Feb 2, 2026
Tired of sifting through conflicting information about psoriasis management? You are not alone. Millions of people live with this chronic autoimmune condition, watching their skin cycle through painful flares and brief periods of calm. The standard treatments help some people. They fail others entirely. Up to 40 percent of patients do not respond adequately to anti-TNF therapy, and many who do respond eventually see their results fade. That leaves a significant population searching for alternatives, reading contradictory advice online, and feeling more frustrated than when they started.
Here is what the research actually shows.
Peptides, short chains of amino acids that serve as signaling molecules throughout the body, are emerging as a genuinely promising area of investigation for inflammatory skin conditions like psoriasis. These are not miracle cures. They are not replacements for medical supervision. But the science behind certain peptides and their interactions with the immune pathways that drive psoriasis is compelling, specific, and growing more robust every year. From KPV, a tripeptide that directly suppresses the NF-kB pathway central to psoriatic inflammation, to GHK-Cu, a copper peptide that modulates cytokine production while simultaneously supporting tissue repair, researchers are identifying mechanisms that address psoriasis at its immunological roots rather than merely suppressing surface symptoms. SeekPeptides has been tracking this research closely, and the evidence is worth understanding in detail. If you want to learn what peptides actually are before diving into psoriasis-specific applications, start there. Otherwise, keep reading. This guide covers the immunology, the specific peptides under investigation, the dosing protocols documented in research, and the practical considerations anyone exploring this area needs to know. Every claim links back to a mechanism. Every recommendation connects to published data. No hype. Just science you can evaluate for yourself.
Understanding psoriasis at the immune system level
Psoriasis is not a skin disease. That statement surprises most people, but it is the foundational truth that shapes everything else in this guide. Psoriasis is a systemic autoimmune condition that happens to manifest most visibly on the skin. The plaques, the scaling, the redness, those are downstream consequences of an immune system that has lost the ability to distinguish between genuine threats and the body own tissue.
To understand why peptides matter for psoriasis, you need to understand the immunological cascade that creates those plaques in the first place.
The IL-23/TH17 axis
At the center of psoriasis pathology sits the IL-23/TH17 axis. This is not a single pathway but rather an interconnected network of immune signals that, in psoriasis, become trapped in a self-amplifying loop. Here is how it works. Dendritic cells in the skin become activated by various triggers, including stress, infection, or injury. Once activated, these dendritic cells release interleukin-23, a cytokine that drives the differentiation and expansion of TH17 cells. TH17 cells then produce their own cytokines, primarily IL-17A and IL-22, which act directly on keratinocytes, the cells that make up the outer layer of skin.
Those keratinocytes respond by proliferating at an abnormal rate. Normal skin cell turnover takes about 28 to 30 days. In psoriatic skin, that cycle compresses to just 3 to 5 days. The result is the characteristic thick, silvery plaques that define the condition. But the keratinocytes do something else too. They release their own inflammatory signals, including chemokines that recruit even more immune cells to the area. This creates what researchers describe as a feed-forward inflammatory loop, a cycle that sustains itself once initiated. Understanding this cycle is essential for anyone researching peptides for autoimmune diseases broadly.
TNF-alpha as the upstream initiator
Tumor necrosis factor alpha sits upstream of the entire cascade. It is one of the first inflammatory signals released when the immune system perceives a threat, and in psoriasis, it acts as a master switch that amplifies virtually every other inflammatory pathway involved. TNF-alpha activates dendritic cells. It promotes TH17 cell differentiation. It directly stimulates keratinocyte proliferation. Research has identified 160 genes that are synergistically regulated by the combined action of IL-17 and TNF-alpha, meaning these two cytokines together produce effects far greater than either one alone.
This is why anti-TNF biologics were among the first targeted therapies developed for psoriasis. And they work, for some people. The problem is that TNF-alpha is so broadly important to immune function that blocking it entirely creates its own complications. The 40 percent non-response rate tells us something important: psoriasis is not driven by a single pathway but by a network, and interrupting one node does not always collapse the entire system.
NF-kB: the transcription factor that controls everything
If the IL-23/TH17 axis is the engine of psoriasis and TNF-alpha is the ignition switch, then NF-kB is the control room. Nuclear factor kappa-light-chain-enhancer of activated B cells, mercifully shortened to NF-kB, is a transcription factor that regulates the expression of hundreds of genes involved in inflammation, immune response, and cell survival. In psoriatic skin, NF-kB is constitutively activated, meaning it stays turned on even without an ongoing trigger.
This matters enormously for peptide research. Several of the most promising peptides under investigation for psoriasis work specifically by modulating NF-kB activity.
They do not shut it down completely, which would compromise immune function, but rather dial it back toward normal levels. That distinction between suppression and modulation is critical and represents one of the key advantages peptides may offer over conventional immunosuppressive therapies. For a deeper understanding of how peptides work at the molecular level, that guide breaks down the signaling mechanisms involved.
The pathogenic triad
Researchers now describe the core of psoriasis pathology as a pathogenic triad consisting of dendritic cells, TH17 cells, and keratinocytes. Each vertex of this triangle communicates with and amplifies the other two.
Dendritic cells release IL-23, which activates TH17 cells. TH17 cells release IL-17 and IL-22, which activate keratinocytes. Keratinocytes release chemokines and antimicrobial peptides, including LL-37, which activate dendritic cells. Round and round it goes. Breaking this cycle at any point can theoretically resolve a flare, but sustainable improvement requires addressing the cycle at multiple points simultaneously, or finding an intervention that modulates the central controlling mechanisms like NF-kB.
The JAK-STAT signaling pathway adds another layer. Many of the cytokines involved in psoriasis signal through Janus kinase and signal transducer and activator of transcription proteins. JAK inhibitors represent another class of targeted therapy, but like anti-TNF agents, they come with significant immunosuppressive effects. The search for interventions that can modulate these pathways more selectively continues, and peptides sit squarely in that investigation. Understanding how the best peptides for immune system support interact with these pathways provides important context.
The role of LL-37 and cathelicidin
One peptide already plays a central role in psoriasis, though not a beneficial one. LL-37, also known as cathelicidin, is an antimicrobial peptide that is dramatically overexpressed in psoriatic skin. Under normal circumstances, LL-37 helps defend against bacterial infection. In psoriasis, however, it takes on a destructive role.
LL-37 forms complexes with self-DNA and self-RNA released from damaged skin cells. These complexes activate toll-like receptors 7 and 9 on dendritic cells, essentially tricking the immune system into treating the body own genetic material as a foreign invader. This is one of the key mechanisms through which psoriasis initiates and sustains itself. Intriguingly, LL-37 also has anti-inflammatory properties in certain contexts, making it a dual-natured molecule. Vitamin D regulates LL-37 production, which partly explains why vitamin D analogs have some efficacy in psoriasis treatment and why sun exposure often improves symptoms.
This dual nature of LL-37 illustrates something important about psoriasis: the immune system is not simply overactive. It is dysregulated. The goal is not broad suppression but precise recalibration. That is exactly what the most promising peptide research aims to achieve.
How peptides interact with psoriasis pathways
Peptides are short chains of amino acids, typically between 2 and 50 amino acids in length, that function as signaling molecules in the body. Unlike large protein biologics that must be administered intravenously and carry significant immunosuppressive risks, peptides offer several distinct advantages for inflammatory skin conditions. They are smaller, more targeted, and in many cases can be administered through multiple routes including topical application, subcutaneous injection, or oral delivery. The getting started with peptides guide covers the fundamentals for anyone new to this field.
Why peptides are different from biologics
Biologics like adalimumab, secukinumab, and ustekinumab are large monoclonal antibodies that block specific cytokines with extraordinary precision. They have transformed psoriasis treatment for many patients. But they come with significant drawbacks: high cost, injection site reactions, increased infection risk due to immune suppression, and the development of anti-drug antibodies over time that can reduce efficacy.
Peptides operate through different mechanisms. Rather than blocking a single cytokine outright, many peptides modulate upstream regulatory pathways. KPV, for instance, does not just block TNF-alpha. It suppresses NF-kB, which controls the expression of TNF-alpha along with dozens of other inflammatory mediators simultaneously. This broader but still targeted approach means peptides can potentially address the network nature of psoriatic inflammation rather than just one node within it. For those comparing different therapeutic approaches, the comparison between peptides versus sarms highlights how peptides differ from other bioactive compounds.
Multiple mechanisms of action
The peptides under investigation for psoriasis do not all work the same way. Some, like KPV, primarily target inflammatory signaling. Others, like BPC-157, focus on tissue repair and angiogenesis. GHK-Cu addresses both inflammation and tissue remodeling simultaneously. Thymosin alpha-1 works at the level of immune cell regulation, promoting the development of regulatory T cells that keep autoimmune responses in check.
This diversity matters because psoriasis is not a single-mechanism disease. A comprehensive approach might combine peptides that address inflammation, tissue repair, and immune regulation simultaneously. The concept of peptide stacking becomes particularly relevant here, though any such approach should be guided by research and medical supervision.
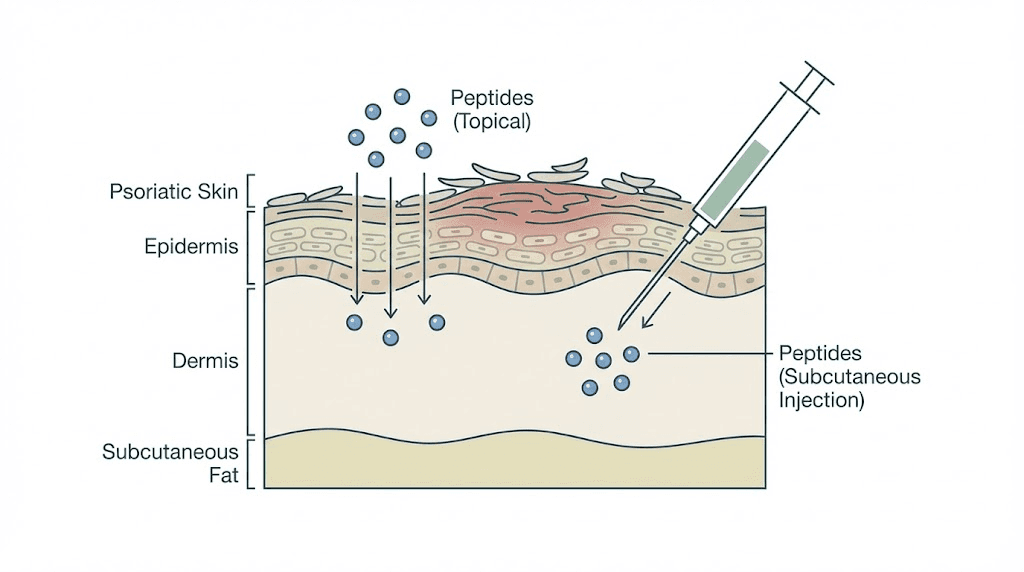
Bioavailability and delivery challenges
One significant challenge with peptides is getting them to where they need to go. The skin is designed to be a barrier, and many peptides are hydrophilic, meaning they dissolve in water rather than in the lipids that make up the skin barrier. GHK-Cu, for example, has poor skin penetration on its own, which is why researchers have explored liposomal encapsulation to improve delivery.
Different administration routes solve different problems. Topical application delivers peptides directly to the affected skin but struggles with penetration depth. Subcutaneous injection bypasses the skin barrier entirely but delivers the peptide systemically rather than locally. Oral delivery is the most convenient but subjects peptides to digestive enzymes that can break them down before they reach the bloodstream. The comparison between injectable versus oral peptides breaks down these tradeoffs in detail.
For psoriasis specifically, topical application has obvious appeal since the condition manifests on the skin surface. But because psoriasis is a systemic immune condition, systemic delivery may be necessary to address the underlying immune dysregulation. Many researchers are investigating combination approaches, using topical peptides to manage local inflammation while administering other peptides systemically to modulate the immune response.
KPV: the leading peptide for psoriasis research
If one peptide deserves the title of most promising for psoriasis research, it is KPV. This tripeptide, consisting of just three amino acids, Lysine, Proline, and Valine, punches far above its weight in terms of anti-inflammatory potency. It is derived from the C-terminal end of alpha-melanocyte-stimulating hormone, and it retains the parent molecule anti-inflammatory properties without the melanogenic (skin-darkening) effects.
How KPV works against psoriatic inflammation
KPV targets the NF-kB pathway directly. When NF-kB is activated in immune cells, it translocates to the nucleus and switches on the transcription of inflammatory genes. KPV interferes with this translocation, keeping NF-kB in the cytoplasm where it remains inactive. The downstream result is a significant reduction in pro-inflammatory cytokine release, including TNF-alpha, IL-1beta, IL-6, and IL-8, all of which are elevated in psoriatic skin.
But KPV does more than just suppress NF-kB. It also interacts with melanocortin receptors, particularly MC1R, which are expressed on immune cells including macrophages, dendritic cells, and T cells. Activation of these receptors shifts immune cells toward an anti-inflammatory phenotype. Macrophages, for instance, shift from the M1 (pro-inflammatory) state to the M2 (anti-inflammatory, tissue-repair) state. This receptor-mediated action adds a second layer of immune modulation on top of the direct NF-kB suppression.
For anyone wanting to explore the full range of KPV research, the KPV peptide benefits guide covers its applications beyond psoriasis. The KPV peptide for inflammation article goes deeper into the anti-inflammatory mechanisms specifically.
KPV dosing protocols from research
Research has documented several administration routes for KPV, each with distinct dosing ranges.
Topical application uses concentrations between 0.005 and 0.1 percent in a cream base, applied twice daily to affected areas. This route delivers the peptide directly to inflamed skin and has shown improvements in plaque reduction and skin texture in early investigations. The advantage is direct, localized delivery with minimal systemic exposure.
Subcutaneous injection protocols typically use 200 to 400 micrograms daily. This route provides systemic delivery that can address the underlying immune dysregulation driving psoriasis, not just the local skin manifestation. It is particularly relevant for patients with widespread plaques or psoriatic arthritis where systemic immune modulation is desirable. The KPV peptide dosage guide provides more detailed breakdowns of these protocols.
Oral administration follows a ramping protocol: 250 micrograms daily for the first three weeks, increasing to 500 micrograms for maintenance. While oral delivery subjects peptides to digestive enzymes, KPV is a remarkably small molecule, just three amino acids, which appears to give it better oral bioavailability than larger peptides. The peptide capsules guide covers oral peptide delivery in greater depth.
Clinical observations with KPV and psoriasis
One documented case study describes a 55-year-old male with chronic plaque psoriasis who used KPV as part of his management protocol. The reported results included reduction in flare frequency, decreased plaque formation, and improved skin texture over the course of several weeks. Skin conditions in general show measurable improvement within 3 to 6 weeks of consistent KPV use, based on available reports.
These are not randomized controlled trials. That distinction matters. But the mechanistic rationale is strong, the safety profile appears favorable, and the early observational data aligns with what the biochemistry predicts. KPV directly addresses the NF-kB dysregulation and cytokine overproduction that define psoriasis at the molecular level. The question is not whether the mechanism makes sense but whether clinical trials will confirm the magnitude of benefit suggested by preclinical and early observational data. For those also interested in KPV research in oncology contexts, the KPV peptide cancer research guide explores that adjacent area.
Why KPV may succeed where other treatments struggle
Remember that 40 percent non-response rate with anti-TNF therapy? Part of the reason is that anti-TNF agents block one specific cytokine while leaving the rest of the inflammatory network intact. The network can often route around a single blockade. KPV operates differently. By suppressing NF-kB, it affects the master regulatory switch that controls the expression of many inflammatory mediators simultaneously. It does not just block TNF-alpha. It reduces TNF-alpha production along with IL-1beta, IL-6, IL-8, and other cytokines at the transcriptional level.
This broader mechanism of action may be more effective against a network disease like psoriasis. It also may explain why KPV appears to have a favorable safety profile compared to more aggressive immunosuppressive approaches. It modulates rather than suppresses. The broader guide on inflammation peptides places KPV within the larger context of peptide-based anti-inflammatory research.
GHK-Cu and skin inflammation management
GHK-Cu, or copper tripeptide, occupies a unique position among peptides relevant to psoriasis. It is both an anti-inflammatory agent and a tissue remodeling peptide, addressing two distinct aspects of the condition simultaneously. In psoriasis, you need to calm the immune response and repair the damaged tissue. GHK-Cu does both.
The science behind GHK-Cu and inflammation
GHK-Cu reduces TNF-alpha-induced IL-6 secretion in dermal fibroblasts. That sentence is dense but important. In psoriatic skin, TNF-alpha triggers fibroblasts to release IL-6, which perpetuates the inflammatory cycle. GHK-Cu interrupts this specific link in the chain. It also suppresses NF-kB p65 and p38 MAPK signaling, two pathways that are hyperactivated in psoriasis.
The p38 MAPK pathway is particularly interesting. It is involved in the production of inflammatory cytokines and in keratinocyte proliferation, the excessive skin cell turnover that creates psoriatic plaques. By suppressing this pathway, GHK-Cu may simultaneously reduce inflammation and slow the abnormal keratinocyte proliferation that characterizes psoriasis. The comprehensive copper peptides GHK-Cu guide covers these mechanisms in detail.
Tissue repair and collagen remodeling
Psoriasis does not just inflame the skin. It damages it. Chronic inflammation degrades the extracellular matrix, disrupts collagen architecture, and impairs the skin barrier function. GHK-Cu directly addresses this damage.
It increases collagen production by up to 70 percent. Read that number again. Seventy percent. It also promotes the synthesis of decorin, a proteoglycan that organizes collagen fibers into functional structures. Beyond collagen, GHK-Cu promotes TGF-beta production, an anti-inflammatory mediator that also plays crucial roles in tissue repair and immune regulation.
The combination of anti-inflammatory and tissue repair properties makes GHK-Cu uniquely suited for a condition like psoriasis where both processes are needed simultaneously. While other peptides may be more potent anti-inflammatory agents, few match GHK-Cu dual functionality. Understanding how copper peptides affect skin provides additional context for their dermatological applications.
GHK-Cu levels decline with age
Here is a fact that adds urgency to this research. GHK-Cu levels in the body decline dramatically with age. At age 20, plasma levels average around 200 nanograms per milliliter. By age 60, that number drops to approximately 80 nanograms per milliliter. That is a 60 percent decline.
Psoriasis can onset at any age, but many patients experience worsening symptoms as they age. The parallel decline in endogenous GHK-Cu levels is suggestive, though causation has not been established. What is clear is that supplementing GHK-Cu restores levels of a peptide that naturally modulates inflammation and supports tissue repair, both of which are impaired in psoriasis.
Administration and penetration challenges
GHK-Cu presents a practical challenge for psoriasis treatment: it is hydrophilic, meaning it does not easily penetrate the lipid-rich skin barrier. This limits the effectiveness of simple topical application. Several approaches are being investigated to overcome this limitation.
Liposomal encapsulation wraps GHK-Cu in lipid vesicles that can fuse with the skin barrier, delivering the peptide to deeper layers. This approach has shown improved penetration in research settings. Microneedling combined with topical GHK-Cu is another approach, creating tiny channels through the stratum corneum that allow the peptide to reach the dermis. Subcutaneous injection bypasses the penetration problem entirely but delivers the peptide systemically rather than locally. The GHK-Cu peptide injection dosage guide covers injection protocols specifically, while the GHK-Cu peptide dosage guide addresses all routes. For topical use in skincare, the copper peptides skincare routine article is a practical resource.
Those exploring GHK-Cu should also understand how it compares to other copper-containing peptides. The GHK-Cu versus other copper peptides comparison clarifies important distinctions, and the best copper peptide serum guide covers topical product selection. It is also worth noting that some users have reported negative initial reactions, which the copper peptides ruined my skin guide and GHK-Cu making skin worse article address transparently.
The GHK-Cu peptide dosage chart provides quick reference for various protocols, and the how to use GHK-Cu peptide injection guide walks through the injection process.
BPC-157 and tissue repair in psoriatic skin
BPC-157 is one of the most widely studied peptides in the healing and recovery space, and its properties make it a compelling candidate for psoriasis-related research. This 15-amino-acid peptide, derived from a protein found naturally in human gastric juice, has demonstrated remarkable tissue repair capabilities across multiple systems. Its relevance to psoriasis lies not in direct immune modulation but in its ability to restore damaged tissue and support the healing processes that chronic inflammation disrupts.
Mechanisms relevant to psoriatic skin
BPC-157 promotes angiogenesis, the formation of new blood vessels, through increased expression of vascular endothelial growth factor. In psoriatic skin, blood vessel architecture is abnormal. The dermal papillae contain tortuous, dilated capillaries that contribute to the redness characteristic of plaques. BPC-157 ability to modulate angiogenesis, promoting healthy vessel formation rather than the disorganized vasculature seen in psoriasis, represents one mechanism of potential benefit. The comprehensive BPC-157 overview covers the full spectrum of this peptide research.
The peptide also reduces oxidative stress, which is significantly elevated in psoriatic skin. Reactive oxygen species contribute to tissue damage and perpetuate the inflammatory cycle. By modulating oxidative stress pathways, BPC-157 may help break one of the self-sustaining loops that keep psoriasis active.
Perhaps most relevant to psoriasis is BPC-157 ability to restore epithelial barriers. The skin barrier is compromised in psoriasis, allowing irritants and microbes to penetrate more easily, which in turn triggers more immune activation. BPC-157 has shown the ability to accelerate epithelial healing and restore barrier integrity in various tissue models. For detailed information on what BPC-157 is and how it was discovered, that guide provides thorough background.
Anti-inflammatory properties of BPC-157
While BPC-157 is primarily known as a healing peptide, it also possesses meaningful anti-inflammatory activity. It reduces the production of pro-inflammatory cytokines at the site of tissue damage and modulates the local immune response toward repair rather than continued inflammation. This anti-inflammatory activity is more localized than that of KPV or thymosin alpha-1, which may be either an advantage or a limitation depending on the context.
For someone with localized psoriatic plaques, the combination of tissue repair and local anti-inflammatory activity could be beneficial. For someone with widespread psoriasis or systemic involvement like psoriatic arthritis, BPC-157 alone would likely be insufficient, but it could serve as a valuable component of a broader protocol. The tissue repair peptides guide places BPC-157 within the larger landscape of healing-focused peptides.
BPC-157 dosing considerations
BPC-157 is typically administered at dosages ranging from 200 to 500 micrograms per day, either through subcutaneous injection near the affected area or orally. The BPC-157 5mg dosing guide provides specific calculations, and the how do you take BPC-157 article walks through administration methods.
One important consideration is that only three published human clinical trials exist for BPC-157. The vast majority of evidence comes from animal studies, which are promising but cannot be directly extrapolated to human outcomes.
This is an area where the mechanism is well-characterized, the animal data is robust, and the safety profile appears favorable, but rigorous human trial data remains limited. Understanding peptide safety and risks is essential context for evaluating any peptide with limited human trial data.
Combining BPC-157 with other peptides
BPC-157 is frequently discussed in the context of combination protocols. The BPC-157 versus TB-500 comparison is one of the most common discussions in the peptide community, and for good reason. TB-500, another tissue repair peptide, works through different mechanisms than BPC-157, and the two are often stacked for synergistic healing effects. The detailed BPC-157 and TB-500 stacking guide covers how these two peptides complement each other.
TB-500 itself has anti-inflammatory and tissue repair properties that are relevant to psoriasis. It modulates actin, a protein involved in cell structure and migration, and promotes the migration of endothelial cells and keratinocytes needed for wound healing. The TB-500 benefits guide and the full TB-500 overview provide more background on this peptide. For those exploring alternatives, the TB-500 alternatives article covers other options in the same category.
For psoriasis specifically, a theoretical protocol might combine KPV for systemic anti-inflammatory action with BPC-157 for local tissue repair at plaque sites. This is speculation based on known mechanisms, not a clinically validated protocol. The peptide stack calculator can help with dosing calculations for combination protocols, and the how many peptides you can take at once article addresses safety considerations for stacking.
Thymosin alpha-1 and immune rebalancing
Thymosin alpha-1 approaches psoriasis from a fundamentally different angle than the peptides discussed so far. Rather than directly suppressing inflammation or repairing tissue, it works at the level of immune system regulation, promoting the development and function of regulatory T cells that keep autoimmune responses in check.
The thymus connection
Thymosin alpha-1 is a 28-amino-acid peptide originally isolated from the thymus gland. The thymus is where T cells mature and learn to distinguish self from non-self, a process that when it goes wrong, leads to autoimmune conditions like psoriasis. Thymosin alpha-1 is not just a thymic byproduct; it is an active regulator of T cell development and function.
In the context of psoriasis, one finding stands out: patients with psoriatic arthritis have significantly lower levels of thymosin alpha-1 compared to healthy controls. This deficit is not merely correlational. The reduced thymosin alpha-1 levels correspond to measurable impairment in regulatory T cell function, and regulatory T cell dysfunction is a recognized driver of psoriasis pathology. The thymalin peptide benefits article covers the broader family of thymic peptides and their immune-regulating properties.
Regulatory T cells and psoriasis
Regulatory T cells, commonly abbreviated as Tregs, are the immune system braking mechanism. They prevent excessive immune activation and suppress autoreactive T cells that would otherwise attack the body own tissues. In psoriasis, Treg function is impaired. There are fewer of them, and those that exist are less effective at suppressing the TH17 cells that drive the disease.
Thymosin alpha-1 promotes Treg differentiation and enhances their suppressive function. This represents a fundamentally different approach to treating psoriasis. Instead of blocking inflammatory cytokines after they are produced or suppressing the immune cells that produce them, thymosin alpha-1 strengthens the natural regulatory mechanisms that should be preventing the autoimmune response in the first place.
Think of it this way.
Most psoriasis treatments are like turning down the volume on a stereo that is too loud. Thymosin alpha-1 is more like fixing the automatic volume control so the stereo regulates itself.
Cytokine modulation
Beyond its effects on Tregs, thymosin alpha-1 directly reduces the production of IL-1beta and TNF-alpha, two cytokines central to psoriasis pathology. This provides a dual mechanism: enhanced immune regulation through Tregs plus direct cytokine suppression. The combination is potent because it addresses psoriasis at both the regulatory level, why the immune system is attacking, and the effector level, the specific inflammatory signals doing the damage.
Clinical context and dosing
Thymosin alpha-1 is approved in more than 35 countries for the treatment of hepatitis B and C, giving it a well-established safety record. The standard dosing protocol is 1.6 to 3.2 milligrams per week, administered subcutaneously. This is a well-characterized peptide with decades of clinical use, though not yet specifically for psoriasis.
No clinical trials have been conducted specifically for psoriasis, which is a significant gap. The mechanistic rationale is strong, the safety profile is established, and the observation that psoriatic arthritis patients have lower thymosin alpha-1 levels is suggestive. But without psoriasis-specific trial data, its use remains theoretical for this indication. The peptide research and studies overview tracks the state of clinical evidence across various peptides.
The broader category of bioregulator peptides includes thymosin alpha-1 and other compounds that work by restoring normal regulatory function rather than forcing specific outcomes. This approach aligns well with the nature of autoimmune conditions where the fundamental problem is regulatory failure.
Emerging peptide research for psoriasis
Beyond the established peptides like KPV, GHK-Cu, BPC-157, and thymosin alpha-1, several newer peptide-based interventions are generating excitement in psoriasis research. These are earlier in development but offer tantalizing glimpses of what may be possible.
PEPITEM-derived tripeptides
PEPITEM is a 14-amino-acid peptide released by B lymphocytes that has shown remarkable results in psoriasis models. What makes PEPITEM-derived tripeptides particularly noteworthy is the magnitude and speed of their effects. Topical application for just seven days produced a 50 percent reduction in Psoriasis Area and Severity Index scores. That level of improvement is comparable to Clobetasol Propionate 0.05 percent, a potent topical corticosteroid, but achieved through a completely different mechanism without the side effects associated with prolonged steroid use.
Even more intriguing are the systemic effects observed with topical application. PEPITEM-derived tripeptides reduced splenomegaly, the enlargement of the spleen that indicates systemic inflammation, and limited the expansion of CD4+, CD8+, TH1, and TH17 cell populations. A topical agent producing systemic immune modulation is unusual and suggests these peptides may be absorbed sufficiently to affect immune function beyond the site of application.
This research is still in early stages, but the combination of topical convenience, rapid onset, steroid-comparable efficacy, and systemic immune modulation makes PEPITEM-derived tripeptides one of the most exciting developments in psoriasis peptide research.
Caveolin-1 (CAV-1) peptides
Caveolin-1 is a protein involved in signal transduction and membrane trafficking. Water-soluble peptides derived from the caveolin scaffolding domain have shown promising results in psoriasis models. The CSD peptides improved skin phenotype, meaning they reduced the visible and histological signs of psoriasis, and suppressed psoriasis-related cytokine expression.
Among the various subregions tested, the sB subregion showed the best results. This is important because it suggests the anti-psoriatic activity can be localized to a specific portion of the larger protein, potentially allowing the development of small, targeted peptide therapeutics. The mechanism involves modulation of signaling pathways that control keratinocyte behavior, making CAV-1 peptides a complement to, rather than a replacement for, peptides that primarily target immune cell function.
Muramyl peptide (Licopid)
Muramyl peptide, marketed as Licopid in some countries, has the most robust clinical data among the emerging peptide treatments for psoriasis. A study of 86 patients reported significant improvement in 58.1 percent of cases. But the most striking finding was the effect on relapse frequency. Patients treated with muramyl peptide experienced flares approximately once per year, compared to two to three times per year in control groups. The treatment doubled the relapse-free period.
No side effects or complications were reported in this study. That safety profile, combined with meaningful clinical improvement and dramatically reduced relapse frequency, makes muramyl peptide one of the most evidence-supported peptide treatments for psoriasis currently available. It works by modulating innate immune responses, activating macrophages in a way that promotes immune regulation rather than unchecked inflammation.
What these emerging peptides mean for the field
The diversity of peptide-based approaches under investigation for psoriasis reflects the complexity of the disease itself. PEPITEM-derived tripeptides modulate adaptive immune responses. CAV-1 peptides affect keratinocyte signaling. Muramyl peptide modulates innate immunity. Each targets a different aspect of the pathogenic triad, and collectively they suggest that the future of psoriasis management may involve precise, multi-targeted peptide protocols tailored to the specific immunological profile of each patient.
This is speculative, but it is grounded in the direction current research is heading. The complete peptide list on SeekPeptides tracks both established and emerging peptides across all categories.
Collagen peptides and skin barrier support
Collagen peptides deserve mention in any discussion of psoriasis management, though their role is supportive rather than directly therapeutic. They do not address the immune dysregulation that drives psoriasis. What they do is support the structural integrity of skin that chronic inflammation has damaged.
How collagen peptides support psoriatic skin
Psoriatic skin has compromised collagen architecture.
The chronic inflammatory environment degrades existing collagen faster than the body can replace it, and the disorganized keratinocyte proliferation further disrupts normal tissue structure. Collagen peptides, when ingested, are broken down into smaller fragments that serve as both building blocks for new collagen synthesis and as signaling molecules that stimulate fibroblasts to produce more collagen.
This is not going to clear a plaque. Let us be clear about that. But it can support the skin repair process that must occur alongside immune modulation for full resolution of psoriatic lesions. Think of collagen peptides as the construction materials that allow the body to rebuild once the inflammatory fire has been contained.
The peptides for skin complete guide covers the full range of skin-relevant peptides, including collagen. For those specifically interested in skin tightening and structural support, the peptides for skin tightening article addresses that angle. And the comparison between collagen hydrolysate and collagen peptides clarifies an often-confused distinction.
Skin barrier repair
The skin barrier in psoriasis is functionally impaired. Transepidermal water loss is increased, and the barrier ability to keep irritants and pathogens out is reduced. This barrier dysfunction contributes to the inflammatory cycle because substances that penetrate the compromised barrier trigger additional immune activation.
Collagen peptides contribute to barrier repair by supporting the dermal matrix that underlies the epidermal barrier. While they do not directly restore the lipid lamellae of the stratum corneum, they do support the structural foundation upon which barrier function depends. The natural peptides for skin guide covers endogenous peptides that support barrier function, providing context for how supplemental collagen peptides fit into the larger picture.
Practical considerations for collagen peptides
Collagen peptides are among the most accessible and well-tolerated peptides available. They are typically taken orally as a powder or capsule, with dosages ranging from 5 to 15 grams daily. Side effects are rare and generally mild. The question of whether collagen peptides count as protein is relevant for anyone tracking macronutrient intake, and the potential for collagen peptides to cause acne is worth understanding, though it is uncommon.
For psoriasis specifically, collagen peptides are best viewed as one component of a comprehensive approach rather than a standalone treatment. They support the structural repair that other peptides, like BPC-157 and GHK-Cu, promote through more direct mechanisms.
Building a peptide protocol for psoriasis management
Understanding individual peptides is useful. Understanding how they might work together is where real practical value emerges. This section outlines the principles behind constructing a research-informed peptide protocol for psoriasis management. This is not medical advice. It is a framework for understanding how the mechanisms discussed throughout this guide could theoretically be combined.
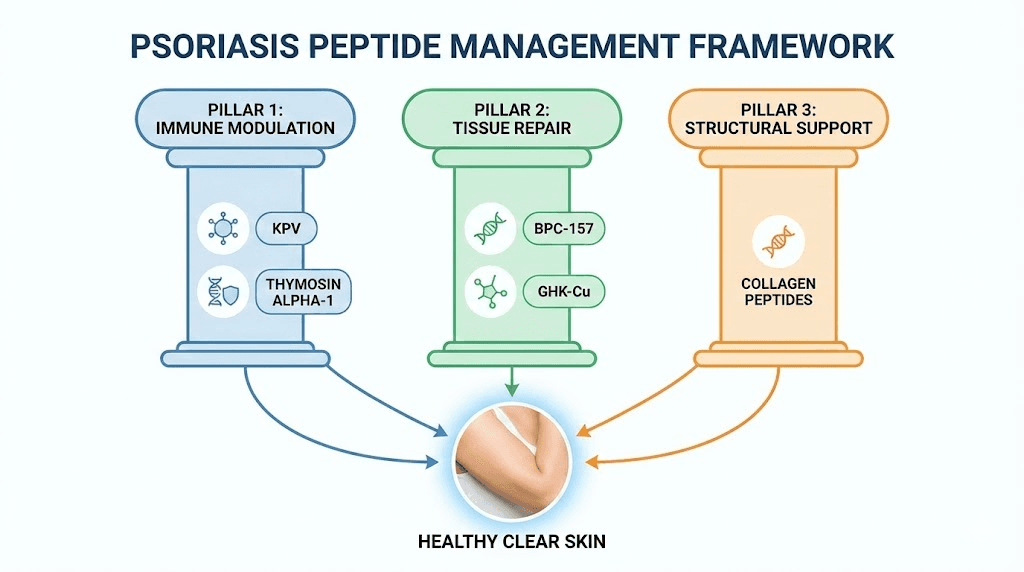
The three pillars of peptide-based psoriasis management
A comprehensive peptide approach to psoriasis would address three distinct aspects of the condition.
First: immune modulation. Calming the overactive immune response that drives the disease. KPV and thymosin alpha-1 are the primary candidates here. KPV provides direct NF-kB suppression and cytokine reduction, while thymosin alpha-1 enhances the regulatory T cell function that should be preventing the autoimmune response in the first place. Together, they address psoriasis at both the effector and regulatory levels of immune function.
Second: tissue repair. Healing the damage that chronic inflammation has caused. BPC-157 and GHK-Cu are the leading candidates for this pillar. BPC-157 promotes angiogenesis, reduces oxidative stress, and restores epithelial barriers. GHK-Cu bridges both pillars by providing anti-inflammatory activity alongside collagen remodeling and tissue regeneration.
Third: structural support. Maintaining the integrity of healing skin and supporting barrier function. Collagen peptides and continued GHK-Cu use address this pillar, providing the raw materials and signaling necessary for sustained tissue quality.
The peptide cycle planning guide provides frameworks for structuring peptide protocols over time, and the peptide dosing guide covers dosing principles that apply across peptide types.
Sequencing and cycling considerations
Not all peptides need to be used simultaneously. A phased approach might start with immune modulation (KPV, potentially thymosin alpha-1) to calm the acute inflammatory response, then introduce tissue repair peptides (BPC-157, GHK-Cu) once the immune environment is more favorable for healing. Collagen peptides could be used throughout as a baseline support measure.
Cycling, the practice of using peptides for a defined period and then taking a break, is standard practice in peptide protocols. The can you cycle different peptides guide addresses the rationale and methods for cycling. The peptide stacks guide covers the broader principles of combining peptides for complementary effects.
Monitoring and adjustment
Any peptide protocol for psoriasis should include regular monitoring. PASI scores, photographic documentation, and symptom tracking provide objective measures of progress. Blood work including inflammatory markers (CRP, ESR), cytokine panels, and immune cell subsets can provide deeper insight into whether the protocol is modulating the underlying immune dysfunction.
Adjustments should be data-driven. If inflammatory markers improve but plaques persist, the tissue repair component may need strengthening. If plaques improve but flares continue, the immune modulation component may need adjustment. The peptides before and after results article discusses realistic timelines for seeing changes, and how long peptides take to work addresses the patience required.
The peptide calculator on SeekPeptides helps with precise dosing calculations, and the peptide cost calculator provides realistic budgeting for protocols that may run weeks or months.
Administration routes and practical considerations
Understanding which peptides might help with psoriasis is only half the equation. The other half is getting those peptides into the body effectively. Administration route affects bioavailability, onset of action, duration of effect, and practicality for long-term use. For a condition like psoriasis that requires sustained management, practical considerations matter enormously.
Topical application
Topical delivery makes intuitive sense for a skin condition. You apply the peptide directly where it is needed. For psoriasis, topical peptides can deliver high concentrations directly to inflamed plaques without significant systemic exposure.
KPV topical formulations at 0.005 to 0.1 percent concentrations have shown promise. PEPITEM-derived tripeptides demonstrated their remarkable results through topical application. GHK-Cu, while challenged by penetration issues, can be effective in liposomal formulations or when combined with penetration enhancers.
The limitation of topical delivery is coverage. For patients with widespread psoriasis affecting large body surface areas, topical application to every plaque becomes impractical. It works best for localized disease or as a complement to systemic treatment for targeted management of persistent plaques.
Subcutaneous injection
Subcutaneous injection is the most common administration route for peptides used systemically. The peptide injections guide covers the technique in detail, and the peptide injection pen guide addresses a common delivery device. The list of injectable peptides provides a comprehensive catalog.
For psoriasis, subcutaneous injection offers several advantages. It bypasses the skin barrier problem entirely. It provides consistent systemic delivery. And it allows precise dosing. KPV at 200 to 400 micrograms daily, thymosin alpha-1 at 1.6 to 3.2 milligrams weekly, and BPC-157 at 200 to 500 micrograms daily are all commonly administered via subcutaneous injection.
The disadvantage is the need for injection, which some people find uncomfortable or inconvenient. Proper technique, including site rotation and sterile preparation, is important for safety and comfort. The common peptide mistakes beginners make article covers pitfalls to avoid.
Oral administration
Oral delivery is the most convenient route but presents bioavailability challenges. Most peptides are partially or fully degraded by digestive enzymes before reaching the bloodstream. However, certain peptides, particularly very small ones like KPV (just three amino acids), appear to retain meaningful bioavailability when taken orally.
BPC-157 is also used orally, which makes sense given that it is naturally found in gastric juice and appears to have evolved to function in the digestive environment. The oral route for BPC-157 may be particularly relevant for psoriasis patients who also have gut involvement, as the gut-skin axis is increasingly recognized as a factor in psoriatic disease.
The peptide capsules guide provides detailed information on oral peptide delivery, and the comparison of injectable versus oral peptides helps in choosing the right route for each peptide.
Nasal spray delivery
Nasal spray delivery offers an alternative route that avoids both the skin barrier and digestive degradation. The nasal mucosa is thin and highly vascularized, allowing relatively efficient absorption of peptides into the bloodstream. The nasal spray peptides guide covers this administration route comprehensively.
For psoriasis, nasal delivery could be relevant for peptides that need systemic distribution but are poorly absorbed orally. KPV and certain immune-modulating peptides are candidates for nasal delivery, though specific psoriasis protocols using this route have not been formally studied.
Reconstitution and storage
Most research peptides come in lyophilized (freeze-dried) powder form and must be reconstituted before use. Proper reconstitution and storage are critical for maintaining peptide potency and safety. The how to reconstitute peptides guide walks through the process step by step. Bacteriostatic water is the standard reconstitution vehicle, and the how to mix peptides with bac water article covers the specifics.
Storage requirements vary by peptide and form. The peptide storage guide provides general principles. More specific questions like how long peptides last in the fridge, how long they last in powder form, and how long they last at room temperature are addressed in dedicated articles. After reconstitution, proper storage becomes even more important, as covered in the how to store peptides after reconstitution guide and the specific question of how long reconstituted peptides last in the fridge. Understanding whether peptides expire is also important for anyone maintaining a long-term protocol.
The comparison between lyophilized and liquid peptides is relevant for understanding shelf life and stability. The peptide reconstitution calculator on SeekPeptides makes dosing math straightforward once reconstituted.
Safety, side effects, and what to watch for
Safety must be the foundation of any peptide protocol, particularly for a chronic condition that requires sustained use. Psoriasis patients are often on other medications, may have comorbidities, and are managing a condition that involves immune dysfunction. Adding peptides to this picture requires careful consideration.
General peptide safety profile
Most of the peptides discussed in this guide have favorable safety profiles based on available data. KPV, as a fragment of a naturally occurring hormone, is generally well-tolerated. BPC-157 is derived from a protein found naturally in gastric juice. GHK-Cu is an endogenous peptide that the body produces on its own. Thymosin alpha-1 is approved in over 35 countries with decades of safety data.
That said, "generally safe" does not mean "universally safe for everyone in all circumstances." Individual reactions vary. Drug interactions can occur. Pre-existing conditions can change the risk-benefit calculation. The peptide safety and risks guide provides a comprehensive overview of safety considerations across peptide types.
Immune modulation risks in autoimmune conditions
Here is where psoriasis adds a layer of complexity. Psoriasis is fundamentally an immune dysregulation disorder. Peptides that modulate immune function are intervening in a system that is already malfunctioning. While the goal is to restore normal regulation, there is a theoretical risk of pushing the immune system in an unintended direction.
Thymosin alpha-1, for example, can both stimulate and regulate immune function depending on context. In a healthy immune system, it tends to enhance function. In a dysregulated system, it tends to promote regulation. This context-dependent behavior is one of its strengths, but it also means responses may not be entirely predictable. Monitoring immune markers during any protocol involving immune-modulating peptides is advisable.
Patients on immunosuppressive medications (methotrexate, cyclosporine, biologics) should exercise particular caution with immune-modulating peptides. Combining immune stimulants with immune suppressants can produce unpredictable results. Medical supervision is not just recommended; it is essential.
Potential side effects by peptide
KPV side effects are generally mild when reported. Injection site reactions including redness and mild swelling are the most common. Topical application occasionally causes local irritation, particularly in already-inflamed skin. Starting with lower concentrations and increasing gradually can minimize this risk.
GHK-Cu topical use can sometimes worsen skin conditions initially. This is well-documented and addressed in the copper peptides and skin reactions guide. The initial worsening typically resolves within one to two weeks as the skin adapts, but it can be distressing for psoriasis patients whose skin is already compromised. Starting with low concentrations and limited application areas is prudent. The copper peptides before and after article provides realistic expectations for timelines and results.
BPC-157 has minimal reported side effects in human use. Nausea, dizziness, and headache have been occasionally reported. Given the limited number of human clinical trials (only three published), the full side effect profile may not be completely characterized.
Thymosin alpha-1, with its extensive clinical use history, has a well-documented side effect profile. Injection site reactions are most common. Fatigue and mild flu-like symptoms can occur, particularly early in treatment. Serious adverse events are rare.
Drug interactions to consider
Peptides that modulate inflammatory pathways could theoretically interact with anti-inflammatory medications, biologics, or immunosuppressants. KPV NF-kB suppression combined with methotrexate anti-inflammatory effects could potentially produce excessive immune suppression. Similarly, combining thymosin alpha-1 immune regulation with biologic therapy could produce unpredictable immune effects.
No formal drug interaction studies exist for most research peptides in combination with psoriasis medications. This is a significant gap in the literature and represents a genuine safety concern. Anyone considering peptides alongside conventional psoriasis treatment should do so under medical supervision with appropriate monitoring. Understanding whether peptides are legal in your jurisdiction and knowing about peptides and drug testing are also practical considerations.
Quality and sourcing concerns
Peptide quality varies significantly between suppliers, and for a condition requiring sustained use, quality consistency is critical. The best peptide vendors guide covers what to look for in a supplier, and the peptide testing labs guide explains how to verify peptide purity and identity. The distinction between research and pharmaceutical grade peptides is important context for anyone evaluating sources.
Contaminants, incorrect concentrations, and degraded peptides can all compromise both safety and efficacy. Third-party testing certificates, HPLC analysis, and mass spectrometry verification should be standard requirements for any peptide used in a health-related protocol. The peptide therapy online guide covers the landscape of online peptide access, including how to evaluate quality claims.
Complementary strategies that support peptide protocols
Peptides do not work in isolation. Their effectiveness is influenced by the overall environment in which they operate. Several complementary strategies can support and enhance peptide-based approaches to psoriasis management.
The gut-skin axis
Research increasingly links gut health to psoriasis outcomes. Gut dysbiosis, or an imbalance in gut microbial populations, is more common in psoriasis patients than in the general population. The gut microbiome influences systemic immune function, and disruptions to gut health can exacerbate autoimmune conditions.
BPC-157 is particularly interesting in this context because of its origin in gastric juice and its demonstrated gut-healing properties. Using BPC-157 may address both gut and skin aspects of psoriasis simultaneously. The peptides for gut health category page covers the range of peptides relevant to gastrointestinal wellness.
Stress and neuroimmune factors
Stress is one of the most common triggers for psoriasis flares. The neuroimmune connection, the pathway through which psychological stress translates into immune activation, is well-established. Stress increases cortisol, which initially suppresses inflammation but ultimately leads to cortisol resistance and rebound inflammation. It also activates the sympathetic nervous system, which directly stimulates immune cells.
Peptides like Selank and Semax, primarily known for their anxiolytic and cognitive effects, may have indirect benefits for psoriasis through stress reduction. By modulating the stress response, they could reduce the neuroimmune triggers that initiate psoriasis flares. The Selank peptide dosage and Semax peptide dosage guides cover these peptides in detail. The peptides for anxiety guide provides broader context, and the best peptides for brain function covers the neurological dimension.
Sleep, exercise, and vitamin D
Three lifestyle factors have documented effects on psoriasis outcomes that are relevant to peptide protocol optimization.
Sleep deprivation increases inflammatory markers and impairs immune regulation. Adequate sleep, typically seven to nine hours per night, supports the immune rebalancing that peptides like thymosin alpha-1 promote.
Regular moderate exercise reduces systemic inflammation and improves immune regulation. It also promotes blood flow to the skin, potentially improving peptide delivery to affected areas. The best peptide for energy guide covers peptides that can support exercise capacity, and the peptides for muscle growth and peptides for fat loss categories are relevant for those addressing metabolic factors that influence psoriasis.
Vitamin D is particularly important for psoriasis. It regulates LL-37 production, modulates immune function, and has direct effects on keratinocyte proliferation and differentiation. Many psoriasis patients are vitamin D deficient, and supplementation has shown benefits in some studies. Vitamin D optimization supports the same immune regulatory pathways that peptides like thymosin alpha-1 and KPV target.
Addressing psoriasis comorbidities with peptides
Psoriasis rarely exists in isolation. It is associated with a range of comorbidities that peptides may also address. Psoriatic arthritis, affecting up to 30 percent of psoriasis patients, involves joint inflammation that tissue repair peptides like BPC-157 are specifically studied for. The best peptides for joint pain and best peptides for tendon repair guides are directly relevant here. The peptides for shoulder pain article covers joint-specific applications, and the best peptide for pain guide addresses pain management broadly.
Bone density concerns are another comorbidity, as psoriasis patients have elevated rates of osteoporosis. The peptides for osteoporosis guide covers this specifically, while the peptides for bone healing and peptides for bone and cartilage repair articles address the underlying mechanisms. For those with neurological impacts of chronic autoimmune conditions, the peptides for multiple sclerosis guide covers another autoimmune condition where peptide research is active.
Cardiovascular risk is elevated in psoriasis patients due to chronic systemic inflammation. The SS-31 peptide and other mitochondrial-targeted peptides are being studied for their cardiovascular protective properties. The longevity peptides guide covers peptides that address the systemic inflammation and oxidative stress that link psoriasis to cardiovascular disease. Additional resources include the peptides for hair loss and peptides for hair growth guides for scalp psoriasis patients, and the peptides for weight loss guide for metabolic considerations.
Skincare and topical combinations
Beyond peptide-specific formulations, overall skincare matters for psoriasis management. Maintaining the skin barrier through appropriate moisturization reduces the triggers that initiate inflammatory cascades. The interaction between vitamin C and peptides is relevant for skin health, as is the combination of peptides and retinol for those using retinoids. The hyaluronic acid and peptide article covers another common combination for skin hydration support. The copper peptides and vitamin C interaction guide is also important for those using GHK-Cu topically.
For those dealing with specific skin concerns alongside psoriasis, SeekPeptides offers guides on peptides for scars, peptides for dark circles, peptides for wrinkles, and peptides for acne. The biomimetic peptides guide and the Snap-8 peptide article cover specific synthetic peptides for skin repair. The collagen peptides for cellulite guide addresses another skin-structural concern. For women navigating psoriasis alongside hormonal changes, the best peptides for women guide and peptides for menopause provide relevant context, while the broader topic of peptides for hormone balance covers hormonal modulation.
Comparing peptides with conventional psoriasis treatments
To put peptide research in proper context, it helps to understand how these compounds compare with established psoriasis treatments.
Topical steroids
Topical corticosteroids remain the first-line treatment for mild to moderate psoriasis. They work by broadly suppressing immune function in the skin. They are fast-acting and effective, but long-term use causes skin thinning, telangiectasia (visible blood vessels), and tachyphylaxis (reduced effectiveness over time). The PEPITEM-derived tripeptides mentioned earlier produced comparable efficacy to Clobetasol Propionate 0.05 percent, one of the strongest topical steroids, without the steroid-associated side effects. This comparison, if confirmed in larger studies, would be significant.
Biologics
Biologic therapies represent the most targeted conventional treatments available. They block specific cytokines (TNF-alpha, IL-17, IL-23) with high precision. They are highly effective for many patients but carry risks including increased infection susceptibility, injection site reactions, and the development of anti-drug antibodies.
Peptides differ from biologics in several ways. They are generally smaller molecules with broader mechanisms of action. They tend to modulate rather than completely block pathways. And their side effect profiles, based on available data, appear milder. However, peptides lack the large-scale randomized controlled trial evidence that supports biologics. This evidence gap is the single largest limitation of peptide-based approaches.
Cost considerations
Biologic therapies for psoriasis can cost tens of thousands of dollars annually. Research peptides are generally much less expensive, though costs vary significantly by source and quality. The peptide therapy cost guide and how much peptides cost article provide realistic cost breakdowns. The peptide cost calculator helps estimate costs for specific protocols.
Cost should never be the primary factor in choosing a treatment approach, but accessibility matters. If peptide-based approaches can provide meaningful benefit at lower cost and with fewer side effects, that combination would make them attractive as either standalone or adjunctive therapy.
The integration approach
The most pragmatic approach for many patients may not be "peptides instead of conventional treatment" but rather "peptides alongside conventional treatment." Using KPV to modulate NF-kB while on a biologic, for example, could theoretically enhance the biologic effectiveness or allow dose reduction. Using BPC-157 to support tissue repair while corticosteroids manage acute flares could accelerate healing. These combinations are theoretical and require medical supervision, but they represent a practical path forward while peptide-specific evidence accumulates.
The what are peptides used for overview provides broader context for the range of conditions being investigated, and the peptides for eczema guide covers a closely related inflammatory skin condition where similar peptides are being studied. For practical protocol planning, the BPC-157 dosage calculator is useful for those incorporating BPC-157, and the wolverine stack peptide article covers a popular healing-focused combination. The klow peptides and glow peptides guides cover emerging formulations relevant to skin health. Additional context is available in the peptides for anti-aging category and the fast injury healing category, both of which overlap with psoriasis-relevant research.
Frequently asked questions
What is the most studied peptide for psoriasis?
KPV is the most extensively studied peptide specifically for psoriasis-related mechanisms. Its direct suppression of NF-kB, the master inflammatory transcription factor involved in psoriasis, gives it the strongest mechanistic rationale. Muramyl peptide (Licopid) has the most robust clinical data, with a study of 86 patients showing 58.1 percent significant improvement. Both are worth investigating, though for different reasons. KPV has the stronger mechanistic story, while muramyl peptide has the stronger clinical evidence.
Can peptides replace biologic therapy for psoriasis?
Not based on current evidence. Biologics have extensive clinical trial data supporting their use in moderate to severe psoriasis. Peptides, while mechanistically promising, lack comparable clinical evidence. The more pragmatic question is whether peptides can complement biologic therapy, potentially improving outcomes or allowing dose reduction. This combination approach is theoretically sound but has not been formally studied.
How long does it take for peptides to show results in psoriasis?
Available data suggests timelines of 3 to 6 weeks for noticeable improvement with KPV. PEPITEM-derived tripeptides showed results in as little as 7 days in preclinical studies. BPC-157 tissue repair effects can begin within days but full structural repair takes weeks to months. Thymosin alpha-1 immune rebalancing effects may take 4 to 8 weeks to manifest clinically. These timelines are estimates based on limited data and individual responses will vary significantly.
Are peptides safe to use alongside methotrexate or other immunosuppressants?
No formal interaction studies exist for research peptides combined with conventional psoriasis medications. Theoretically, combining immune-modulating peptides with immunosuppressive drugs could produce excessive immune suppression. This is particularly concerning with KPV (NF-kB suppression) and thymosin alpha-1 (immune regulation). Medical supervision is essential if considering peptides alongside any conventional psoriasis treatment.
Can I use topical peptides directly on psoriatic plaques?
Topical KPV and PEPITEM-derived tripeptides have been studied for direct application to affected skin. GHK-Cu can be applied topically though penetration is limited. BPC-157 is not typically used topically for skin conditions. When applying any topical product to psoriatic skin, patch testing on a small area first is advisable, as compromised skin may react differently than healthy skin. Some users report initial irritation that resolves with continued use.
Which administration route is best for psoriasis peptide protocols?
It depends on the peptide and the extent of disease. Topical application works well for localized plaques with peptides like KPV and GHK-Cu. Subcutaneous injection is more appropriate for systemic immune modulation with thymosin alpha-1 or when treating widespread disease. Oral delivery is convenient for maintenance protocols with KPV or BPC-157. Many protocols combine routes, using systemic delivery for immune modulation and topical delivery for direct plaque treatment.
Is there a best peptide stack specifically for psoriasis?
No clinically validated psoriasis peptide stack exists. A theoretically sound approach based on known mechanisms would combine KPV for NF-kB suppression, thymosin alpha-1 for immune regulation, BPC-157 or GHK-Cu for tissue repair, and collagen peptides for structural support. This combination has not been studied as a protocol and should only be explored under medical supervision.
Do peptides for psoriasis require a prescription?
Regulatory status varies by country and by specific peptide. Thymosin alpha-1 is available by prescription in over 35 countries. Other peptides like KPV and BPC-157 are available as research compounds in many jurisdictions. Collagen peptides are widely available as dietary supplements. Understanding the legal status of specific peptides in your jurisdiction is important before obtaining them.
Can peptides help with psoriatic arthritis, not just skin symptoms?
Several of the peptides discussed have potential relevance to psoriatic arthritis. BPC-157 and TB-500 are extensively studied for joint and tissue repair. Thymosin alpha-1 immune regulation would theoretically benefit the systemic autoimmune process driving both skin and joint involvement. KPV NF-kB suppression would affect inflammation regardless of location. The systemic nature of these peptide mechanisms means they could potentially address both skin and joint manifestations of psoriatic disease.
What should I look for in peptide quality when sourcing for a psoriasis protocol?
Look for third-party testing certificates including HPLC purity analysis and mass spectrometry identity verification. Purity should be 98 percent or higher for research-grade peptides. Proper lyophilization, appropriate packaging (amber vials to protect from light), and verifiable cold chain shipping are all quality indicators. Avoid suppliers who do not provide testing documentation or who make therapeutic claims.
For members of SeekPeptides, the journey from research to practical application becomes considerably easier. The platform provides detailed protocol guides, dosage calculators, peptide stacking frameworks, and regularly updated research summaries that translate complex immunological findings into actionable information. If you are managing psoriasis and exploring peptide research, membership gives you access to the tools and knowledge base needed to make informed decisions alongside your healthcare provider. From the peptide calculator to the reconstitution calculator, from beginner guides to advanced protocol planning, SeekPeptides exists to make peptide education accessible, accurate, and practical.
In case I do not see you, good afternoon, good evening, and good night. Join us here.



