Jan 22, 2026
Before you administer another dose of MOTS-c, you need to understand exactly what might happen next.
This is not fear-mongering. This is practical information that separates informed researchers from those flying blind. MOTS-c, the mitochondrial-derived peptide that has captured attention for its metabolic benefits, comes with a safety profile that remains largely unexplored in human clinical trials. The FDA has not approved it. No major health authority has given it the green light. And the people using it are, in many ways, conducting their own experiments.
That does not mean MOTS-c is dangerous. It means the safety landscape is complex, nuanced, and requires careful navigation. Some users report nothing more than mild injection site reactions. Others experience fatigue, heart palpitations, and gastrointestinal distress that disrupts their daily routines. The difference often comes down to dosing protocols, individual physiology, and the quality of the product being used.
SeekPeptides exists to help researchers navigate exactly these kinds of questions. Understanding MOTS-c side effects is not optional. It is essential for anyone considering this peptide as part of their research protocol. This guide covers every documented side effect, the mechanisms behind them, contraindications that could put you at risk, drug interactions you must consider, and practical strategies for minimizing adverse reactions while maximizing the potential benefits of this fascinating mitochondrial peptide.
Understanding MOTS-c and why side effects occur
MOTS-c is not like other peptides. It is a mitochondrial-derived peptide encoded by mitochondrial DNA, not nuclear DNA like most hormones and signaling molecules your body produces. This fundamental difference shapes everything about how it works, why it produces certain effects, and where side effects originate.
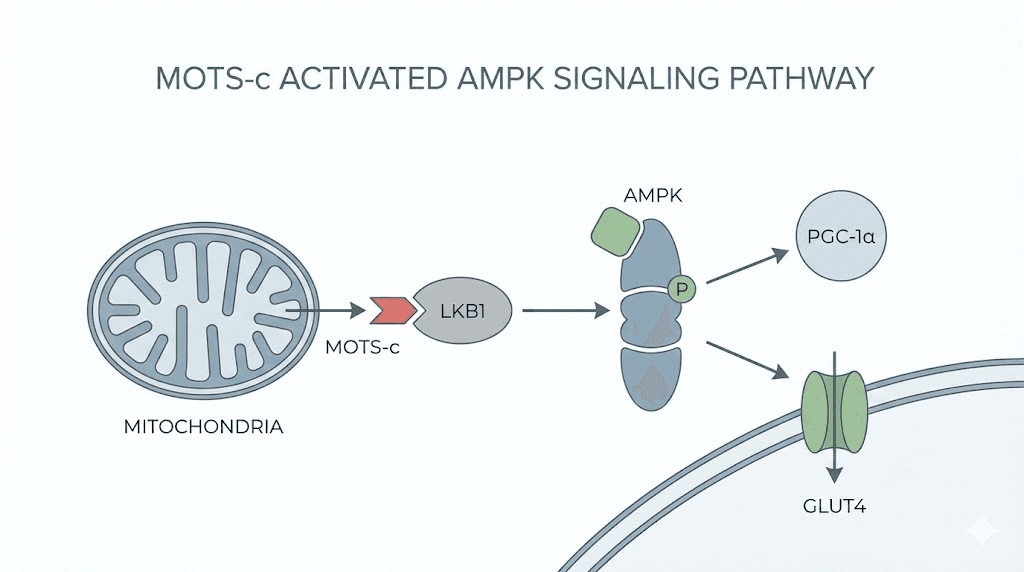
The peptide consists of just 16 amino acids. Small. Compact. But incredibly powerful in its effects on cellular metabolism. When MOTS-c enters your system, it activates AMPK, the master regulator of cellular energy that influences everything from fat burning to muscle preservation to longevity pathways.
AMPK activation is the key to understanding both the benefits and the side effects. This enzyme responds to energy stress. Low cellular energy. High energy demands. Exercise. Fasting. All of these trigger AMPK naturally. MOTS-c essentially mimics and amplifies these signals, telling your cells that energy is scarce and metabolic adaptation is necessary.
The mechanism behind metabolic side effects
When you introduce exogenous MOTS-c, you are essentially overriding your body's natural energy sensing systems. The peptide acts through the folate-AICAR-AMPK pathway, a signaling cascade that influences energy production, glucose metabolism, and cellular stress responses.
Within 30 minutes of administration, MOTS-c translocates to the nucleus. It interacts with stress response transcription factors, including NRF2, which controls antioxidant gene expression. This nuclear translocation explains why some side effects appear quickly while others develop over time as gene expression patterns shift.
The side effects you experience depend largely on how your body interprets these signals. For someone with metabolic dysfunction, MOTS-c might restore balance. For someone with already optimal metabolism, the same dose could create excessive stress responses. Understanding this context matters enormously when evaluating your own risk profile.
Why individual responses vary so dramatically
Two researchers can take identical doses of MOTS-c and experience completely different side effect profiles. One reports nothing. The other experiences fatigue, nausea, and blood sugar fluctuations that persist for days.
Several factors explain this variation. Baseline metabolic health matters enormously. Someone with insulin resistance may respond differently than someone with excellent glucose control. Peptide quality and storage affects potency and purity. Research-grade products can contain impurities that trigger immune responses independent of the MOTS-c itself.
Age plays a role as well. Circulating MOTS-c levels naturally decline with age. Blood levels in young people are approximately 11% higher than middle-aged individuals and 21% higher than older adults. Supplementing when baseline levels are already low might produce different effects than supplementing when levels are normal.
Your concurrent medications, peptide stacks, and supplements also influence outcomes. MOTS-c interacts with anything else that activates AMPK. Metformin. Berberine. Even aspirin. Combining multiple AMPK activators can amplify both benefits and adverse reactions.
Common MOTS-c side effects and how they present
The most frequently reported side effects fall into predictable categories based on the mechanisms we just discussed. Understanding what is common versus rare helps you calibrate expectations and recognize when something requires attention.
Injection site reactions
Nearly every injectable peptide causes some degree of local reaction. MOTS-c is no exception. Users commonly report redness, minor swelling, and irritation at the injection site. These reactions typically resolve within hours to days without intervention.
The clinical trial of CB4211, a MOTS-c analog, was actually paused due to persistent injection site reactions. Participants developed painless bumps that lasted longer than expected. The protocol was modified and the trial resumed, but this highlights that injection site issues can be more than trivial.
Proper reconstitution technique and injection protocols minimize these reactions. Using bacteriostatic water, rotating injection sites, and ensuring sterile technique all reduce the likelihood and severity of local reactions.
Fatigue and energy fluctuations
This side effect confuses many users because MOTS-c is supposed to enhance energy and metabolism. Yet fatigue is among the most commonly reported adverse reactions, particularly in the initial weeks of use.
The explanation lies in cellular adaptation. When you suddenly amplify AMPK signaling, your cells shift metabolic priorities. Fat oxidation increases. Glucose uptake changes. Mitochondrial function adjusts.
This transition period creates temporary energy deficits even as your cells become more efficient long-term.
Some users describe this as feeling like they have the flu without actually being sick. Muscle weakness accompanies the fatigue. Energy dips that were not there before. This typically resolves as adaptation progresses, usually within one to three weeks.
The key distinction is between adaptation fatigue and problematic fatigue. If fatigue persists beyond three weeks, worsens progressively, or accompanies other concerning symptoms, that warrants reassessment of your protocol and possibly consultation with a healthcare provider who understands peptide therapy.
Gastrointestinal disturbances
Nausea, bloating, and digestive upset affect a subset of MOTS-c users. These symptoms usually appear shortly after injection and resolve within hours. They tend to be mild to moderate rather than severe.
MOTS-c affects glucose metabolism throughout the body, including the gut. The peptide also influences inflammatory pathways that can temporarily disrupt digestive function. Staying well-hydrated and taking doses with food often mitigates these effects.
For researchers already using other gut-affecting peptides like BPC-157 or KPV, combining with MOTS-c may either help or exacerbate GI symptoms depending on individual response. Starting MOTS-c alone before adding other compounds helps isolate cause and effect.
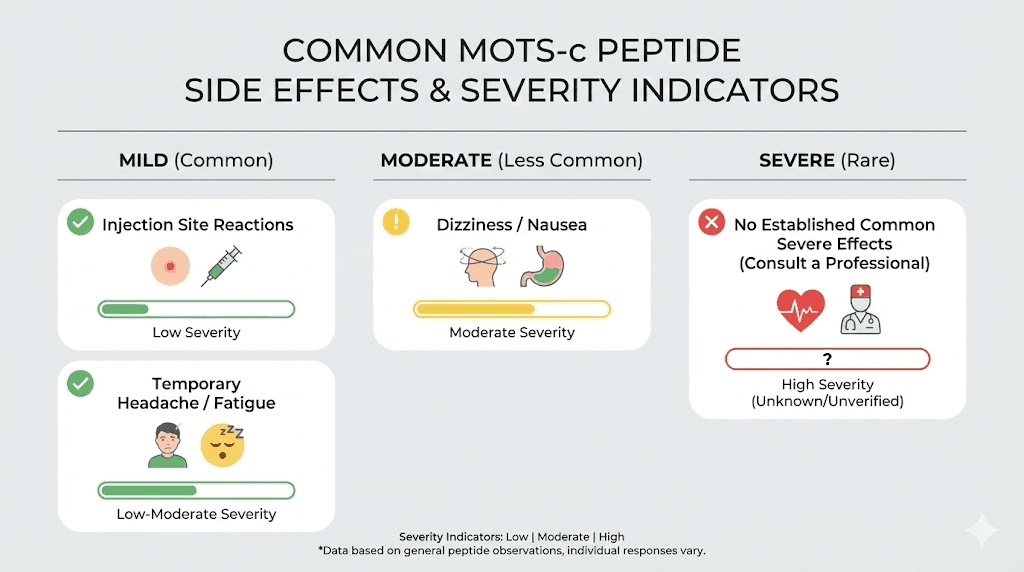
Blood sugar fluctuations
MOTS-c directly influences glucose metabolism. It increases GLUT4 translocation, enhancing glucose uptake into muscle cells. It improves insulin sensitivity.
These metabolic shifts can cause temporary blood sugar fluctuations, especially in the adjustment period.
Symptoms of blood sugar fluctuations include headaches, dizziness, lightheadedness, and shakiness. These are particularly likely if you dose while fasting or have naturally lower blood sugar levels. The peptide essentially accelerates glucose clearance, potentially dropping blood sugar faster than your body expects.
People with diabetes or prediabetes need to be especially careful. While MOTS-c may ultimately improve glucose control, the transition period can create unpredictable swings that require monitoring. Fat loss peptides that also affect metabolism, like semaglutide or retatrutide, combined with MOTS-c could amplify blood sugar effects.
Cardiovascular effects
Heart palpitations and increased heart rate appear in some user reports. The U.S. Anti-Doping Agency specifically lists these among the side effects reported by people who purchase MOTS-c online.
The cardiovascular effects likely relate to metabolic activation. AMPK signaling affects heart tissue directly. The peptide also influences systemic metabolism in ways that can increase metabolic rate and, consequently, heart rate. For most users, these effects are mild and temporary.
Anyone with pre-existing cardiovascular conditions should approach MOTS-c with caution. The lack of long-term safety data means we cannot definitively say whether these cardiovascular effects pose risks for vulnerable populations. Consulting with a cardiologist before starting MOTS-c is prudent if you have any history of heart problems.
Insomnia and sleep disturbances
Some users report difficulty sleeping after starting MOTS-c. This makes metabolic sense. The peptide tells your cells that energy is needed, that metabolic activity should increase, that the body needs to mobilize resources. These are not signals that promote rest.
Timing your doses earlier in the day often resolves sleep issues. Taking MOTS-c in the morning rather than evening allows the metabolic activation to coincide with your natural circadian rhythm rather than fighting against it.
Sleep quality matters for the benefits you are seeking from MOTS-c anyway. Poor sleep undermines metabolic health, muscle recovery, and virtually every outcome peptide researchers pursue. If MOTS-c disrupts your sleep, the net effect might be negative even if other markers improve.
Appetite changes
Decreased appetite and unintentional weight loss represent another category of common side effects. MOTS-c enhances fat oxidation and alters metabolic signaling in ways that can suppress hunger.
For researchers pursuing fat loss, this might seem like a welcome side effect rather than a problem. But excessive appetite suppression can lead to inadequate nutrition, muscle loss, and metabolic slowdown that ultimately works against your goals.
Monitoring caloric intake and ensuring adequate protein consumption for muscle preservation becomes important during MOTS-c protocols. The goal is controlled fat loss, not starvation-induced metabolic damage.
Serious side effects and safety concerns
Beyond the common side effects, MOTS-c carries several serious safety considerations that every researcher must understand before starting a protocol.
The cancer question
This is perhaps the most concerning and least understood aspect of MOTS-c safety. Some sources make contradicting claims about whether MOTS-c could promote prostate and breast cancer development.
The concern stems from MOTS-c's effects on cellular proliferation and metabolism. Cancer cells have altered metabolic profiles. They rely heavily on glucose. They often have dysfunctional mitochondria. Introducing a peptide that modulates these very systems raises theoretical concerns.
Research suggests MOTS-c may actually have anti-cancer properties in some contexts. The peptide influences inflammatory pathways and oxidative stress that contribute to cancer progression. But until more research clarifies the relationship, anyone with active cancer should avoid MOTS-c entirely unless specifically directed otherwise by their oncologist.
If you have a family history of hormone-sensitive cancers, discuss MOTS-c with your healthcare provider before starting. The precautionary principle applies here. When long-term effects are unknown, avoiding potential risks in high-risk populations makes sense.
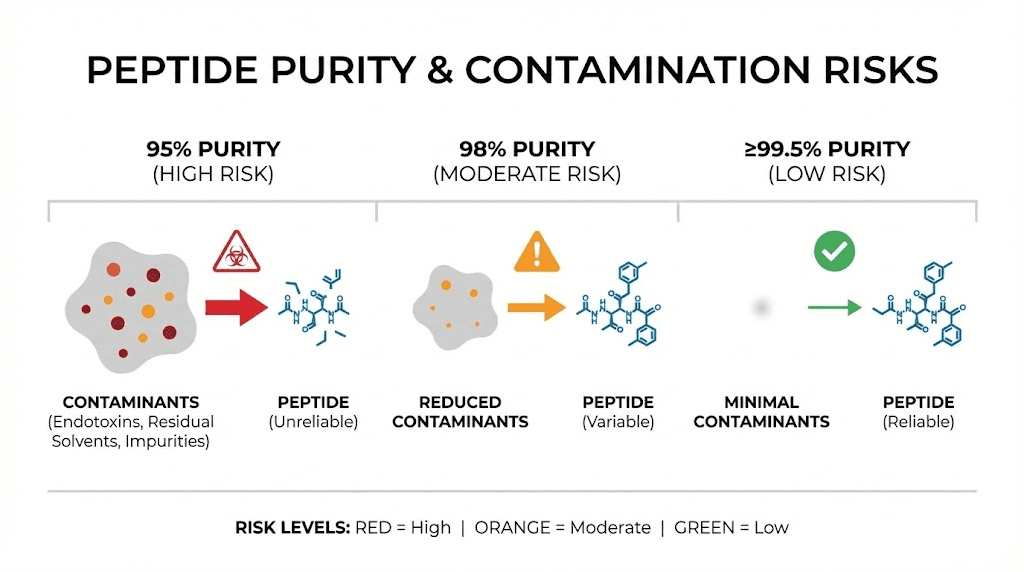
Immunogenicity and purity risks
Research-grade peptides, the type available online without prescription, often lack the quality controls of pharmaceutical products. Studies show some research peptides have purity as low as 60%. That means 40% of what you are injecting is not the peptide you intended.
Impurities pose two problems. First, they can be directly toxic. Unknown compounds entering your body create unpredictable reactions. Second, impurities can trigger immunogenicity, a condition where your immune system mounts a response against the foreign substances.
Immunogenic reactions can range from mild allergic symptoms to life-threatening anaphylaxis. They can also create antibodies that neutralize not just the impurities but the actual peptide, making future doses ineffective.
This is why peptide testing and vendor verification matter so much. The side effects you experience might have nothing to do with MOTS-c itself and everything to do with contaminated product. SeekPeptides provides resources for evaluating research peptide quality and identifying reliable sources.
Long-term effects remain unknown
This is the elephant in the room for all MOTS-c users. We simply do not know what happens with chronic, long-term use. The longest human trials lasted only weeks. The animal studies, while promising, cannot fully predict human responses.
Chronic AMPK activation carries theoretical risks. Excessive catabolism could break down muscle tissue even while promoting fat loss. Interference with natural energy signaling could create dependencies. Cellular dysregulation from continuously elevated MOTS-c might have consequences that take years to manifest.
None of these outcomes are proven. But none have been disproven either. Anyone using MOTS-c is accepting uncertainty about long-term effects as part of their informed consent.
Cycling protocols, where you use MOTS-c for defined periods followed by breaks, may reduce long-term risks by preventing chronic adaptation. Most users follow protocols of four to eight weeks on, followed by similar periods off. Whether this actually mitigates risk is speculative but physiologically plausible.
MOTS-c contraindications and who should not use it
Certain populations face elevated risks from MOTS-c that make avoidance the appropriate choice. Understanding these contraindications could prevent serious harm.
Pregnancy and breastfeeding
MOTS-c should not be used during pregnancy or while breastfeeding. No safety data exists for these populations. The peptide crosses into systemic circulation and could theoretically affect fetal development or pass into breast milk.
The metabolic effects of MOTS-c, particularly on glucose handling and cellular energy, could disrupt the carefully regulated metabolic environment required for healthy pregnancy. This is a clear contraindication with no exceptions.
Active cancer
As discussed, the relationship between MOTS-c and cancer remains unclear. Until research clarifies whether the peptide promotes or inhibits cancer growth, anyone with an active cancer diagnosis should avoid it entirely.
This extends to those in remission from recent cancers. The metabolic changes induced by MOTS-c could theoretically influence recurrence risk.
A five-year remission period might be a reasonable threshold before considering MOTS-c, but this should be an individual decision made with oncologist input.
Severe metabolic disorders
While MOTS-c shows promise for metabolic conditions, severe diabetes, uncontrolled thyroid disorders, or other serious metabolic dysfunction create unpredictable risk profiles. The peptide's effects on glucose and energy metabolism could cause dangerous fluctuations in already unstable systems.
If you have a metabolic disorder, work with a healthcare provider who understands both your condition and peptide therapy. They can help you determine whether the potential benefits outweigh the risks given your specific situation.
Adolescents
Young people should not use MOTS-c. The developing metabolic and endocrine systems of adolescents could be disrupted in unpredictable ways. There is no research on MOTS-c in pediatric populations, and the potential for developmental interference makes this a clear contraindication.
Wait until physical maturation is complete, typically early to mid-twenties, before considering metabolic peptides like MOTS-c. The patience pays off in reduced risk.
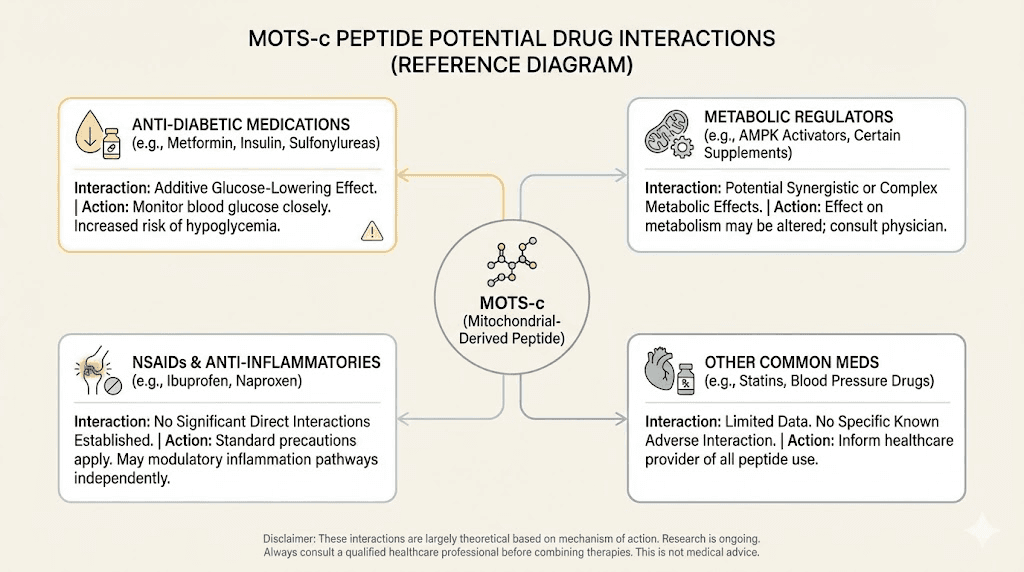
Drug interactions with MOTS-c
MOTS-c interacts with several medication categories through its effects on AMPK and metabolic pathways. Understanding these interactions prevents dangerous combinations.
AMPK-activating medications
The most significant interactions involve other AMPK activators. Metformin, the most commonly prescribed diabetes drug, works primarily through AMPK activation. Combining metformin with MOTS-c creates additive or synergistic AMPK activation that could produce excessive effects.
Thiazolidinediones, another class of diabetes drugs, also activate AMPK. Aspirin at higher doses has AMPK-activating properties. Even some supplements like berberine and resveratrol work through these pathways.
If you take any of these medications, either avoid MOTS-c or work with a healthcare provider to adjust dosing carefully. The combination could cause:
Severe hypoglycemia from excessive glucose clearance
Metabolic imbalances from over-activation of energy sensing pathways
Muscle catabolism from excessive AMPK signaling
Fatigue and weakness from energy depletion
Some protocols intentionally combine metformin and MOTS-c at reduced doses, but this requires careful monitoring and should not be attempted without medical supervision.
Insulin and insulin-sensitizing agents
MOTS-c enhances insulin sensitivity and glucose uptake. Adding it to insulin therapy or other insulin-sensitizing medications creates risk of hypoglycemia, a potentially dangerous drop in blood sugar.
Symptoms of hypoglycemia include shakiness, sweating, confusion, rapid heartbeat, and in severe cases, loss of consciousness. If you use insulin, insulin secretagogues, or GLP-1 agonists, combining with MOTS-c requires extreme caution and glucose monitoring.
Statin medications
Statins affect mitochondrial function. Since MOTS-c is a mitochondrial-derived peptide, theoretical interactions exist. No clinical data confirms problems, but the overlap in cellular targets suggests caution.
If you take statins, monitor for increased muscle pain or weakness when adding MOTS-c.
Both can affect muscle tissue through different mechanisms, and combined effects might amplify issues.
Other peptides and research compounds
Stacking MOTS-c with other peptides requires understanding how their mechanisms interact. Some combinations make sense. Others create redundancy or conflict.
BPC-157 for healing and MOTS-c for metabolism work through different pathways and likely pose minimal interaction risk. TB-500 similarly works through distinct mechanisms.
But stacking MOTS-c with other metabolic peptides like AOD-9604 or growth hormone secretagogues like CJC-1295 and Ipamorelin creates overlapping metabolic effects that need careful consideration. The peptide stacking calculator can help evaluate combinations, but ultimately individual response determines whether a stack is appropriate.
Managing and minimizing MOTS-c side effects
Most side effects are manageable with proper protocols. Here is how experienced researchers minimize adverse reactions while maintaining effective dosing.
Start low and titrate slowly
The single most effective strategy for minimizing side effects is conservative initial dosing. Rather than jumping to common protocol doses, start at 50-75% of typical doses and increase gradually over two to three weeks.
Common starting protocols suggest 5mg twice weekly.
A conservative approach might start at 2.5mg twice weekly for the first week, then 5mg once weekly the second week, before moving to 5mg twice weekly if tolerated.
This gradual approach allows your metabolic systems to adapt. Side effects that would be overwhelming at full dose often become tolerable when the body has time to adjust.
Optimize timing
When you take MOTS-c matters for both efficacy and side effect profile. Morning dosing tends to produce fewer sleep disturbances than evening dosing.
Taking doses on workout days, particularly before exercise, can enhance the synergy between exogenous MOTS-c and exercise-induced AMPK activation.
Some protocols suggest dosing during fasted states to maximize AMPK activation. But this also increases risk of blood sugar fluctuations. If you experience hypoglycemic symptoms, switching to fed-state dosing often helps.
Support metabolic adaptation
The side effects of MOTS-c largely reflect metabolic stress as your body adapts. Supporting this adaptation through nutrition and lifestyle choices reduces both severity and duration of side effects.
Adequate protein intake protects against muscle catabolism during enhanced fat oxidation. Complex carbohydrates provide sustained glucose that smooths out blood sugar fluctuations. Staying hydrated supports all metabolic processes and helps clear any metabolic waste products.
Sleep quality becomes even more important during MOTS-c protocols. The metabolic adaptations happen largely during rest. Poor sleep undermines these adaptations and can amplify fatigue side effects.
Ensure product quality
As discussed, many side effects may relate to impurities rather than MOTS-c itself. Sourcing high-quality peptides from verified vendors dramatically reduces contamination-related adverse events.
Look for certificates of analysis from third-party testing. Verify that purity exceeds 98%. Check for proper cold-chain shipping to maintain peptide stability. These quality controls cost more but provide assurance that what you are injecting is actually what you intend.
Proper storage after purchase maintains quality. Reconstitution with appropriate diluent prevents contamination. Following established protocols ensures consistent dosing.
Monitor and document
Keeping detailed records of your protocol, responses, and any side effects allows you to identify patterns and optimize your approach. What time of day did you dose? What did you eat? How did you feel at 1 hour, 4 hours, 24 hours post-dose?
This data becomes invaluable for troubleshooting. If side effects consistently appear under certain conditions, you can adjust those conditions. If certain doses produce problems while others do not, you can find your optimal range.
SeekPeptides members have access to protocol tracking tools that make this documentation easier and more structured.
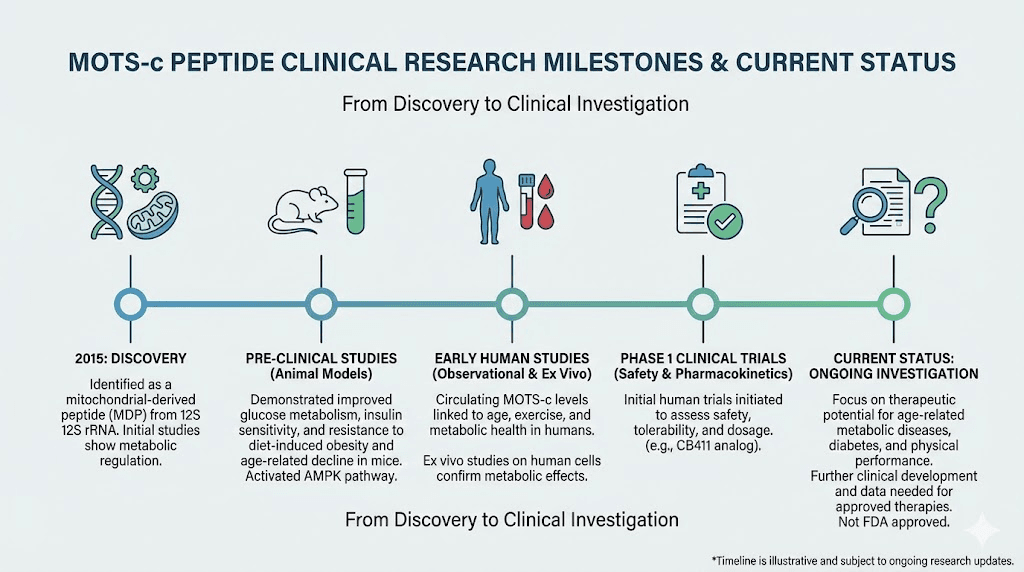
What the clinical research actually shows about MOTS-c safety
Understanding the current state of clinical evidence helps calibrate expectations about what we know versus what remains uncertain about MOTS-c safety.
The CB4211 trial
CB4211, a MOTS-c analog developed by CohBar, represents the most advanced clinical testing of MOTS-c related compounds. The Phase 1a/1b trial tested safety in healthy volunteers and obese individuals with fatty liver disease.
Twenty participants with obesity and at least 10% liver fat received either 25mg CB4211 via daily subcutaneous injection or placebo for 28 days. The trial found CB4211 was generally safe and well-tolerated in short-term use.
However, persistent injection site reactions caused the trial to pause temporarily. Participants developed painless bumps at injection sites that lasted longer than expected. The protocol was modified before resuming.
This trial provides our best human safety data, but it has limitations. The duration was short. The sample size was small. And CB4211 is an analog, not identical to native MOTS-c. We cannot perfectly extrapolate these findings to the MOTS-c peptides available to researchers.
Observational exercise studies
Several studies have examined how endogenous MOTS-c changes with exercise in humans. These provide indirect safety information by showing that natural MOTS-c fluctuations are tolerated normally.
Exercise increases skeletal muscle MOTS-c approximately 11.9-fold. Circulating levels rise about 1.6-fold during exercise and remain elevated for around four hours. These are substantial increases that the body handles without apparent adverse effects.
This suggests that acute elevations in MOTS-c are well-tolerated in healthy individuals. But chronic supplementation might differ from the transient increases produced by exercise. The body's systems might adapt differently to sustained versus intermittent exposure.
Animal study safety signals
Animal studies provide longer-term data, though with obvious limitations in translating to humans. Mice treated with MOTS-c at various doses for periods up to several weeks showed no obvious toxicity. Some studies showed life extension and improved healthspan markers.
Importantly, because MOTS-c is an endogenous peptide that the body naturally produces, it is theoretically less likely to trigger immune responses or off-target effects compared to completely foreign molecules. The body has existing systems for processing and responding to MOTS-c.
However, supplementing to levels above natural production creates uncertainty. The safety of physiological MOTS-c does not guarantee the safety of supraphysiological doses.
Regulatory status and legal considerations
Understanding the regulatory landscape around MOTS-c clarifies both legal concerns and what the regulatory status implies about safety evaluation.
FDA position
The FDA has not approved MOTS-c for any human use. More significantly, the FDA has clarified that MOTS-c is among the peptides that compounding pharmacies cannot legally include in compounded medications.
This means you cannot obtain MOTS-c through legitimate pharmaceutical channels in the United States. Any MOTS-c you acquire comes from research chemical suppliers operating in regulatory gray areas. The FDA explicitly states that compounded drugs containing MOTS-c may pose significant immunogenicity risks for certain routes of administration.
This regulatory stance does not mean MOTS-c is necessarily dangerous. It means MOTS-c has not undergone the rigorous clinical testing required for approval. The FDA is being cautious about allowing distribution of a compound without comprehensive safety data.
International status
Regulatory status varies by country, but no major health authority has approved MOTS-c for human therapeutic use. Some countries have less restrictive regulations around research peptides, but this reflects regulatory approach rather than safety evaluation.
MOTS-c appears on the World Anti-Doping Agency (WADA) prohibited list under Section 4.4 as an AMPK activator.
This means competitive athletes cannot use it regardless of regulatory status for general use. WADA prohibitions often precede and influence broader regulatory actions.
What this means for researchers
Legal access to MOTS-c typically requires purchasing as a research chemical with stated purposes of in vitro research rather than human administration. This creates a gap between how products are sold and how they are actually used.
This situation puts responsibility squarely on individual users. Without regulatory oversight, you must conduct your own due diligence on product quality, appropriate dosing, and risk management. SeekPeptides exists partly to fill this educational gap, providing the information and resources needed for informed decision-making.
Comparing MOTS-c side effects to other metabolic peptides
Context helps evaluate whether MOTS-c's side effect profile is concerning relative to alternatives. How does it compare to other peptides targeting similar outcomes?
Versus GLP-1 agonists
GLP-1 agonists like semaglutide and tirzepatide have become popular for metabolic optimization and weight loss. Their side effect profiles are well-documented from extensive clinical trials.
GLP-1 agonists commonly cause nausea, vomiting, diarrhea, and constipation, particularly when starting or increasing doses. These GI effects are often more pronounced than what MOTS-c users typically report. However, GLP-1 agonists have the advantage of extensive safety data and FDA approval.
MOTS-c may have advantages for those who cannot tolerate GLP-1 GI effects. But the uncertainty around MOTS-c's long-term safety makes direct comparison difficult. We know more about what GLP-1 agonists do long-term, even if short-term tolerability favors MOTS-c for some users.
Versus growth hormone peptides
Growth hormone secretagogues like CJC-1295 and Ipamorelin offer metabolic benefits through different mechanisms. Their side effects include water retention, joint pain, and potential effects on blood sugar through GH's insulin-antagonizing effects.
MOTS-c works through AMPK rather than growth hormone, creating a different side effect profile. There is less concern about joint effects or growth hormone-related risks. But there is also less long-term human data on MOTS-c specifically.
For pure metabolic enhancement without growth hormone involvement, MOTS-c offers a different option. For those seeking the muscle-building and recovery benefits of elevated GH, the growth hormone peptides make more sense despite their different risk profile.
Versus AOD-9604
AOD-9604 targets fat metabolism without the broader metabolic effects of full growth hormone. Its side effect profile is generally mild, with injection site reactions and occasional headaches being most common.
MOTS-c has a broader mechanism affecting overall cellular energy rather than just fat metabolism. This means MOTS-c might offer more comprehensive metabolic benefits but also creates more potential for side effects across various systems.
For targeted fat loss with minimal metabolic disruption, AOD-9604 might have advantages. For broader metabolic optimization where the additional effects are desired, MOTS-c could be more appropriate.
Protocol adjustments for side effect management
When side effects occur, systematic adjustment of your protocol often resolves issues while maintaining benefits. Here are specific modifications for common problems.
For fatigue
If fatigue is your primary issue, consider these adjustments:
Reduce dose by 25-50% until adaptation occurs
Increase carbohydrate intake to support energy needs
Ensure adequate sleep, ideally 7-9 hours nightly
Time doses to morning rather than evening
Add a rest week every 3-4 weeks of use
Consider supporting mitochondrial function with CoQ10 or NAD+ precursors
Fatigue typically resolves within 2-3 weeks if your protocol is otherwise appropriate. Persistent fatigue beyond this suggests either dosing too high for your current metabolic capacity or an underlying issue requiring assessment.
For blood sugar issues
Blood sugar fluctuations respond well to nutritional timing:
Avoid dosing while fasted
Take MOTS-c with a meal containing protein and complex carbs
Keep quick-acting glucose available (fruit, juice) for hypoglycemic symptoms
Consider breaking doses into smaller, more frequent administrations
Monitor glucose if you have any history of blood sugar issues
If you are on diabetes medications, work with your healthcare provider to adjust medications as MOTS-c's effects on glucose metabolism develop.
For GI disturbances
Gastrointestinal issues usually improve with these modifications:
Always dose with food rather than on empty stomach
Stay well-hydrated throughout the day
Consider adding digestive enzymes if issues persist
Ginger or peppermint tea can settle nausea
Reduce dose if GI symptoms are severe
For injection site reactions
Minimize local reactions through proper technique:
Rotate injection sites systematically
Ensure peptide is fully reconstituted with no particles
Use proper needle gauge (typically 29-31 gauge for subcutaneous)
Allow alcohol to fully dry before injecting
Ice the area briefly before and after injection if reactions are bothersome
Verify peptide purity through third-party testing
For sleep disturbances
Timing adjustments usually resolve sleep issues:
Move all doses to morning, no later than early afternoon
Avoid taking on days where sleep is particularly important
Support sleep hygiene with consistent schedule and dark environment
Consider magnesium glycinate in the evening for relaxation
If insomnia persists despite timing changes, reduce overall dose
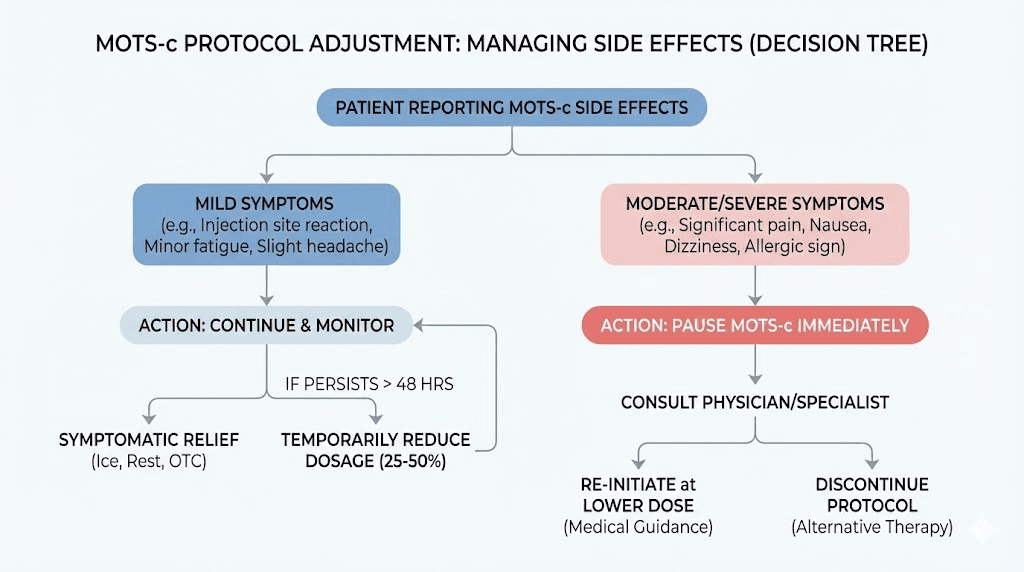
When to stop using MOTS-c
Not all side effects warrant discontinuation, but certain situations require stopping immediately and reassessing whether MOTS-c is appropriate for you.
Stop immediately if you experience
The following warrant immediate discontinuation and medical consultation:
Severe allergic reactions (difficulty breathing, facial swelling, hives)
Severe hypoglycemia that does not respond to glucose intake
Significant cardiovascular symptoms (chest pain, severe palpitations, fainting)
Signs of infection at injection sites (spreading redness, fever, pus)
Severe persistent headaches, especially with vision changes
Any symptoms that feel seriously wrong, even if not listed here
Trust your instincts. If something feels seriously off, stopping the peptide and seeking evaluation is the appropriate response. You can always restart later if the issue resolves and is deemed unrelated.
Consider stopping if
These situations warrant reassessment though not necessarily immediate discontinuation:
Side effects persist beyond 3-4 weeks without improvement
Benefits do not justify the side effects you are experiencing
You cannot maintain consistent dosing due to side effects
Your healthcare provider advises discontinuation
You develop new health conditions that might interact
Temporary breaks versus permanent discontinuation
Sometimes a break is all that is needed.
If you have been using MOTS-c for extended periods and side effects are accumulating, taking 4-8 weeks off allows your systems to reset. Many users find that side effects are less pronounced when they restart after a break.
Permanent discontinuation is appropriate if you consistently cannot tolerate MOTS-c despite protocol adjustments, if your health situation changes to create new contraindications, or if the risk-benefit calculation simply does not favor continued use for your goals.
Questions researchers commonly ask about MOTS-c side effects
How long do MOTS-c side effects typically last?
Most initial side effects resolve within one to three weeks as your body adapts to the metabolic changes MOTS-c induces. Fatigue, GI issues, and energy fluctuations commonly improve by week two or three. Injection site reactions typically resolve within days of each dose. If side effects persist beyond four weeks, reassess your dosing protocol and consider whether MOTS-c is appropriate for your situation.
Can MOTS-c cause permanent damage?
No evidence exists of permanent damage from MOTS-c use at typical research doses.
However, the lack of long-term clinical trials means we cannot definitively rule out cumulative effects. Using cycling protocols rather than continuous long-term use may reduce any theoretical risks of chronic exposure. Severe adverse events would theoretically be reversible upon discontinuation in most cases.
Is MOTS-c safe to use with other peptides?
Many researchers successfully stack MOTS-c with other peptides, but combinations should be approached thoughtfully. Peptides working through different mechanisms like BPC-157 and TB-500 for healing typically combine well. Other metabolic peptides require more caution due to overlapping effects. Start peptides individually before combining, and use the stacking calculator to evaluate proposed combinations.
Does the source of MOTS-c affect side effects?
Product quality significantly impacts side effect profiles. Research-grade peptides with purity below 98% contain impurities that can cause adverse reactions independent of the actual MOTS-c content. Third-party tested products from verified vendors dramatically reduce contamination-related side effects. Always verify certificates of analysis before purchasing.
Should I worry about MOTS-c affecting my hormones?
MOTS-c primarily works through metabolic pathways rather than direct hormonal manipulation. Unlike testosterone-affecting peptides or growth hormone secretagogues, MOTS-c does not directly alter hormonal production. However, improved metabolism can indirectly influence hormonal balance. Blood sugar improvements might affect cortisol. Better body composition could influence estrogen in men. These are generally positive effects rather than side effects.
Can I take MOTS-c if I have diabetes?
MOTS-c shows promise for diabetes through improved glucose metabolism and insulin sensitivity. However, combining with diabetes medications creates hypoglycemia risk. If you have diabetes, work with a healthcare provider who understands both your condition and peptide therapy. You may need medication adjustments as MOTS-c's metabolic effects develop. Never start MOTS-c without medical supervision if you use insulin or insulin secretagogues.
What is the safest way to start MOTS-c?
Start with lower doses than commonly recommended, typically 2.5-3mg rather than 5mg per injection. Begin with once weekly dosing before increasing to twice weekly. Give your body two to three weeks to adapt before increasing doses. Ensure you have high-purity product from a verified source. Document everything so you can identify patterns if side effects occur. Having a healthcare provider familiar with your health history adds safety margin.
Are MOTS-c side effects dose-dependent?
Generally yes. Higher doses produce more pronounced side effects in most users. This is why starting low and titrating up works so well for minimizing adverse reactions. However, some individuals are simply more sensitive regardless of dose. If you experience significant side effects even at minimal doses, MOTS-c may not be appropriate for you despite theoretical benefits.
For researchers serious about understanding peptide safety and optimizing their protocols, SeekPeptides provides comprehensive resources including detailed MOTS-c guides, dosing protocols, and a community of experienced researchers who have navigated these exact questions.
In case I do not see you, good afternoon, good evening, and good night. Join us.



