Jan 29, 2026
Your lungs are aging faster than you realize. Every breath you take, every exposure to pollution, every respiratory infection leaves its mark on delicate bronchial tissue. And unlike a scraped knee or a pulled muscle, lung damage accumulates silently. By the time symptoms appear, years of decline have already occurred.
This is the problem that Russian researchers began tackling decades ago. They asked a simple question: what if you could give aging lung cells the signal to repair themselves? What if you could reverse the clock on respiratory decline before it became irreversible?
The answer they developed was bronchogen. A four-amino-acid peptide so small it seems almost impossible that it could do anything meaningful. Yet research spanning three decades suggests this tiny molecule may hold remarkable potential for respiratory health, from chronic obstructive pulmonary disease to age-related lung decline.
This guide examines everything researchers need to know about bronchogen. How it works at the cellular level. What the studies actually show. How it compares to other respiratory interventions. And what the future might hold for this fascinating bioregulator peptide.
SeekPeptides provides the most comprehensive resources available for understanding peptide bioregulators like bronchogen, with detailed protocols and research summaries that go far beyond surface-level information.
What is bronchogen and how does it work
Bronchogen belongs to a class of compounds called peptide bioregulators. These are short chains of amino acids, typically just two to four units long, that appear to regulate gene expression in specific tissues. Think of them as molecular keys that fit only certain locks. Bronchogen fits the locks in bronchial and lung tissue.
The specific sequence is Ala-Glu-Asp-Leu. Four amino acids. Alanine, glutamic acid, aspartic acid, leucine. That is it. This simplicity is both its strength and the source of skepticism from researchers unfamiliar with bioregulator science. How could something so small have meaningful biological effects?
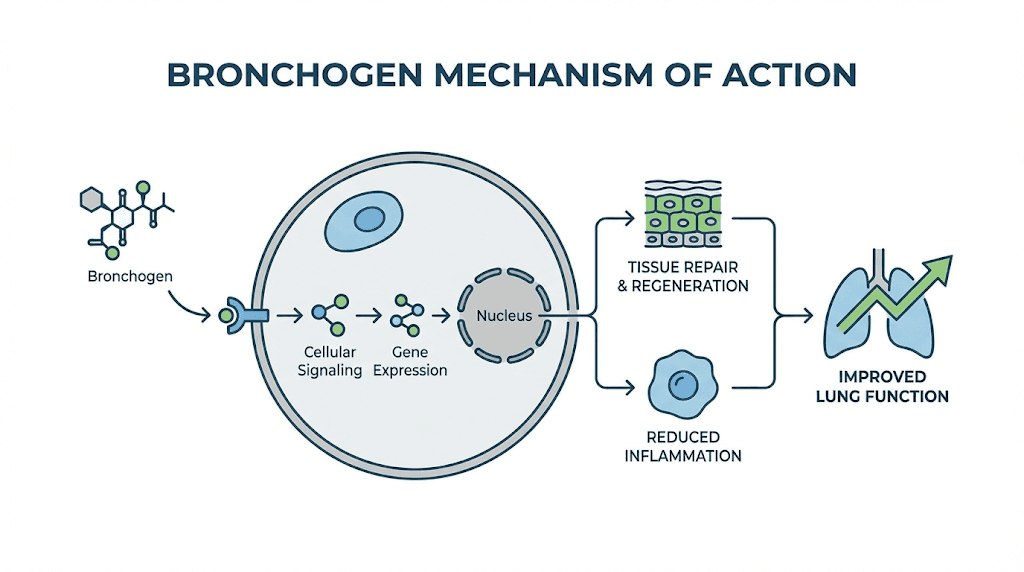
The answer lies in gene regulation. Unlike pharmaceutical drugs that block or activate specific receptors, bioregulator peptides appear to work by influencing which genes get expressed and which remain silent. They do not force the body to do something unnatural. They encourage it to do what it should be doing anyway.
The science behind peptide bioregulation
Vladimir Khavinson, the researcher most associated with bioregulator peptide development, has been studying these compounds since the 1970s. His work, conducted primarily at the Saint Petersburg Institute of Bioregulation and Gerontology, has produced over 775 scientific publications and 196 patents.
The central theory is elegant. As we age, gene expression patterns change. Genes that should be active become silent. Genes that should be silent become overactive. This dysregulation accelerates aging and disease. Bioregulator peptides, according to Khavinson research, can help restore youthful gene expression patterns.
For bronchogen specifically, this means targeting genes involved in bronchial epithelial cell function, mucus production, ciliary activity, and local immune response. The peptide appears to have specific affinity for the NKX2-1, SCGB1A1, SCGB3A2, FOXA1, and FOXA2 genes, all of which play crucial roles in maintaining healthy respiratory tissue.
In microcalorimetry studies, bronchogen has been shown to increase DNA thermostability in bronchial cells. This suggests the peptide actually interacts with genetic material directly, stabilizing the double helix structure and potentially protecting against DNA damage.
Research findings on bronchogen and lung health
The most compelling research on bronchogen comes from studies on chronic obstructive pulmonary disease models. COPD affects hundreds of millions of people worldwide and represents one of the leading causes of death globally. Current treatments manage symptoms but do nothing to reverse the underlying tissue damage.
In a key study published in the Bulletin of Experimental Biology and Medicine, researchers induced COPD in rats through 60 days of intermittent nitrogen dioxide exposure. This mimics the chronic irritation that causes COPD in humans, whether from smoking, pollution, or occupational hazards.
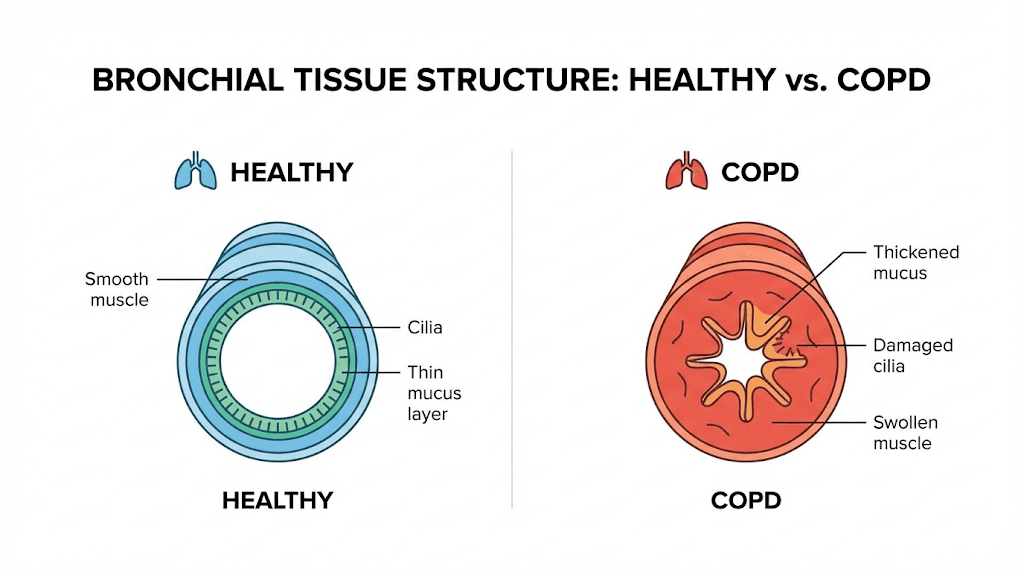
The damage was significant. Goblet cell hyperplasia. Squamous metaplasia. Lymphocytic infiltration. Emphysema. The bronchial epithelium was essentially being destroyed and replaced with dysfunctional tissue. This is exactly what happens in human COPD patients over years of exposure.
Then came the intervention. Rats received bronchogen for one month. The results were remarkable. The symptoms of bronchial remodeling that define COPD began to reverse. Ciliated cells, the tiny hair-like structures that sweep mucus and debris out of the airways, were restored. Goblet cell hyperplasia normalized. The tissue was healing.
Immune function improvements
Beyond structural repair, the studies showed functional improvements in local immunity. Secretory IgA, an antibody that serves as the first line of defense in respiratory mucosa, increased significantly in treated animals. This is not a minor finding. Secretory IgA deficiency is associated with recurrent respiratory infections and poor outcomes in chronic lung disease.
The profile of inflammatory markers also normalized. Pro-inflammatory cytokines that were elevated during the disease state returned to baseline levels. Neutrophilic inflammation, a hallmark of COPD that drives much of the tissue destruction, decreased substantially.
These findings suggest bronchogen does not simply mask symptoms. It appears to address underlying pathological processes. The anti-inflammatory effects combined with tissue regeneration represent a fundamentally different approach than current COPD medications.
Comparison to standard treatments
Standard COPD treatment involves bronchodilators to open airways, inhaled corticosteroids to reduce inflammation, and oxygen therapy in advanced cases. These treatments help patients breathe more easily but do not reverse disease progression. Lung function continues to decline. Tissue continues to deteriorate.
The bronchogen research suggests a different paradigm. Rather than managing symptoms while the disease progresses, the peptide appears to promote actual tissue restoration. The distinction matters enormously for long-term outcomes. Managing decline is not the same as reversing it.
Of course, significant caveats apply. All bronchogen COPD research to date has been conducted in animal models. We do not have human clinical trials establishing equivalent effects in people. The jump from rat lungs to human lungs is substantial, and many promising animal findings fail to translate to clinical practice.
How bronchogen supports respiratory tissue repair
Understanding how bronchogen promotes tissue repair requires examining several interconnected mechanisms. The peptide does not work through a single pathway. Instead, it appears to orchestrate multiple cellular processes that together result in improved bronchial health.
First, bronchogen influences fibroblast activity. Fibroblasts are the cells responsible for producing collagen and other structural proteins in connective tissue. In damaged lungs, fibroblast activity often becomes dysregulated, either producing too little matrix for proper repair or too much matrix leading to fibrosis.
Research suggests bronchogen helps normalize this balance. In models of bleomycin-induced pulmonary fibrosis, the peptide reduced fibrotic changes while supporting healthy tissue structure. This dual action, promoting repair while preventing pathological scarring, is unusual and valuable.
Second, bronchogen supports epithelial cell differentiation. The bronchial epithelium contains multiple cell types, each serving specific functions. Ciliated cells move mucus. Goblet cells produce mucus. Club cells help detoxify inhaled substances. When damage occurs, the balance of these cell types often shifts inappropriately.
In COPD, you see goblet cell hyperplasia, meaning too many mucus-producing cells, combined with loss of ciliated cells. This creates the characteristic COPD problem of excessive mucus that cannot be cleared effectively. Bronchogen appears to help restore the proper cellular balance, reducing goblet cell overgrowth while promoting ciliated cell regeneration.
Surfactant production and alveolar function
Beyond the bronchial airways, bronchogen may also support alveolar function. The alveoli are the tiny air sacs where gas exchange actually occurs. They are lined with a substance called surfactant that reduces surface tension and prevents the alveoli from collapsing.
Research has shown bronchogen treatment increases levels of surfactant protein B in animal models. This protein is essential for proper surfactant function. Low surfactant protein B is associated with respiratory distress and poor lung compliance. By supporting surfactant production, bronchogen may help maintain the mechanical properties of lung tissue.
The combined effects on bronchial epithelium and alveolar surfactant suggest bronchogen acts throughout the respiratory system, not just in the larger airways. This comprehensive activity makes sense given the peptide's proposed mechanism of gene regulation rather than receptor-specific action.
Angiogenesis and tissue perfusion
Healthy lung tissue requires adequate blood supply. Chronic lung disease often involves vascular damage and reduced perfusion, which limits the delivery of nutrients and oxygen to healing tissue while impairing the removal of metabolic waste and inflammatory mediators.
While bronchogen research focuses primarily on epithelial and immune effects, the peptide likely influences vascular components as well. Gene regulation in one tissue type inevitably affects neighboring tissues through paracrine signaling. Improved epithelial health creates a better environment for vascular maintenance and repair.
For researchers interested in tissue repair mechanisms, bronchogen offers a model of how small regulatory peptides can produce broad systemic effects through targeted gene expression modulation.
Bronchogen versus other respiratory peptides
Bronchogen is not the only peptide studied for respiratory applications. Understanding how it compares to other options helps researchers make informed decisions about their protocols.
Thymalin is another Khavinson bioregulator that affects respiratory health, though indirectly. Thymalin targets thymus function and immune regulation rather than bronchial tissue specifically. For researchers focused on the immune component of respiratory disease, thymalin may complement bronchogen's direct tissue effects.
KPV peptide offers potent anti-inflammatory effects that could benefit respiratory conditions. However, KPV works through different mechanisms than bronchogen and targets different inflammatory pathways. The two peptides address overlapping but distinct aspects of respiratory inflammation.
BPC-157, widely known for its healing properties, has some research suggesting respiratory applications. However, BPC-157 is a larger peptide with different tissue specificity. Its primary effects are on gastrointestinal and musculoskeletal tissue rather than bronchial epithelium specifically.
Bioregulator peptide comparisons
Within the bioregulator category, several peptides target related systems. Crystagen affects immune function. Cartalax targets cartilage and connective tissue. Each bioregulator has specific tissue affinity based on its amino acid sequence.
Bronchogen stands alone in its direct targeting of bronchial and lung tissue. No other peptide in the bioregulator family specifically addresses respiratory epithelium the way bronchogen does. This makes it unique for researchers focused on lung health.
The comparison also extends to delivery methods. Many bioregulators were originally studied in oral capsule form. Bronchogen research has examined both injectable and oral administration. The small size of bioregulator peptides may allow for some oral absorption, though injectable delivery typically provides more consistent bioavailability.
Peptide | Primary Target | Mechanism | Respiratory Relevance |
|---|---|---|---|
Bronchogen | Bronchial epithelium | Gene regulation | Direct |
Thymalin | Thymus/immune system | Immune modulation | Indirect |
KPV | Multiple tissues | Anti-inflammatory | Indirect |
BPC-157 | GI/musculoskeletal | Growth factor modulation | Minimal |
Crystagen | Immune cells | Gene regulation | Indirect |
Understanding bronchogen protocols in research settings
Research protocols using bronchogen vary based on the model being studied and the specific outcomes being measured. The animal research provides some guidance on timing and duration, though translation to human applications remains theoretical.
In the COPD studies, bronchogen administration continued for one month following induction of lung damage. This duration appeared sufficient to produce measurable tissue restoration and functional improvement. Shorter durations might produce less pronounced effects, while longer durations have not been systematically studied.
The research literature does not establish precise dose-response relationships in humans because human clinical trials have not been conducted. Animal studies use doses scaled to body weight, but simple weight-based scaling from rodents to humans often proves unreliable due to differences in metabolism and tissue distribution.
Researchers investigating bronchogen typically use it as part of broader protocols that may include other longevity peptides and supportive interventions. The bioregulator approach emphasizes comprehensive support rather than single-compound solutions.
Combining bronchogen with other interventions
The bioregulator philosophy developed by Khavinson emphasizes using multiple tissue-specific peptides together. Just as the body has multiple organ systems that all age simultaneously, the argument goes that addressing only one system leaves other weaknesses unaddressed.
For respiratory focus, combining bronchogen with immune-supporting bioregulators like thymalin makes theoretical sense. The immune system plays a crucial role in respiratory health, both in defending against infection and in modulating inflammatory responses that can damage tissue.
Lifestyle factors also matter enormously. No peptide will overcome continued exposure to respiratory irritants. Smoking cessation, air quality improvement, and infection prevention remain foundational for any respiratory health protocol. Bronchogen is not a substitute for these interventions but potentially an adjunct to them.
Researchers should also consider the broader context of anti-aging protocols. Respiratory decline is one aspect of aging, but it occurs alongside cardiovascular changes, metabolic shifts, neurological aging, and musculoskeletal deterioration. Comprehensive approaches address multiple systems simultaneously.
Safety profile and potential concerns
One of the most consistent findings across bioregulator research is the favorable safety profile of these small peptides. Bronchogen is no exception. Animal studies have not revealed significant adverse effects at research doses.
This makes sense from a mechanistic perspective. Bioregulators work by modulating gene expression toward healthier patterns rather than by forcing unnatural biochemical states. They do not block receptors. They do not inhibit enzymes. They do not introduce foreign molecules that the body must detoxify.
However, the absence of observed toxicity in limited studies does not guarantee safety in all contexts. Several considerations apply to bronchogen research.
First, the research base is relatively narrow. Most studies come from a single research group in Russia. Independent replication by other laboratories in other countries would strengthen confidence in both efficacy and safety findings.
Second, long-term effects remain unknown. The animal studies typically last weeks to a few months. We do not know what happens with years of use. Chronic administration of any compound, even a naturally derived peptide, could produce unexpected effects over extended timeframes.
Third, individual variation matters. Genetic differences, existing health conditions, and concurrent medications could all influence how a person responds to bronchogen. What produces no adverse effects in one individual might cause problems in another.
Contraindications and cautions
No formal contraindications have been established for bronchogen because it remains a research compound rather than an approved medication. However, common sense suggests certain populations should exercise particular caution.
Anyone with active cancer should approach bioregulators carefully. While some research suggests geroprotective effects that might actually oppose cancer development, compounds that influence gene expression and cell proliferation warrant careful consideration in oncology contexts.
Pregnant and nursing women have not been studied. The general principle of avoiding unnecessary compounds during pregnancy and lactation applies here as with most research peptides.
People with autoimmune conditions affecting the lungs should consider whether immunomodulatory effects could potentially exacerbate their condition. The immune-enhancing effects of bronchogen might be problematic if autoimmune inflammation is already an issue.
Medical supervision is strongly recommended for anyone considering bronchogen, particularly those with existing respiratory conditions or those taking medications for lung disease. Peptide therapy works best when integrated with comprehensive medical care rather than pursued in isolation.
Bronchogen and age-related respiratory decline
Aging affects the respiratory system in multiple ways. Lung capacity decreases. Elastic recoil diminishes. The diaphragm weakens. Ciliary function declines. Immune surveillance in the respiratory tract becomes less effective. These changes combine to make older adults more vulnerable to respiratory infections and less able to recover from respiratory illness.
The concept of geroprotection, protecting against aging-related decline, is central to bioregulator research. Bronchogen specifically may help address several aspects of respiratory aging.
Ciliary dysfunction is a major problem in aging lungs. The cilia that line the airways become shorter, beat more slowly, and coordinate less effectively. This impairs mucociliary clearance, the mechanism by which the respiratory tract removes inhaled particles and pathogens. Bronchogen research showing restoration of ciliated cells in damaged tissue suggests potential relevance for age-related ciliary decline.
Secretory IgA production also decreases with age. This immunological aging leaves the respiratory tract more vulnerable to infection. The research showing bronchogen increases secretory IgA production addresses this specific vulnerability directly.
Lung tissue elasticity depends on the extracellular matrix produced by fibroblasts. Age-related changes in matrix composition contribute to decreased lung compliance and impaired breathing mechanics. Bronchogen's effects on fibroblast activity and matrix organization could theoretically support maintenance of tissue elasticity.
The geroprotective hypothesis
Khavinson's broader theory proposes that peptide bioregulators can extend healthy lifespan by maintaining organ function that would otherwise decline with age. For bronchogen specifically, this means potentially preserving respiratory function beyond what would occur naturally.
If the research findings in animal models translate to humans, bronchogen could help maintain respiratory capacity, immune function, and tissue integrity throughout the lifespan. This is not about treating disease. It is about preventing the decline that makes disease more likely and more severe.
The distinction matters for how researchers approach bronchogen. Rather than waiting for respiratory problems to develop and then attempting treatment, the geroprotective approach suggests earlier intervention to maintain healthy function. Prevention is easier than reversal.
This paradigm aligns with growing interest in longevity science and proactive health maintenance. Rather than accepting age-related decline as inevitable, researchers increasingly ask whether interventions can slow, stop, or reverse specific aspects of aging. Bronchogen represents one possible tool in this broader effort.
Practical considerations for researchers
Researchers interested in bronchogen face several practical considerations regarding sourcing, storage, and experimental design.
Quality matters enormously with peptide research. Impure preparations, degraded peptides, or incorrect sequences will produce unreliable results. Researchers should verify peptide identity through mass spectrometry and assess purity through HPLC analysis. Third-party testing provides additional confidence in compound quality.
Storage conditions affect peptide stability. Bronchogen, like most peptides, should be stored frozen in lyophilized form until use. Once reconstituted, refrigeration and protection from light help maintain potency. Repeated freeze-thaw cycles should be avoided as they can degrade the peptide.
The reconstitution process requires appropriate solvent selection. Bacteriostatic water is commonly used for peptide preparation. The concentration should be calculated based on the planned dose and injection volume to ensure accurate administration.
Documentation and record-keeping
Rigorous documentation supports meaningful research. Recording lot numbers, storage conditions, preparation dates, and administration details enables troubleshooting if results are unexpected and ensures reproducibility.
Baseline measurements establish the starting point against which changes can be assessed. For respiratory research, this might include pulmonary function testing, inflammatory marker levels, symptom scores, or quality of life assessments depending on the research context.
Timing of assessments matters as well. Effects may not be immediate. The animal research showed structural improvements after one month of treatment. Researchers should plan assessment schedules that allow sufficient time for potential effects to manifest.
Comparison groups or baseline comparison periods strengthen research design. Without a control condition, it is impossible to distinguish treatment effects from natural variation, placebo effects, or spontaneous improvement. Even in N-of-1 designs, systematic variation of treatment periods can help isolate treatment-specific effects.
The future of bronchogen research
Bronchogen research stands at an interesting juncture. Decades of animal studies have produced intriguing findings. The mechanisms are plausible. The safety profile appears favorable. Yet human clinical data remains essentially nonexistent.
This gap reflects broader challenges facing peptide bioregulator research. The compounds were developed primarily in Russia and have not received the same attention from Western pharmaceutical companies and regulatory bodies that would drive large-scale clinical trials. The path from promising animal research to approved human therapy is long, expensive, and uncertain.
However, several factors could accelerate bronchogen research in coming years.
First, the growing global burden of respiratory disease creates urgent need for new approaches. COPD alone affects over 300 million people worldwide and causes over 3 million deaths annually. Existing treatments are inadequate. The market for effective respiratory therapies is enormous.
Second, increased interest in longevity science brings attention to geroprotective compounds. As lifespan extension moves from science fiction to serious research priority, compounds that address specific aspects of aging become more attractive for investigation.
Third, advances in personalized medicine and biomarker development enable more sophisticated assessment of treatment effects. We can now measure respiratory function, inflammatory status, and tissue health with greater precision than ever before. These tools could accelerate bronchogen research by enabling detection of effects that might have been missed with cruder measurements.
Questions requiring further research
Many important questions about bronchogen remain unanswered.
Do the findings in rodent models translate to humans? This is the fundamental question for any preclinical research. Rodent lungs share basic features with human lungs but also differ in important ways. Effects seen in rats may or may not occur in people.
What is the optimal protocol for human use? Even if bronchogen works in humans, we do not know the best dose, timing, duration, or administration route. These parameters require systematic investigation.
How does bronchogen interact with existing respiratory medications? Many people with lung disease take multiple medications. Understanding potential interactions is essential for safe integration of bronchogen into comprehensive treatment plans.
Can bronchogen prevent respiratory disease, or only treat it once established? The geroprotective hypothesis suggests preventive use, but this has not been tested. Prevention trials are particularly challenging because they require following healthy individuals for years to assess disease development.
What are the very long-term effects of bronchogen use? Current studies measure effects over weeks to months. Lifetime effects remain unknown. This gap is concerning because respiratory health is a lifelong concern, not a short-term problem.
Combining bronchogen with lifestyle optimization
No peptide works in isolation. The biological context in which bronchogen operates includes diet, exercise, sleep, stress, environmental exposures, and dozens of other factors that influence respiratory health. Optimizing this context maximizes the potential benefit of any intervention.
Exercise improves respiratory function directly. Cardiovascular training increases lung capacity and strengthens respiratory muscles. The enhanced recovery and adaptation supported by appropriate peptides may complement exercise-induced respiratory improvements.
Diet affects inflammation throughout the body, including in respiratory tissue. Anti-inflammatory dietary patterns rich in vegetables, fruits, omega-3 fatty acids, and antioxidants create a favorable environment for tissue health. Pro-inflammatory diets high in processed foods, sugar, and industrial seed oils work against respiratory health.
Sleep provides the primary window for tissue repair and regeneration. Growth hormone release during deep sleep supports repair processes throughout the body. Sleep deprivation impairs immune function and tissue healing. Adequate quality sleep is foundational for respiratory health.
Stress affects the immune system in complex ways. Chronic stress promotes inflammation and impairs immune surveillance. Stress management through whatever methods work for the individual, whether meditation, exercise, social connection, or professional support, contributes to respiratory wellness.
Environmental optimization
Air quality matters enormously for respiratory health. Indoor air often contains more pollutants than outdoor air due to off-gassing from furniture, cleaning products, and building materials. Air purification, proper ventilation, and reducing chemical exposures in the home environment support lung health.
Outdoor air quality varies dramatically by location and time. Avoiding exercise during high-pollution periods, checking air quality indices, and considering air filtration for homes in polluted areas are practical steps that protect respiratory tissue.
Occupational exposures deserve attention as well. Many jobs involve respiratory hazards, from obvious ones like mining and construction to less obvious ones like cleaning, hairdressing, and office work with poor ventilation. Appropriate respiratory protection and workplace modifications reduce cumulative damage.
Smoking cessation is the single most important intervention for respiratory health in those who smoke. No peptide can overcome the ongoing damage from inhaling smoke. Cessation reverses some damage over time and prevents continued deterioration. Bronchogen might theoretically support recovery after cessation, though this has not been specifically studied.
Bioregulator peptides in the broader context of peptide research
Bronchogen represents one approach within the broader field of peptide therapeutics. Understanding how bioregulators fit into this landscape helps contextualize their unique properties and limitations.
Traditional peptide drugs like insulin and growth hormone work by replacing or supplementing natural hormones. They are identical or nearly identical to the body's own signaling molecules and produce effects by activating the same receptors those natural hormones activate.
Synthetic peptides like BPC-157 and TB-500 work through growth factor pathways to promote tissue repair and regeneration. They have specific effects on healing processes and have attracted significant research interest for musculoskeletal applications.
Secretagogue peptides like ipamorelin and CJC-1295 stimulate the release of endogenous hormones, particularly growth hormone. They work by activating specific receptors in the pituitary to increase natural hormone production.
Bioregulator peptides like bronchogen represent yet another paradigm. Rather than activating specific receptors or replacing hormones, they appear to work through gene regulation. This is a more subtle mechanism that may produce broader, more physiological effects but is also more difficult to study and verify.
The bioregulator approach emphasizes tissue specificity. Each bioregulator targets a particular organ or tissue type. This selectivity distinguishes bioregulators from compounds with systemic effects throughout the body.
The advantages and limitations of bioregulators
Bioregulators offer potential advantages including excellent safety profiles, physiological rather than pharmacological effects, and tissue-specific action. They may be particularly suited for long-term use and preventive applications where aggressive pharmacological intervention would be inappropriate.
The limitations are also significant. The research base is smaller than for mainstream pharmaceuticals. Independent verification is limited. The mechanisms, while theoretically plausible, are not as well characterized as receptor-mediated drug effects. And regulatory approval pathways are unclear in most countries.
For researchers, this means approaching bioregulators with both openness and skepticism. The research is interesting and the safety profile favorable. But the evidence base is not as robust as for better-studied compounds. Critical evaluation and careful documentation of personal results are essential.
SeekPeptides members gain access to detailed comparisons of different peptide approaches, helping researchers understand where bioregulators fit within their broader protocols and which compounds might best address their specific goals.
Frequently asked questions
What is bronchogen peptide made of?
Bronchogen consists of four amino acids in the sequence Ala-Glu-Asp-Leu, meaning alanine, glutamic acid, aspartic acid, and leucine. This makes it a tetrapeptide, which is typical for bioregulator peptides that often contain just two to four amino acids. The small size allows for potential oral absorption and may contribute to the favorable safety profile observed in research.
Is bronchogen approved for human use?
Bronchogen is not approved by the FDA or most other regulatory agencies for human therapeutic use. It remains a research compound. In Russia and some Eastern European countries, peptide bioregulators are available as dietary supplements, but this does not constitute medical approval for treating disease. Researchers should understand the regulatory status in their jurisdiction before proceeding.
How does bronchogen differ from other lung support supplements?
Unlike herbal supplements or vitamins that provide general nutritional support, bronchogen appears to work through direct gene regulation in bronchial tissue. This tissue-specific mechanism distinguishes it from compounds with broader, less targeted effects. However, the research supporting bronchogen is more limited than the research behind some nutritional interventions.
Can bronchogen help with asthma or allergies?
The research on bronchogen has focused primarily on COPD and age-related respiratory decline rather than allergic conditions. While the anti-inflammatory effects might theoretically benefit asthma or allergies, this has not been specifically studied. People with allergic respiratory conditions should consult healthcare providers before considering bronchogen.
How long does bronchogen take to show effects?
The animal research showing tissue restoration used treatment periods of approximately one month. Effects may not be immediate, and structural tissue repair takes time. Researchers should plan assessment schedules that allow sufficient time for potential effects to manifest rather than expecting rapid changes.
Can bronchogen be combined with other peptides?
The bioregulator approach developed by Khavinson often involves using multiple tissue-specific peptides together. Combining bronchogen with immune-supporting peptides or other bioregulators is consistent with this philosophy. However, specific interaction data is limited, and researchers should document their experiences carefully when combining compounds. The peptide stack calculator can help plan combination protocols.
External resources
For researchers serious about understanding peptide bioregulators and their applications for respiratory health, SeekPeptides offers the most comprehensive resource available, with detailed protocols, research summaries, and a community of experienced researchers sharing insights and results.
In case I do not see you, good afternoon, good evening, and good night. May your airways stay clear, your lungs stay strong, and your breathing stay effortless.



