Feb 3, 2026
Your antihistamine is a bandage. It masks the itch, dries the sinuses, dulls the reaction. But the moment it wears off, the sneezing returns. The eyes water. The throat tightens. Nothing has changed underneath. The immune system still overreacts to harmless proteins, still floods your tissues with histamine at the faintest trace of pollen or pet dander, still treats the outside world like a battlefield. And you are still reaching for the same pill, day after day, season after season, wondering if there is a better way.
There is. Peptides offer something antihistamines cannot. They do not just block symptoms. They address the immune dysfunction driving those symptoms in the first place. From KPV, a tripeptide that stabilizes mast cells and suppresses the inflammatory cascade, to BPC-157, which outperformed traditional antihistamines in preventing anaphylactoid reactions in animal studies, to thymosin alpha-1, which fine-tunes the entire adaptive immune response, peptides represent a fundamentally different approach to allergies. One that works with your biology rather than against it.
This guide covers every peptide with documented relevance to allergic conditions. You will find specific mechanisms, research findings with actual numbers, practical protocols, and honest assessments of what the evidence supports and where gaps remain. Whether you are dealing with seasonal rhinitis, food sensitivities, mast cell activation syndrome, or chronic inflammatory responses that refuse to resolve, the information here will help you understand what peptides can and cannot do for your situation. SeekPeptides members already use many of these protocols. The research is compelling. But it requires nuance, and that is exactly what we will provide.
How allergies actually work at the cellular level
Before you can understand how peptides help with allergies, you need to understand what goes wrong in the first place. Most people think allergies are simple. Pollen enters nose. Body reacts. Sneeze happens. But the reality involves a complex chain of immune events that begins long before any symptom appears.
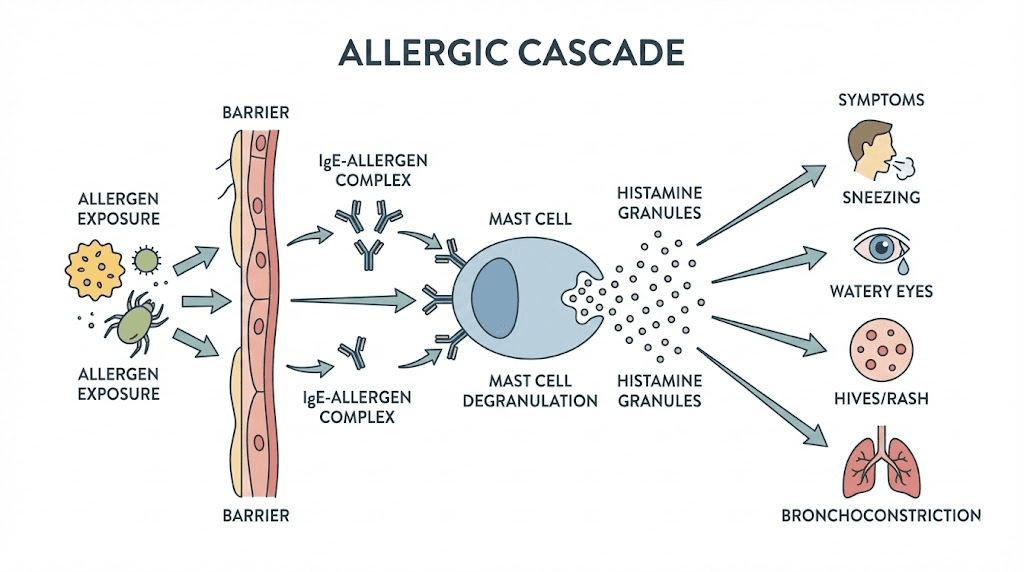
Allergies are a case of mistaken identity. Your immune system encounters a harmless substance, a protein from pollen, dust mites, pet dander, or certain foods, and misidentifies it as dangerous. This triggers a cascade that involves multiple immune cell types, signaling molecules, and feedback loops. Understanding this process reveals exactly where peptides intervene and why they can be so effective.
The sensitization phase
The first time you encounter an allergen, nothing visible happens. No sneezing. No rash. But internally, your immune system is taking notes. Dendritic cells capture the allergen and present it to T-helper cells. In people prone to allergies, these T-cells differentiate into Th2 cells rather than Th1 cells, creating an imbalance that favors inflammatory responses. The Th2 cells release cytokines, specifically IL-4 and IL-13, that instruct B-cells to produce immunoglobulin E (IgE) antibodies specific to that allergen.
These IgE antibodies then attach to receptors on mast cells throughout your tissues. Mast cells are sentinel immune cells found in skin, airways, gut lining, and other boundary tissues. Once armed with IgE, they become primed landmines. They are waiting.
The activation phase
The second exposure is when symptoms explode. The allergen binds to the IgE molecules sitting on mast cell surfaces. When two or more IgE molecules are cross-linked by the allergen, the mast cell degranulates. This is the critical moment. The cell releases its stored contents: histamine, tryptase, prostaglandins, leukotrienes, and a cocktail of other inflammatory mediators.
Histamine alone causes vasodilation, increased vascular permeability, smooth muscle contraction, mucus secretion, and nerve stimulation. That is why you experience swelling, redness, itching, sneezing, and congestion. All from one molecule released by one cell type.
But it does not stop there.
The late-phase response
Hours after the initial reaction, a second wave arrives. Mast cells and Th2 cells recruit eosinophils, basophils, and additional inflammatory cells to the site. These cells release their own mediators, causing prolonged inflammation that can last days. This late-phase response explains why allergy symptoms often persist long after allergen exposure ends and why chronic allergic conditions like eczema and asthma involve ongoing tissue damage.
The entire process is regulated by NF-kB, a master transcription factor that controls the expression of hundreds of inflammatory genes. This is where peptides like KPV become relevant, because they target NF-kB directly. Traditional antihistamines only block one downstream effect. Peptides can modulate the upstream signals that drive the entire allergic cascade.
KPV: the mast cell stabilizer peptide
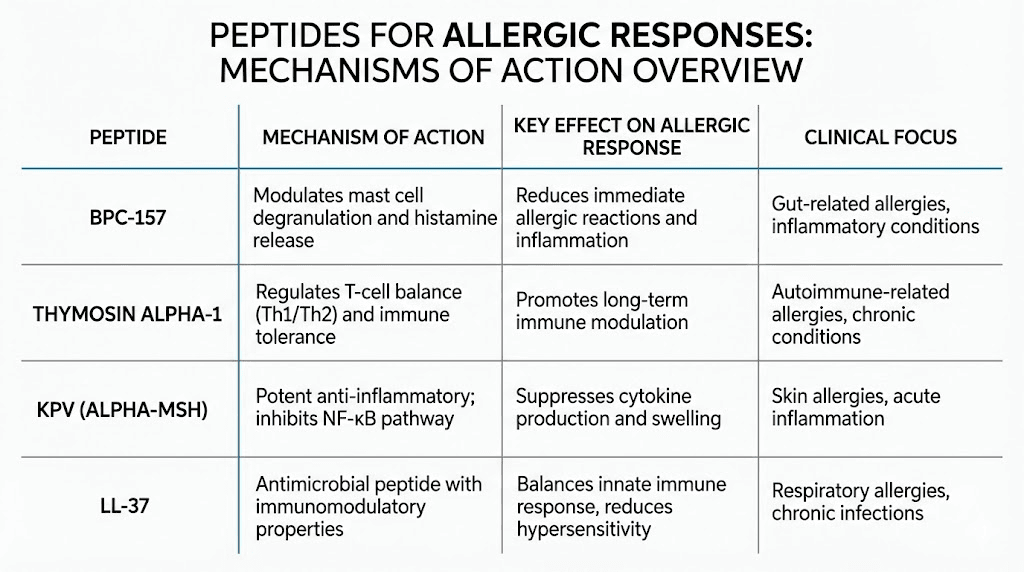
If you could pick one peptide specifically designed for allergic conditions, KPV would be it. This tripeptide, just three amino acids (lysine-proline-valine), packs an outsized punch against the inflammatory machinery that drives allergic reactions. Derived from alpha-melanocyte stimulating hormone (alpha-MSH), KPV retains all the anti-inflammatory benefits of its parent molecule without affecting pigmentation or hormone levels.
How KPV works against allergies
KPV operates through several mechanisms that are directly relevant to allergic conditions. The most important is its ability to inhibit NF-kB, the master switch of inflammation. In human bronchial epithelial cells, researchers found that KPV translocates to the cell nucleus and competitively blocks the interaction between importin-alpha-3 and the p65 subunit of NF-kB. This is not a minor modulation. It is a direct interruption of the central signaling pathway that drives inflammatory responses throughout the body.
By suppressing NF-kB, KPV reduces the expression of pro-inflammatory cytokines including IL-8 and monocyte chemoattractant protein-1 (MCP-1). It also reduces mast cell degranulation, which is the pivotal event in allergic reactions. Fewer degranulating mast cells means less histamine release, fewer prostaglandins, fewer leukotrienes, and a dramatically reduced allergic response.
KPV also interacts with melanocortin-1 receptors (MC1R), a class of G-protein-coupled receptors on immune cells. This binding suppresses TNF-alpha and IL-6 while modulating downstream inflammatory pathways. The result is a coordinated reduction in the inflammatory tone that underlies allergic disease.
KPV for mast cell activation syndrome
Mast cell activation syndrome (MCAS) represents one of the most challenging allergic-spectrum conditions. In MCAS, mast cells become hyperactive, degranulating in response to triggers that should not provoke a reaction. Temperature changes. Stress. Certain foods. Even physical pressure on the skin. The symptoms are wide-ranging and often debilitating: flushing, hives, abdominal pain, brain fog, heart palpitations, and severe fatigue.
KPV has gained significant attention in the MCAS community precisely because it stabilizes mast cells without suppressing immune function entirely. Unlike immunosuppressive drugs, KPV modulates the immune system. It brings overactive mast cells back toward baseline without compromising your ability to fight infections. Practitioners working with MCAS patients sometimes combine KPV with BPC-157 for a synergistic effect: KPV calms the mast cells while BPC-157 repairs the tissue damage that chronic inflammation has caused, particularly in the gut.
KPV for airway inflammation
Research published in the journal Biological Chemistry demonstrated that KPV and its related melanocortin peptide gamma-MSH suppress NF-kB signaling in airway epithelium through two distinct mechanisms: inhibition of p65 nuclear import (KPV) and epithelial MC3R activation (gamma-MSH). The authors concluded that melanocortin peptides provide a robust mechanism for targeting airway inflammation in lung disease.
This has direct implications for allergic asthma and allergic rhinitis, two conditions where airway inflammation drives symptoms. If KPV can suppress the inflammatory signaling in bronchial epithelial cells, it may help reduce the hyperreactivity that makes allergic airways so sensitive to triggers.
KPV for histamine intolerance
Histamine intolerance differs from classical allergies but shares the same cellular machinery. In histamine intolerance, the body cannot break down histamine efficiently, often due to low levels of diamine oxidase (DAO) enzyme or impaired methylation pathways. The result is a buildup of histamine that causes allergy-like symptoms even without IgE-mediated reactions.
KPV helps here by reducing the overall histamine burden. By stabilizing mast cells and preventing unnecessary degranulation, KPV decreases the amount of histamine entering the system in the first place. Combined with gut-healing protocols, this approach addresses histamine intolerance from both the production and clearance sides. Many SeekPeptides members report significant improvement in histamine-related symptoms when adding KPV to their protocols.
KPV research protocols
Research protocols for KPV in allergic conditions typically use subcutaneous administration. Common research dosing ranges from 200 to 500 mcg per day, though some protocols use oral or sublingual routes for gut-targeted effects. The peptide has shown an excellent safety profile with minimal reported side effects, partly because it is a naturally occurring fragment of a human peptide.
For seasonal allergies, some researchers begin KPV protocols 2 to 4 weeks before allergy season starts, aiming to stabilize mast cells before peak pollen exposure. For MCAS and year-round histamine issues, longer-term protocols are more common. Exact KPV dosing depends on the specific condition, body weight, and individual response. The peptide calculator can help determine starting amounts, but monitoring and adjustment are essential.
BPC-157: outperforming antihistamines in research
BPC-157 is not typically the first peptide people associate with allergies. Known primarily for its remarkable tissue-healing properties, this 15-amino-acid peptide derived from human gastric juice has a surprising body of research supporting its use in allergic and anaphylactoid conditions. And the results are not subtle.
The anaphylaxis study
In a study published in the European Journal of Pharmacology, researchers induced anaphylactoid reactions in anesthetized mice and rats using intravenous dextran and white egg protein. The untreated animals developed prominent edema of the face, lips, snout, paws, and scrotum, along with extreme cyanosis, poor respiration, and fatalities. This is about as severe as allergic reactions get in an animal model.
BPC-157 was administered at doses ranging from 10 micrograms per kilogram down to just 10 picograms per kilogram. The results were striking. BPC-157 regimens effectively prevented anaphylactoid reactions and could rescue animals already experiencing them. Upper and lower lip edema, snout swelling, cyanosis, and paw and scrotal edema were all markedly attenuated. Poor respiration and fatalities were not observed in BPC-157 treated animals.
Here is the most significant finding. Unlike H1 and H2 histamine receptor antagonists, which showed inconsistent results across the different animal models, BPC-157 had a strong beneficial effect in both rats and mice. Traditional antihistamines were effective in some models but not others. BPC-157 worked across the board.
How BPC-157 modulates allergic responses
BPC-157 appears to interact with allergic pathways through multiple mechanisms. First, it has been observed to prevent mast cell activation more effectively than antihistamines. This is upstream of histamine release, meaning it prevents the problem rather than managing its consequences. Second, BPC-157 prevents and reverses mitochondrial damage, which is significant because mitochondrial dysfunction contributes to mast cell hyperactivation in conditions like MCAS.
Third, BPC-157 has powerful tissue-repair properties that address the downstream damage allergic inflammation causes. Chronic allergies damage mucosal tissues in the airways and gut. Tissue repair is essential for breaking the cycle where damaged tissues become more reactive, which triggers more inflammation, which causes more damage. BPC-157 promotes angiogenesis (new blood vessel formation) and granulation tissue formation, both critical for repairing inflamed tissues.
BPC-157 and gut-mediated allergies
A significant percentage of allergic conditions have a gut component. The gut houses approximately 70% of the immune system. When the intestinal barrier becomes compromised, a condition sometimes called leaky gut, undigested food proteins and other molecules can cross into the bloodstream and trigger immune reactions. This contributes to food allergies, food sensitivities, and systemic inflammatory responses.
BPC-157 was originally discovered for its gut-protective properties. It is a fragment of a protein found naturally in gastric juice. Research has demonstrated its effectiveness in healing gastric and duodenal lesions, protecting intestinal mucosa, and reducing gut inflammation. In the context of allergies, this means BPC-157 can help restore the intestinal barrier that is supposed to prevent allergens from reaching the immune system in the first place.
The combination of mast cell stabilization and gut barrier repair makes BPC-157 particularly valuable for people dealing with food sensitivities and food-related immune reactions. Some researchers combine it with KPV to address both the immune overreaction and the tissue damage simultaneously.
BPC-157 research protocols for allergic conditions
Standard BPC-157 dosing in research protocols typically ranges from 200 to 300 mcg administered subcutaneously twice daily. For gut-specific applications, oral administration is also used, as BPC-157 is stable in gastric juice (unlike most peptides). Researchers studying allergic conditions often use the BPC-157 dosage calculator to determine weight-based amounts.
Protocols for allergic conditions typically run 4 to 8 weeks, with some researchers reporting improvements within the first 1 to 2 weeks. The anaphylaxis study mentioned above showed effects at doses as low as 10 picograms per kilogram, suggesting that BPC-157 may be effective at remarkably small amounts for allergic modulation specifically. However, most practical protocols use higher doses for broader therapeutic benefit.
One important note: some users have reported increased gut histamine initially when starting BPC-157. This appears to be a transient effect related to the healing process rather than a worsening of the condition. If you are highly histamine-sensitive, starting with lower doses and increasing gradually may be advisable. Proper reconstitution and storage are essential for maintaining peptide potency.

Thymosin alpha-1: fine-tuning the immune system
While KPV targets mast cells and BPC-157 addresses tissue repair and acute allergic reactions, thymosin alpha-1 (Ta1) operates at a deeper level. This 28-amino-acid peptide, originally isolated from the thymus gland, modulates the immune system at its foundation. It does not simply suppress or stimulate. It regulates. And for allergies, which are fundamentally a disorder of immune regulation, this distinction matters enormously.
The bidirectional immune modulator
What makes thymosin alpha-1 unique among immune-modulating peptides is its bidirectional nature. It can both enhance and suppress immune responses depending on what the body needs. When the immune system is underperforming, Ta1 boosts it. When it is overreacting, as in allergies, Ta1 calms it down. This is not something most drugs can do.
The mechanism involves Toll-like receptors on dendritic cells, which are the immune system's scouts. Ta1 activates both myeloid and plasmacytoid dendritic cells through these receptors, initiating signaling pathways that lead to appropriate (not excessive) immune activation. Critically, Ta1 also works through an indoleamine 2,3-dioxygenase (IDO) dependent pathway that can attenuate the inflammatory activity of dendritic cells. This qualifies it as a genuine immune regulatory peptide capable of fine-tuning the quality of the immune response.
Thymosin alpha-1 and regulatory T cells
One of the most relevant mechanisms for allergy sufferers is Ta1s support of regulatory T cells (Tregs). Tregs are the immune system's peacekeepers. They suppress excessive immune responses and maintain tolerance to harmless substances. In allergic individuals, Treg function is often impaired, allowing Th2-dominant responses to run unchecked.
Ta1 has been shown to promote Treg differentiation and function. By restoring the Treg population, it helps rebalance the Th1/Th2 ratio that is skewed in allergic disease. This is the kind of fundamental immune correction that antihistamines cannot achieve. It addresses the root cause of allergic overreaction rather than just managing downstream symptoms.
Thymosin alpha-1 and autoimmune overlap
Many people with severe allergies also have autoimmune tendencies. The same Treg dysfunction that allows allergic responses can also permit autoimmune attacks. Researchers have studied Ta1 in patients with psoriatic arthritis, rheumatoid arthritis, and systemic lupus erythematosus, finding that it influences multiple components of the inflammatory and autoimmune cascade.
For people dealing with both allergies and autoimmune conditions, Ta1 offers the possibility of addressing both simultaneously through its central immune-regulatory mechanism. This is particularly relevant for conditions where the line between allergy and autoimmunity blurs, such as eosinophilic esophagitis or chronic urticaria with autoimmune features.
Thymosin alpha-1 research protocols
The synthetic form of Ta1, thymalfasin (marketed as Zadaxin), is approved in over 35 countries for various immune conditions. Standard research protocols use 1.6 mg administered subcutaneously, typically twice weekly. The peptide has an excellent safety profile with only minor side effects reported across decades of clinical use.
One limitation is its short serum half-life of approximately 2 hours. Researchers have been working on solutions including Fc fusion proteins that extend the half-life to around 25 hours in mouse models. For practical research use, the twice-weekly dosing schedule accounts for the short half-life while still maintaining therapeutic benefit through the lasting immunological changes each dose initiates.
For allergy-specific applications, some researchers combine Ta1 with other peptides. A common approach pairs Ta1 for foundational immune rebalancing with KPV for acute mast cell stabilization. The theory is that Ta1 addresses the underlying Th2 skew while KPV manages the immediate inflammatory output. This kind of peptide stacking requires careful planning and monitoring, and SeekPeptides provides detailed stacking protocols for members navigating these decisions.
Peptide immunotherapy: allergen-derived peptides for tolerance
While the peptides discussed above modulate general immune function, there is an entirely separate category of peptide therapy designed specifically for allergies: peptide immunotherapy. This approach uses short synthetic peptides derived from the allergens themselves to retrain the immune system and achieve genuine tolerance.
Why peptide immunotherapy is different from traditional allergy shots
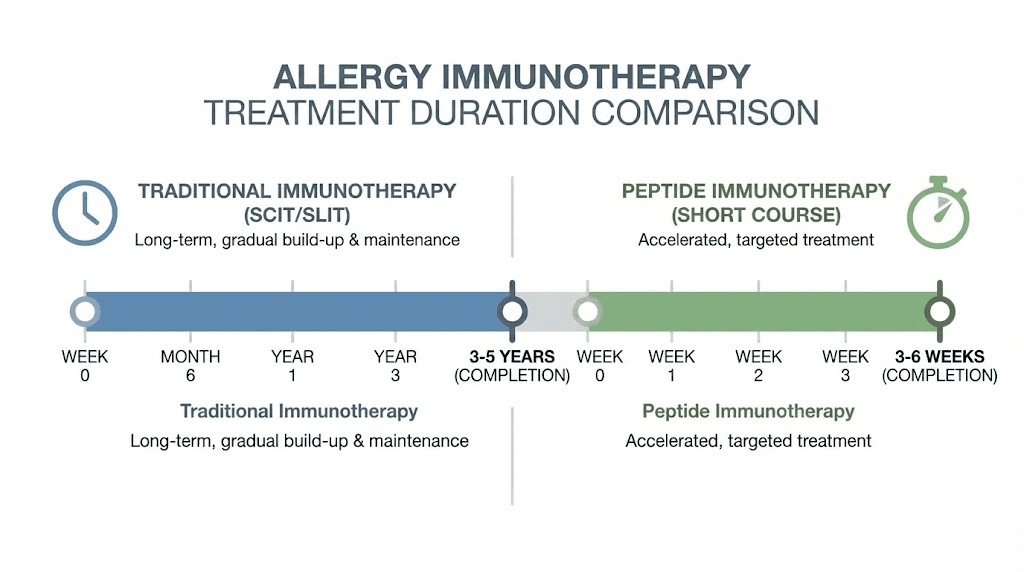
Traditional allergen immunotherapy (allergy shots) has been used for over a century. It works by exposing the immune system to gradually increasing doses of whole allergen proteins, eventually inducing tolerance. The problem is that whole proteins can cross-link IgE on mast cells and trigger the very allergic reactions you are trying to treat. This means treatment must start with tiny doses, increase slowly, and continue for 3 to 5 years. Side effects, including anaphylaxis, remain a real risk.
Peptide immunotherapy solves this problem elegantly. Instead of whole proteins, it uses short synthetic peptides (less than 20 amino acids) that contain only the immunodominant T-cell epitopes of the allergen. These peptides are too short to cross-link IgE molecules on mast cell surfaces, so they cannot trigger mast cell degranulation. But they can still interact with T-cells to induce tolerance.
The result? Higher doses can be given safely. Treatment courses are dramatically shorter. And the risk of anaphylaxis drops substantially.
Grass pollen peptide immunotherapy
The most advanced peptide immunotherapy research involves grass pollen, which affects an estimated 400 million people worldwide. A randomized, double-blind, placebo-controlled trial enrolled 554 adults with grass pollen allergic rhinoconjunctivitis. Participants received 8 subcutaneous injections of grass allergen peptide hydrolysates (LPP) over just 3 weeks.
The results showed a 15.5% reduction in combined symptom and medication scores during peak pollen season and a 17.9% reduction over the entire season, compared to placebo. These improvements came from just 4 clinic visits over 3 weeks, compared to years of treatment with traditional allergy shots.
Even more promising, follow-up data showed continued symptom relief 2 years after treatment. There is preliminary evidence suggesting that a short 4 to 6 week course of peptide immunotherapy may confer sustained clinical benefit lasting 1 to 3 years without additional treatment.
Cat allergen peptide therapy
Researchers treated cat-allergic volunteers with a mixture of seven peptides representing immunodominant epitopes of Fel d 1, the major cat allergen. Baseline symptoms were compared with identical cat allergen exposure 4 to 5 months later. The peptide-treated group showed significant symptom reduction compared to controls, demonstrating that the approach works across different allergen types.
Peanut allergy peptide therapy
One of the most exciting developments is PVX108, a peptide therapy being developed for peanut allergies. This therapy is designed to render basophils and mast cells inoperative while containing only the small peptide components of peanut protein needed for T-cell tolerance. Clinical trials showed a favorable safety and tolerability profile with no serious adverse events. Treatment-related effects were mild or moderate.
Given that peanut allergy can cause life-threatening anaphylaxis, a peptide-based approach that achieves tolerance without risking severe reactions represents a genuine breakthrough. This is disease modification at its most meaningful.
Olive pollen and emerging research
T-cell epitopes derived from Ole e 1, the major olive pollen allergen, have shown the ability to modulate allergic responses in vitro. Researchers are exploring synthetic peptides based on these epitopes as safe preventive tools for olive pollen allergy. Similar approaches are being investigated for dust mite, birch pollen, and other common allergens.
How peptide immunotherapy achieves tolerance
The mechanisms include induction of specific T-cell anergy (functional inactivation of allergen-reactive T-cells), differentiation of naive T-cells into regulatory T-cells, immune deviation (shifting the Th2/Th1 ratio toward Th1), and allergen-specific Th2 cell deletion. Studies also provide evidence for fast suppression of basophil responses, modulation of T-cell and B-cell subsets, induction of FoxP3-positive regulatory T-cells, and production of IgG4 blocking antibodies that compete with IgE for allergen binding.
These are the same mechanisms that traditional immunotherapy uses, but peptide immunotherapy appears to achieve them faster and with fewer risks. The key question remaining is whether long-term outcomes match those of traditional immunotherapy, which can provide decades-long remission in some patients.
VIP: the airway-protective peptide
Vasoactive intestinal peptide (VIP) occupies a unique position among therapeutic peptides. It is an endogenous neuropeptide found naturally in airway nerve terminals and mast cells, with potent anti-inflammatory and bronchodilatory properties. For allergic asthma and allergic airway disease, VIP addresses the condition from an angle that other peptides do not.
VIP and airway function
VIP is essential for normal lung function. When researchers created VIP knockout mice (animals genetically unable to produce VIP), those mice spontaneously developed features of asthma. They showed peribronchiolar airway inflammation, accumulation of lymphocytes and eosinophils, elevated IL-5 and IL-6, and airway hyperresponsiveness to inhaled methacholine. In other words, without VIP, the lungs default to an asthmatic, allergic state.
This suggests that VIP deficiency may contribute to allergic airway disease in humans. And indeed, studies have found altered VIP levels in people with asthma. Restoring appropriate VIP signaling could help normalize airway function in allergic individuals.
VIP and immune cell modulation
VIP interacts with multiple immune cells through specific receptors (VPAC-1, VPAC-2, CRTH2, and PAC1) expressed on eosinophils, mast cells, neutrophils, lymphocytes, dendritic cells, NK cells, and macrophages. This broad receptor distribution means VIP can modulate virtually every immune cell type involved in allergic responses.
Of particular relevance for allergies, VIP has been proposed as a therapeutic option for inflammatory allergic disorders including asthma, rhinitis, atopic dermatitis, and eosinophil-associated gastrointestinal disorders. That is a wide range of allergic conditions, all potentially addressable through a single peptide pathway.
VIP for food allergies
A study published in Immunopharmacology and Immunotoxicology found that VIP administration inhibited food allergy responses through restoration of immune suppressive functions in B regulatory cells. Specifically, VIP alleviates food allergy by upregulating expression of TSP1 to stabilize IL-10 expression in B regulatory cells and recover their immune regulatory functions.
IL-10 is a key anti-inflammatory cytokine, and B regulatory cells are critical for maintaining immune tolerance. By restoring these functions, VIP addresses food allergy at a fundamental regulatory level rather than just blocking symptoms.
VIP delivery challenges
The main limitation of VIP as a therapeutic peptide is its extremely short plasma half-life. Systemic administration also causes side effects including hypotension and decreased heart rate. These challenges have driven research into alternative delivery methods.
Inhaled VIP formulations bypass many systemic side effects while delivering the peptide directly to the airways. Researchers have developed dry powder inhalation systems and VIP conjugated with alpha-alumina nanoparticles (alpha-AN-VIP) that significantly reduced eosinophil counts, serum IgE, Th2 cytokines, and airway hyperresponsiveness in asthmatic animal models. The nanoparticle formulation outperformed both beclomethasone (a standard inhaled corticosteroid) and unconjugated VIP alone.
Current research continues developing stable, long-acting VIP agonists that could make this peptide practical for clinical use in allergic conditions. While not yet widely available in research settings, VIP remains one of the most promising peptides for allergic airway disease.
Additional peptides with allergy-relevant mechanisms
Beyond the primary peptides discussed above, several others have mechanisms relevant to allergic conditions. Each approaches the problem from a different angle, and understanding these options helps build a comprehensive picture of how peptide research is reshaping allergy management.
LL-37 (cathelicidin)
LL-37 is a 37-amino-acid peptide formed from the cathelicidin precursor hCAP18. It is produced by white blood cells (especially neutrophils) and keratinocytes. LL-37 plays a complex dual role in allergic inflammation.
On one hand, LL-37 is a potent mast cell chemoattractant. It recruits mast cells to sites of inflammation through a dose-dependent, Gi protein-phospholipase C signaling pathway. It also directly stimulates mast cell degranulation and promotes the release of pruritogens including histamine and prostaglandins. LL-37 induces mast cells to release IL-31, a cytokine associated with itch and inflammatory skin diseases.
On the other hand, when LL-37 coexists with bacterial components like LPS, it switches mast cell function away from Th2 allergic responses and toward innate immune defense. The Th2 cytokine upregulation caused by LL-37 alone is cancelled by LPS, while pro-inflammatory cytokine production for pathogen defense remains active. This suggests LL-37 acts as an alarm system, redirecting mast cells from allergic mode to infection-fighting mode when bacteria are present.
Elevated LL-37 levels have been found in patients with chronic rhinosinusitis, where it is associated with increased neutrophil and mast cell infiltration. Understanding LL-37 is important not as a therapeutic peptide for allergies, but as a key player in the biology of how allergic inflammation develops and resolves. Research into LL-37 modulation could eventually lead to new therapeutic approaches.
Selank
Selank is a synthetic peptide based on the naturally occurring immunomodulatory peptide tuftsin. While primarily known for its anxiolytic and nootropic properties, Selank has documented effects on immune function that may be relevant to allergic conditions. It modulates IL-6, a cytokine involved in both allergic inflammation and the stress response. Since stress and anxiety can exacerbate allergic conditions through neuroimmune pathways, Selank may offer indirect allergy benefits through stress modulation.
Semax
Semax, another synthetic peptide derived from ACTH, has immunomodulatory properties that include modulation of inflammatory cytokine expression. Like Selank, its primary applications are neurological, but the immune-modulating effects could contribute to a comprehensive protocol addressing the neuroimmune aspects of allergic disease.
DSIP (delta sleep-inducing peptide)
DSIP promotes deep sleep, and sleep quality directly affects immune regulation. Poor sleep increases inflammatory cytokines, shifts the Th1/Th2 balance toward Th2 (pro-allergic), and impairs regulatory T-cell function. By improving sleep architecture, DSIP may indirectly support better immune regulation in people with allergic conditions.
Epitalon
Epitalon, the synthetic version of epithalamin, stimulates pineal gland function and melatonin production. Melatonin has documented anti-inflammatory and immunomodulatory effects, including inhibition of NF-kB and modulation of mast cell function. While not a direct allergy treatment, epitalon may contribute to immune homeostasis through its effects on circadian rhythm and melatonin, both of which influence allergic responses. Many allergy sufferers notice symptom fluctuation based on time of day, a pattern partly mediated by circadian immune cycling.
Building an allergy-focused peptide protocol
Understanding individual peptides is one thing. Combining them into an effective protocol requires a different level of analysis. Allergic conditions vary enormously in their underlying mechanisms, triggers, and severity. A protocol for seasonal hay fever looks nothing like one for MCAS or food allergies. The key is matching peptide mechanisms to your specific immune dysfunction.
Protocol 1: seasonal allergic rhinitis (hay fever)
Goal: Reduce seasonal allergy symptoms by stabilizing mast cells and modulating immune response before and during pollen season.
Primary peptide: KPV at 200 to 400 mcg subcutaneously daily
Support peptide: Thymosin alpha-1 at 1.6 mg subcutaneously twice weekly
Implementation schedule:
Pre-season (4 weeks before): Begin KPV daily and Ta1 twice weekly to stabilize mast cells and rebalance Th1/Th2 ratio before allergen exposure peaks
During season: Continue both peptides at the same doses throughout the allergy season
Post-season: Taper KPV over 2 weeks. Continue Ta1 for an additional 4 weeks for lasting immune rebalancing
Expected timeline:
Week 1 to 2: Gradual reduction in baseline inflammation
Week 3 to 4: Noticeable decrease in reactivity to allergens
During season: Reduced sneezing, congestion, and eye symptoms compared to previous years
Monitoring: Track symptom scores daily using a standardized allergy symptom diary. Note medication use (antihistamines, nasal sprays) as a secondary measure. Proper peptide reconstitution and storage are critical for consistent results.
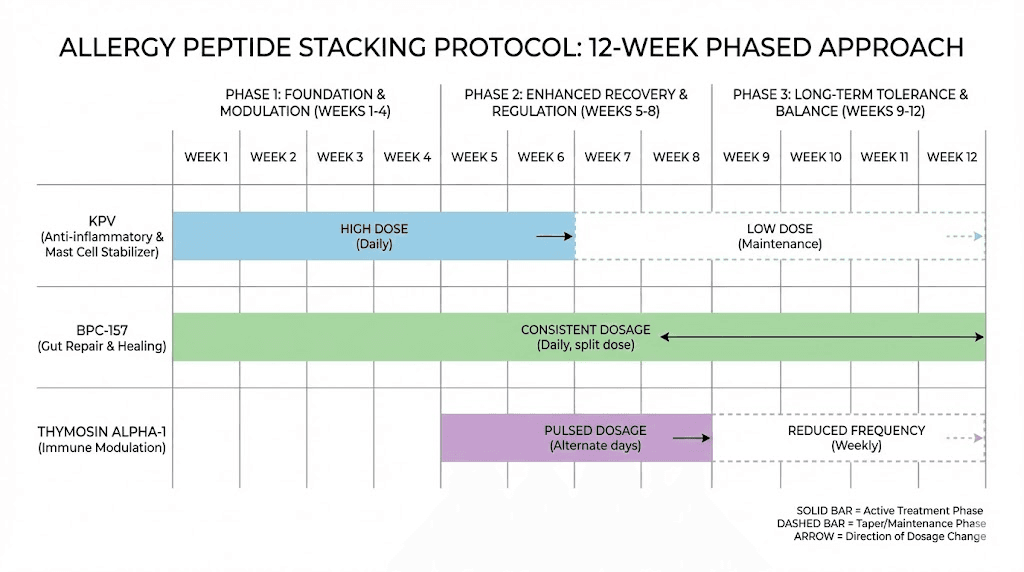
Protocol 2: mast cell activation syndrome (MCAS)
Goal: Stabilize hyperactive mast cells, reduce chronic inflammatory burden, and repair damaged mucosal tissues.
Primary peptides:
KPV at 300 to 500 mcg subcutaneously daily (mast cell stabilization)
BPC-157 at 250 mcg subcutaneously twice daily (tissue repair, additional mast cell stabilization)
Support peptide: Ta1 at 1.6 mg subcutaneously twice weekly (immune regulation)
Implementation schedule:
Week 1: Start KPV alone at lower dose (200 mcg) to assess tolerance
Week 2: Add BPC-157 at 200 mcg twice daily. Increase KPV to target dose
Week 3: Add Ta1 twice weekly. Increase BPC-157 to target dose
Weeks 4 to 12: Maintain all three peptides at target doses
Week 12+: Reassess. Some researchers reduce to maintenance dosing
Important considerations: MCAS patients are often highly sensitive. Start low and increase slowly. Monitor for any paradoxical reactions. The initial BPC-157 dose may temporarily increase gut histamine in some individuals. Having a clear dosing plan and tracking symptoms daily is essential.
Protocol 3: food sensitivities and gut-mediated allergies
Goal: Repair intestinal barrier, reduce gut inflammation, and decrease immune reactivity to food antigens.
Primary peptide: BPC-157 at 250 mcg orally twice daily (gut-specific delivery)
Support peptide: KPV at 200 to 300 mcg orally or subcutaneously daily
Implementation schedule:
Weeks 1 to 4: BPC-157 orally to target gut lining repair. Add KPV for mast cell stabilization
Weeks 5 to 8: Continue both. Begin reintroducing previously reactive foods one at a time, monitoring for reactions
Weeks 9 to 12: Assess which food reactions have diminished. Adjust protocol based on progress
Expected timeline:
Week 2 to 3: Reduced gut inflammation symptoms (bloating, cramping)
Week 4 to 6: Improved tolerance to some previously reactive foods
Week 8 to 12: Broader food tolerance, reduced systemic inflammation
This protocol works best combined with an elimination diet and careful food reintroduction. The peptides support the immune and structural changes needed, but dietary management provides the environment for healing. Use the peptide calculator for precise dosing.
Protocol 4: allergic asthma support
Goal: Reduce airway hyperreactivity, decrease eosinophilic inflammation, and improve breathing.
Primary peptide: KPV at 300 to 500 mcg subcutaneously daily (NF-kB suppression in airway epithelium)
Support peptide: Ta1 at 1.6 mg subcutaneously twice weekly (immune rebalancing)
Duration: 8 to 12 weeks minimum, with reassessment
Important note: Peptide protocols for asthma should complement, not replace, prescribed controller medications. Never discontinue inhaled corticosteroids or other asthma medications without physician guidance. The goal of peptide support is to reduce the underlying inflammation that drives asthma severity, potentially allowing medication reduction over time under medical supervision.
Stacking considerations
When combining multiple peptides for allergic conditions, timing and compatibility matter. KPV and BPC-157 can typically be administered at the same time without interaction concerns. Ta1 is usually given on a separate schedule (twice weekly rather than daily). The peptide stack calculator helps plan multi-peptide protocols, and cycling considerations apply for long-term use.
Some researchers also include supportive supplements alongside peptide protocols: quercetin as a natural mast cell stabilizer, vitamin D for immune regulation, and omega-3 fatty acids for anti-inflammatory support. These complement peptide mechanisms without interfering with them.
Comparing peptides for different allergy types
Not all allergies are created equal, and not all peptides are equally suited for every allergic condition. This comparison helps match the right peptide approach to your specific situation.
Allergy Type | Best Primary Peptide | Key Mechanism | Evidence Strength | Timeline to Results |
|---|---|---|---|---|
Seasonal rhinitis | KPV | Mast cell stabilization, NF-kB inhibition | Moderate (preclinical + clinical analogs) | 2 to 4 weeks |
Food allergies | BPC-157 + KPV | Gut barrier repair + mast cell stabilization | Moderate (animal studies) | 4 to 8 weeks |
MCAS | KPV + BPC-157 | Mast cell stabilization + tissue repair | Moderate (preclinical + clinical reports) | 2 to 6 weeks |
Allergic asthma | KPV + Ta1 | Airway NF-kB suppression + immune rebalancing | Moderate (preclinical) | 4 to 8 weeks |
Atopic dermatitis | KPV | Anti-inflammatory, mast cell stabilization | Moderate (preclinical) | 3 to 6 weeks |
Histamine intolerance | KPV + BPC-157 | Reduced histamine release + gut repair | Moderate (preclinical + clinical reports) | 2 to 4 weeks |
Contact allergies | Ta1 | Immune regulation, Treg support | Low to moderate (extrapolated) | 4 to 8 weeks |
Drug allergies | Ta1 | Immune tolerance promotion | Low (theoretical) | Variable |
A few important notes about this table. Evidence strength ratings reflect the current state of research, which is heavily weighted toward animal studies for most peptide-allergy applications. "Moderate" means there are multiple preclinical studies showing clear mechanisms plus some human clinical data or clinical reports. "Low" means the connection is primarily theoretical based on known mechanisms. Timeline estimates are based on reported outcomes in research settings and will vary between individuals.
The science of mast cell stabilization
Mast cells sit at the center of allergic disease. Stabilizing them, preventing inappropriate degranulation while maintaining their legitimate immune functions, is the holy grail of allergy treatment. Several peptides achieve this through different pathways, and understanding these mechanisms helps explain why peptide approaches can be more effective than traditional antihistamines.
Why antihistamines fall short
Traditional H1 antihistamines (cetirizine, loratadine, fexofenadine) block histamine receptors on target tissues. The histamine is still released. Mast cells still degranulate. The inflammatory cascade still fires. You just do not feel the specific effects of histamine binding. But histamine is only one of dozens of mediators released during mast cell degranulation. Tryptase, prostaglandins, leukotrienes, and various cytokines continue to cause inflammation, tissue damage, and immune activation even when histamine receptors are blocked.
This is why many allergy sufferers find antihistamines only partially effective. They are blocking one output of a much larger process.
How peptides stabilize mast cells
Peptides like KPV work upstream of degranulation. By inhibiting NF-kB signaling, KPV reduces the mast cell's tendency to degranulate in the first place. Fewer degranulation events mean less release of all inflammatory mediators, not just histamine. This is a fundamentally more complete approach.
BPC-157 adds another dimension by preventing the mitochondrial damage that contributes to mast cell hyperactivation. Damaged mitochondria produce excessive reactive oxygen species, which can trigger mast cell degranulation independently of IgE. By protecting mitochondrial function, BPC-157 removes one of the non-allergic triggers that can keep mast cells in a hyperactive state.
Thymosin alpha-1 works at yet another level, restoring the regulatory T-cell populations that normally keep mast cell activation in check. When Tregs function properly, they produce IL-10 and TGF-beta, cytokines that actively suppress mast cell degranulation. Ta1 restores this natural braking mechanism.
Together, these three peptides address mast cell hyperactivation from three different angles: direct NF-kB suppression (KPV), mitochondrial protection (BPC-157), and regulatory T-cell restoration (Ta1). This multi-layered approach explains why peptide stacking can be more effective than any single intervention.
The role of the gut-mast cell axis
The gut contains the highest concentration of mast cells in the body. When the intestinal barrier is compromised, increased antigen exposure activates these mast cells, creating a systemic inflammatory state that amplifies allergic reactivity everywhere. This gut-mast cell axis explains why many people with seasonal allergies also have food sensitivities, and why gut health is fundamental to allergy management.
BPC-157 directly addresses this axis by repairing the intestinal barrier while simultaneously stabilizing gut mast cells. KPV, when taken orally, can also reach the gut lining and provide local anti-inflammatory effects. Some researchers report that addressing gut barrier integrity significantly reduces the severity of respiratory and skin allergies, even when the gut was not the primary complaint.
Peptide reconstitution and storage for allergy protocols
Peptide potency matters enormously for allergy protocols. An underdosed or degraded peptide will not stabilize mast cells effectively. Proper reconstitution and storage are not optional steps. They are essential for therapeutic outcomes.
Reconstitution basics
Most peptides used for allergy protocols come as lyophilized (freeze-dried) powder in sealed vials. Reconstitution involves adding bacteriostatic water to create an injectable solution. The peptide reconstitution calculator determines the exact amount of water needed based on the peptide quantity and your desired concentration per unit of volume.
Key reconstitution rules:
Use bacteriostatic water (not sterile water) for multi-use vials
Add water slowly along the vial wall, never directly onto the powder
Swirl gently to dissolve. Never shake
Allow the solution to clear completely before drawing a dose
Use an alcohol swab on the vial stopper before every needle insertion
Storage requirements
Reconstituted peptides must be refrigerated at 2 to 8 degrees Celsius. Most reconstituted peptides remain stable for 3 to 4 weeks under proper refrigeration, though some are more fragile. Lyophilized powder can be stored longer, ideally in a freezer for extended storage.
For allergy protocols that may run for months, plan your peptide supply carefully. Order enough for 4 to 6 weeks at a time, reconstituting fresh batches as needed. Proper storage practices ensure consistent dosing throughout your protocol.
Injection technique for allergy protocols
Subcutaneous injection is the standard route for KPV, BPC-157, and Ta1 in allergy protocols. Use insulin syringes (29 to 31 gauge) for comfortable injections. Rotate injection sites between the abdomen (around the navel), thighs, and upper arms. Clean the injection site with an alcohol swab and allow it to dry before injecting.
For BPC-157 in gut-focused allergy protocols, oral administration is also an option. Simply hold the reconstituted solution under the tongue for 60 seconds before swallowing. BPC-157 is uniquely stable in gastric fluid, making oral delivery viable, a property most peptides do not share.
The peptide injection guide covers technique in detail, and the peptide calculator ensures accurate dosing regardless of reconstitution volume.
What the research does and does not support
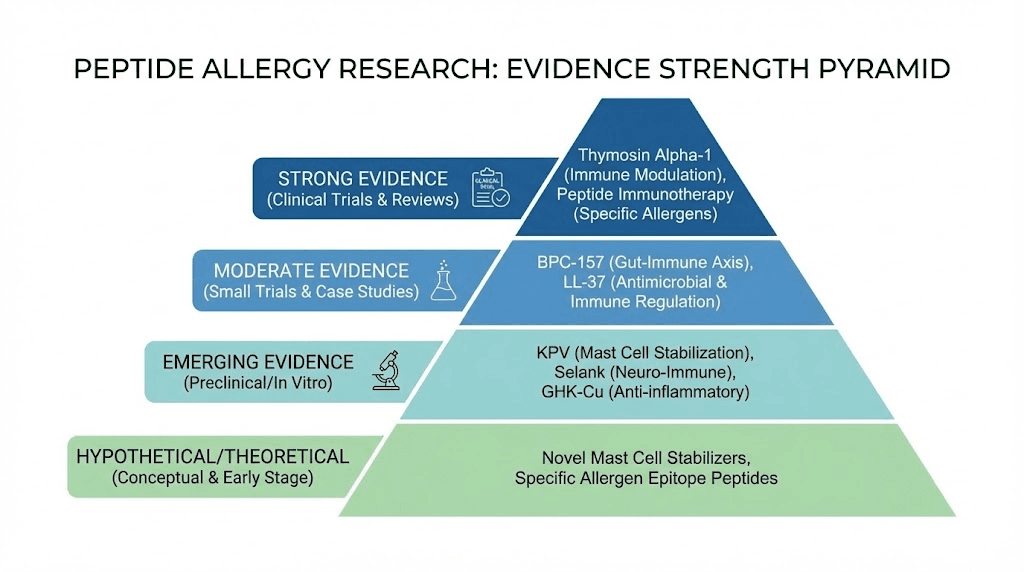
Honesty matters more than hype. Peptides for allergies represent a genuinely promising area of research, but the evidence varies significantly depending on which peptide and which allergic condition you are considering. Here is a straightforward assessment.
Strong evidence
Peptide immunotherapy (allergen-derived peptides) has the strongest evidence base. Multiple randomized, double-blind, placebo-controlled trials have demonstrated efficacy for grass pollen allergies, with sustained benefits lasting years after short treatment courses. Cat allergen and peanut allergen peptide therapies are progressing through clinical trials with encouraging results.
BPC-157 for anaphylactoid reactions showed clear, dose-dependent protection across multiple animal models, outperforming traditional antihistamines. While these are animal studies, the consistency and the comparison to established treatments make this evidence particularly compelling.
Moderate evidence
KPV for mast cell stabilization and NF-kB inhibition is supported by preclinical studies demonstrating clear mechanisms in relevant cell types (bronchial epithelial cells, mast cells). Clinical experience from practitioners treating MCAS and histamine intolerance adds human evidence, though formal clinical trials for allergic conditions specifically are still needed.
Thymosin alpha-1 for immune regulation has extensive clinical data supporting its immunomodulatory effects, with approval in over 35 countries for immune-related conditions. Its application to allergic disease specifically relies partly on extrapolation from its known mechanisms (Treg support, Th1/Th2 rebalancing), which are directly relevant to allergy pathophysiology.
Emerging evidence
VIP for allergic asthma has strong preclinical support but clinical application is limited by delivery challenges. The VIP knockout mouse studies provide compelling evidence for VIP's role in airway health, and novel delivery systems (nanoparticle conjugates, inhaled formulations) are making clinical translation more feasible.
Peptide combinations (stacking) for allergies are supported by mechanistic rationale and clinical reports but lack formal comparative trials. The idea of combining KPV, BPC-157, and Ta1 is logical based on their complementary mechanisms, but controlled studies of this specific combination for allergic conditions have not been conducted.
What we do not know yet
Long-term outcomes of peptide protocols for allergies remain understudied. Most research involves short-term endpoints. Whether peptide-induced improvements in allergic disease persist after discontinuation, and for how long, is an open question for most peptides (except allergen-derived peptide immunotherapy, which does have multi-year follow-up data).
Optimal dosing for allergic conditions specifically is often extrapolated from research in other contexts. The BPC-157 anaphylaxis study used a wide dose range (from picograms to micrograms per kilogram), suggesting that allergy-specific dosing may differ from standard tissue-repair dosing. More research is needed to optimize protocols.
Individual variation in response is significant and poorly characterized. Why some people respond dramatically to KPV while others see modest improvement likely relates to their specific type of immune dysfunction, genetic factors, and the complexity of their allergic condition. Personalized approaches, like those available through SeekPeptides membership protocols, help address this variation.
Safety considerations and contraindications
Peptides used for allergic conditions generally have favorable safety profiles, but they are not without considerations. Understanding potential risks and contraindications is essential for responsible use.
General peptide safety
KPV, derived from a naturally occurring human peptide (alpha-MSH), has shown minimal side effects in research settings. Its small size (only three amino acids) and endogenous origin contribute to its excellent tolerability. The most common issue reported is mild injection site irritation.
BPC-157 has been studied in safety trials for inflammatory bowel disease and multiple sclerosis, with no toxic effects observed. Its status as a naturally occurring fragment of a gastric juice protein supports its safety profile. However, as noted earlier, some individuals experience temporary increases in gut histamine when starting BPC-157, which can be concerning for highly histamine-sensitive people.
Thymosin alpha-1 has the most extensive human safety data of any peptide discussed here, with decades of clinical use across 35+ countries. Side effects are generally minor, including occasional injection site reactions and mild flu-like symptoms.
Specific cautions for allergy patients
If you have severe allergies or MCAS, your immune system may be more reactive to new substances, including peptides. Start with the lowest recommended dose and increase gradually. Monitor for any unusual reactions in the first 24 to 48 hours after starting a new peptide.
BPC-157 has been shown to promote angiogenesis (new blood vessel formation). While this is beneficial for tissue repair, people with conditions where angiogenesis is undesirable should discuss this with their healthcare provider.
Thymosin alpha-1, because it enhances immune function, should be used cautiously in people with overactive immune conditions. For allergies, its bidirectional nature (it can suppress overactive responses) generally makes it appropriate, but monitoring is important.
Drug interactions
Limited formal drug interaction studies exist for most therapeutic peptides. However, some general considerations apply.
Immunosuppressive medications may conflict with immunomodulatory peptides like Ta1. If you are taking immunosuppressants for an autoimmune condition, adding Ta1 requires careful medical oversight.
Antihistamines and peptides can generally be used together. In fact, many researchers continue antihistamines while starting peptide protocols, then gradually reduce antihistamine use as peptide effects become established.
Mast cell stabilizer medications (cromolyn sodium, ketotifen) may have additive effects with KPV. This can be beneficial but may also indicate a need for dose adjustment of one or both.
Who should avoid these peptides
Pregnant or breastfeeding women should avoid all therapeutic peptides due to insufficient safety data in these populations.
People with active cancer should consult their oncologist before using any immunomodulatory peptide, including Ta1 (despite its use in cancer immunotherapy in some countries) and BPC-157 (due to angiogenesis promotion).
Children should not use these peptides without specific medical guidance, as pediatric dosing and safety data are limited.
Lifestyle factors that amplify peptide effectiveness
Peptides do not work in isolation. The environment you create for your immune system determines how effectively these compounds can do their job. Several lifestyle factors directly influence allergic inflammation and can either amplify or undermine your peptide protocol.
Diet and gut health
The gut-immune connection is not theoretical. It is measurable. Approximately 70% of immune tissue resides in the gut, and the composition of your gut microbiome directly influences systemic inflammation and allergic reactivity. A diet high in processed foods, sugar, and industrial seed oils promotes gut dysbiosis and intestinal permeability, both of which increase allergic sensitivity.
Support your peptide protocol with an anti-inflammatory diet rich in omega-3 fatty acids (fatty fish, walnuts, flaxseed), polyphenols (berries, green tea, dark chocolate), and prebiotic fiber (garlic, onions, asparagus). Fermented foods (sauerkraut, kimchi, kefir) support microbiome diversity, though histamine-sensitive individuals should introduce these cautiously as fermented foods can be high in histamine.
Stress management
Stress hormones, particularly cortisol and norepinephrine, directly modulate mast cell function. Chronic stress keeps mast cells in a primed, hyperactive state, making allergic reactions more frequent and severe. This is why allergy symptoms often worsen during stressful periods.
Regular stress management practices (meditation, deep breathing, exercise, adequate social connection) reduce mast cell hyperactivation through neuroimmune pathways. When combined with mast cell-stabilizing peptides like KPV, stress management can produce synergistic improvements that neither approach achieves alone.
Sleep quality
Poor sleep disrupts immune regulation in ways that specifically worsen allergies. Sleep deprivation increases IL-4 and IL-13 (Th2 cytokines that drive allergic inflammation) while decreasing IL-10 and IFN-gamma (anti-allergic cytokines). It also impairs regulatory T-cell function, the same cells that Ta1 works to support.
Prioritize 7 to 9 hours of quality sleep. Address sleep disorders aggressively. Sleep-supporting peptides like DSIP and epitalon may complement allergy protocols when poor sleep is a contributing factor.
Environmental optimization
Reducing allergen exposure while your peptide protocol takes effect makes practical sense. HEPA filters, allergen-proof bedding, and regular cleaning reduce the daily allergic burden on your immune system. This means your mast cells face fewer triggers while KPV is working to stabilize them, giving the peptide a better chance of achieving lasting improvement.
Nasal saline irrigation removes allergens from nasal passages and can reduce the need for pharmacological intervention. Some researchers use nasal spray peptide formulations for direct airway delivery.
Frequently asked questions
Can peptides replace antihistamines for allergies?
For some people, yes, over time. Peptides like KPV and BPC-157 address the underlying immune dysfunction rather than just blocking histamine receptors. Many researchers report gradually reducing antihistamine use as peptide protocols take effect. However, this should be a gradual process guided by symptom monitoring, not an abrupt switch. Keep antihistamines available during the transition period.
How long do peptide allergy protocols take to work?
Most people notice initial improvements within 2 to 4 weeks. Mast cell stabilization with KPV can begin within days, but meaningful clinical improvement in allergy symptoms typically takes 2 to 6 weeks depending on the severity and type of allergic condition. For long-term immune rebalancing with thymosin alpha-1, allow 4 to 8 weeks for the full regulatory T-cell effects to develop.
Are peptides safe for people with severe allergies?
The peptides discussed in this guide have favorable safety profiles. However, people with severe allergies, particularly MCAS, may be more reactive to any new substance. Start with the lowest recommended dose, increase gradually, and monitor closely. Have your rescue medications (epinephrine auto-injector if prescribed) available when starting any new protocol.
Can I use peptides alongside allergy immunotherapy?
There is no established evidence of negative interactions between therapeutic peptides (KPV, BPC-157, Ta1) and standard allergen immunotherapy (allergy shots or sublingual drops). Some researchers hypothesize that immunomodulatory peptides could enhance the tolerance-inducing effects of immunotherapy, though this has not been formally studied. Discuss with your allergist if you are considering combining approaches.
Which peptide is best for seasonal allergies specifically?
KPV is the top choice for seasonal allergies due to its direct mast cell stabilizing and NF-kB suppressing effects in airway epithelium. Starting a KPV protocol 2 to 4 weeks before allergy season provides the best results. Adding thymosin alpha-1 for deeper immune rebalancing improves outcomes further.
Do peptides work for pet allergies?
The mechanisms that make peptides effective for pollen allergies (mast cell stabilization, NF-kB suppression, immune regulation) apply to pet allergies as well. Cat allergen peptide immunotherapy using Fel d 1 epitopes has been specifically studied with positive results. For general mast cell stabilization, KPV does not distinguish between different allergen triggers, so it should help reduce reactivity regardless of the specific allergen.
Can children use peptides for allergies?
Pediatric use of therapeutic peptides lacks sufficient safety and dosing data. Most research has been conducted in adult populations. Children with allergies should work with pediatric allergists using established treatments. The allergen-derived peptide immunotherapy approach is being studied in children, but general therapeutic peptides like KPV and BPC-157 do not yet have pediatric protocols.
What is the difference between peptides for allergies and peptides for autoimmune conditions?
Allergies and autoimmune diseases both involve immune dysregulation, but in different directions. Allergies typically involve excessive Th2 responses against external proteins, while autoimmune diseases involve Th1 or Th17 attacks on self-tissues. However, the regulatory mechanisms that keep both in check overlap significantly. Peptides like thymosin alpha-1 that support regulatory T-cell function can benefit both conditions. Immune balance is the shared goal.
External resources
Inhibition of Cellular and Systemic Inflammation by Melanocortin-Related Peptides (PMC)
Thymosin Alpha 1: A Comprehensive Review of the Literature (World Journal of Experimental Medicine)
Short Course of Grass Allergen Peptides Immunotherapy (Clinical and Experimental Allergy)
Vasoactive Intestinal Peptide Inhaled Agonists: Role in Respiratory Therapeutics (PMC)
For researchers serious about optimizing their allergy management through peptide protocols, SeekPeptides offers the most comprehensive resource available. Members access evidence-based guides, proven protocols for allergic conditions, personalized dosing calculators, and a community of thousands who have navigated these exact questions.
In case I do not see you, good afternoon, good evening, and good night. May your mast cells stay stable, your airways stay clear, and your immune system stay balanced. Learn more.



