Feb 3, 2026
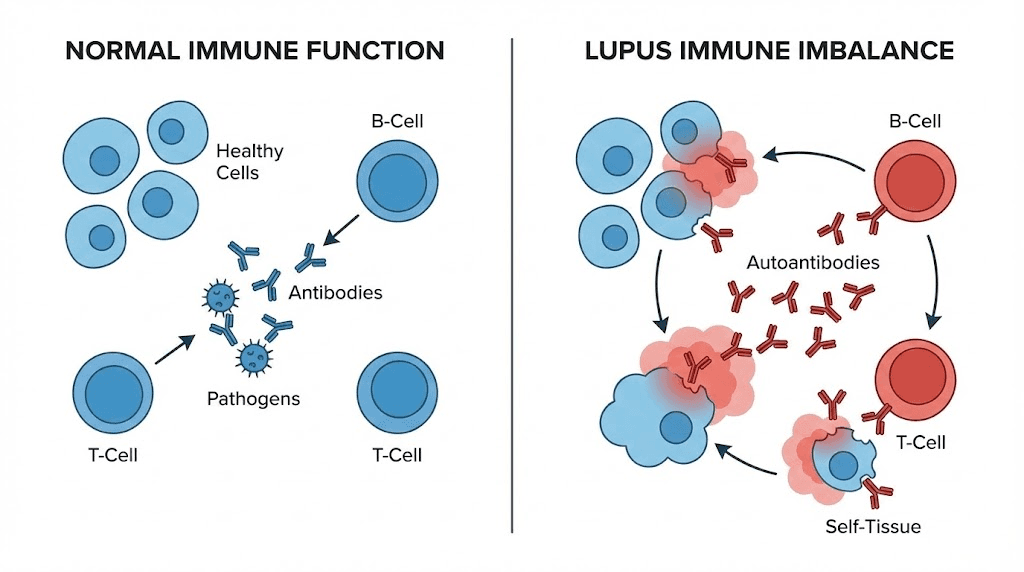
At the cellular level, lupus begins with a betrayal. The immune system, designed to protect the body from infection and disease, turns inward. It manufactures autoantibodies that attack healthy tissue. Skin. Joints. Kidneys. The heart. Even the brain.
This is systemic lupus erythematosus, a condition where the body defense network cannot distinguish between foreign invaders and its own cells. The result is chronic inflammation, tissue damage, and a cycle of flares that can disrupt every aspect of daily life. For the estimated 1.5 million Americans living with lupus, and millions more worldwide, conventional treatments often involve a difficult tradeoff: suppress the immune system broadly to reduce attacks on healthy tissue, but leave the body vulnerable to infections and long-term complications. Corticosteroids reduce inflammation but carry serious side effects over time. Immunosuppressants like mycophenolate and azathioprine dial down immune activity, yet they do not restore balance. They simply quiet the entire system. This is where peptide research offers a fundamentally different approach. Rather than suppressing immunity wholesale, certain peptides appear to modulate immune function, correcting the specific imbalances that drive autoimmune activity while preserving the body ability to fight real threats. From thymosin alpha-1 and its capacity to rebalance T-cell populations, to KPV and its targeted anti-inflammatory action through NF-kB inhibition, to experimental peptides like P140/Lupuzor that have reached Phase III clinical trials specifically for lupus, the landscape of peptide-based immune modulation is expanding rapidly. This guide examines the science behind each peptide with relevance to lupus, explores practical considerations for researchers, and provides evidence-based context for understanding how these molecules interact with autoimmune pathology.
Whether you are a researcher exploring peptides for autoimmune diseases or someone seeking deeper understanding of immune modulation, the information ahead covers mechanisms, protocols, safety considerations, and the current state of clinical evidence.
Understanding lupus and the immune system
Lupus is not one disease. It is a spectrum. Systemic lupus erythematosus, the most common and severe form, can affect virtually any organ system in the body. The hallmark of SLE is the production of anti-nuclear antibodies, proteins that target the body own DNA, histones, and other nuclear components. These autoantibodies form immune complexes that deposit in tissues, triggering complement activation and chronic inflammation. The damage accumulates over time, particularly in the kidneys, where lupus nephritis remains one of the most serious complications.
The demographics tell a striking story. Women develop lupus at roughly nine times the rate of men, with onset most common between ages 15 and 45. Black, Hispanic, and Asian populations face disproportionately higher risk and more severe disease courses. These patterns point to complex interactions between hormonal factors, genetic susceptibility, and environmental triggers that researchers are still working to untangle.
Understanding how peptides work in the context of lupus requires grasping what goes wrong immunologically. In a healthy immune system, regulatory T cells keep autoreactive immune cells in check. Dendritic cells present antigens appropriately. B cells produce antibodies only against genuine threats. In lupus, these checkpoints fail. Regulatory T cells lose their suppressive function. Plasmacytoid dendritic cells produce excessive type I interferons, driving a cascade of immune activation. B cells escape tolerance mechanisms and churn out autoantibodies. The entire system shifts into overdrive.
Current treatment limitations
Conventional lupus treatments have improved survival dramatically over the past several decades. Hydroxychloroquine remains a cornerstone, reducing flares and improving long-term outcomes. But it does not work for everyone, and it does not address the underlying immune dysregulation. Corticosteroids like prednisone provide powerful anti-inflammatory effects, yet chronic use leads to bone loss, weight gain, diabetes, and increased infection risk. Immunosuppressants such as mycophenolate mofetil and azathioprine reduce disease activity but carry risks of serious infections, liver toxicity, and malignancy.
Newer biologics represent progress. Belimumab targets B-lymphocyte stimulator protein, reducing B cell survival and autoantibody production. Anifrolumab blocks the type I interferon receptor, addressing a key driver of lupus pathology. Rituximab depletes CD20-positive B cells. These agents offer more targeted approaches than traditional immunosuppressants, but they still involve significant immune suppression and come with substantial costs and potential side effects.
The gap in current treatment is clear. What lupus patients need is not broader suppression but smarter modulation, an approach that corrects specific immune imbalances without leaving the rest of the immune system compromised. This is precisely the theoretical promise that certain peptides bring to autoimmune research.
How peptides work differently from conventional lupus treatments
The distinction between immunosuppression and immunomodulation is not just semantic. It represents a fundamentally different approach to treating autoimmune disease. Traditional immunosuppressants work like a volume knob turned all the way down. They reduce overall immune activity, which decreases autoimmune attacks but also weakens defense against infections, reduces cancer surveillance, and impairs wound healing. Peptide-based immunomodulation, by contrast, aims to work more like an equalizer, adjusting specific frequencies while leaving others intact.
Peptides serve many functions in the body. In the context of autoimmunity, the most relevant are those that influence T-cell differentiation, cytokine production, inflammatory signaling pathways, and immune cell communication. Rather than suppressing the entire immune response, these peptides can potentially shift the balance between pro-inflammatory and anti-inflammatory processes, promote regulatory T cell function, and restore tolerance mechanisms that have broken down in lupus.
Consider the difference in practical terms. A patient on high-dose prednisone experiences reduced lupus flares but also faces increased susceptibility to every cold, flu, and opportunistic infection. A peptide that enhances regulatory T cell function, if effective, would theoretically reduce the autoimmune component while preserving or even enhancing the body ability to fight genuine infections. This is the paradigm that drives peptide research in autoimmune conditions.
Tolerogenic approaches
One of the most exciting frontiers in lupus research is the concept of immune tolerance restoration. In healthy individuals, the immune system learns early in life to tolerate the body own tissues. In lupus, this tolerance breaks down. Tolerogenic peptides aim to re-educate the immune system, teaching it once again to recognize self-antigens as non-threatening.
This approach differs from both traditional immunosuppression and even newer biologics. Instead of blocking specific immune pathways or depleting certain cell populations, tolerogenic strategies attempt to restore the fundamental regulatory mechanisms that prevent autoimmunity in the first place.
Several peptides under investigation for lupus fall into this category, including P140/Lupuzor and certain histone-derived peptides that have shown promise in preclinical studies. Understanding peptide research and clinical studies helps contextualize where these approaches currently stand in the development pipeline.
The best peptides for immune system support in autoimmune conditions share certain characteristics. They tend to reduce excessive inflammatory signaling without eliminating protective immunity. They often work through multiple mechanisms simultaneously. And they generally demonstrate favorable safety profiles compared to conventional immunosuppressants, though long-term data in autoimmune populations remains limited for most of these compounds.
Thymosin alpha-1 and lupus immune regulation
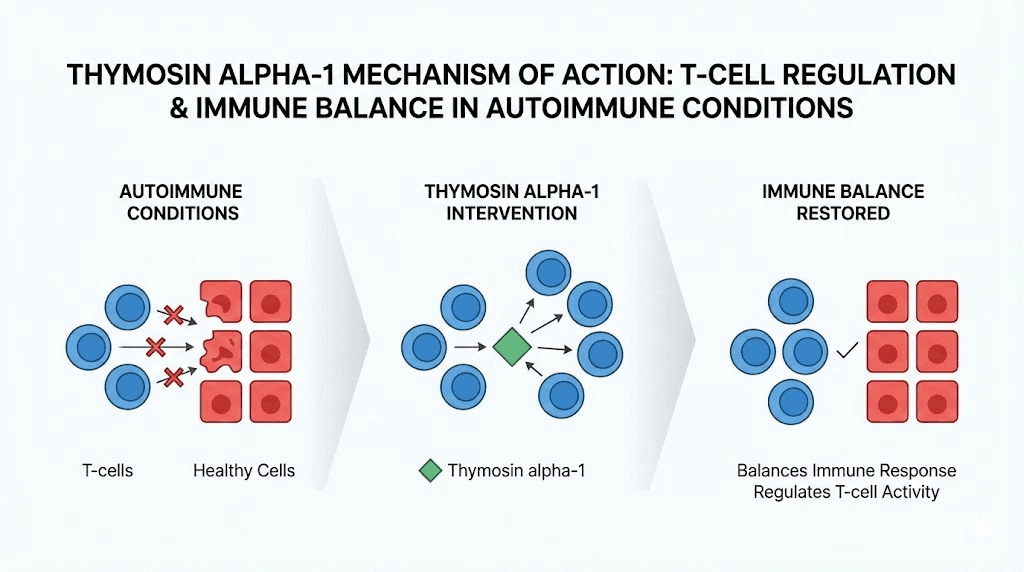
Thymosin alpha-1 stands out among immune-modulating peptides for a simple reason. It does not just suppress or stimulate. It balances. This 28-amino-acid peptide, originally isolated from thymic tissue, has been studied extensively for its ability to enhance immune function in immunocompromised states while simultaneously modulating overactive immune responses in autoimmune conditions. For lupus researchers, this dual capacity makes it particularly interesting.
The mechanism is elegant. Thymosin alpha-1 enhances natural killer cell activity and increases cytotoxic T cell function, both important for fighting infections and cancer. At the same time, it promotes the differentiation of regulatory T cells, the very population that is dysfunctional in lupus. It also reduces apoptosis of immune cells through improved cellular signaling, and it helps balance the Th1/Th2 ratio, a parameter that is frequently skewed in autoimmune conditions.
Research findings relevant to lupus
Several observations connect thymosin alpha-1 directly to lupus pathology. Studies have found that lupus patients tend to have lower serum levels of thymosin alpha-1 compared to healthy controls. This deficit correlates with disease activity, suggesting that reduced thymosin alpha-1 may contribute to the immune dysregulation seen in SLE. Supplementing with exogenous thymosin alpha-1 could theoretically help restore normal immune regulatory function.
The peptide has regulatory approval in over 30 countries for conditions including hepatitis B and C, where it is used as an immune enhancer. Its safety profile is well-established over decades of clinical use. For lupus, the evidence is largely preclinical and observational, but the mechanistic rationale is strong. By enhancing regulatory T cell populations and normalizing immune signaling, thymosin alpha-1 addresses several of the core immune deficits that drive lupus pathology.
Many researchers exploring inflammation-related peptide protocols include thymosin alpha-1 as a foundational component. Its broad immune-modulatory effects make it a natural complement to more targeted anti-inflammatory peptides. Understanding appropriate peptide dosages is essential when considering thymosin alpha-1 for research protocols, as the standard dose used in clinical settings typically ranges from 1.6 mg administered subcutaneously two to three times per week.
Practical considerations for thymosin alpha-1
Researchers interested in thymosin alpha-1 should understand several practical factors. The peptide is typically supplied as a lyophilized powder requiring reconstitution with bacteriostatic water. Proper peptide reconstitution technique is critical for maintaining potency and sterility. Once reconstituted, storage conditions significantly affect stability, and researchers should consult peptide storage guidelines to ensure proper handling.
The peptide reconstitution calculator on SeekPeptides can help determine the correct amount of bacteriostatic water to add for desired concentrations. This matters because incorrect reconstitution can lead to either subtherapeutic dosing or excessive concentrations that may affect stability. For those new to peptide handling, the guide on how to mix peptides with bacteriostatic water provides step-by-step instructions.
Administration is typically subcutaneous, with peptide injection protocols following standard practices. Some researchers cycle thymosin alpha-1, while others maintain continuous low-dose protocols.
The decision often depends on the specific research goals and the individual immune profile being studied. For general guidance on peptide cycle planning, understanding both on-cycle and off-cycle periods helps optimize outcomes.
BPC-157 and the gut-immune connection in lupus
The gut is not just where food gets digested. It is the largest immune organ in the body. Approximately 70% of the immune system resides in gut-associated lymphoid tissue, making intestinal health inseparable from immune function. For lupus patients, this connection carries particular significance. Intestinal permeability, often called "leaky gut," has been increasingly linked to autoimmune disease initiation and flare activity. When the intestinal barrier breaks down, bacterial products and undigested proteins enter the bloodstream, triggering immune activation that can worsen autoimmune responses throughout the body.
BPC-157 enters this picture as one of the most studied peptides for gastrointestinal protection and tissue repair. This 15-amino-acid peptide, derived from a protein found in human gastric juice, has demonstrated remarkable protective effects on the gastrointestinal tract in numerous preclinical studies.
It promotes angiogenesis, accelerates wound healing, reduces inflammation, and appears to protect against various forms of GI damage including those caused by NSAIDs, a class of drugs frequently used by lupus patients for joint pain and inflammation.
The gut-immune axis in lupus
Research has revealed that lupus patients frequently exhibit altered gut microbiome compositions and increased intestinal permeability. This dysbiosis can drive immune activation through several mechanisms. Bacterial translocation across a compromised intestinal barrier triggers toll-like receptor activation. Molecular mimicry between bacterial antigens and self-antigens may initiate or perpetuate autoimmune responses. Reduced production of short-chain fatty acids from altered microbiome composition impairs regulatory T cell function in the gut.
BPC-157 addresses several of these pathways simultaneously. Its ability to accelerate healing of gastric and intestinal mucosal damage could help restore barrier integrity. Its anti-inflammatory properties reduce local inflammation that contributes to barrier breakdown. And its effects on nitric oxide pathways and growth factor expression support tissue repair processes throughout the GI tract. For researchers studying peptides for gut health, BPC-157 represents one of the most extensively studied options available.
Understanding what BPC-157 is and how it functions helps contextualize its potential relevance to lupus. The peptide does not directly suppress immune function. Instead, by supporting GI tract integrity, it may help reduce one of the upstream drivers of autoimmune activation. This indirect approach to immune modulation through gut health represents a complementary strategy rather than a direct treatment for lupus itself.
BPC-157 dosing considerations for autoimmune research
Standard research protocols for BPC-157 typically involve doses ranging from 200 to 500 mcg administered once or twice daily. The BPC-157 dosage calculator can help determine weight-based dosing for specific research applications. For autoimmune contexts, many researchers start with conservative doses and adjust based on response, following principles outlined in BPC-157 dosing guides.
Administration route matters. Subcutaneous injection near the abdomen targets gut-related effects more directly, while oral peptide capsules offer an alternative delivery method that directly contacts the GI mucosa. Some researchers combine BPC-157 with TB-500 for enhanced tissue repair effects, a combination explored in detail in the BPC-157 and TB-500 stacking guide. The comparison between these two healing peptides is covered thoroughly in the BPC-157 vs TB-500 analysis.
For those considering BPC-157 as part of autoimmune research, proper handling remains essential. The peptide should be stored according to powder form storage guidelines before reconstitution, and reconstituted peptide shelf life varies based on storage temperature and preparation technique. Using proper bacteriostatic water and following sterile reconstitution practices are non-negotiable aspects of any peptide research protocol.
KPV peptide for lupus-related inflammation
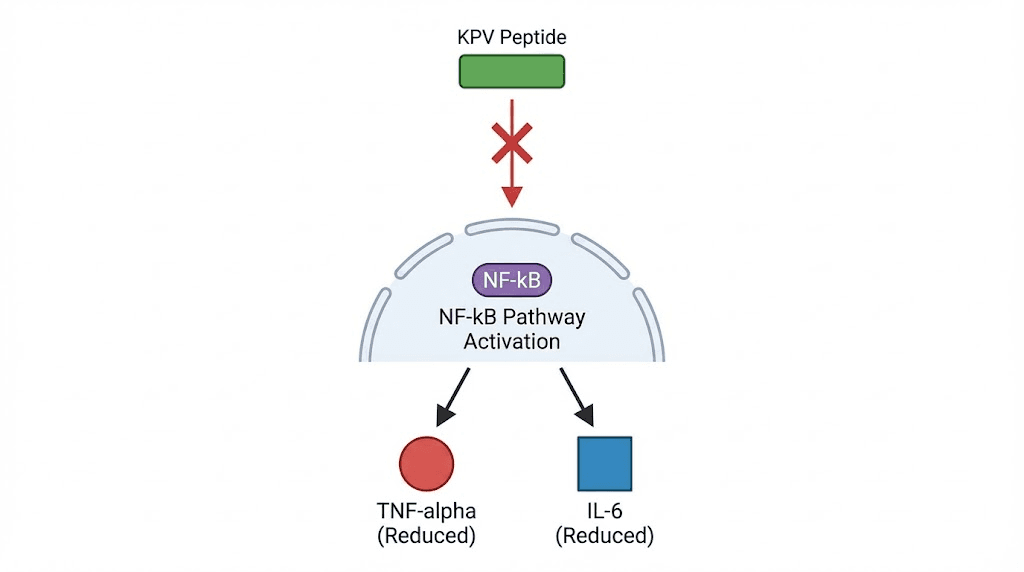
Three amino acids. That is all KPV is. Lysine-Proline-Valine, a tripeptide fragment derived from alpha-melanocyte stimulating hormone. Yet this tiny molecule demonstrates anti-inflammatory potency that belies its simplicity. For lupus researchers, KPV offers something rare: the ability to reduce inflammatory signaling through specific pathway inhibition without broadly suppressing immune function.
The primary mechanism behind KPV anti-inflammatory action is inhibition of the NF-kB pathway. NF-kB is a master transcription factor that controls the expression of hundreds of genes involved in inflammation, immune activation, and cell survival. In lupus, NF-kB is constitutively activated in multiple immune cell types, driving the persistent inflammatory state that characterizes the disease. By interfering with NF-kB nuclear translocation, KPV reduces the production of key pro-inflammatory cytokines including TNF-alpha, IL-1beta, and IL-6, all of which are elevated in active lupus.
KPV mechanism in detail
The anti-inflammatory properties of KPV extend beyond simple cytokine reduction. The peptide also reduces neutrophil infiltration into inflamed tissues, a process that contributes significantly to tissue damage in lupus. Neutrophils in lupus patients undergo increased NETosis, releasing nuclear material that further drives autoantibody production and immune complex formation. By reducing neutrophil recruitment, KPV may help interrupt this self-perpetuating cycle of inflammation and immune activation.
KPV also promotes epithelial repair, which connects to the gut-immune considerations discussed in the BPC-157 section. The peptide has shown particular promise in models of intestinal inflammation, where it reduces mucosal damage while preserving immune surveillance capacity. The comprehensive KPV peptide benefits guide covers these mechanisms in greater detail, including the peptide effects on various inflammatory pathways.
What makes KPV especially attractive for autoimmune research is its safety profile. Because it targets specific inflammatory pathways rather than broadly suppressing immunity, the side effect profile appears minimal based on available research. This contrasts sharply with corticosteroids and traditional immunosuppressants, which carry extensive lists of potential adverse effects. The KPV dosage guide provides detailed information on research protocols and administration approaches.
KPV and lupus flare management
Lupus flares represent periods of increased disease activity, often triggered by stress, sun exposure, infections, or hormonal changes. During flares, inflammatory cytokine levels spike, complement is consumed, and tissue damage accelerates. The ability to rapidly reduce inflammatory signaling during these acute episodes is critical for preventing permanent organ damage.
KPV theoretical utility during flares stems from its mechanism of action. By inhibiting NF-kB activation, it could potentially reduce the inflammatory cascade that drives flare severity without compromising the immune response to concurrent infections, a common concern during lupus flares when patients may also be on immunosuppressive medications. Research into KPV and immune-related research provides additional context on how this peptide interacts with immune signaling pathways.
Researchers studying KPV for autoimmune applications often consider administration routes carefully. Nasal spray delivery offers rapid absorption and may provide systemic effects, while subcutaneous administration follows more traditional peptide research protocols. The choice depends on the specific research objectives and the inflammatory targets being addressed. For understanding the general principles of peptide injection administration, comprehensive resources are available to guide proper technique.
Selank and immune tolerance in autoimmune conditions
Selank occupies a unique position in the peptide landscape. Developed at the Institute of Molecular Genetics of the Russian Academy of Sciences, this synthetic peptide is based on the naturally occurring immunomodulatory peptide tuftsin, with additional amino acids added to improve stability and bioavailability.
While selank is primarily known for its anxiolytic and nootropic properties, its immunomodulatory effects make it relevant to autoimmune research in ways that are often overlooked.
The connection between selank and autoimmunity runs through cytokine regulation. Selank has been shown to normalize the production of key cytokines, including IL-6 and interferon-gamma, both of which play central roles in lupus pathology. Rather than simply suppressing these cytokines, selank appears to promote their production within normal physiological ranges, reducing both excessive elevation and inappropriate suppression. This normalizing effect is the hallmark of true immunomodulation.
Selank immune mechanisms
Research on selank demonstrates several mechanisms relevant to lupus. The peptide promotes T-cell proliferation and differentiation, potentially supporting the regulatory T cell populations that are deficient in autoimmune conditions. It modulates the expression of genes involved in immune signaling, including those related to the IL-6 pathway that is consistently dysregulated in SLE. And it influences the hypothalamic-pituitary-adrenal axis, which connects immune regulation to stress response, an important consideration given that psychological stress is a known trigger for lupus flares.
The stress connection deserves elaboration. Lupus patients frequently report that emotional and physical stress precedes disease flares. The HPA axis mediates the body stress response, and dysregulation of this axis can amplify immune activation. Selank ability to modulate HPA axis function while simultaneously normalizing cytokine production offers a dual mechanism that addresses both the trigger (stress) and the response (immune dysregulation). For researchers interested in the broader cognitive and emotional effects, the selank dosage and injection guide provides detailed protocol information.
The relationship between peptides and anxiety management is particularly relevant for lupus patients, who experience anxiety at rates significantly higher than the general population. Selank may address both the immunological and psychological dimensions of living with a chronic autoimmune condition. Similarly, research on peptides for depression and anxiety highlights the interconnection between neurological health and immune function.
Building immune tolerance with selank
The concept of immune tolerance is central to understanding autoimmune disease. In lupus, the immune system has lost tolerance to self-antigens, treating the body own proteins as foreign invaders. Restoring this tolerance is the holy grail of autoimmune treatment, and selank ability to calm overactive immune signaling while promoting T-cell function may contribute to this goal.
Selank does not achieve tolerance restoration directly. That is important to understand. But by normalizing the cytokine environment and supporting proper T-cell differentiation, it may create conditions more favorable for tolerance induction. This is why some researchers combine selank with other immunomodulatory peptides in comprehensive protocols, using each peptide strengths to address different aspects of the autoimmune cascade. The principles of peptide stacking apply here, though autoimmune applications require particular caution and careful monitoring.
For lupus-related research protocols, selank is often paired with peptides that address the inflammatory component more directly, such as KPV or thymosin alpha-1. Understanding how many peptides can be combined safely is essential for designing multi-target protocols. The peptide stack calculator on SeekPeptides can help researchers evaluate potential combinations and identify interactions.
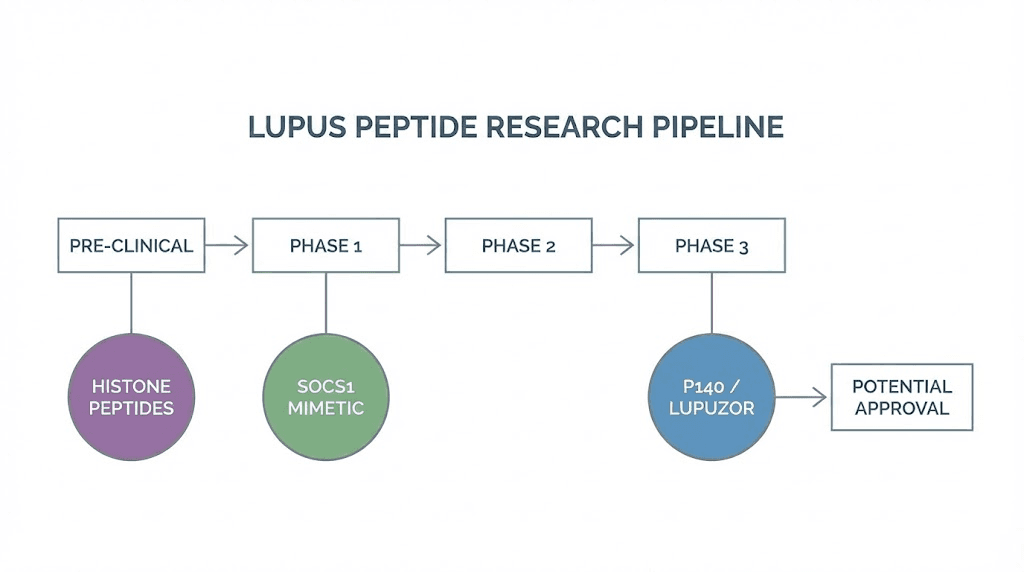
Research-stage peptides with lupus potential
Beyond the peptides already discussed, several molecules in various stages of research show specific promise for lupus. These represent the cutting edge of peptide-based autoimmune research, and while most have not yet reached widespread clinical application, their mechanisms and preliminary results warrant serious attention from researchers in this field.
P140/Lupuzor: the lupus-specific peptide
P140, also known as Lupuzor, represents the most advanced peptide therapy developed specifically for systemic lupus erythematosus. This synthetic phosphopeptide reached Phase III clinical trials, making it one of the few peptide-based therapies to advance that far in autoimmune disease research. The mechanism is fascinating and highly specific to lupus pathology.
P140 works by binding to HSPA8/HSC70, a chaperone protein involved in chaperone-mediated autophagy. In lupus, this autophagy pathway becomes hyperactivated, leading to excessive processing and presentation of self-antigens that fuel the autoimmune response. By interfering with this hyperactivated process, P140 reduces the presentation of self-antigens to T cells, effectively dampening the autoimmune drive at one of its key control points.
Phase IIb clinical trial results were encouraging. The peptide demonstrated safety and met primary efficacy endpoints, showing reductions in disease activity scores compared to placebo. Importantly, P140 appears to selectively deplete hyper-activated autoreactive T and B cells while sparing normal immune cells. This selectivity is what sets it apart from conventional immunosuppressants, which reduce all immune cell populations indiscriminately. The research community continues to watch P140 development closely, as it represents a proof of concept for peptide-based tolerogenic approaches to lupus.
SOCS1 peptide
Research from the University of Florida has produced a peptide that mimics the function of Suppressor of Cytokine Signaling 1, a key regulatory protein in immune responses. In healthy individuals, SOCS1 acts as a brake on immune activation, preventing excessive cytokine signaling. In lupus, SOCS1 function is often impaired, contributing to the uncontrolled immune activation that characterizes the disease.
The SOCS1-mimicking peptide reduced lupus-like symptoms in mouse models, demonstrating the potential to regulate an immune response that runs on overdrive. By restoring SOCS1 function at the molecular level, this peptide addresses a specific regulatory deficit rather than broadly suppressing immune activity. While still in preclinical stages, the SOCS1 peptide represents an important conceptual advance in targeted autoimmune therapy.
Histone-derived peptides
Histone proteins are among the primary targets of autoantibodies in lupus. Anti-histone antibodies are found in a significant proportion of SLE patients and are particularly associated with drug-induced lupus. Researchers have developed peptides derived from histone sequences that, paradoxically, can induce immune tolerance to these same proteins.
These histone-derived peptides work by promoting the expansion of CD4+ and CD8+ regulatory T cell populations. In mouse models of lupus, treatment with these peptides reduced lupus nephritis severity and decreased B cell activation. The tolerogenic mechanism is elegant: by presenting histone epitopes in a context that promotes regulatory rather than effector T cell responses, these peptides essentially re-educate the immune system to tolerate its own nuclear components.
hCDR1/Edratide
Edratide, also known as hCDR1, is a peptide based on the complementarity-determining region of a human anti-DNA antibody. It was designed to specifically target the autoimmune response against double-stranded DNA, one of the hallmark autoantibody targets in lupus. In clinical trials, edratide showed effects on immune biomarkers relevant to lupus, though clinical efficacy results were mixed. The approach demonstrates how peptides can be designed to target specific aspects of lupus autoimmunity rather than the immune system as a whole.
Nanoparticle-delivered peptides
One of the challenges with peptide therapies is delivery. Peptides are rapidly degraded in the body, often requiring frequent dosing to maintain therapeutic levels. Nanoparticle technology offers solutions. PLGA nanoparticles, lipid nanoparticles, and mesoporous silica nanoparticles have all been explored as delivery vehicles for lupus-relevant peptides. These platforms can protect peptides from degradation, target them to specific tissues or immune cell populations, and provide sustained release over extended periods.
Particularly exciting are magnetic nanoparticles designed to selectively target lupus B cells, delivering tolerogenic peptides directly to the cells responsible for autoantibody production. mRNA lipid nanoparticles, similar to those used in vaccine technology, can potentially enable in vivo production of therapeutic peptides by the body own cells. And hydrogel systems offer sustained release formulations that could reduce dosing frequency from daily injections to weekly or even monthly administrations. These delivery innovations may solve many of the practical limitations that currently constrain peptide therapy for chronic conditions like lupus. For current options in peptide administration, researchers can explore the differences between injectable versus oral peptide delivery.
Understanding LL-37 dual role in lupus
Not every peptide relevant to lupus is straightforwardly beneficial. LL-37, also known as cathelicidin, presents a complex picture that researchers must understand fully before incorporating it into any autoimmune research framework. This antimicrobial peptide plays dual roles that can either protect against or contribute to lupus pathology, depending on context.
In healthy individuals, LL-37 serves as a first-line defense against infection, killing bacteria, viruses, and fungi through membrane disruption. It also modulates immune responses, promoting wound healing and regulating inflammation. These protective functions are valuable and important. But in lupus, LL-37 takes on a different character.
The pathogenic side of LL-37 in lupus
In lupus patients, LL-37 has been found to form complexes with self-DNA released from dying cells. These LL-37/DNA complexes are potent activators of plasmacytoid dendritic cells, triggering massive production of type I interferons, the same cytokines that drive the "interferon signature" characteristic of active lupus. In essence, LL-37 converts normally non-immunogenic self-DNA into a powerful trigger for autoimmune activation.
Furthermore, LL-37 itself can become a target of autoantibodies in lupus. Anti-LL-37 antibodies have been detected in SLE patients, and their presence correlates with disease activity. This creates a paradoxical situation where a peptide designed by the body for protection becomes both a driver and a target of autoimmune pathology.
For lupus researchers, the LL-37 story provides important lessons. Not all peptides with immune-modulating properties are appropriate for autoimmune conditions. Context matters enormously. A peptide that enhances immune function in an immunocompromised patient may exacerbate disease in someone with lupus. This is why understanding the safety profile and risks of individual peptides is essential before designing any research protocol for autoimmune applications.
The lesson extends beyond LL-37 itself. Any peptide that strongly activates innate immune pathways or promotes interferon production requires careful evaluation in the lupus context. Researchers should consider whether a peptide mechanism of action could theoretically worsen the specific immune pathways that are already overactive in SLE. This kind of thoughtful analysis distinguishes responsible autoimmune peptide research from approaches that simply assume all immune-modulating peptides are beneficial.
Bioregulator peptides and long-term immune support
The Khavinson bioregulator peptides represent a distinct category of short peptides, typically just two to four amino acids in length, that are theorized to interact directly with DNA and regulate gene expression in specific organs and tissues. Developed by Professor Vladimir Khavinson over decades of research at the Saint Petersburg Institute of Bioregulation and Gerontology, these peptides offer a different paradigm for immune support that focuses on restoring optimal organ function rather than directly modulating immune cell activity.
For lupus researchers, bioregulator peptides are relevant because lupus is not just an immune disease. It is a disease that damages organs. Kidneys, heart, lungs, brain, blood vessels, skin, all can be affected by chronic autoimmune inflammation. Supporting the health and regenerative capacity of these organs while addressing the immune component of disease may offer a more comprehensive approach than targeting immunity alone. The comprehensive bioregulator peptides guide and the overview of Khavinson peptides provide extensive background on this class of compounds.
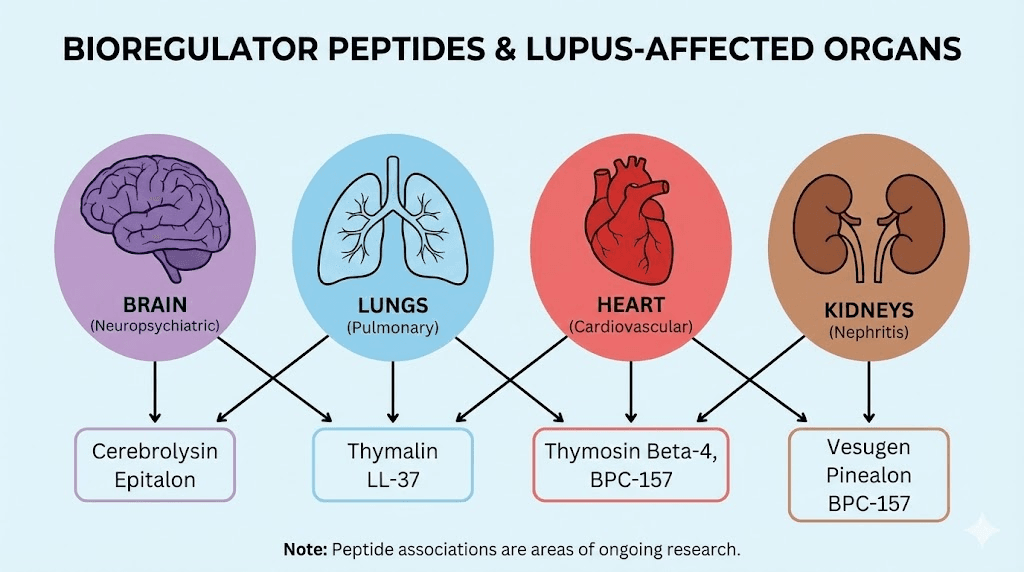
Organ-specific bioregulators relevant to lupus
Several bioregulator peptides target organs commonly affected by lupus. Thymalin, the thymic bioregulator, supports thymic function and T-cell maturation, addressing the immune regulatory deficits that drive autoimmune pathology. The thymus plays a central role in establishing immune tolerance, and thymic involution (shrinkage with age) has been linked to increased autoimmune risk. By supporting thymic function, thymalin may help maintain the regulatory T cell populations that keep autoimmunity in check.
Cortagen targets the cerebral cortex and may support neurological function in lupus patients who experience neuropsychiatric symptoms, a feature present in up to 75% of SLE patients at some point in their disease course. Cardiogen supports cardiac tissue and may be relevant for lupus patients with cardiac involvement, including pericarditis and myocarditis. Vesugen targets vascular tissue, which matters because accelerated atherosclerosis is a leading cause of morbidity and mortality in long-standing lupus.
The kidney-targeted bioregulator deserves particular attention given that lupus nephritis affects up to 60% of lupus patients and remains one of the most dangerous complications. Chonluten and other organ-specific bioregulators represent a strategy of supporting tissue health alongside immune modulation, addressing both the cause and the consequences of autoimmune inflammation simultaneously.
Other bioregulators that may support lupus management include Livagen for liver support (important given the hepatotoxicity of many lupus medications), Vilon for immune regulation, Ovagen for liver and GI support, Pancragen for pancreatic function, and Bronchogen for respiratory support in cases where lupus affects the lungs.
Long-term versus acute approaches
Bioregulator peptides are typically used in cyclical protocols, with courses lasting 10 to 30 days followed by breaks of several months. This differs from the continuous or semi-continuous protocols used with peptides like BPC-157 or thymosin alpha-1. The rationale is that bioregulators aim to restore optimal gene expression patterns rather than provide continuous pharmacological effects. Once the regulatory signals have been delivered, the body maintains the improved function for extended periods before another course may be needed.
For lupus researchers, this raises interesting questions about protocol design. A comprehensive autoimmune protocol might include continuous immune-modulating peptides like thymosin alpha-1 or KPV for ongoing immune regulation, combined with periodic bioregulator courses to support organ health and recovery. Understanding how to cycle different peptides is essential for designing such protocols, and the peptide dosing guide provides foundational information on protocol structure.
The longevity peptide category overlaps significantly with bioregulators, as many of the same compounds that support organ health also demonstrate anti-aging properties. Epitalon, the pineal bioregulator, has been studied for its effects on telomerase activation and melatonin production, both of which may be relevant to the accelerated aging processes observed in lupus patients. And peptides for anti-aging more broadly offer tools for addressing the premature cellular aging that chronic inflammation drives.
Practical considerations for lupus researchers
Moving from theory to practice requires addressing the logistical realities of peptide research. Reconstitution, storage, administration, and cycling all affect outcomes, and errors in any of these areas can compromise both safety and efficacy. For autoimmune applications, where the margin between helpful modulation and harmful stimulation may be narrow, getting these details right matters even more than in other contexts.
Reconstitution and storage
Most research peptides arrive as lyophilized (freeze-dried) powders that must be reconstituted before use. The process is straightforward but requires attention to detail. Understanding how much bacteriostatic water to add determines the final concentration, which in turn affects dosing accuracy. Too little water creates a highly concentrated solution that is difficult to dose precisely. Too much creates a dilute solution that may require larger injection volumes.
The reconstitution calculator simplifies this process by calculating the exact water volume needed for a desired concentration. After reconstitution, proper storage becomes critical. Reconstituted peptides are far less stable than their lyophilized counterparts. Understanding how to store peptides after reconstitution and refrigerated shelf life expectations prevents potency loss that could compromise research results.
Temperature sensitivity varies by peptide. Some remain stable at room temperature for hours, while others begin degrading almost immediately outside refrigeration. The guide on room temperature peptide stability helps researchers plan their workflow to minimize exposure to non-optimal conditions. For peptides that will not be used immediately, understanding lyophilized versus liquid peptide stability characteristics informs purchasing and storage decisions.
Administration routes
Different peptides favor different administration routes, and the choice can significantly affect both bioavailability and the distribution of effects throughout the body. Subcutaneous injection remains the most common route for research peptides, offering reliable absorption and the ability to target specific anatomical regions. The complete peptide injection guide covers technique, site selection, and best practices.
For peptides targeting gut-related aspects of lupus, oral delivery may offer advantages by directly contacting the intestinal mucosa. Peptide capsules provide an alternative that some researchers prefer, though bioavailability differs significantly from injectable forms. Nasal spray peptides offer yet another option, particularly for peptides with neurological targets like selank, where intranasal delivery provides relatively direct access to the central nervous system.
The comparison between injectable and oral peptides becomes particularly relevant for autoimmune protocols, where consistency of dosing and predictability of effects are essential. Researchers should consider which route best serves their specific objectives and understand the tradeoffs involved with each approach.
Cycling and protocol design
Peptide cycling, alternating periods of use with rest periods, serves multiple purposes. It prevents receptor desensitization, allows the body to integrate peptide-induced changes, and reduces the risk of immune system adaptation that could diminish effectiveness over time. For autoimmune applications, cycling also provides opportunities to assess disease activity off peptide and determine whether improvements are sustained.
The cycle planning guide outlines general principles that apply across peptide types. Common cycle structures include 4 weeks on, 2 weeks off; 8 weeks on, 4 weeks off; or continuous low-dose with periodic higher-dose phases. For autoimmune research, longer cycles with gradual dose adjustments may be preferable to avoid the immune system fluctuations that abrupt changes can trigger.
Understanding how long peptides take to produce effects helps set realistic expectations for autoimmune research protocols. Unlike acute pain medications that work within hours, immune-modulating peptides typically require weeks to months before meaningful changes in disease activity become apparent. Patience and consistent protocol adherence are essential.
Safety, side effects, and what to watch for
Safety in autoimmune peptide research requires a different mindset than safety in other peptide applications. When the immune system is already dysregulated, introducing compounds that modulate immune function carries inherent risks. The same peptide that calms overactive immunity in one context could theoretically trigger a flare or unexpected immune reaction in another. Vigilance is not optional.
Autoimmune-specific cautions
The most important caution for lupus researchers is this: immune-stimulating peptides must be approached with extreme care. While thymosin alpha-1 promotes immune balance rather than simple stimulation, other peptides with immune-enhancing properties could theoretically worsen lupus by amplifying the already overactive immune response. Before adding any peptide to a lupus research protocol, its mechanism must be evaluated specifically for compatibility with autoimmune pathology.
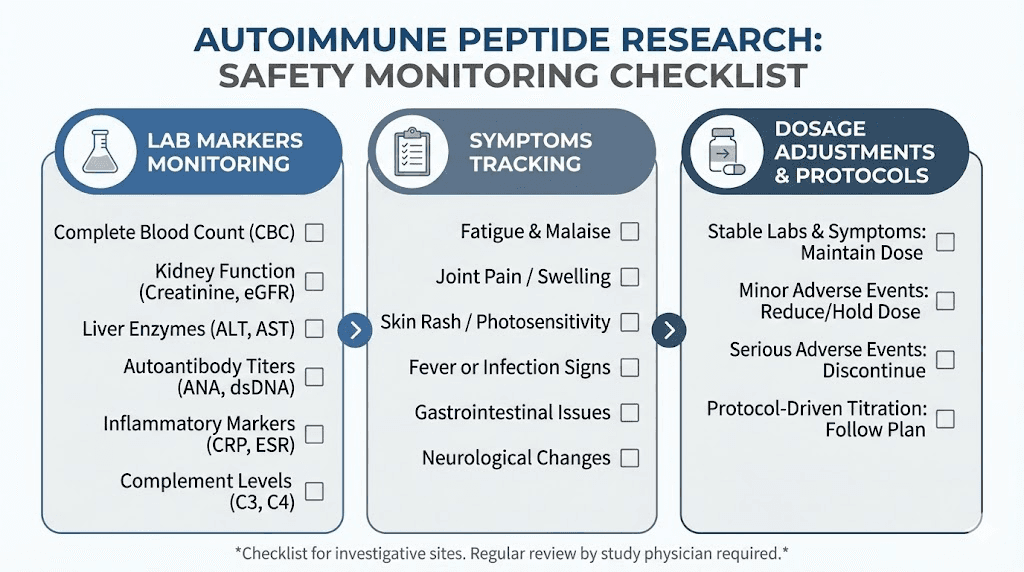
Flare management presents another challenge. Lupus flares can be triggered by infections, stress, hormonal changes, and, theoretically, by changes in immune signaling that peptide introduction might cause. Researchers should have clear protocols for monitoring disease activity markers and responding to signs of increased disease activity. This means tracking symptoms, laboratory values, and functional measures throughout any research protocol.
The comprehensive overview of peptide safety and risks provides foundational information, though autoimmune applications require additional considerations beyond those covered in general safety guides. Understanding common peptide mistakes helps avoid errors that could be particularly consequential in autoimmune contexts.
Drug interactions and concurrent therapies
Most lupus patients are on multiple medications, and potential interactions between these drugs and research peptides must be considered carefully. Immunosuppressants like mycophenolate or azathioprine could theoretically interact with immune-modulating peptides in unpredictable ways. Corticosteroids alter immune signaling pathways that peptides may also target. Biologics like belimumab or anifrolumab have specific immune targets that could overlap with or be affected by peptide mechanisms.
Working with healthcare providers who understand both conventional lupus treatment and peptide research is essential. The guide on finding peptide therapy providers and understanding online peptide therapy options can help connect researchers with knowledgeable practitioners. Additionally, understanding physician prescribing capabilities for research-grade peptides provides clarity on the regulatory landscape.
The cost dimension also matters for lupus patients, who often face significant financial burden from their disease. Understanding peptide costs and using the peptide cost calculator helps plan budgets for research protocols. The comprehensive peptide therapy cost guide provides additional context on pricing factors and considerations.
Monitoring and adjustment
Any lupus-related peptide protocol should include regular monitoring. This means tracking both disease-specific markers (complement levels, anti-dsDNA titers, inflammatory markers, urine protein) and general health parameters. Changes in any of these should prompt protocol review and possible adjustment.
Starting with one peptide at a time allows researchers to attribute any changes, positive or negative, to specific compounds. While stacking multiple peptides can offer synergistic benefits, introducing them sequentially provides clearer information about individual effects and tolerability. The getting started with peptides guide emphasizes this principle of methodical introduction, which is doubly important in autoimmune contexts.
Sourcing also affects safety. The quality and purity of research peptides vary significantly between suppliers. Using verified, tested peptides from reputable sources reduces the risk of contamination or mislabeled products that could cause adverse effects. The best peptide vendors guide and information on peptide testing laboratories help researchers identify reliable sources. Understanding the nuances of research versus pharmaceutical grade peptides and the landscape of grey market peptides provides important context for sourcing decisions.
Building a peptide protocol for autoimmune support
Protocol design for autoimmune conditions requires a different approach than protocols for muscle building, fat loss, or general wellness. The stakes are higher. The immune system is already compromised. And the goal is not simply enhancement but restoration of balance. With these considerations in mind, the following sample protocols illustrate different approaches to peptide-based autoimmune support, ranging from conservative to comprehensive.
Protocol 1: Conservative foundation (beginners)
Goal: Gentle immune modulation with minimal risk
Components:
Thymosin alpha-1: 1.6 mg subcutaneously, twice weekly
BPC-157: 250 mcg subcutaneously, once daily (abdominal injection for gut support)
Schedule:
Monday and Thursday: Thymosin alpha-1
Daily: BPC-157 in the morning
Duration: 8 weeks on, 4 weeks off
Monitoring: Baseline labs before starting, repeat at week 4 and week 8. Track symptoms daily. Watch for any signs of flare activity.
Expected timeline:
Weeks 1-2: Minimal observable changes, body adjusting
Weeks 3-4: Possible reduction in baseline inflammation
Weeks 5-8: Gradual improvement in energy, reduction in minor symptoms
This protocol focuses on two well-studied peptides with complementary mechanisms. Thymosin alpha-1 addresses immune regulation directly while BPC-157 supports gut integrity, addressing the gut-immune axis component of autoimmune pathology. For researchers new to peptides, the beginner guide provides essential foundation knowledge.
Protocol 2: Intermediate approach (experienced researchers)
Goal: Multi-target immune modulation with anti-inflammatory support
Components:
Thymosin alpha-1: 1.6 mg subcutaneously, three times weekly
KPV: 200-500 mcg subcutaneously, once daily
BPC-157: 250 mcg subcutaneously, twice daily
Selank: 250-500 mcg intranasally, once daily
Schedule:
Monday, Wednesday, Friday: Thymosin alpha-1
Daily AM: KPV + BPC-157
Daily PM: BPC-157
Daily: Selank nasal spray (morning or midday)
Duration: 12 weeks on, 4 weeks off
Monitoring: Baseline labs, repeat every 4 weeks. Daily symptom tracking. Inflammatory markers at baseline and weeks 6 and 12.
Expected timeline:
Weeks 1-3: Body adaptation, selank anxiolytic effects may be noticeable
Weeks 4-6: Reduction in inflammatory markers, possible decrease in minor flare frequency
Weeks 7-12: Cumulative benefits in energy, joint comfort, and immune stability
This protocol adds KPV for targeted anti-inflammatory action and selank for immune modulation plus stress management. The four components address different aspects of autoimmune pathology simultaneously. The peptide calculator can help determine precise dosing based on body weight, and the stacking guide covers interaction considerations.
Protocol 3: Comprehensive approach (advanced, with medical supervision)
Goal: Full-spectrum immune modulation with organ support
Phase 1 (Weeks 1-4): Foundation
Thymosin alpha-1: 1.6 mg subcutaneously, three times weekly
BPC-157: 300 mcg subcutaneously, twice daily
Phase 2 (Weeks 5-12): Add modulation
Continue Phase 1 peptides
Add KPV: 300-500 mcg subcutaneously, once daily
Add Selank: 500 mcg intranasally, once daily
Phase 3 (Weeks 13-16): Organ support cycle
Continue Phase 2 peptides
Add Thymalin: 10 mg course over 10 days
Add targeted bioregulators based on affected organs (kidney, cardiac, vascular as needed)
Phase 4 (Weeks 17-20): Rest and assess
Discontinue all peptides
Monitor disease activity markers
Assess sustained benefits
Plan next cycle based on results
Monitoring: Full labs every 4 weeks, including CBC, CMP, complement levels, anti-dsDNA, CRP, ESR, urine protein/creatinine ratio. Weekly symptom assessments. Immediate reporting of any flare symptoms.
This protocol is designed for advanced researchers working under medical supervision. The phased approach allows each component to be evaluated individually before adding complexity. The organ support phase addresses tissue damage and recovery, complementing the immune modulation of earlier phases. Use the cost calculator to budget for comprehensive protocols, which involve multiple peptides over extended periods.
For all protocols, sourcing quality peptides is fundamental. The peptide vial research guide helps researchers understand what to look for, and understanding peptide legality ensures compliance with applicable regulations. Researchers should also be aware of drug testing considerations if relevant to their situation, and stay informed about peptide regulation changes that may affect access.
Comparing lupus-relevant peptides
Peptide | Primary mechanism | Lupus relevance | Evidence level | Best for |
|---|---|---|---|---|
Thymosin alpha-1 | T-cell regulation, Th1/Th2 balance | Directly addresses immune dysregulation | Moderate (clinical use for other conditions) | Foundation immune modulation |
BPC-157 | Tissue repair, GI protection | Gut-immune axis support | Moderate (extensive preclinical) | GI involvement, medication-related GI damage |
KPV | NF-kB inhibition, cytokine reduction | Reduces key lupus inflammatory pathways | Moderate (preclinical) | Active inflammation, flare management |
Selank | Cytokine normalization, HPA axis | Immune tolerance support, stress management | Moderate (clinical use in Russia) | Stress-triggered flares, anxiety |
P140/Lupuzor | Chaperone-mediated autophagy modulation | Lupus-specific, depletes autoreactive cells | High (Phase III trials) | Direct lupus treatment (not yet available) |
SOCS1 | Cytokine signaling regulation | Restores immune braking mechanism | Low (preclinical only) | Future research direction |
Thymalin | Thymic function support | T-cell maturation, tolerance maintenance | Moderate (clinical use in Russia) | Long-term immune support cycles |
This comparison table provides a quick reference for researchers evaluating which peptides to prioritize for lupus-related protocols. The evidence levels reflect the current state of research specifically in autoimmune applications, not the overall evidence base for each peptide. For a comprehensive overview of all available compounds, the complete peptide list provides an extensive reference, and the peptide formula guide covers the molecular science behind these compounds.
Additional peptides worth investigating for lupus
While the peptides discussed above represent the strongest candidates for lupus research, several others merit mention for their potential contributions to autoimmune management. These peptides may not target lupus directly, but their mechanisms address symptoms or complications common in the disease.
Peptides for energy and fatigue
Fatigue is one of the most debilitating and persistent symptoms of lupus, affecting over 80% of patients. It often persists even when other disease markers improve. SS-31 (elamipretide) targets mitochondrial function directly, improving cellular energy production at its source. By stabilizing cardiolipin in the inner mitochondrial membrane, SS-31 enhances electron transport chain efficiency and reduces oxidative stress, both of which are impaired in chronic inflammatory conditions like lupus.
The best peptides for energy overview covers additional options that may help address lupus-related fatigue. Multiple energy-supporting peptides exist, each working through different mechanisms. For lupus patients, those that improve mitochondrial function or reduce inflammatory burden are most likely to address the root causes of fatigue rather than simply providing temporary stimulation.
Peptides for joint and musculoskeletal symptoms
Joint pain and arthritis are among the most common lupus symptoms, affecting up to 95% of patients at some point. While BPC-157 and TB-500 provide general tissue repair support, specific peptides target joint-related inflammation and cartilage health more directly. The guide to peptides for joint pain covers these options comprehensively.
TB-500 promotes systemic tissue repair and reduces inflammation through unique mechanisms involving upregulation of actin. The TB-500 dosage calculator helps determine appropriate research doses, and understanding how TB-500 complements BPC-157 can enhance outcomes. For lupus-related joint involvement, both peptides address different aspects of tissue damage and inflammation.
Researchers interested in broader pain management approaches can explore peptides for pain relief and options for specific conditions like fibromyalgia, which shares symptom overlap with lupus. Shoulder-specific protocols and spinal applications may also be relevant for lupus patients with musculoskeletal involvement.
Peptides for neuropsychiatric lupus
Neuropsychiatric lupus affects up to 75% of patients and can manifest as cognitive dysfunction ("lupus fog"), mood disorders, seizures, psychosis, and peripheral neuropathy. Peptides that support neurological function may help address these dimensions of the disease.
Dihexa promotes synaptic connections and has been studied for cognitive enhancement at extremely low doses. Cerebrolysin, a mixture of neurotrophic peptides, has demonstrated neuroprotective effects in various neurological conditions. The BDNF peptide guide covers compounds that support brain-derived neurotrophic factor production, essential for neuroplasticity and cognitive function. And peptides for brain repair offer broader perspective on neurological applications.
The best peptides for brain function guide provides a comprehensive overview of cognitive support options, while Pinealon targets central nervous system regulation specifically. DSIP may help with the sleep disturbances common in lupus, addressing yet another dimension of the disease that affects quality of life. The Semax dosage guide covers another peptide with neuroprotective and nootropic properties that may support cognitive function in lupus patients experiencing neurological symptoms.
Peptides for skin involvement
Cutaneous lupus, including the characteristic butterfly rash and discoid lesions, affects the majority of SLE patients. GHK-Cu promotes skin healing, collagen production, and anti-inflammatory effects that may benefit lupus-related skin involvement. The GHK-Cu dosage guide and injection protocol cover practical application details. More broadly, peptides for skin health offer multiple approaches to supporting skin recovery and reducing inflammatory damage.
Hair loss is another common and distressing symptom of lupus. The guide to peptides for hair loss covers options that may support hair regrowth in autoimmune-related alopecia, including GHK-Cu which has demonstrated effects on hair follicle function.
Considerations for women with lupus
Given that lupus disproportionately affects women, gender-specific peptide considerations matter. The guides on best peptides for women and safe peptide protocols for women address hormonal interactions and female-specific dosing considerations. For women with lupus who are also managing hormonal transitions, resources on peptides for menopause, perimenopause support, and peptides for women over 40 provide relevant context.
Hormonal balance plays a significant role in lupus disease activity. Estrogen can enhance autoimmune responses, which partly explains the female predominance of the disease. The peptides for hormone balance guide explores compounds that may help manage hormonal influences on immune function. Similarly, understanding the relationship between peptides and hormone replacement provides context for lupus patients considering hormonal interventions alongside peptide research.
Related autoimmune conditions
Lupus frequently overlaps with other autoimmune conditions, and peptide research in related diseases provides valuable insights. Research on peptides for multiple sclerosis addresses shared autoimmune mechanisms. Studies on peptides for psoriasis and peptides for eczema inform understanding of immune-mediated skin conditions that may co-occur with lupus. And peptides for allergies explores immune modulation approaches relevant to the hypersensitivity reactions seen in autoimmune conditions.
The broader context of tissue repair peptides and healing applications through injury healing protocols provides additional perspective on how peptides can support recovery from the tissue damage that lupus causes. Similarly, peptides for bone healing may be relevant for lupus patients dealing with steroid-induced osteoporosis, a common consequence of long-term corticosteroid use. The strength protocol guide offers additional context for maintaining physical function alongside autoimmune management.
Understanding peptide quality and sourcing for autoimmune research
When peptides are being used in the context of autoimmune disease research, quality is not just important. It is critical. Impure peptides may contain bacterial endotoxins, residual solvents, or incorrect amino acid sequences, any of which could trigger unpredictable immune responses in someone whose immune system is already dysregulated. A contaminated peptide that might cause minor irritation in a healthy individual could potentially trigger a serious flare in a lupus patient.
Third-party testing provides the strongest assurance of quality. Certificates of analysis should show purity levels above 98%, and independent testing through accredited laboratories adds another layer of verification. The peptide testing labs guide identifies reputable analytical services. Understanding the difference between research and pharmaceutical grade peptides helps researchers make informed decisions about sourcing.
For those tracking results, peptide before and after documentation provides frameworks for systematic outcome tracking. And growth hormone secretagogues like ipamorelin, while not directly targeting autoimmunity, may support recovery and tissue repair processes that benefit lupus patients dealing with chronic inflammation and tissue damage.
The anti-aging peptides category also intersects with lupus management, as the accelerated cellular aging driven by chronic inflammation may be partially addressed through peptides that support telomere maintenance, mitochondrial function, and cellular repair processes. Exploring the full range of peptides versus alternative compounds like SARMs helps researchers understand the relative advantages and limitations of different molecular approaches.
Frequently asked questions
Can peptides cure lupus?
No. Lupus currently has no cure, and no peptide should be presented as curative. However, certain peptides show potential for modulating the immune dysfunction that drives lupus, potentially reducing symptoms and flare frequency. P140/Lupuzor reached Phase III clinical trials specifically for SLE, demonstrating that peptide-based approaches are being seriously investigated as therapeutic options. For comprehensive background, the peptides for autoimmune diseases guide covers the current state of research.
Are peptides safe to use alongside lupus medications?
This depends entirely on the specific peptides and medications involved. Immune-modulating peptides could theoretically interact with immunosuppressants, biologics, and corticosteroids in unpredictable ways. Any peptide research involving concurrent medications should be conducted under the supervision of a healthcare provider familiar with both lupus management and peptide pharmacology. Review the peptide safety guide for foundational information.
Which peptide should a lupus researcher start with?
Thymosin alpha-1 is often considered the most logical starting point due to its well-established safety profile, its direct immune-modulatory mechanism, and the finding that lupus patients tend to have lower endogenous levels. BPC-157 is another conservative starting option, particularly for researchers whose lupus involves GI symptoms or who want to address the gut-immune axis. The beginner guide to peptides covers fundamental protocols.
How long before peptides show effects on autoimmune symptoms?
Immune modulation is a gradual process. Most researchers report that meaningful changes in autoimmune-related symptoms require 4 to 12 weeks of consistent use. Some effects, like BPC-157 GI protection, may become apparent sooner, while deeper immune changes from thymosin alpha-1 or selank may take longer to manifest. Understanding peptide timelines helps set realistic expectations.
Can peptides trigger lupus flares?
This is a legitimate concern. Any compound that modulates immune function could theoretically trigger flare activity in autoimmune conditions. This is why starting with one peptide at a time, using conservative doses, and monitoring closely are essential practices. Immune-stimulating peptides should be approached with particular caution, and any signs of increased disease activity should prompt immediate protocol review.
What is the difference between immunosuppression and immunomodulation?
Immunosuppression reduces overall immune activity broadly, which decreases autoimmune attacks but also weakens defense against infections and cancer. Immunomodulation aims to restore normal immune balance by adjusting specific pathways without broadly weakening the immune system. Peptides like thymosin alpha-1 and KPV are considered immunomodulators because they normalize immune function rather than simply suppressing it. The immune system peptides guide explores this distinction in detail.
Where can I learn more about specific peptide protocols?
SeekPeptides provides comprehensive protocol guides, dosing calculators, and research resources for individual peptides. The dosage chart offers quick reference information, while detailed guides for specific peptides cover mechanisms, protocols, and safety considerations in depth.
Is P140/Lupuzor available for research?
P140/Lupuzor completed Phase IIb trials with positive results and entered Phase III trials for SLE. As of the most recent available information, it is in advanced clinical development but not yet commercially available as a research peptide in the way that thymosin alpha-1 or BPC-157 are. Researchers should monitor peptide regulation news for updates on availability.
External resources
Lupus Foundation of America - Comprehensive patient and research resources for systemic lupus erythematosus
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) - Lupus - NIH research and clinical information
ClinicalTrials.gov - Search for current peptide and lupus clinical trials
PubMed - Access peer-reviewed research on peptides and autoimmune conditions
American College of Rheumatology - Professional guidelines for lupus management and research
For researchers serious about optimizing peptide protocols for autoimmune support, SeekPeptides offers the most comprehensive resource available. Members access evidence-based protocols for immune-modulating peptides, detailed safety guides for autoimmune-specific considerations, dosing calculators tailored to individual needs, and a community of researchers navigating the complex intersection of peptide science and autoimmune disease management.
In case I do not see you, good afternoon, good evening, and good night. May your immune balance stay restored, your inflammation stay controlled, and your protocols stay evidence-based.



