, that provided powerful cytoprotective effects. The implications extended far beyond neuroprotection to fundamental aging biology.
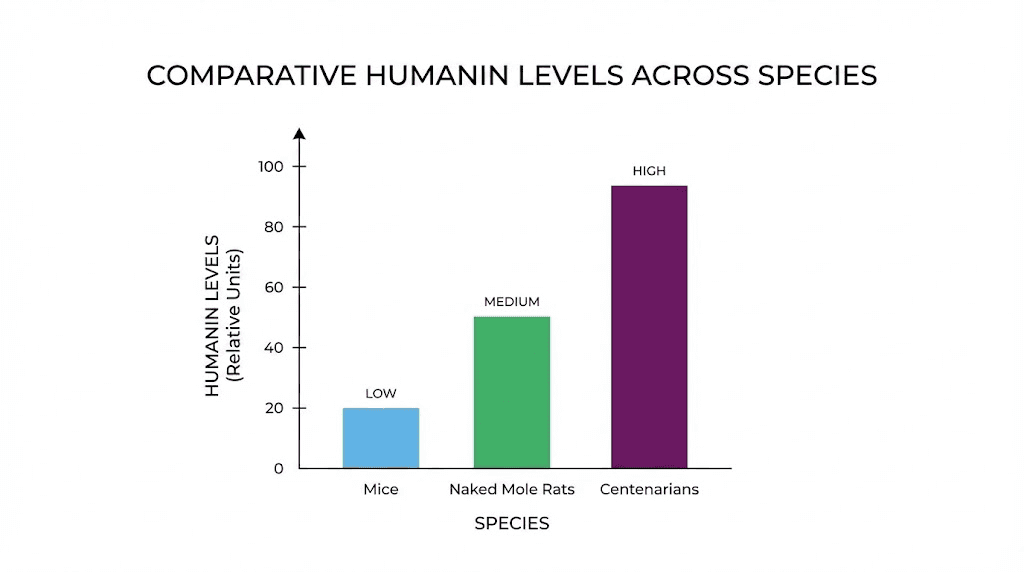
The connection to longevity became apparent when researchers measured Humanin levels across species and ages. Naked mole rats, the famously long-lived rodents that resist cancer and show minimal age-related decline over their 30-year lifespans, maintain high Humanin levels throughout life with minimal decline. Mice, which live only 2-3 years, experience a 40 percent drop in Humanin by 18 months. Primates show similar dramatic declines with age.
Even more compelling, children of centenarians have significantly higher circulating Humanin levels than age-matched controls. This correlation between Humanin and exceptional longevity suggested the peptide might not just be a biomarker but a functional regulator of lifespan.
Experimental evidence supports this hypothesis. In the model organism C. elegans, overexpression of Humanin is sufficient to extend lifespan. The effect depends on daf-16/FOXO, the same transcription factor that mediates lifespan extension from caloric restriction and insulin signaling mutations. This places Humanin squarely within established longevity pathways.
In mice, treatment with HNG, a potent Humanin analogue, produces dramatic improvements in metabolic health. Middle-aged mice receiving HNG twice weekly showed decreased visceral fat, increased lean body mass, improved glucose metabolism, and reduced inflammatory markers. These are precisely the changes associated with healthy aging and extended healthspan.
The mechanisms underlying Humanin effects are multifaceted. The peptide reduces oxidative stress by neutralizing free radicals and preventing cellular damage. It inhibits apoptosis by blocking signals that trigger programmed cell death. It preserves mitochondrial function, maintaining the integrity of these essential organelles even under stress conditions. And it provides neuroprotection, reducing the neurotoxicity of amyloid beta peptides associated with Alzheimer disease.
For cognitive applications, Humanin shows particular promise. Beyond Alzheimer disease, the peptide may protect against age-related cognitive decline more broadly by maintaining neuronal health and supporting synaptic function. Research continues to explore specific protocols for cognitive preservation.
The challenge with Humanin is clinical translation. Like many mitochondrial peptides, it faces bioavailability and stability issues. The peptide has a short half-life in circulation and may not reach target tissues at effective concentrations after typical administration. Researchers have developed synthetic analogues with improved pharmacokinetic profiles, but human clinical data remains limited.
What we do know suggests Humanin represents a fundamentally important longevity signal. Its mitochondrial origin, its correlation with exceptional lifespan across species and individuals, and its documented effects on metabolism, inflammation, and cellular protection all point to a peptide with genuine healthspan-extending potential. As delivery methods improve and clinical trials progress, Humanin may become a cornerstone of peptide longevity protocols.
SS-31 Elamipretide: targeting the inner mitochondrial membrane
SS-31, also known as Elamipretide, represents a different approach to mitochondrial intervention. Rather than being encoded in mitochondrial DNA like MOTS-c or Humanin, SS-31 is a synthetic tetrapeptide designed specifically to accumulate in the inner mitochondrial membrane, where it interacts with cardiolipin to optimize electron transport and ATP production.
Cardiolipin is a phospholipid unique to mitochondria, concentrated in the inner membrane where the electron transport chain proteins reside. With age and under stress conditions, cardiolipin becomes oxidized and loses its normal structure. This impairs mitochondrial function, reduces energy production, and increases the generation of reactive oxygen species. SS-31 binds to cardiolipin and stabilizes its structure, preserving mitochondrial function even in aged or damaged cells.
The effects of SS-31 on aging parameters are striking. In aged mice, eight weeks of treatment substantially reversed diastolic dysfunction, a prominent feature of cardiac aging in both mice and humans. Exercise tolerance improved. Skeletal muscle function returned toward youthful levels. The improvements occurred without increases in mitochondrial content, suggesting SS-31 enhances the function of existing mitochondria rather than triggering new mitochondrial production.
Clinical development of SS-31 has progressed further than most longevity peptides. The compound received FDA Fast Track designation for primary mitochondrial myopathy and orphan drug designation for Barth syndrome, a rare condition characterized by mitochondrial dysfunction, cardiomyopathy, and shortened life expectancy. Recent FDA approval for Barth syndrome treatment marks a significant milestone for mitochondrial-targeted peptide therapies.
The clinical trial data provides insight into human effects. In Barth syndrome patients, 48 weeks of SS-31 treatment produced significant improvements in the 6-minute walk test, symptom scores, and cardiac physiology. While this population has a specific genetic mitochondrial defect, the results demonstrate that mitochondrial-targeted peptides can produce measurable functional improvements in humans.
For broader aging applications, the preclinical data is compelling. Studies show benefits in models of cognitive impairment, muscle aging, atherosclerosis, osteoarthritis, diabetes, and glaucoma. However, clinical trials in heart failure and primary mitochondrial myopathy have yielded mixed results, with some not meeting their primary endpoints. This highlights the complexity of translating promising preclinical findings to human therapeutics.
The safety profile of SS-31 appears favorable. The peptide accumulates in the inner mitochondrial membrane without entering the matrix, and it has no effects on normal mitochondria. Human studies reported injection site reactions as the most common adverse effects, including redness, itching, and occasional hives, most of which were mild. No serious adverse reactions or fatalities have been observed in clinical development.
Dosing in clinical trials typically uses subcutaneous administration. The specific protocols vary by indication, but the general approach involves regular injections over periods ranging from weeks to months. For researchers interested in longevity applications, the key insight is that mitochondrial function can be improved through targeted peptide intervention, and SS-31 demonstrates one validated approach to achieving this.
SS-31 peptide protocols continue to evolve as clinical data accumulates. The compound represents one of the most clinically advanced longevity peptides, making it particularly valuable for researchers seeking compounds with established human safety data.
Thymosin alpha-1: restoring immune function in aging
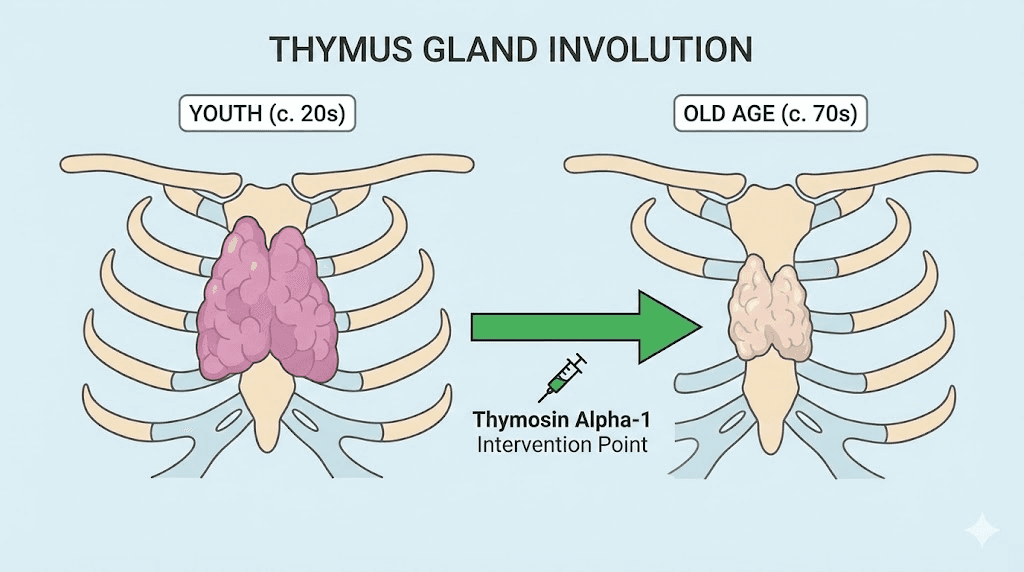
The thymus gland is the training center for your immune system. T cells mature there, learning to distinguish self from non-self, friend from foe. But here is the problem: after puberty, the thymus begins to shrink. By middle age, it is a fraction of its youthful size. By old age, it barely functions at all. This process, called thymic involution, directly causes much of the immune decline that characterizes aging.
Thymosin alpha-1 is a 28-amino acid peptide fragment derived from prothymosin alpha, a protein naturally produced by the thymus. As the thymus involutes, production of thymic hormones including thymosin alpha-1 declines precipitously. This decline contributes to immunosenescence, the age-related deterioration of immune function that increases susceptibility to infections, reduces vaccine responses, and impairs cancer surveillance.
The therapeutic rationale is straightforward: replace what the body no longer produces adequately. Thymosin alpha-1 supplementation aims to restore immune function toward youthful levels, countering the effects of thymic involution.
Clinical evidence supports this approach. The peptide has been used therapeutically for decades, primarily in Asia, for conditions including hepatitis B and C, certain cancers, and immune deficiencies. The FDA approved thymalfasin, a synthetic version of thymosin alpha-1, as an orphan drug for malignant melanoma and hepatocellular carcinoma, acknowledging its immunomodulatory and anti-tumor effects.
For aging specifically, the evidence centers on immune restoration and vaccine enhancement. Elderly individuals respond poorly to vaccines because their aged immune systems cannot mount robust antibody responses. Studies show that thymosin alpha-1 administration improves vaccine responses in older adults, potentially addressing one of the key vulnerabilities of immune aging.
The mechanisms involve multiple immune cell types. Thymosin alpha-1 promotes T cell maturation and differentiation. It enhances thymic output of naive T cells, the cells needed to respond to new pathogens and vaccines. It modulates dendritic cell and macrophage activity, improving antigen presentation. And it enhances natural killer cell function, important for tumor surveillance.
Beyond acute immune function, thymosin alpha-1 may address chronic inflammation. Immunosenescence is characterized not by simple immune decline but by immune dysregulation, with reduced responses to new challenges but increased baseline inflammation. By restoring proper immune regulation, thymosin alpha-1 may help resolve this inflammaging state.
Research also shows benefits in sepsis, where mortality from multiple organ failure is significantly reduced in patients receiving thymosin alpha-1. This suggests the peptide helps coordinate complex immune responses, not just boost overall immune activity.
One important consideration for thymosin alpha-1 effectiveness is zinc status. The peptide requires zinc to activate T cells and natural killer cells. Zinc deficiency, which is common in older adults and those with chronic illness, may blunt the benefits of thymosin alpha-1 supplementation. Ensuring adequate zinc intake is a practical prerequisite for optimal results.
Standard dosing protocols typically use 1.6 milligrams administered subcutaneously, with frequency varying by application from twice weekly to daily. Thymic peptide protocols often combine thymosin alpha-1 with other bioregulators for comprehensive immune support.
The Khavinson bioregulators: organ-specific longevity peptides
Russian gerontologist Vladimir Khavinson spent over four decades developing what he called peptide bioregulators, short-chain peptides that target specific organs and tissue types. His research produced a remarkable body of evidence, including clinical trials spanning 6-12 years with hundreds of participants documenting reduced mortality and improved organ function.
The bioregulator approach differs from other peptide strategies. Rather than targeting systemic processes like telomere maintenance or mitochondrial function, bioregulators aim to restore specific organs by regulating gene expression within those tissues. The theory holds that each organ has characteristic peptides that maintain its function, and age-related decline in these peptides contributes to organ deterioration.
The clinical evidence is substantial. In one landmark study, 266 elderly patients received either peptide bioregulator treatment or placebo and were followed for 6-8 years. The treatment group, which received thymic and pineal bioregulators, showed mortality rates roughly half that of controls. They had lower rates of cardiovascular disease, respiratory illness, and malignancy. Multiple organ function markers improved and remained improved for years after treatment.
Animal studies showed even more dramatic effects. Long-term treatment with thymus and pineal extracts increased mean lifespan in rodents by 20-40 percent. Some animals reached their species maximum lifespan. Biomarkers of aging slowed. Tumor development decreased. The results were not marginal improvements but fundamental alterations in aging trajectories.
Specific bioregulators target different organ systems. Epithalamin and its synthetic form Epitalon target the pineal gland. Thymalin targets the thymus. Cardiogen targets the heart. Vesugen targets blood vessels. Pancragen targets the pancreas. The list extends to cover essentially every major organ system.
These are di-, tri-, and tetrapeptides, very short amino acid chains that can penetrate cells and interact directly with DNA. Unlike longer peptides that work through receptor binding, bioregulators function at the genetic level, influencing which genes are expressed in their target tissues. This epigenetic mechanism allows them to produce sustained effects that persist long after the peptide itself has been cleared from the body.
The research comes primarily from the St. Petersburg Institute of Bioregulation and Gerontology, which Khavinson directed until his death in 2024. While this concentration of research in one institution has raised questions about independent replication, the studies themselves were rigorous, double-blinded, and published in peer-reviewed journals. Recent international interest has led to replication efforts in other laboratories.
For practical application, bioregulators are typically used in cycles. A common protocol involves taking specific organ-targeting peptides for 10-20 days, then cycling off for several months before repeating. The effects appear to be cumulative, with each cycle reinforcing the previous one. Long-term users report sustained improvements in energy, cognitive function, and disease resistance.
The complete bioregulator guide covers individual peptides, combination strategies, and protocols based on specific health goals. SeekPeptides provides access to the most comprehensive bioregulator information available in English, synthesizing decades of Russian research into actionable protocols.
BPC-157 and TB-500: the regenerative healing peptides
While BPC-157 and TB-500 are often discussed in the context of injury recovery and athletic performance, their relevance to longevity is increasingly recognized. Aging is characterized by declining regenerative capacity. Tissues that once healed quickly now repair slowly or incompletely. Chronic damage accumulates. The ability to regenerate is fundamental to maintaining function over time.
BPC-157 is a pentadecapeptide originally isolated from human gastric juice. The name stands for Body Protection Compound, reflecting its broad protective and regenerative properties. In animal studies, BPC-157 accelerates healing of muscles, tendons, ligaments, bone, and even internal organs. It promotes angiogenesis, the formation of new blood vessels that supply healing tissues. It reduces inflammation without suppressing beneficial immune responses.
The mechanisms involve multiple pathways. BPC-157 activates VEGFR2 and nitric oxide signaling, promoting blood vessel formation and tissue oxygenation. It engages growth factor signaling, particularly for VEGF and the EGF receptor. It modulates the dopaminergic and serotonergic systems, which may explain its effects on gut-brain axis function and mental health parameters observed in some studies.
For longevity, the gut connection is particularly relevant. BPC-157 originates from gastric secretions and has powerful effects on gut health. It protects the gut lining, reduces intestinal inflammation, and helps repair damage from ulcers and inflammatory bowel conditions. Given the emerging understanding of gut health's role in systemic aging and inflammation, supporting gut integrity may have broad longevity implications.
TB-500, or Thymosin Beta-4, complements BPC-157's effects. This peptide promotes cell migration and differentiation, essential processes for tissue repair. It helps new blood vessels form. It reduces inflammation and fibrosis, the scarring that impairs tissue function after injury. Athletes have used TB-500 to accelerate recovery, but the same mechanisms support maintenance of tissue integrity with aging.
The combination of BPC-157 and TB-500 is popular among researchers because their mechanisms are complementary. BPC-157 emphasizes growth factor signaling and angiogenesis. TB-500 emphasizes cellular migration and differentiation. Together, they provide comprehensive support for tissue regeneration that neither achieves alone.
Dosing varies by application. For systemic regenerative support, common protocols use 250-500 micrograms of BPC-157 once or twice daily, often combined with similar doses of TB-500. Local injection near injury sites may use higher concentrations for targeted effects. Cycles typically run 4-8 weeks, though some researchers use longer continuous protocols.
One human study in patients with chronic knee pain found that 7 of 12 participants experienced relief lasting more than six months after a single BPC-157 injection. While limited, this provides some human evidence for the regenerative effects documented extensively in animal models.
The longevity relevance of regenerative peptides is conceptually simple. Aging is, in part, an accumulation of damage that the body cannot fully repair. By enhancing regenerative capacity, peptides like BPC-157 and TB-500 may slow this accumulation, maintaining tissue function longer. They do not address telomeres or mitochondria directly. But they support the physical integrity of tissues that those cellular processes depend on.
NAD+ peptides and the cellular energy crisis of aging
NAD+, nicotinamide adenine dinucleotide, is not a peptide. It is a coenzyme essential for hundreds of metabolic reactions, DNA repair processes, and the function of sirtuins, the so-called longevity proteins. But NAD+ levels decline dramatically with age, and peptide-based approaches to restoring those levels are emerging as a longevity strategy.
The decline is severe. By middle age, NAD+ levels in many tissues are roughly half what they were in youth. By old age, the decline may reach 70-80 percent. This creates a cellular energy crisis. Enzymes that depend on NAD+ function less efficiently. DNA repair slows. Sirtuins cannot perform their regulatory functions. Mitochondria struggle to produce ATP.
The consequences manifest as multiple hallmarks of aging. Lower NAD+ impairs sirtuin function, which normally regulates inflammation, stress resistance, and cellular repair. Insufficient NAD+ hinders DNA repair, leading to genomic instability. Mitochondrial function declines, causing both energy deficits and increased oxidative stress. The decline in NAD+ is not just a biomarker of aging. It is a driver.
Restoration strategies include NAD+ precursors like NMN and NR, which the body can convert to NAD+. These compounds have shown benefits in animal studies and early human trials. However, peptide-based approaches offer potential advantages in targeting specific tissues and enhancing the efficiency of NAD+ utilization.
Research on NAD+ peptide therapies explores several angles. Some focus on enhancing the enzymes that synthesize NAD+ from precursors. Others target the sirtuins directly, bypassing NAD+ limitations. Still others address mitochondrial NAD+ pools specifically, which may be most relevant for energy production.
The connection to other longevity peptides is worth noting. MOTS-c activates AMPK, which promotes NAD+ synthesis. SS-31 improves mitochondrial function, potentially enhancing NAD+ utilization efficiency. Epitalon affects multiple metabolic pathways that intersect with NAD+ biology. A comprehensive longevity peptide protocol may address NAD+ through multiple complementary mechanisms rather than relying on a single intervention.
Preclinical data on NAD+ restoration is compelling. Mice with NAD+ levels restored to youthful concentrations show cardiovascular improvements, reversed metabolic conditions, and improved muscle function and endurance. The effects appear to reverse age-related decline rather than merely slowing it.
Human translation is ongoing. Clinical trials of NAD+ precursors have shown safety and some evidence of efficacy, though results vary. Peptide-based NAD+ therapies are earlier in development but represent a promising frontier. The goal is moving beyond precursor supplementation to more targeted interventions that optimize NAD+ specifically where it is most needed.
For researchers building longevity protocols, understanding NAD+ biology is essential. Many peptide interventions work better when cellular energy is adequate. Addressing NAD+ decline may enhance the effects of other longevity peptides by providing the metabolic foundation they require to function optimally.
CJC-1295 and Ipamorelin: growth hormone optimization for aging
Growth hormone production declines substantially with age, a process called somatopause. By middle age, growth hormone secretion may be half what it was in youth. By old age, the decline is even more severe. This decline correlates with increased body fat, reduced muscle mass, thinner skin, slower healing, and diminished exercise capacity.
The question of whether to replace growth hormone in aging has been debated for decades. Direct growth hormone supplementation carries risks including insulin resistance and potential effects on cancer development. But peptides that stimulate the body's own growth hormone production offer a potentially safer approach, maintaining pulsatile secretion patterns that mirror physiological regulation.
CJC-1295 is a growth hormone releasing hormone analogue. It stimulates the pituitary gland to release growth hormone in a pattern similar to natural secretion. The compound has a modified structure that extends its half-life, allowing less frequent dosing than native GHRH while maintaining effectiveness.
Ipamorelin is a growth hormone secretagogue that works through a different mechanism, binding ghrelin receptors to stimulate growth hormone release. It is highly selective for growth hormone, causing less release of cortisol and prolactin than other secretagogues. This selectivity reduces side effects and makes Ipamorelin one of the mildest growth hormone peptides available.
The combination of CJC-1295 and Ipamorelin is synergistic. They work through different receptors and pathways, and when used together, they produce greater growth hormone increases than either alone. This combination has become the most popular approach for growth hormone optimization among researchers seeking age-related benefits.
The evidence for growth hormone peptides in aging centers on body composition. Studies show increases in lean mass and decreases in body fat. Skin quality may improve as collagen synthesis increases. Sleep often improves, since growth hormone is naturally secreted primarily during deep sleep. Exercise recovery accelerates. These effects address many of the functional declines associated with somatopause.
However, the relationship between growth hormone and longevity is complex. In certain contexts, reduced growth hormone signaling extends lifespan. Dwarf mice with growth hormone deficiencies live longer than their normal counterparts. This has led some researchers to question whether boosting growth hormone in aging is advisable for longevity specifically.
The resolution may lie in the distinction between constant elevation and pulsatile secretion. Natural growth hormone release occurs in pulses, primarily at night. This pulsatile pattern may be important for benefits without risks. Peptides that stimulate natural release maintain this pulsatile pattern, unlike continuous growth hormone infusion or injections of the hormone itself.
CJC-1295 protocols typically use 100-300 micrograms injected before bed, often 1-2 times per week due to the extended half-life. Ipamorelin is usually dosed at 200-300 micrograms 1-3 times daily, with the pre-sleep dose considered most important for mimicking natural secretion patterns. The combination may use reduced doses of each compared to standalone use.
For longevity applications specifically, growth hormone peptides may be most relevant when clear symptoms of somatopause are present: stubborn body fat especially centrally, difficulty maintaining muscle despite exercise, slow recovery from exertion, poor sleep quality, and thin or sagging skin. In these contexts, restoring more youthful growth hormone patterns may improve quality of life and functional capacity.
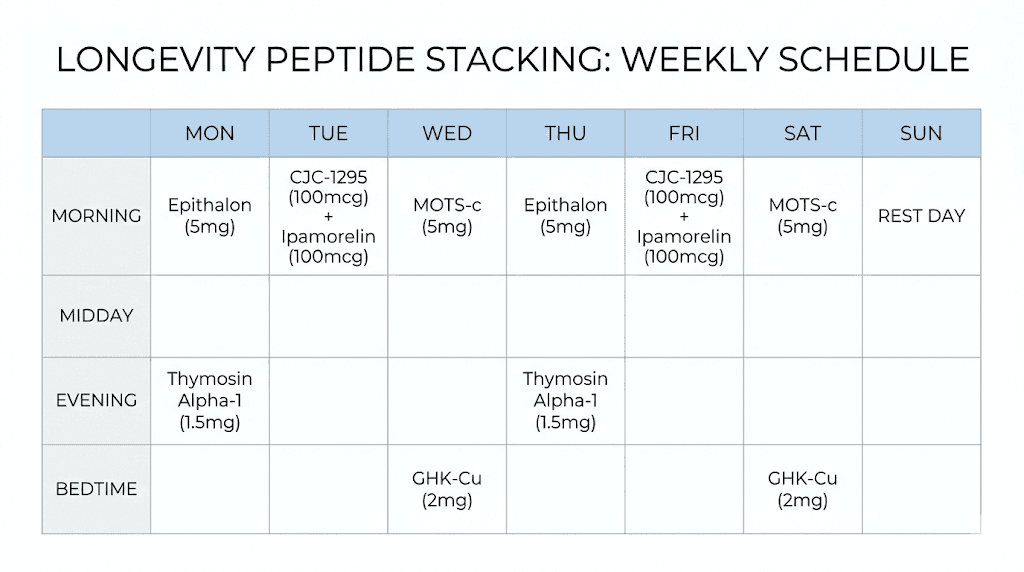
Combining longevity peptides: stacking strategies and protocols
Individual peptides target specific mechanisms. But aging involves multiple mechanisms simultaneously. This raises the question of how to combine peptides effectively, maximizing synergies while avoiding problematic interactions.
The first principle is mechanistic complementarity. An effective stack addresses different hallmarks of aging rather than redundantly targeting the same pathway. For example, combining Epitalon for telomere maintenance with MOTS-c for mitochondrial function addresses two distinct aging mechanisms. Adding Thymosin alpha-1 addresses immune decline. This three-peptide combination covers telomeres, mitochondria, and immune function, three major domains of age-related deterioration.
The second principle is temporal separation. Some peptides are best taken at specific times. Growth hormone peptides work most effectively before bed, when natural GH secretion peaks. MOTS-c may work best around physical activity, given its role as an exercise-induced signal. Organizing a protocol around optimal timing for each peptide can enhance results.
The third principle is cycling. Most peptides are not meant for continuous indefinite use. The body may downregulate receptors or develop tolerance. Cycling, using peptides for defined periods followed by breaks, maintains sensitivity and may produce more sustained benefits. Common cycles range from 4-12 weeks on, followed by similar off periods. Some bioregulators use even shorter cycles of 10-20 days with months between.
A basic longevity stack might include:
Epitalon for 10-20 days once or twice yearly for telomere support
GHK-Cu topically or via injection for gene expression reset
Thymosin alpha-1 seasonally for immune support
BPC-157 as needed for regenerative support and gut health
A more comprehensive stack might add:
MOTS-c or SS-31 for mitochondrial function
CJC-1295/Ipamorelin for growth hormone optimization
Organ-specific bioregulators based on individual needs
The key is individualization. Not everyone needs every peptide. Someone with excellent mitochondrial function biomarkers might skip MOTS-c. Someone with strong immune function might reduce thymosin alpha-1 frequency. Someone not experiencing growth hormone deficiency symptoms might omit GH secretagogues entirely. The goal is addressing actual deficits, not applying every available intervention indiscriminately.
Monitoring becomes important with complex stacks. Baseline testing before beginning and periodic retesting during protocols helps assess whether interventions are producing intended effects. Markers like telomere length, inflammatory cytokines, IGF-1 levels, and comprehensive metabolic panels can guide protocol adjustments.
Peptide stacking calculators help researchers plan combinations based on their specific goals. SeekPeptides provides comprehensive stacking guidance, including synergy information, timing recommendations, and cycling schedules for common longevity goals.
Safety considerations and what the research does not yet show
The enthusiasm around longevity peptides should be tempered with realistic assessment of what we know and do not know. While many compounds have promising preclinical and even clinical data, longevity specifically, measured as actual lifespan extension in humans, has not been demonstrated for any peptide. What we have are improvements in biomarkers and risk factors associated with aging, not proof that using these peptides will make humans live longer.
This distinction matters. Improving telomere length is not the same as proving someone will live longer. Enhancing mitochondrial function is not the same as preventing age-related disease. The hallmarks of aging are correlated with lifespan, but correlation and causation are not identical. Interventions that modify these hallmarks may or may not translate to actual longevity benefits.
The safety data, while generally reassuring for most peptides, is also incomplete. Long-term human studies are rare. Most peptides have been used clinically for specific indications, not for decades-long longevity protocols. Potential effects of very long-term use are unknown. The absence of documented problems is not the same as proof of safety.
Specific concerns apply to specific peptides. For Epitalon and telomerase activators, there is theoretical concern about promoting cancer. Telomerase is active in most cancer cells, enabling their unlimited proliferation. Activating telomerase systemically could theoretically promote malignant transformation. However, no clinical evidence suggests Epitalon increases cancer risk, and some data suggests it may actually reduce tumor development. The concern remains theoretical.
For growth hormone peptides, the relationship to cancer is also debated. IGF-1, which rises with growth hormone stimulation, is associated with increased cancer risk in epidemiological studies. However, these associations are with endogenously high IGF-1, not with peptide-stimulated increases. Whether peptide use affects cancer risk is unknown. Prudent researchers may avoid GH peptides if they have personal or family history of hormone-sensitive cancers.
Many peptides remain unapproved by regulatory agencies. The FDA has flagged numerous peptide bulk substances as potential safety concerns in compounding, including BPC-157, LL-37, DSIP, Epitalon, injectable GHK-Cu, and thymosin beta-4 fragments. This does not necessarily mean these peptides are dangerous, but it does mean they lack the safety documentation that FDA approval would require.
Quality and purity are significant concerns. Peptides from unverified sources may be contaminated, degraded, mislabeled, or counterfeit. Testing by independent laboratories is essential for researchers using peptides obtained outside regulated channels. Proper handling and reconstitution also affects peptide integrity.
Interactions with medications are largely unstudied. Researchers taking prescription drugs should consider potential interactions, particularly for peptides affecting immune function, metabolic processes, or growth factor signaling. Medical supervision is advisable for complex protocols, especially in individuals with existing health conditions.
The honest summary is that longevity peptides show genuine promise based on available evidence, but that evidence has limitations. Responsible use involves understanding both what the research shows and what it does not yet establish. Benefits are plausible but not guaranteed. Risks are generally low but not zero. Individual judgment and, ideally, medical guidance should inform decisions about peptide use for longevity purposes.
Practical protocols for longevity peptide research
Moving from theory to practice requires specific, actionable protocols. Here are evidence-based approaches for key longevity peptides, drawn from clinical research and experienced practitioner protocols.
Epitalon Protocol:
Dose: 5 milligrams once daily for 20 consecutive days, or 10 milligrams once daily for 10 consecutive days. Administration: subcutaneous injection, typically in the evening to support circadian melatonin production. Frequency: 1-2 cycles per year. Some researchers use the longer 20-day protocol once annually, while others prefer shorter cycles twice yearly.
MOTS-c Protocol:
Dose: Research in animals uses 5-15 mg/kg, but human translation suggests much lower absolute doses may be effective given body weight scaling. Common research protocols use 5-10 milligrams 3-5 times weekly. Administration: subcutaneous injection, often timed around physical activity. Duration: 8-12 week cycles with similar off periods.
GHK-Cu Protocol:
Topical: Products with 2-10 percent concentration applied once or twice daily. Effects visible in 4-8 weeks, with optimal results around 12 weeks of consistent use. Injectable: Doses extrapolated from animal studies suggest 1-2 milligrams daily for research purposes, with concurrent zinc supplementation. Cycles typically 4-8 weeks.
Thymosin Alpha-1 Protocol:
Dose: 1.6 milligrams is the standard clinical dose. Administration: subcutaneous injection. Frequency: twice weekly for ongoing immune support, or daily for 2-4 weeks during acute immune challenges. Ensure adequate zinc status for optimal effectiveness.
BPC-157 Protocol:
Dose: 250-500 micrograms once or twice daily for systemic effects. Higher doses may be used for local injection near injury sites. Administration: subcutaneous or intramuscular injection. Duration: typically 4-8 weeks, though some protocols run longer for chronic conditions.
CJC-1295/Ipamorelin Combination:
Dose: CJC-1295 at 100-300 micrograms 1-2 times weekly; Ipamorelin at 200-300 micrograms before bed nightly or 1-3 times daily. When combined, some researchers reduce individual doses. Administration: subcutaneous injection. Timing: Ipamorelin before bed, CJC-1295 evening or night. Duration: 8-16 week cycles with 4-8 week breaks.
These protocols represent starting points, not rigid prescriptions. Individual responses vary. Adjustments based on subjective response and objective testing are appropriate. Peptide calculators can help determine appropriate doses based on body weight and goals.
SeekPeptides members get access to detailed protocol databases, including variations for different goals, adjustment guidelines, and tracking tools to monitor progress. The complexity of longevity peptide protocols makes comprehensive resources valuable for researchers seeking to optimize their approaches.
Frequently asked questions
What is the best peptide for longevity?
There is no single best peptide because aging involves multiple mechanisms. Epitalon has the longest research history and documented human clinical data on telomere extension and mortality reduction. MOTS-c and SS-31 target mitochondrial function, which declines universally with age. GHK-Cu influences thousands of genes in patterns associated with younger cells. The optimal approach combines peptides targeting different aging mechanisms rather than relying on any single compound.
How long does it take to see results from longevity peptides?
Timeframes vary by peptide and outcome measured. Subjective improvements in energy, sleep, or skin quality may appear within weeks. Objective biomarker changes like improved metabolic panels or reduced inflammatory markers typically require 2-3 months. Structural changes like significant skin collagen improvement or body composition shifts often take 3-6 months. Telomere or longevity effects would require years to assess, which is why researchers rely on biomarker proxies.
Are longevity peptides safe for long-term use?
Safety data for most longevity peptides is reassuring but incomplete. Russian bioregulator studies followed participants for 6-12 years without significant adverse effects.
SS-31 has the most extensive clinical development with FDA approval for one indication. However, multi-decade safety data for longevity protocols specifically does not exist. Prudent practice involves cycling rather than continuous use, monitoring relevant biomarkers, and maintaining medical supervision for complex protocols.
Can longevity peptides be combined with each other?
Yes, and combination protocols are common in longevity research. Effective stacking typically combines peptides that target different mechanisms, such as Epitalon for telomeres plus MOTS-c for mitochondria plus Thymosin alpha-1 for immune function. Temporal separation may optimize results, with different peptides taken at different times of day or on different days. Avoid combining peptides that target identical pathways, which provides redundancy without additional benefit.
Do I need to inject longevity peptides or are oral forms available?
Most research-supported longevity peptides require injection because peptides are typically degraded in the digestive system before absorption. GHK-Cu is an exception with demonstrated topical efficacy and oral bioavailability for some applications. Some nasal spray formulations exist for certain peptides like Epitalon, offering non-injection delivery with faster absorption than oral routes. However, subcutaneous injection remains the most established and reliable administration method for most compounds.
What testing should I do before and during longevity peptide protocols?
Comprehensive baseline testing provides a reference for assessing effects. Useful markers include: telomere length testing, comprehensive metabolic panel, inflammatory markers like CRP and IL-6, fasting insulin and glucose, lipid panel including advanced markers, IGF-1 levels if using growth hormone peptides, and hormone panels appropriate to age and sex. Retesting every 3-6 months during active protocols helps track changes and guide adjustments.
Are longevity peptides legal?
Legal status varies by jurisdiction and specific peptide. In the United States, many peptides are sold as research chemicals not for human consumption. The FDA has flagged certain peptides as potentially problematic for pharmacy compounding. Some peptides like SS-31 have FDA approval for specific medical indications. The legality of personal use for research purposes exists in a gray area that researchers should understand before obtaining peptides.
External resources
AagingBase: Comprehensive database of anti-aging peptides (PMC)
NAD+ metabolism and roles in cellular aging (Nature Reviews)
MOTS-c is an exercise-induced regulator of physical decline (Nature Communications)
For researchers serious about optimizing their longevity protocols, SeekPeptides provides the most comprehensive resource available. Evidence-based guides, proven protocols, and a community of thousands who navigate these exact questions daily.
The science of longevity peptides is advancing rapidly, and staying current requires dedicated resources that synthesize emerging research into practical application.
In case I do not see you, good afternoon, good evening, and good night. Join us.



