Jan 18, 2026

The human body produces roughly 200 peptides. Most stay in their lane, doing one job, doing it quietly. Then there's IGF-1.
This single peptide sits at the crossroads of nearly every anabolic process in your body. Muscle growth. Tissue repair. Fat metabolism. Cellular regeneration. Even cognitive function.
IGF-1 doesn't just participate in growth. It orchestrates it.
When the pituitary gland releases growth hormone, the liver responds by producing IGF-1. This cascade has been happening in human bodies for millions of years. But researchers have only begun to understand its full implications in the last few decades. What they've found challenges conventional thinking about muscle development, aging, and metabolic health.
The peptide research community has developed several modified versions of IGF-1, each with distinct characteristics that make them interesting subjects of study. Understanding peptides begins with understanding the relationship between structure and function. And IGF-1 variants demonstrate this principle perfectly.
This guide covers everything researchers and enthusiasts need to know about IGF peptides. The mechanisms behind their effects. The differences between variants. The protocols that matter. The safety considerations that can't be ignored. Whether you're exploring peptides for muscle growth or investigating recovery applications, the information ahead will give you a foundation built on research rather than speculation.
What is IGF-1 and why it matters
Insulin-like growth factor 1 is a 70-amino acid peptide that shares structural similarities with insulin. This resemblance isn't coincidental. Both peptides evolved from common ancestors, and they share the ability to bind to similar receptor types. But their functions diverge significantly once they enter the bloodstream.
IGF-1 acts as the primary mediator of growth hormone's anabolic effects. When people talk about HGH building muscle, they're really talking about IGF-1 doing the heavy lifting.
The peptide exists in circulation bound to specific carrier proteins called IGF binding proteins, or IGFBPs. Six different IGFBPs have been identified, each playing a role in regulating how much free IGF-1 remains available to tissues. This binding system extends IGF-1's half-life from minutes to hours, creating a reservoir of growth-promoting activity throughout the body.
Most IGF-1 production occurs in the liver. This accounts for roughly 75% of circulating levels. But here's where it gets interesting. Muscle tissue, bone, and other organs also produce IGF-1 locally in response to mechanical stress and other stimuli. This local production, called autocrine or paracrine signaling, can have effects that circulating IGF-1 never reaches.
The liver-produced IGF-1 acts systemically, affecting tissues throughout the body. Local production targets specific areas. Both matter for different reasons.
How peptides work depends heavily on receptor binding. IGF-1 primarily activates the IGF-1 receptor (IGF-1R), triggering downstream signaling cascades that promote protein synthesis and inhibit protein breakdown. This dual action makes it remarkably efficient at supporting anabolic processes.
The PI3K/Akt pathway represents one of the most important signaling routes activated by IGF-1. This pathway regulates protein synthesis through mTOR activation while simultaneously suppressing catabolic processes. Researchers studying peptide research consistently find this pathway at the center of IGF-1's muscle-building effects.
Beyond muscle, IGF-1 influences virtually every tissue capable of growth or repair. Bone formation. Cartilage maintenance. Nerve regeneration. Skin healing. The breadth of its influence explains why it remains such a significant focus of research across multiple fields.
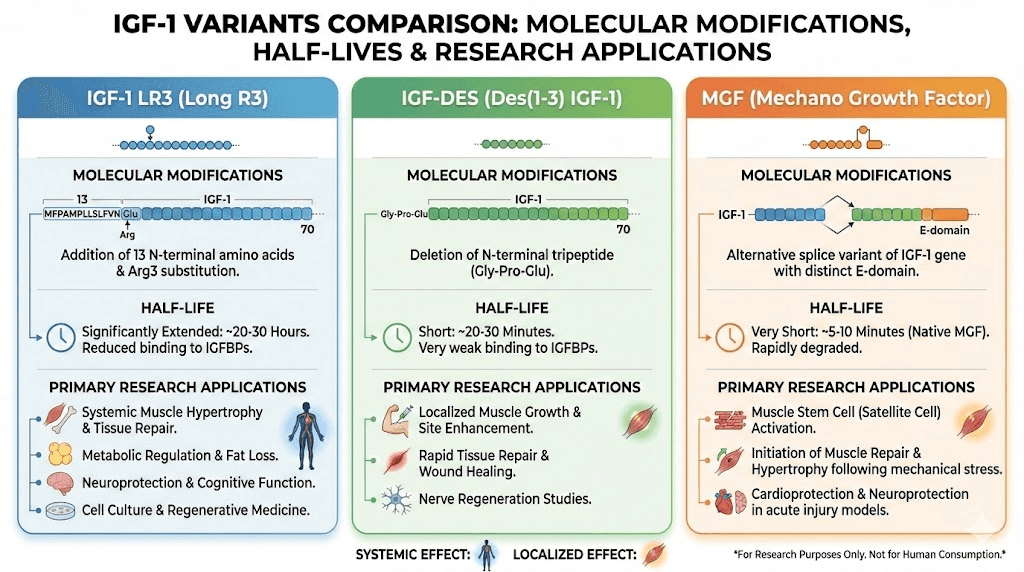
The IGF-1 variants: LR3, DES, and MGF explained
Not all IGF-1 peptides are created equal. Researchers have developed modified versions that alter the original peptide's characteristics in ways that make them more useful for specific applications. Understanding these variants helps clarify why different protocols call for different compounds.
IGF-1 LR3: the long-acting variant
IGF-1 LR3 stands for Long Arg3 IGF-1. This modified version contains 83 amino acids instead of the native 70, with an additional 13 amino acids at the N-terminus and an arginine substitution at position 3.
These modifications serve a critical purpose. They dramatically reduce binding to IGF binding proteins.
Native IGF-1 binds strongly to IGFBPs, which sequester most of the peptide and limit its activity. IGF-1 LR3 largely escapes this binding, remaining active in circulation far longer. Where native IGF-1 has a half-life measured in minutes, IGF-1 LR3 extends this to roughly 20-30 hours. Some researchers estimate it remains active up to 120 times longer than the native form.
This extended activity window changes everything about how the peptide can be used. Single daily doses become practical. Systemic effects become more pronounced. The compound circulates throughout the body rather than being quickly neutralized.
For those exploring peptides for muscle growth, IGF-1 LR3 represents one of the most potent options available. Its ability to stimulate muscle hyperplasia, the actual creation of new muscle cells rather than just enlargement of existing ones, sets it apart from most other anabolic compounds.
IGF-1 DES: the localized specialist
IGF-1 DES (des(1-3)IGF-1) takes the opposite approach from LR3. Instead of adding amino acids, this variant removes three from the N-terminus.
The result is a peptide with a very short half-life, roughly 20-30 minutes, but with approximately 10 times greater receptor binding affinity in the local area where it's applied. This makes IGF-1 DES ideal for targeted applications rather than systemic effects.
Researchers find IGF-1 DES particularly interesting for its ability to bind to receptors that have been affected by lactic acid. During intense exercise, lactic acid accumulates in working muscles. This creates an environment where native IGF-1 binding is impaired. IGF-1 DES appears to work regardless of this acid environment, potentially making it useful for promoting growth even during training.
The localized nature of IGF-1 DES means it's often studied for site-specific applications. Rather than circulating throughout the body, it acts primarily where it's introduced. This characteristic makes it a subject of research for targeted muscle development and localized tissue repair.
Mechano-growth factor: the exercise-responsive isoform
MGF (Mechano-Growth Factor) represents a naturally occurring splice variant of IGF-1, also known as IGF-1Ec in humans. The body produces this variant in response to mechanical stress, particularly the kind experienced during resistance training.
When muscle fibers experience damage from exercise, the IGF-1 gene undergoes alternative splicing to produce MGF alongside standard IGF-1. This MGF then activates dormant satellite cells, the stem cells responsible for muscle repair and growth.
The research on MGF reveals a fascinating mechanism. Standard IGF-1 promotes differentiation of activated satellite cells into mature muscle fibers. MGF, by contrast, primarily stimulates proliferation, increasing the pool of satellite cells available for repair. These functions complement each other in the natural recovery process.
Synthetic versions of the MGF E-domain peptide (the unique portion of MGF) have been developed for research. Studies suggest this peptide can delay satellite cell senescence and increase their fusion potential, potentially enhancing the muscle repair process. For those interested in peptides for injury recovery, MGF represents a compelling area of investigation.
However, the research picture isn't entirely clear. Some studies have failed to demonstrate significant effects from isolated MGF peptides on muscle stem cells. The debate continues about whether synthetic MGF peptides replicate the effects of naturally produced MGF during exercise.
How IGF-1 builds muscle: the mechanisms
Understanding how IGF-1 promotes muscle growth requires examining its effects at the cellular level. The peptide doesn't simply make muscles bigger. It fundamentally alters how muscle tissue responds to stress, recovers from damage, and adapts over time.
Satellite cell activation and hyperplasia
Satellite cells sit dormant between the sarcolemma and basal lamina of muscle fibers. They remain quiescent until called into action by signals indicating muscle damage or stress. IGF-1 is one of the most potent activators of these cells.
When IGF-1 binds to receptors on satellite cells, it triggers their activation. These activated cells then proliferate, creating a larger pool of muscle precursor cells. Eventually, these precursors fuse with existing muscle fibers or form entirely new fibers.
This process, called hyperplasia, distinguishes IGF-1's effects from those of most other anabolic compounds. Anabolic steroids primarily cause hypertrophy, the enlargement of existing muscle cells. IGF-1 can promote both hypertrophy and hyperplasia, potentially creating permanent increases in muscle cell number.
Research in animal models has demonstrated this hyperplastic effect convincingly. When researchers overexpressed IGF-1 locally in muscle tissue, they observed significant increases in muscle mass that persisted even after the stimulus was removed. The new muscle fibers remained.
For people researching peptides for men focused on muscle development, this hyperplastic potential represents a unique advantage. Similarly, those exploring peptides for women find IGF-1's mechanisms applicable regardless of gender, though optimal protocols may differ.
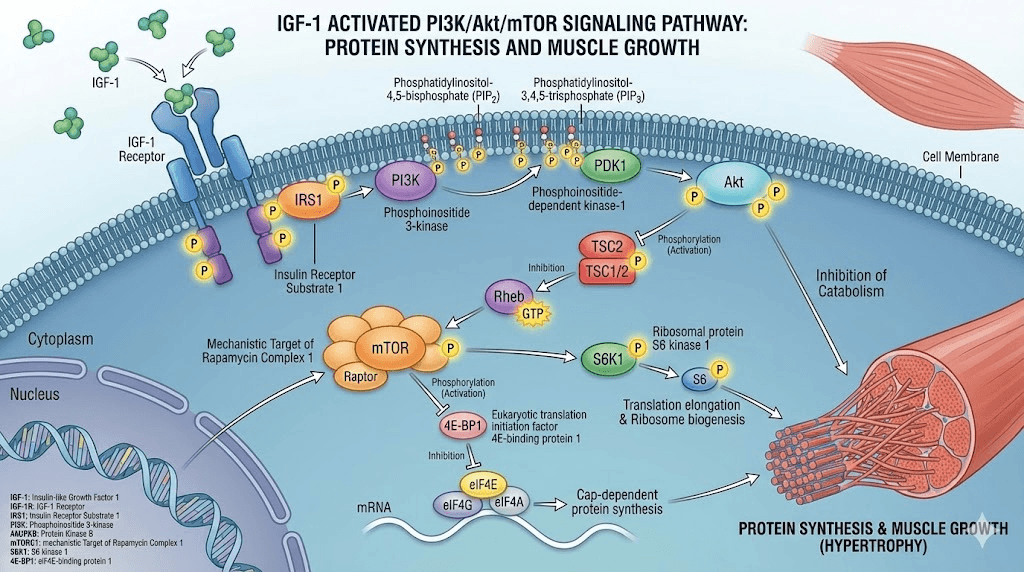
The PI3K/Akt/mTOR pathway
IGF-1 receptor activation initiates a cascade of intracellular signaling. The phosphatidylinositol 3-kinase (PI3K) pathway stands as the primary route through which IGF-1 exerts its anabolic effects.
When IGF-1 binds its receptor, PI3K becomes activated. This enzyme then phosphorylates Akt (also known as protein kinase B), which in turn activates mTOR (mechanistic target of rapamycin). The mTOR complex directly stimulates protein synthesis by promoting ribosome biogenesis and translation of mRNA into proteins.
Simultaneously, Akt inhibits FoxO transcription factors. These factors normally promote the expression of ubiquitin ligases that break down muscle proteins. By suppressing FoxO, IGF-1 reduces muscle protein breakdown while enhancing synthesis. The net result favors muscle growth.
This dual action, increasing synthesis while decreasing breakdown, makes IGF-1 remarkably efficient at shifting the body toward an anabolic state. Few other compounds achieve this balance as effectively.
Understanding these pathways helps explain why peptide stacking can be so effective. Combining IGF-1 with compounds that work through complementary mechanisms can theoretically enhance overall results beyond what either would achieve alone.
Nutrient partitioning effects
IGF-1 doesn't just build muscle. It influences where nutrients go once consumed. This nutrient partitioning effect explains some of the body composition changes associated with elevated IGF-1 levels.
The peptide promotes glucose uptake in muscle tissue while reducing fat storage. Amino acids get shuttled preferentially toward muscle protein synthesis rather than other tissues. This partitioning effect can result in simultaneous muscle gain and fat loss, a combination difficult to achieve through training and nutrition alone.
Research has shown that IGF-1 and IGF-1 LR3 enhance lipolysis, the breakdown of stored fat. At the same time, they may inhibit adipogenesis, the formation of new fat cells. These effects combine to create favorable conditions for improving body composition.
For those researching fat burning peptides or exploring peptides for fat loss, IGF-1's nutrient partitioning effects represent an important consideration. The compound may contribute to fat reduction through mechanisms distinct from dedicated fat loss peptides.
IGF-1 vs HGH: understanding the relationship
The relationship between human growth hormone and IGF-1 causes considerable confusion. Some view them as interchangeable. Others see them as entirely separate compounds. The reality lies somewhere between these extremes, and understanding their relationship helps clarify when each might be preferred.
The growth hormone cascade
Growth hormone itself doesn't directly build muscle. Instead, it acts as a signaling molecule that triggers IGF-1 production, primarily in the liver.
When the pituitary gland releases GH in response to sleep, exercise, or other stimuli, blood levels rise briefly. The liver responds by synthesizing and releasing IGF-1 into circulation. This IGF-1 then travels throughout the body, binding to receptors on muscle, bone, and other tissues.
The anabolic effects most people associate with growth hormone, muscle development, fat metabolism improvements, tissue repair, actually come from IGF-1 acting at target tissues. GH provides the signal. IGF-1 does the work.
This relationship has important implications. Using GH secretagogues like Ipamorelin or CJC-1295 increases natural GH release, which then stimulates natural IGF-1 production. Using IGF-1 directly bypasses this entire cascade, providing the end product without requiring GH release.
Key differences in application
GH and IGF-1 work through different mechanisms despite their relationship. Understanding these differences helps in protocol design.
Growth hormone has significant effects on fat metabolism through direct action on adipose tissue. It promotes lipolysis and opposes insulin's fat-storing effects. These actions occur independently of IGF-1 production. GH also promotes deep sleep, supports immune function, and has effects on connective tissue synthesis.
IGF-1 excels at protein synthesis and muscle cell activation. Its effects on satellite cells and the PI3K/Akt pathway make it more directly anabolic than GH. However, IGF-1 has insulin-like effects that can promote hypoglycemia if nutrition isn't managed properly.
For those comparing options, the choice often comes down to goals. Someone prioritizing fat loss might prefer approaches that emphasize GH release. Someone focused primarily on muscle development might find direct IGF-1 administration more efficient. Many researchers conclude that using both strategically offers advantages over either alone.
The HGH alternatives page provides context for those exploring the broader landscape of growth hormone-related compounds. Meanwhile, understanding how peptides compare to steroids helps place IGF-1 in the broader context of anabolic compounds.
Why some researchers use both
Combining GH-releasing peptides with IGF-1 has become a common approach in research settings. The rationale involves complementary effects.
GH-releasing peptides stimulate natural GH production, which supports fat metabolism, sleep quality, and overall hormonal balance. Adding IGF-1 provides direct anabolic support without relying entirely on the body's conversion process.
Some research suggests that exogenous IGF-1 administration can actually suppress natural GH production through negative feedback. Combining both may help maintain GH levels while still providing the benefits of elevated IGF-1.
For those interested in comprehensive protocols, exploring peptide stacking strategies provides guidance on combining different compounds for specific goals.
Dosing protocols for IGF peptides
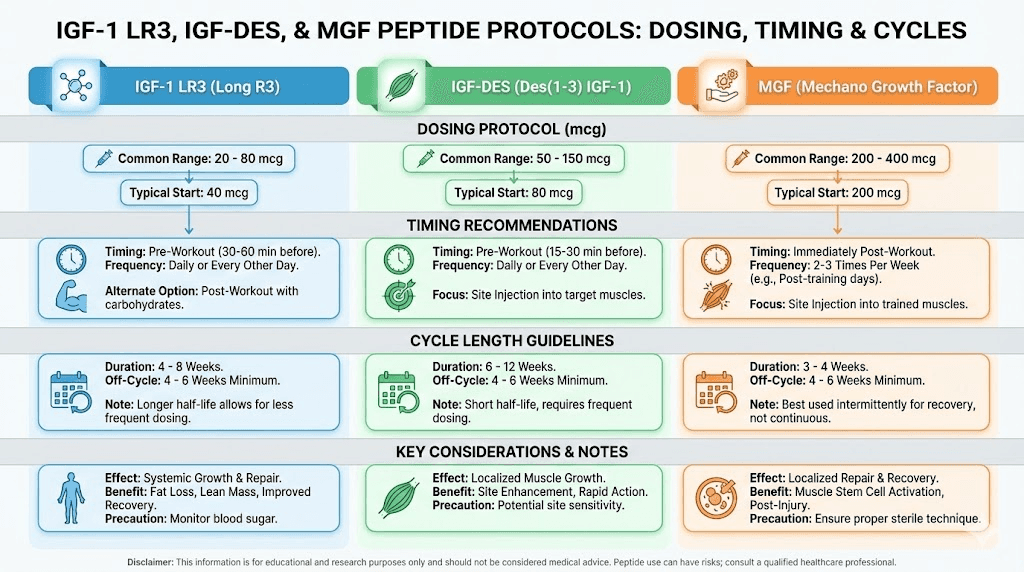
Dosing IGF peptides requires careful attention to the specific variant being used. Each has different characteristics that affect optimal dosing strategies. The following information reflects commonly referenced research protocols, though individual responses vary significantly.
IGF-1 LR3 dosing considerations
Research protocols for IGF-1 LR3 typically reference doses ranging from 20-100 mcg per day. Starting at the lower end allows assessment of individual response before potential increases.
Most protocols suggest daily administration, taking advantage of the extended half-life. Some researchers divide doses, administering morning and post-workout, though the extended activity window makes strict timing less critical than with shorter-acting compounds.
Cycle length typically ranges from 4-6 weeks. Extended use beyond this period may lead to receptor desensitization, reducing the compound's effectiveness. After a cycle, equal time off is commonly recommended before resuming.
For context on dosing calculations, the peptide calculator provides useful tools, though IGF-1 LR3 dosing differs from simpler compounds due to its unique characteristics.
IGF-1 DES dosing considerations
IGF-1 DES operates differently due to its short half-life and localized action. Research protocols typically suggest 20-50 mcg per injection, administered at specific sites rather than systemically.
The short half-life means multiple daily administrations may be preferred for research studying sustained effects. Pre-workout administration is commonly referenced, with the rationale that lactic acid accumulation enhances the peptide's receptor binding during training.
Bilateral injection into target muscle groups represents a common approach in research focused on localized effects. The logic follows that site-specific administration allows the peptide to act before systemic distribution dilutes its concentration.
MGF dosing considerations
Mechano-growth factor protocols in research settings typically reference 100-300 mcg per administration. Some protocols suggest splitting doses bilaterally into targeted muscle groups.
Timing often focuses on the post-workout window, when natural MGF expression increases. The theory suggests that supplementing this natural spike may enhance satellite cell activation beyond what exercise alone achieves.
MGF is sometimes studied in alternating protocols with IGF-1 LR3. The rationale involves MGF's role in proliferating satellite cells (increasing their number) while IGF-1 promotes their differentiation (maturation into functional muscle tissue). Using both in sequence theoretically optimizes both phases of muscle development.
Understanding how to calculate peptide dosages provides foundation knowledge applicable to any peptide protocol. The peptide dosage chart offers reference points for various compounds.
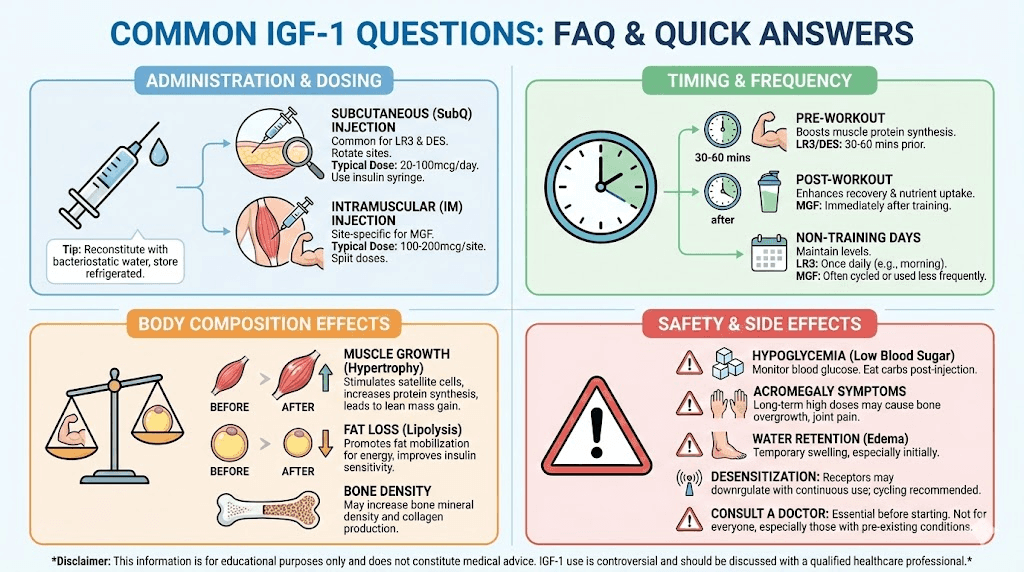
Administration and reconstitution
Proper handling of IGF peptides ensures potency and reduces risks associated with contamination or degradation. These compounds require more careful handling than many other peptides due to their sensitivity.
Reconstitution protocol
IGF peptides typically arrive as lyophilized (freeze-dried) powder. Before use, they must be reconstituted with an appropriate solvent.
Bacteriostatic water serves as the standard reconstitution medium for most peptides. The benzyl alcohol in bacteriostatic water inhibits bacterial growth, extending the usable life of the reconstituted solution.
The reconstitution process requires gentle handling. Direct the stream of water against the vial wall rather than directly onto the powder. Allow the powder to dissolve naturally rather than shaking, which can denature the protein structure. Gentle swirling is acceptable if needed.
For detailed guidance, the peptide reconstitution guide covers the process thoroughly. The peptide reconstitution calculator helps determine appropriate volumes for achieving desired concentrations.
Storage requirements
Unreconstituted IGF peptides should be stored in a freezer to maintain stability. At -20°C, properly sealed vials can remain stable for extended periods.
Once reconstituted, storage moves to refrigeration. Reconstituted IGF peptides should be kept at 2-8°C (standard refrigerator temperature) and used within a limited timeframe. Most sources suggest using reconstituted IGF-1 within 2-4 weeks for optimal potency.
Understanding how long reconstituted peptides last helps with planning and prevents waste. The peptide storage guide provides comprehensive information applicable to all peptide types.
Exposure to light, heat, or agitation can degrade IGF peptides rapidly. Vials should be stored in dark locations, and unnecessary handling should be avoided. Some researchers wrap vials in foil for additional light protection.
Injection considerations
Most IGF peptide research involves subcutaneous administration. Insulin syringes with fine gauge needles (29-31 gauge) minimize discomfort and tissue damage.
For IGF-1 DES, intramuscular administration into targeted muscle groups represents a common approach when localized effects are being studied. The shorter half-life means the peptide acts primarily where introduced rather than distributing systemically.
Rotating injection sites prevents the development of scar tissue that could affect absorption. The abdomen, thigh, and deltoid represent common subcutaneous sites. For intramuscular administration targeting specific muscles, research protocols typically inject directly into the muscle belly.
The peptide injection guide covers techniques applicable to various administration routes. Understanding proper technique reduces risks and improves consistency.
Benefits supported by research
The research literature on IGF-1 spans decades and encompasses multiple fields. While much work has been conducted in animal models or cell cultures, human studies provide relevant context for understanding potential benefits.
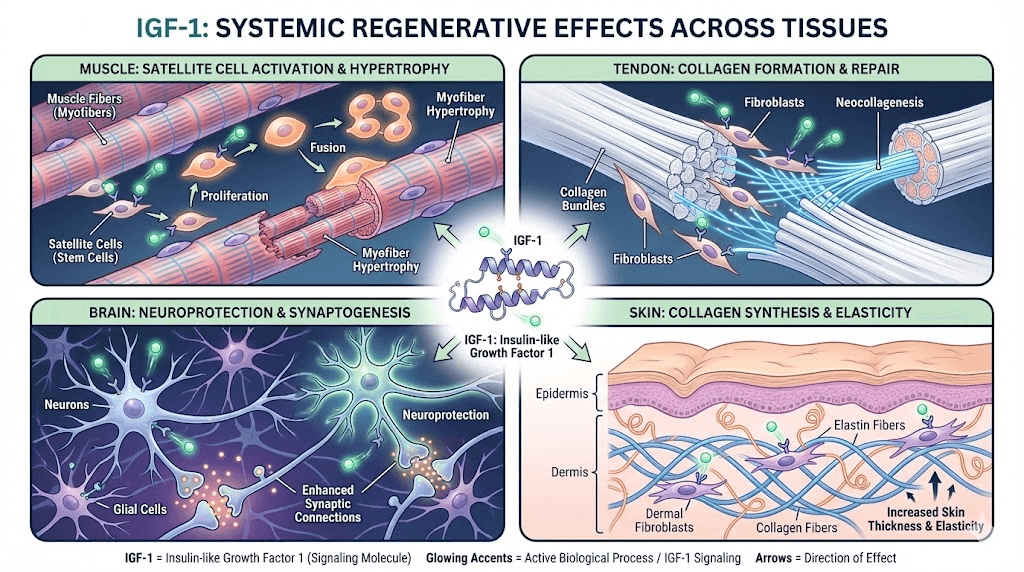
Muscle development and hypertrophy
IGF-1's effects on muscle tissue represent its most extensively studied application. Research consistently demonstrates increased protein synthesis and reduced protein breakdown in muscle tissue exposed to IGF-1.
Animal studies using transgenic mice that overexpress IGF-1 in muscle have shown significant hypertrophy without exercise. More impressively, these effects persisted as the animals aged, suggesting IGF-1 may counter age-related muscle loss.
In humans, research has explored IGF-1's potential for treating muscle wasting conditions. Studies in growth hormone-deficient individuals have shown improvements in lean body mass with IGF-1 administration. Whether similar effects occur in healthy individuals remains an area of ongoing investigation.
For those researching safe peptides for muscle growth, IGF-1 variants warrant consideration alongside other options like BPC-157 and TB-500.
Injury recovery and tissue repair
IGF-1's role in tissue repair extends beyond muscle. Research has investigated its effects on tendons, ligaments, bone, and even nerve tissue.
Tendon healing research has shown promise. Studies demonstrate that IGF-1 promotes tenocyte proliferation and collagen synthesis, the primary structural protein in tendons. Local administration has accelerated functional recovery in animal models of tendon injury.
For bone repair, IGF-1 stimulates osteoblast activity and bone matrix formation. Research in fracture healing models has shown improved bone strength and accelerated healing with IGF-1 treatment. This application area continues to generate significant research interest.
Those exploring peptides for tendon repair or peptides for bone and cartilage repair will find IGF-1 frequently mentioned in the literature alongside dedicated healing peptides.
Anti-aging and longevity research
The relationship between IGF-1 and aging presents a fascinating paradox. High IGF-1 levels promote growth and tissue repair. Yet some longevity research suggests lower IGF-1 signaling may extend lifespan in certain model organisms.
This apparent contradiction may reflect the difference between physiological and supraphysiological levels, or between systemic and local IGF-1 effects. The research continues to evolve.
What seems clear is that IGF-1 plays important roles in maintaining tissue health during aging. Declining IGF-1 levels correlate with sarcopenia (age-related muscle loss), thinning skin, reduced bone density, and slower wound healing. Whether restoring youthful IGF-1 levels provides net benefits remains an active research question.
For skin specifically, IGF-1 supports fibroblast function and collagen production. Research has shown associations between higher IGF-1 levels and reduced facial wrinkling. The peptides for anti-aging category encompasses multiple compounds that may work through IGF-1 related mechanisms.
Cognitive function and neuroprotection
IGF-1 crosses the blood-brain barrier and acts on neurons and glial cells. Research has demonstrated neuroprotective effects in various injury and disease models.
The peptide promotes neuron survival and axonal growth. It supports the formation of new synapses and may enhance neuroplasticity. Animal studies have shown improved learning and memory performance with IGF-1 treatment.
Age-related cognitive decline correlates with falling IGF-1 levels. Some researchers hypothesize that maintaining adequate IGF-1 signaling in the brain may help preserve cognitive function during aging. However, human studies in this area remain limited.
Those interested in peptides for brain function or nootropic peptides will find IGF-1 discussed alongside dedicated cognitive-enhancing compounds.
Side effects and safety considerations
IGF-1 peptides carry significant risks that require careful consideration. Their potent anabolic effects come with potential downsides that must be understood before any research application.
Hypoglycemia risks
IGF-1's structural similarity to insulin extends to its metabolic effects. The peptide promotes glucose uptake into cells, which can lower blood sugar levels rapidly.
Symptoms of hypoglycemia include shakiness, sweating, anxiety, confusion, dizziness, and in severe cases, loss of consciousness.
These risks increase with higher doses, fasted administration, or combination with insulin or insulin-sensitizing compounds.
Managing this risk requires attention to nutrition timing. Administering IGF-1 with meals that contain adequate carbohydrates can help stabilize blood sugar. Monitoring for hypoglycemic symptoms, especially during initial use, allows for protocol adjustment if problems arise.
Those with existing blood sugar regulation issues face elevated risks. The peptide safety guide provides broader context for understanding and managing peptide-related risks.
Potential insulin resistance
Chronic IGF-1 administration may affect insulin sensitivity over time. While short-term use can actually improve insulin sensitivity, prolonged exposure might lead to receptor desensitization or metabolic adaptations that impair glucose metabolism.
This potential for metabolic disruption represents one reason why cycling protocols are typically recommended.
Periodic breaks allow metabolic systems to normalize and may help preserve receptor sensitivity.
Monitoring fasting glucose and insulin levels provides data for detecting early signs of metabolic changes. Adjusting protocols based on this feedback helps manage long-term risks.
Concerns about cell proliferation
IGF-1 promotes cell division and growth. While this drives beneficial effects like muscle development and tissue repair, it raises theoretical concerns about unwanted cell proliferation.
Cancer cells often have elevated IGF-1 receptor expression and respond to IGF-1 signaling. While no clear evidence demonstrates that IGF-1 administration causes cancer, the peptide might theoretically accelerate growth of existing or dormant tumors.
This concern applies particularly to individuals with personal or family histories of hormone-sensitive cancers. Breast, prostate, and colorectal cancers have been associated with elevated IGF-1 levels in some epidemiological studies, though causation remains unestablished.
The FDA-approved IGF-1 medication mecasermin (for severe IGF-1 deficiency) carries warnings about potential cancer risk, reflecting regulatory acknowledgment of this concern.
Water retention and joint discomfort
Like growth hormone, IGF-1 can cause fluid retention. Some individuals experience puffiness, particularly in the face and extremities. This effect typically resolves after discontinuation.
Joint pain represents another commonly reported issue.
The mechanism isn't entirely clear but may relate to rapid changes in connective tissue or fluid dynamics within joints.
Most reports suggest these symptoms are dose-dependent and reversible.
Regulatory status
IGF-1 and its variants are not FDA-approved for general use. Mecasermin, a recombinant human IGF-1, carries approval only for severe primary IGF-1 deficiency, a rare condition where the body cannot produce adequate IGF-1 despite normal or elevated growth hormone levels.
The World Anti-Doping Agency (WADA) prohibits all forms of IGF-1 in athletic competition. This ban reflects both performance enhancement potential and safety concerns.
These regulatory restrictions limit legal availability to research purposes in most jurisdictions. Understanding peptide legality helps navigate this complex landscape.
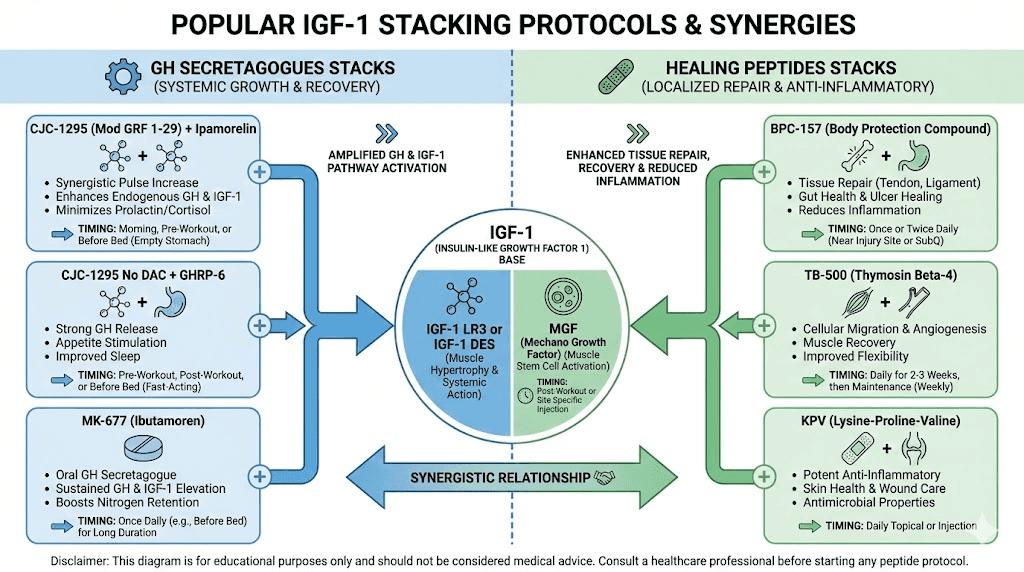
Stacking IGF peptides with other compounds
Researchers often study IGF peptides in combination with other compounds to understand synergistic effects.
Strategic combinations may enhance results while potentially reducing required doses of individual compounds.
IGF-1 with growth hormone secretagogues
Combining IGF-1 with GH-releasing peptides addresses both sides of the growth hormone axis. The secretagogues stimulate natural GH production, supporting fat metabolism, sleep quality, and overall hormonal health. Direct IGF-1 provides targeted anabolic support.
Common combinations include IGF-1 LR3 with Sermorelin, Ipamorelin, or CJC-1295.
These compounds increase endogenous GH release, which then produces its own IGF-1.
Adding exogenous IGF-1 on top of this creates a more robust anabolic environment than either approach alone.
The rationale involves complementary mechanisms. GH secretagogues work through the hypothalamus-pituitary axis, maintaining natural rhythm and feedback. Direct IGF-1 bypasses this system, providing consistent levels regardless of natural GH fluctuations.
IGF-1 with healing peptides
For injury recovery applications, combining IGF-1 with dedicated healing peptides may enhance results. BPC-157 and TB-500 work through different mechanisms than IGF-1, creating potential for synergistic effects.
BPC-157 promotes angiogenesis (new blood vessel formation) and has anti-inflammatory effects. TB-500 enhances cellular migration and differentiation in injured tissues. IGF-1 adds direct growth factor stimulation to this mix.
Research protocols studying injury recovery often incorporate multiple peptides based on this complementary relationship. The fast injury healing approach frequently involves these combinations.
IGF-1 alternating with MGF
The relationship between IGF-1 and MGF suggests a logical sequencing strategy. MGF promotes satellite cell proliferation (increasing the pool of muscle stem cells). Standard IGF-1 or IGF-1 LR3 promotes differentiation of these cells into mature muscle tissue.
Some research protocols alternate these compounds rather than using them simultaneously. The theory suggests that increasing satellite cell numbers first, then promoting their differentiation, might maximize muscle development potential.
A commonly referenced protocol involves MGF administration following training (when natural MGF expression peaks), followed by IGF-1 LR3 during recovery periods to promote the maturation of activated satellite cells.
Considerations for stacking
Combining multiple compounds increases complexity and potential for interactions. Side effects may compound. Metabolic effects may become unpredictable.
For these reasons, research typically establishes baseline responses to individual compounds before exploring combinations. This approach allows identification of which compounds produce desired effects and which cause problems for a given subject.
The peptide stack calculator provides tools for planning multi-compound protocols. Understanding how many peptides can be taken together helps establish reasonable limits.
IGF-1 for specific goals
Different goals call for different approaches to IGF peptide use. Understanding how the compounds relate to specific objectives helps in protocol design.
For muscle building
Maximizing muscle development with IGF peptides typically involves IGF-1 LR3 as the primary compound. Its extended half-life provides sustained anabolic signaling that supports protein synthesis throughout the day.
Protocols focused on muscle growth often incorporate adequate caloric surplus and protein intake. IGF-1's nutrient partitioning effects work best when nutrients are available for directed into muscle tissue. Training stimulus remains essential, as mechanical stress amplifies IGF-1's effects on satellite cells.
Adding MGF pre- or post-workout may enhance results by increasing the satellite cell pool available for IGF-1 to act upon. The alternating protocol mentioned earlier represents one approach to this combination.
Those exploring this application should also consider peptides supporting testosterone, as optimal hormonal environment enhances anabolic responses.
For injury recovery
Tissue repair applications may favor different approaches depending on the injury type and location.
For localized injuries, IGF-1 DES offers targeted effects through site-specific administration. Its short half-life limits systemic exposure while delivering concentrated growth factor activity where needed.
Tendon and ligament injuries benefit from IGF-1's effects on collagen synthesis. Combining with BPC-157 addresses inflammation and blood supply while IGF-1 promotes structural repair.
Muscle injuries may respond well to MGF given its role in satellite cell activation. The natural expression of MGF increases in damaged muscle, and supplementing this response may accelerate recovery.
The best peptides for pain and peptides for back pain provide context for pain-focused applications that may complement IGF-1 use.
For body composition
Improving body composition, building muscle while losing fat, aligns well with IGF-1's nutrient partitioning effects.
IGF-1 LR3's ability to direct nutrients toward muscle tissue while promoting lipolysis creates favorable conditions for recomposition. Combined with appropriate training and nutrition, these effects may allow simultaneous muscle gain and fat loss.
However, IGF-1 alone may not be optimal for those primarily focused on fat loss. Dedicated peptides for visceral fat or weight loss peptides may provide more targeted effects for this goal.
For true recomposition rather than fat loss alone, the combination of IGF-1 with GH secretagogues offers comprehensive support for both muscle anabolism and fat metabolism.
For anti-aging applications
Anti-aging approaches with IGF-1 typically focus on maintaining tissue health rather than maximizing muscle growth. Lower doses aimed at restoring more youthful IGF-1 levels may provide benefits without the risks associated with supraphysiological exposure.
Skin health applications may benefit from both systemic IGF-1 and topical formulations containing GHK-Cu, a copper peptide that influences IGF-1 signaling locally. Peptides for wrinkles often work through IGF-1 related mechanisms.
Cognitive support applications remain more speculative but represent an interesting area of research. The peptides for memory overview covers compounds that may support brain health through various mechanisms.
Comparing IGF-1 to other anabolic options
Understanding how IGF-1 compares to other muscle-building approaches helps contextualize its unique characteristics and potential role in research protocols.
IGF-1 vs anabolic steroids
Anabolic steroids and IGF-1 both promote muscle growth, but through fundamentally different mechanisms.
Steroids primarily cause hypertrophy by increasing protein synthesis within existing muscle cells. They don't significantly affect muscle cell number. Their effects are largely mediated through androgen receptors, creating a ceiling effect once receptors become saturated.
IGF-1 promotes both hypertrophy and hyperplasia. The creation of new muscle cells through satellite cell activation represents a mechanism steroids don't share. This hyperplastic potential may allow for muscle development beyond what steroid-only approaches can achieve.
The side effect profiles differ substantially. Steroids affect the HPTA (hypothalamic-pituitary-testicular axis), suppressing natural testosterone production. IGF-1 doesn't directly affect testosterone but carries risks related to glucose metabolism and cell proliferation.
The peptides vs steroids comparison examines these differences in greater detail.
IGF-1 vs SARMs
Selective androgen receptor modulators (SARMs) attempt to provide steroid-like effects with greater tissue selectivity. They primarily work through androgen receptors, similar to steroids but potentially with fewer systemic effects.
IGF-1 works through completely different pathways. It doesn't interact with androgen receptors at all. This means IGF-1 could theoretically be combined with SARMs for additive effects through complementary mechanisms.
Both categories remain in early stages of research for most applications. Neither has broad FDA approval for muscle-building purposes. The peptides vs SARMs comparison provides additional context.
IGF-1 vs other peptides
Within the peptide category, IGF-1 occupies a unique position due to its direct growth factor activity.
GH secretagogues like Sermorelin or Ipamorelin work indirectly, stimulating natural GH release which then produces IGF-1. This approach maintains physiological feedback but may not achieve the peak levels possible with direct IGF-1 administration.
GHRP-6, GHRP-2, and similar compounds also stimulate GH release but through different receptor mechanisms than GHRH analogs. They can be combined with GHRH analogs for synergistic effects on GH production.
None of these alternatives provide the direct, immediate growth factor exposure that exogenous IGF-1 delivers. For applications requiring maximum anabolic signaling, direct IGF-1 remains the most potent option within the peptide category.
SeekPeptides members gain access to detailed protocol guidance for various peptide applications, helping navigate these complex choices with expert support.
Frequently asked questions about IGF peptides
What is the difference between IGF-1 LR3 and IGF-1 DES?
IGF-1 LR3 has an extended half-life of 20-30 hours and acts systemically throughout the body. IGF-1 DES has a very short half-life of about 20-30 minutes but approximately 10 times greater local potency. LR3 suits protocols requiring sustained systemic effects, while DES targets specific sites with brief but intense activity.
Can IGF-1 cause hypoglycemia?
Yes. IGF-1's structural similarity to insulin means it promotes glucose uptake into cells, potentially lowering blood sugar. Symptoms range from mild shakiness to severe confusion or unconsciousness. Managing this risk requires attention to meal timing and carbohydrate intake around administration.
How long should an IGF-1 cycle last?
Most research protocols reference 4-6 week cycles followed by equal time off. This cycling approach aims to prevent receptor desensitization that may reduce effectiveness with continuous use. Extended cycles may carry increased metabolic and safety risks.
Does IGF-1 need to be injected?
Currently, effective administration requires injection, typically subcutaneous or intramuscular depending on the variant and research goals. Oral IGF-1 degrades in the digestive tract before reaching the bloodstream. Research into alternative delivery methods continues.
Is IGF-1 legal?
IGF-1 peptides exist in a regulatory gray area in most jurisdictions. They're not FDA-approved for general use but may be purchased for research purposes. They're prohibited in competitive sports by WADA and most athletic organizations. Legal status varies by country, and regulations change periodically.
Can IGF-1 be used for fat loss?
IGF-1 promotes nutrient partitioning that favors muscle over fat storage and may enhance lipolysis. However, dedicated fat loss peptides may be more effective for those primarily focused on reducing body fat.
IGF-1's primary strengths lie in anabolic effects rather than direct fat metabolism.
What should IGF-1 be stacked with?
Common research combinations include GH secretagogues for comprehensive growth hormone axis support, healing peptides like BPC-157 and TB-500 for recovery applications, and MGF for optimized satellite cell development. Stacking increases complexity and potential for interactions, requiring careful protocol design.
Does IGF-1 affect testosterone levels?
IGF-1 doesn't directly suppress natural testosterone production the way anabolic steroids do. However, it may influence the HPT axis indirectly through metabolic effects. Most research suggests testosterone levels remain relatively stable during IGF-1 use, though comprehensive hormone panels are advisable for monitoring.
How quickly does IGF-1 work?
Effects on protein synthesis begin within hours of administration. Visible changes in muscle fullness may appear within days to weeks. Significant body composition changes typically require several weeks of consistent use. The timeline varies based on dose, protocol, training, and nutrition factors.
Can women use IGF-1?
Yes. IGF-1's mechanisms don't depend on androgens, making it applicable regardless of gender. Dosing may differ, with women typically using the lower end of research ranges. The safe peptides for women overview provides additional gender-specific considerations.
Research timeline and expectations
Understanding realistic timelines helps set appropriate expectations for IGF peptide research. Results don't appear overnight, and patience combined with consistency produces the best outcomes.
First week observations
During the initial days of IGF-1 use, researchers often note increased muscle fullness and pumps during training. This likely reflects enhanced nutrient uptake and water movement into muscle cells rather than actual tissue growth.
Hypoglycemia risk may be highest during this period as the body adjusts to altered glucose dynamics. Careful attention to nutrition and monitoring for symptoms is particularly important early in a protocol.
Weeks two through four
This period typically shows measurable changes in body composition for those using appropriate doses with good training and nutrition. Increased strength often precedes visible muscle changes.
Recovery between workouts may improve noticeably. The ability to train with higher frequency or volume without excessive fatigue suggests enhanced recovery capacity.
Side effects, if they occur, usually become apparent during this window. Joint discomfort, water retention, or metabolic symptoms that persist warrant protocol adjustment.
Week four and beyond
By the end of a standard cycle, cumulative effects become more evident. Muscle mass changes that began subtly may now be clearly visible.
Body composition shifts, assuming appropriate nutrition, show measurable fat loss alongside muscle gain.
Those considering extended use should weigh the diminishing returns as receptors potentially desensitize against the increased risks of prolonged exposure. Most research suggests cycling off provides benefits that outweigh potential gains from continuous use.
Knowing how long peptides take to work in general provides context for these IGF-specific timelines.
Post-cycle considerations
What happens after an IGF-1 cycle matters nearly as much as the cycle itself. Proper management of the post-cycle period helps preserve gains and supports metabolic recovery.
Maintaining gains
Muscle gained through IGF-1's hyperplastic effects (new muscle cells) may be more permanent than gains from other anabolic compounds. However, maintaining the size and function of these cells requires continued training stimulus and adequate nutrition.
Protein intake should remain elevated during the post-cycle period. The new tissue still needs amino acids for maintenance. Dropping protein too quickly may compromise retention.
Training intensity and volume can typically be maintained if recovery allows. The enhanced recovery capacity of the cycle period won't persist, so training may need adjustment based on how recovery feels.
Metabolic recovery
Insulin sensitivity should normalize during time off from IGF-1. Any metabolic adaptations that occurred during the cycle typically reverse with sufficient break time.
Blood glucose monitoring during the post-cycle period can confirm normalization. Persistent abnormalities might indicate longer-term effects requiring medical attention.
Allowing equal time off as the cycle lasted represents standard practice. A six-week cycle followed by six weeks off provides adequate recovery time for most before considering another cycle.
Evaluating results
Honest assessment of results helps inform future protocols. D
id the approach achieve desired goals?
Were side effects manageable?
What would you do differently?
Keeping records of doses, timing, body composition changes, training performance, and side effects creates valuable data for optimizing future research. What works varies between individuals, and personal records help identify what works for you.
Common mistakes to avoid
Learning from others' errors saves time and reduces risks. Several common mistakes appear repeatedly in discussions of IGF peptide use.
Starting with excessive doses
The temptation to start high, assuming more equals faster results, leads many researchers into problems. Side effects scale with dose. Beginning at the lower end of research ranges allows assessment of individual response before any increases.
IGF-1 LR3 at 20 mcg daily represents a conservative starting point. Jumping straight to 100 mcg invites unnecessary risk when the lower dose might have produced excellent results with fewer side effects.
Ignoring nutrition
IGF-1 partitions nutrients toward muscle. But it can't partition nutrients that aren't there. Failing to eat adequately, especially protein and carbohydrates, severely limits potential results.
Carbohydrates particularly matter given hypoglycemia risks. Very low carbohydrate diets combined with IGF-1 administration increase the chance of blood sugar problems.
Neglecting training
IGF-1 enhances the body's response to training stimulus. Without that stimulus, much of its potential remains unrealized. The peptide doesn't build muscle by itself. It amplifies the growth response to appropriate training.
Those expecting IGF-1 to produce results without serious training consistently find disappointment. The compound enhances what training provides, it doesn't replace it.
Poor storage and handling
IGF peptides degrade rapidly when mishandled. Heat exposure, light exposure, or rough handling during reconstitution can reduce or eliminate potency. What appears to be a non-responder might actually be using degraded product.
Proper storage at correct temperatures, gentle handling during reconstitution, and protection from light ensure the product retains its activity. The peptide expiration guide covers stability factors in detail.
Combining too many compounds
Complex stacks with multiple compounds make it impossible to know what's working and what's causing problems. When issues arise, there's no way to identify the source.
Starting with single compounds, establishing baseline response, then adding others systematically allows proper evaluation of each element. This approach takes longer but produces more reliable information about what actually works.
The common peptide mistakes article covers additional errors that apply broadly to peptide use.

The future of IGF-1 research
IGF-1 research continues to evolve as scientists explore new applications and refine understanding of existing ones. Several areas show particular promise for future development.
Targeted delivery systems
Current administration methods deliver IGF-1 systemically, regardless of whether localized effects are desired. Research into targeted delivery mechanisms, including nanoparticle encapsulation and sustained-release formulations, aims to improve specificity and reduce systemic exposure.
Hydrogel systems that slowly release IGF-1 at injury sites represent one promising direction.
These could provide sustained local effects for tissue repair without the metabolic implications of systemic administration.
Combination therapies
Understanding how IGF-1 interacts with other growth factors opens possibilities for optimized combination approaches. Research combining IGF-1 with PDGF, TGF-beta, and other factors shows synergistic effects in certain applications.
For muscle development, combinations targeting both proliferation (satellite cell expansion) and differentiation (maturation into muscle tissue) may prove more effective than single-factor approaches.
MGF followed by IGF-1 represents a crude version of this strategy that may be refined with further research.
Safer analogs
The side effect profile of current IGF-1 variants limits their applicability. Research into modified peptides that retain anabolic effects while reducing metabolic and proliferative risks could expand the potential user base significantly.
Work on receptor-selective analogs aims to activate muscle-specific pathways while minimizing effects on other tissues. Success in this area would address many current safety concerns.
Age-related applications
The relationship between declining IGF-1 levels and age-related tissue deterioration makes this peptide particularly interesting for gerontology research.
Understanding how to safely restore more youthful IGF-1 signaling without increasing cancer or metabolic risks remains a significant research priority.
As the global population ages, effective interventions for sarcopenia, cognitive decline, and tissue frailty become increasingly important.
IGF-1 may play a role in addressing these challenges.
Getting started with IGF peptides
For those new to IGF peptide research, taking a methodical approach sets the foundation for success. These compounds deserve respect, and careful preparation pays dividends.
Education first
Before acquiring any peptides, develop a solid understanding of their mechanisms, risks, and proper handling. Reading this guide represents a good start. Consulting additional sources, including peer-reviewed research when possible, builds a more complete picture.
Understanding what you're working with prevents costly mistakes and improves outcomes. The investment in education returns value throughout your research journey.
Getting started with peptides provides foundational knowledge applicable to all peptide research. The complete peptide list offers an overview of the broader landscape.
Sourcing considerations
Peptide quality varies dramatically between sources. Impure or degraded products produce inconsistent results at best and pose safety risks at worst. Researching suppliers before purchasing helps ensure you're working with legitimate products.
Third-party testing, proper documentation, and community reputation provide indicators of supplier reliability. The peptide vendor guide discusses what to look for in a reputable source.
Setting up properly
Having the right supplies before beginning prevents problems.
Bacteriostatic water, appropriate syringes, alcohol swabs, and proper storage containers should all be ready before your peptides arrive.
The injectable peptide guide covers necessary supplies and preparation. Having everything organized beforehand allows you to focus on the research itself rather than scrambling for materials.
Establishing baselines
Before starting any peptide protocol, establish baseline measurements. Body weight, body composition if possible, training performance metrics, and photographs all provide reference points for evaluating progress.
Blood work establishing baseline glucose, insulin, and IGF-1 levels provides valuable medical context. Changes from these baselines during research offer insight into the body's response.
SeekPeptides offers members access to comprehensive protocol guidance, helping navigate the complexity of peptide research with expert support and community knowledge.
External resources
For those seeking additional scientific context on IGF-1 peptides, these authoritative sources provide peer-reviewed research and medical information:
PMC: Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy - Comprehensive review of IGF-1's molecular mechanisms in muscle tissue
PubMed: Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation - Research on IGF-1's role in satellite cell activation
Frontiers: IGF-1 Empowering Tendon Regenerative Therapies - Recent research on IGF-1 for tendon healing
These resources provide scientific foundation for understanding IGF-1 beyond the practical applications covered in this guide.
Cross-referencing multiple sources builds comprehensive knowledge.
The journey into IGF peptide research offers fascinating insights into how the body regulates growth and repair.
These compounds represent some of the most potent anabolic tools available, and with that potency comes responsibility. Used thoughtfully, with proper preparation and respect for their power, IGF peptides can support research goals in muscle development, recovery, and beyond.
Whether you're exploring peptide cycle planning for the first time or refining existing protocols, the principles remain consistent: educate yourself thoroughly, start conservatively, monitor carefully, and adjust based on response. This approach maximizes results while minimizing risks.
In case I don't see ya, good afternoon, good evening, and good night.