Jan 22, 2026
Four days. That is all it takes for PE-22-28 to double the rate of new neuron formation in the hippocampus.
Traditional antidepressants require three to four weeks to achieve the same neurogenic effect. This is not a subtle difference. This is not a marginal improvement. This represents a fundamental shift in how researchers approach nootropic peptides and their potential for brain health.
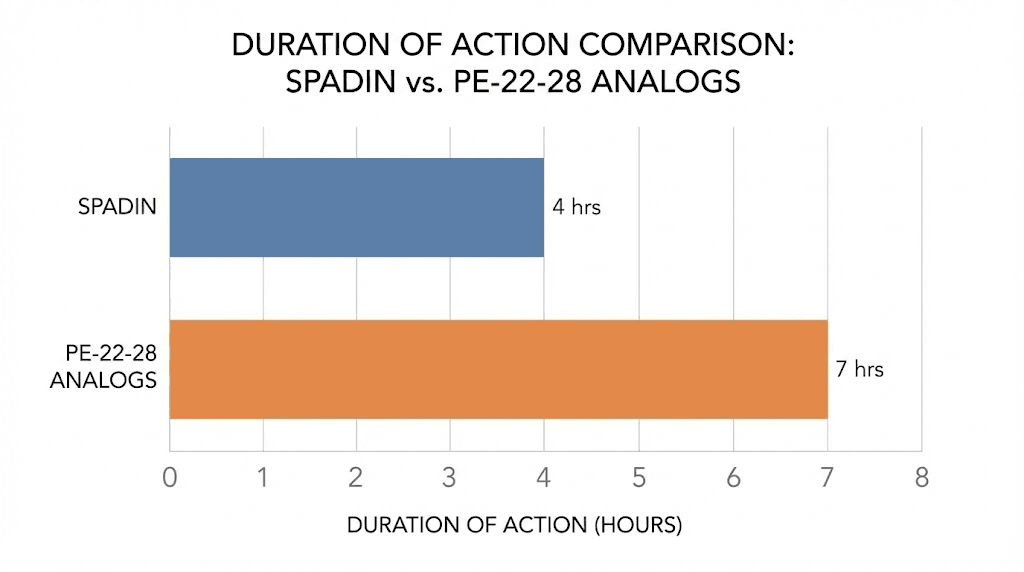
The story of PE-22-28 begins with a larger molecule called spadin. Researchers discovered that mice lacking the TREK-1 potassium channel displayed resistance to depression and showed enhanced serotonin transmission. They found spadin, a natural peptide that blocks this channel. But spadin had problems. Its effects lasted only seven hours. Its potency was modest. It degraded quickly in the bloodstream.
So scientists did what scientists do. They analyzed the breakdown products of spadin in blood samples, looking for fragments that retained activity. What they discovered changed everything about this field of research. A seven amino acid fragment, positions 22 through 28 of the original propeptide, displayed 300 times greater affinity for the TREK-1 channel than spadin itself. The duration of action extended from seven hours to nearly twenty-four. And the neurogenic effects, the ability to stimulate the birth of new neurons, remained fully intact.
This guide explores every aspect of PE-22-28 that researchers need to understand.
We will examine how it works at the molecular level, what the research studies actually show, how it compares to other cognitive peptides, and what considerations matter for laboratory applications. SeekPeptides has compiled the most comprehensive analysis available, drawing from published literature and documented research protocols.
Understanding TREK-1 and why blocking it matters
The TREK-1 channel belongs to a family called two-pore domain potassium channels. These proteins sit in neuron membranes and regulate electrical excitability. When TREK-1 opens, potassium flows out of the cell, making the neuron less likely to fire. Think of TREK-1 as a brake on neural activity.
Depression changes this system. Chronic stress increases TREK-1 expression in the hippocampus, the brain region critical for memory and mood regulation. More TREK-1 means more braking. More braking means reduced neural firing. Reduced neural firing correlates with the cognitive fog, emotional flatness, and memory problems that characterize depressive states.
The evidence supporting TREK-1 as a depression target comes from multiple sources. Genetic studies showed that mice lacking the kcnk2 gene, which codes for TREK-1, display a depression-resistant phenotype. They show enhanced serotonin neurotransmission in the dorsal raphe nucleus. They exhibit increased neurogenesis in the hippocampal dentate gyrus. They respond differently to stress challenges compared to normal mice.
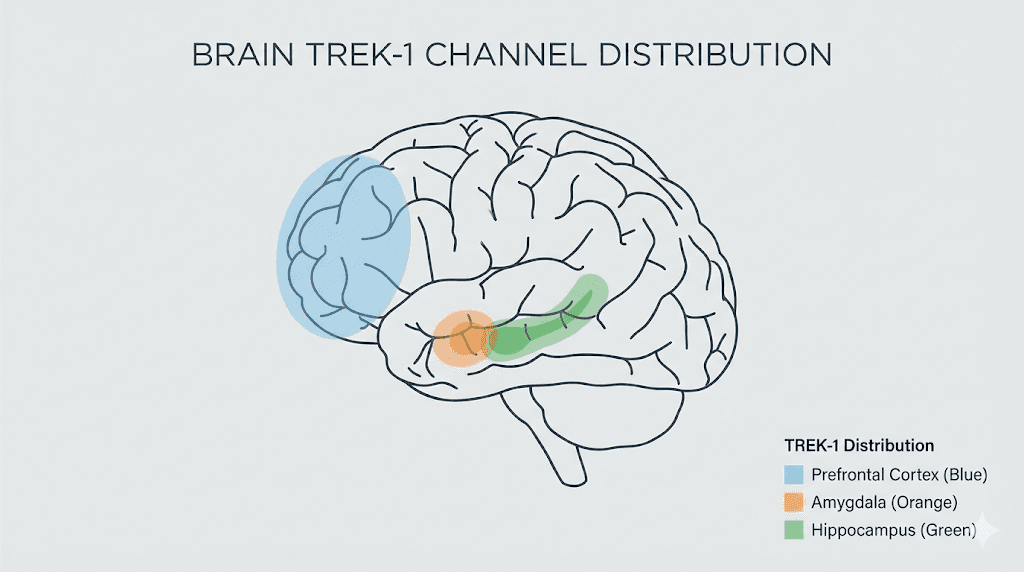
Where TREK-1 channels concentrate in the brain
Location matters for understanding PE-22-28 effects. TREK-1 channels show high expression in the prefrontal cortex, the amygdala, and the hippocampus. These three regions form the core circuit for mood regulation, anxiety processing, and memory consolidation. The prefrontal cortex handles executive function and emotional regulation. The amygdala processes threat detection and fear responses. The hippocampus manages memory formation and spatial navigation.
This distribution pattern explains why TREK-1 inhibitors produce their characteristic effects. Block the channel in the prefrontal cortex and you may see improvements in decision making and emotional control. Block it in the amygdala and anxiety responses may diminish. Block it in the hippocampus and neurogenesis increases while memory function potentially improves.
TREK-1 also exists outside the brain. The channel appears in heart tissue, smooth muscle, kidney, and skeletal muscle. This raises obvious questions about selectivity and side effects. One advantage of spadin and its analogs like PE-22-28 is their apparent selectivity for the channel in its activated state, particularly when stimulated by arachidonic acid. This may explain why safety profiles in animal studies appear favorable.
The connection between TREK-1 and serotonin
TREK-1 inhibition increases serotonergic transmission.
This occurs through effects on the dorsal raphe nucleus, the brain structure that produces most serotonin. When TREK-1 is blocked, neurons in this region fire more readily.
More firing means more serotonin release.
But here is where PE-22-28 differs from traditional antidepressants. Selective serotonin reuptake inhibitors (SSRIs) work by preventing serotonin reabsorption after it is released. They do not change how much serotonin neurons produce or how often they fire. TREK-1 inhibitors work upstream of this process. They affect the fundamental excitability of serotonin-producing neurons.
This mechanistic difference may explain the speed advantage. SSRIs require weeks for adaptive changes to accumulate. TREK-1 inhibitors produce immediate changes in neural firing patterns. The downstream effects on neuroplasticity and neurogenesis appear to follow more quickly.
The molecular profile of PE-22-28
PE-22-28 consists of seven amino acids.
This makes it substantially smaller than spadin, which contains seventeen amino acids. Smaller peptides often show improved stability and bioavailability. They may cross biological membranes more easily. They are certainly easier and cheaper to synthesize.
The IC50 value for PE-22-28 at the TREK-1 channel measures 0.12 nanomolar. Compare this to spadin at 40-60 nanomolar. This represents roughly a 300-fold improvement in binding affinity. Lower IC50 means the peptide achieves its effect at lower concentrations. Lower required concentrations mean smaller doses may be effective.
Researchers also developed modified versions of PE-22-28 to study structure-activity relationships. G/A-PE 22-28, which substitutes glycine for alanine at a key position, shows an IC50 of 0.10 nanomolar. Even better binding. A biotinylated version allows tracking in tissues and shows an IC50 of 1.2 nanomolar, still substantially more potent than spadin.
How PE-22-28 binds to the TREK-1 channel
The binding mechanism involves a specific conformational state of the TREK-1 channel. Pharmacological and mutational analysis suggests PE-22-28 binds when the channel adopts a down conformation.
The binding site appears distinct from the C-terminal domain. Evidence points to location on the extracellular face of the channel protein.
This binding mode has practical implications. TREK-1 channels respond to multiple signals including arachidonic acid, mechanical stretch, temperature changes, and pH shifts. PE-22-28 specifically antagonizes the arachidonic acid activation pathway. It does not simply block the channel under all conditions. It modulates activity in a context-dependent manner.
Such selectivity may explain why PE-22-28 and related compounds appear to avoid interfering with other TREK-1 functions. The channel plays roles in pain perception, neuroprotection during ischemia, and seizure threshold regulation. Animal studies with spadin showed no effects on these functions at doses producing antidepressant activity. The channel still works for its other jobs. Only the depression-relevant signaling pathway gets modified.
Duration of action compared to spadin
One of the most significant improvements PE-22-28 offers over spadin is duration of effect. Despite having a relatively short half-life in serum when measured in vitro, the antidepressant efficacy persists for nearly 24 hours in vivo.
The numbers tell the story clearly. Spadin at 100 micrograms per kilogram showed a half-time of effect of only 6 hours. G/A-PE 22-28 at just 3.2 micrograms per kilogram achieved a half-time of 14 hours. At 32 micrograms per kilogram, this extended to 21 hours. The biotinylated version at 40 micrograms per kilogram reached 23 hours.
This extended duration transforms potential research applications. A compound requiring multiple daily administrations presents different challenges than one with once-daily activity. For researchers studying chronic effects, the logistical simplification matters enormously.
Neurogenesis research findings
The hippocampus produces new neurons throughout adult life. This process, called adult neurogenesis, occurs primarily in the dentate gyrus subregion. Depression correlates with reduced hippocampal volume and decreased neurogenesis. Effective antidepressants, regardless of their primary mechanism, tend to restore neurogenic capacity.
Classical antidepressants require two to four weeks of administration before neurogenesis increases become measurable. This timeline matches the clinical observation that patients need similar periods before experiencing symptom relief. The correlation suggests neurogenesis may be causally important for antidepressant effects, not merely a coincidental biomarker.
PE-22-28 accelerates this timeline dramatically. In mouse studies, four days of treatment at 3.0-4.0 micrograms per kilogram per day significantly increased BrdU-positive cells in the hippocampus. BrdU is a marker that labels dividing cells, allowing researchers to count new neurons.
Quantifying the neurogenic effect
The numbers from published research are striking. Saline-injected control mice showed 899 plus or minus 109 BrdU-positive cells.
PE-22-28 treated mice showed 1,736 plus or minus 126 cells. G/A-PE 22-28 produced 2,110 plus or minus 132 cells. The biotinylated version yielded 1,809 plus or minus 267 cells. All differences reached high statistical significance with p values less than 0.0001.
These numbers represent roughly a doubling of new neuron formation. In just four days. This rate of neurogenic stimulation has not been reported with any other antidepressant intervention. The speed challenges fundamental assumptions about how quickly brain structure can change in response to pharmacological intervention.
The fate of these new neurons matters too. Producing neural precursor cells is one thing. Having them mature into functional neurons is another. Studies tracking synaptogenesis markers suggest the new cells do mature properly. PSD-95, a protein that marks functional synaptic connections, increased significantly by 36 hours of treatment. Levels approximately doubled compared to baseline.
Hippocampal volume and depression
Patients with major depressive disorder consistently show reduced hippocampal volume on brain imaging studies.
This shrinkage correlates with illness duration and severity. Successful treatment tends to reverse the volume loss over time.
If PE-22-28 doubles neurogenesis rate, it might theoretically accelerate hippocampal volume recovery. No human imaging studies exist yet to test this hypothesis. But the preclinical data provides a plausible mechanism. More new neurons forming means more raw material for volume restoration.
This connects PE-22-28 to broader concepts in cellular regeneration and brain health. The hippocampus is not merely a mood center. It handles memory consolidation, spatial navigation, and contextual learning. Interventions that support hippocampal health have implications beyond depression treatment.
Synaptogenesis and neural connectivity
New neurons need connections to function. The process of forming these connections, synaptogenesis, determines whether newborn neurons integrate into existing circuits. PE-22-28 appears to promote both neurogenesis and synaptogenesis simultaneously.
PSD-95 serves as the key marker.
This protein concentrates at postsynaptic densities, the receiving end of synaptic connections. More PSD-95 expression indicates more synaptic infrastructure. In studies with PE-22-28 analogs, PSD-95 levels increased to approximately twice baseline within 36 hours of treatment.
This dual action, stimulating both new neuron birth and new synapse formation, distinguishes PE-22-28 from interventions that affect only one process. Some neurogenic stimuli produce immature neurons that fail to integrate. Some synaptogenic signals work only on existing neurons. PE-22-28 appears to coordinate both processes.
How synaptogenesis supports cognitive function
Synaptic density correlates with cognitive performance. More connections mean more potential pathways for information processing. Learning and memory depend on the brain ability to form new synaptic connections and strengthen existing ones.
Depression impairs synaptic plasticity. Stress hormones damage existing connections. Reduced neuronal activity leads to synaptic pruning. The net effect is a brain that becomes progressively less connected, less flexible, less capable of the neural gymnastics that underlie normal cognition.
Interventions that restore synaptic density may restore cognitive function. This is one reason researchers have interest in PE-22-28 beyond pure antidepressant applications. The cognitive symptoms of depression, the difficulty concentrating, the memory problems, the mental fog, may respond to synaptic restoration even before mood symptoms improve.
Users report improvements in clarity, focus, and short-term memory with peptide interventions targeting these pathways. These cognitive effects may result from increased synaptic density and enhanced neural network efficiency. The brain becomes better connected and communication between regions improves.
Antidepressant testing in animal models
Researchers assess antidepressant potential using several standardized behavioral tests. Each measures different aspects of depression-relevant behavior. Together they provide converging evidence about a compound therapeutic profile.
The forced swim test measures behavioral despair. Mice are placed in water-filled cylinders and their activity is recorded. Depressed animals give up swimming quickly and float passively. Antidepressants reduce this immobility time, keeping animals active longer.
PE-22-28 treated animals showed significantly reduced immobility. Control mice given saline displayed 161.7 plus or minus 6.49 seconds of immobility. PE-22-28 reduced this to 91.8 plus or minus 6.1 seconds. The G/A variant showed 110.2 plus or minus 6.6 seconds. The biotinylated version showed 140.7 plus or minus 7.1 seconds.
All represented statistically significant improvements.
Novelty suppressed feeding test results
This test measures anxiety-related behavior. Hungry mice are placed in a novel environment with food in the center. Anxious animals hesitate to approach the food despite their hunger. Antidepressants reduce this latency to eat.
Sub-chronic treatment with PE-22-28 significantly reduced the latency to eat the food pellet. Treated animals reached the food in 129.2 plus or minus 15.28 seconds. Control animals required 226.1 plus or minus 34.97 seconds. This roughly 40 percent reduction suggests anxiolytic effects alongside antidepressant activity.
The novelty suppressed feeding test is particularly relevant because it responds specifically to chronic antidepressant treatment. Acute doses of SSRIs do not change feeding latency. Only after weeks of treatment do effects emerge. That PE-22-28 shows effects after sub-chronic dosing, just days of treatment, underscores its accelerated timeline of action.
Learned helplessness paradigm
In this model, animals exposed to inescapable stress subsequently fail to escape from avoidable stress. They have learned that their actions do not matter. This helplessness parallels core features of human depression where patients feel incapable of changing their circumstances.
Four-day treatment with PE-22-28 analogs significantly reduced escape latencies in this paradigm. Animals learned that escape was possible and acted on this learning. The reversal of learned helplessness suggests effects on motivation and agency, not just passive mood changes.
Across all three behavioral paradigms, PE-22-28 and its analogs performed comparably to or better than spadin while requiring substantially lower doses. The improved potency and extended duration translate into more robust behavioral effects with simpler dosing regimens.
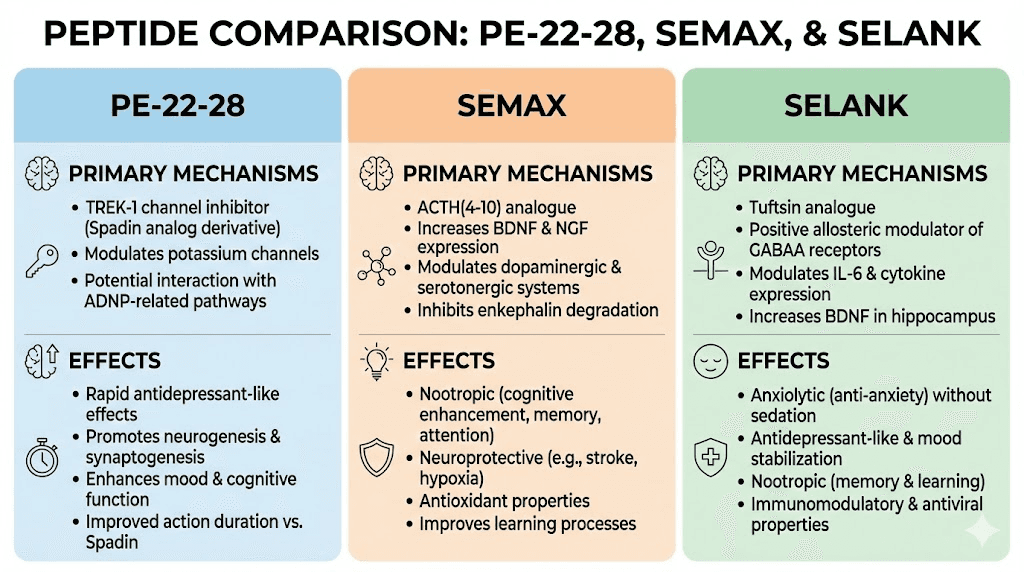
Comparing PE-22-28 to other cognitive peptides
The nootropic peptide field includes several established compounds. Semax and Selank have longer histories of human use, particularly in Russia where they have regulatory approval. Understanding how PE-22-28 fits into this landscape helps researchers select appropriate tools for their questions.
Semax derives from ACTH, the adrenocorticotropic hormone. It consists of seven amino acids and primarily targets cognitive enhancement. Effects center on attention, learning, and memory. The mechanism involves BDNF modulation and dopaminergic and serotonergic pathway effects. Semax does not produce stimulant or sedative effects. It simply sharpens cognitive function.
Selank derives from tuftsin, an immune-modulating tetrapeptide. It contains seven amino acids and targets anxiety reduction and stress resilience. Effects include anxiolytic action without sedation, emotional stabilization, and immune support. The mechanism involves GABA receptor modulation and serotonin system effects.
PE-22-28 occupies a distinct niche. While all three peptides affect brain function, their primary targets and effect profiles differ substantially.
Mechanism differences between the three peptides
Semax works through BDNF (brain-derived neurotrophic factor) modulation.
BDNF supports neuron survival, encourages new neuron growth, and facilitates synaptic plasticity. By increasing BDNF levels, Semax creates conditions favorable for learning and memory consolidation.
Selank modulates GABA receptors and influences the serotonin system. GABA is the brain primary inhibitory neurotransmitter. Enhancing GABAergic signaling produces calming effects without the sedation associated with benzodiazepines. The serotonin effects contribute to mood stabilization.
PE-22-28 targets the TREK-1 potassium channel directly. By blocking this channel, it increases neuronal excitability, particularly in serotonin-producing neurons. But it also appears to function as a BDNF-mimetic, activating TrkB receptors that BDNF normally binds. This dual action, combining ion channel effects with growth factor signaling, may explain its rapid neurogenic effects.
The BDNF-mimetic aspect is particularly interesting. BDNF itself is a large protein that cannot easily be administered as a therapeutic. It does not cross the blood-brain barrier well. It is unstable. PE-22-28 may provide some of the benefits of enhanced BDNF signaling through a small, stable, bioavailable peptide.
Which peptide for which research goal
Selection depends on the research question. For pure cognitive enhancement, attention improvement, and learning studies, Semax offers the most established evidence base. Its effects on focus and information processing are well documented.
For anxiety models and stress response studies, Selank provides targeted anxiolytic effects. Its ability to reduce worry and emotional reactivity without sedation makes it valuable for behavioral research where baseline activity levels matter.
For depression models, neurogenesis studies, and neuroplasticity research, PE-22-28 offers unique advantages. Its rapid neurogenic effects and extended duration make it particularly suitable for time-course studies. The ability to see effects in four days rather than four weeks accelerates research timelines substantially.
Some researchers combine peptides. Pinealon for brain health, Selank for stress reduction, and PE-22-28 for neurogenesis and mood can theoretically work together. But combination studies add complexity and interpretation challenges. Single-agent studies usually precede combination experiments.
Research protocols and methodological considerations
Laboratory studies of PE-22-28 have employed various dosing regimens.
Understanding these protocols helps researchers design appropriate experiments and interpret published findings.
Acute studies typically used intraperitoneal injection at doses of 3.0 to 4.0 micrograms per kilogram. This route ensures reliable systemic absorption for dose-response characterization. Higher doses up to 32-40 micrograms per kilogram were used in duration studies to establish upper bounds of effect persistence.
Sub-chronic studies for neurogenesis assessment ran for four consecutive days. This minimal treatment period proved sufficient to observe doubled BrdU-positive cell counts. Longer treatment periods have not been extensively characterized in published literature, representing an area for further investigation.
Oral administration has been explored. Doses of 1 milligram per kilogram via gavage produced antidepressant effects in behavioral testing. This oral activity is notable because many peptides show poor oral bioavailability due to gastrointestinal degradation. The small size and particular sequence of PE-22-28 may confer some protection against enzymatic breakdown.
Considerations for reconstitution and storage
Like all peptides, PE-22-28 requires proper handling to maintain stability. Lyophilized powder form offers the best shelf stability. Reconstitution should use appropriate diluents such as bacteriostatic water for research applications.
Temperature matters significantly for peptide stability. Proper storage typically involves freezing lyophilized material and refrigerating reconstituted solutions.
The duration peptides remain stable depends on storage conditions, with frozen material lasting longest.
Light exposure can degrade peptides. Amber vials or foil wrapping provides protection from photodegradation. Room temperature storage should be minimized, with reconstituted solutions returned to refrigeration promptly after use.
Dosing calculations for research
Researchers can use peptide calculators to convert between concentration units and determine volumes for specific doses. Understanding peptide dosage calculations prevents errors that can invalidate experiments.
The peptide dosing fundamentals remain consistent across compounds. Body weight-based dosing, typical in animal studies, requires accurate weight measurement. Concentration verification ensures delivered doses match intended doses.
SeekPeptides provides reconstitution calculators to simplify these computations. Accurate preparation is foundational. Without correct dosing, experimental results become uninterpretable.
Safety profile from preclinical studies
The safety data for PE-22-28 comes primarily from animal studies and from understanding the parent compound spadin. No human clinical trials have been published.
This limits what can be stated definitively about human safety.
In animal studies, PE-22-28 and its analogs appeared well tolerated at effective doses. No effects on hERG channels were observed, suggesting cardiac safety at the ion channel level. Heart rate and blood pressure remained stable. Glucose regulation was unaffected. Pain perception showed no changes at antidepressant doses.
The absence of TREK-1-related side effects deserves emphasis. The channel serves multiple functions beyond mood regulation. TREK-1 contributes to pain processing, seizure threshold, and neuroprotection during ischemia. Spadin studies specifically evaluated these functions and found no interference at therapeutic doses.
Reported effects and tolerability
Based on preclinical data and limited observations, PE-22-28 shows several favorable characteristics. No sedative effects have been noted. This contrasts with many psychoactive compounds that impair alertness. No dependency or withdrawal phenomena were observed. Sleep-wake cycles remained normal up to high doses. EEG rhythms showed no changes regardless of sleep-wake state.
Memory function was preserved and potentially enhanced. Unlike some agents that impair cognition while affecting mood, spadin and its analogs showed no negative effects on short or long-term memory in animal testing. This aligns with the neurogenic and synaptogenic effects that would theoretically support rather than impair memory.
The contrast with traditional antidepressants is notable. SSRIs commonly cause sexual dysfunction, weight changes, emotional blunting, and discontinuation syndromes. SNRIs add blood pressure effects. Tricyclics bring anticholinergic side effects. Ketamine produces dissociation. PE-22-28 analogs showed none of these issues in preclinical evaluation.
Potential concerns and unknowns
The preclinical safety profile, while favorable, does not guarantee human safety. Species differences in drug metabolism, receptor distribution, and physiological responses mean animal data always requires cautious interpretation.
Long-term exposure data is limited. Most studies lasted days to weeks. Chronic effects over months or years remain uncharacterized. Effects on development, pregnancy, or nursing have not been systematically studied.
Individual variation in response is unknown. Human populations are far more genetically diverse than laboratory mouse strains. Some individuals might respond differently or experience effects not seen in animals.
PE-22-28 remains a research compound. It has not received FDA approval or similar regulatory clearance for any therapeutic use. It is not approved by the World Anti-Doping Agency. Human consumption for performance enhancement or self-treatment purposes falls outside approved uses.
BDNF mimetic properties and TrkB activation
Beyond TREK-1 inhibition, PE-22-28 appears to function as a BDNF mimetic. This means it activates some of the same signaling pathways that brain-derived neurotrophic factor activates. The implications extend beyond what TREK-1 inhibition alone would predict.
BDNF binds to TrkB receptors on neurons. This binding triggers intracellular signaling cascades that promote cell survival, encourage neurite outgrowth, and facilitate synaptic plasticity. BDNF is often called a fertilizer for neurons. It creates conditions where neurons thrive and connections strengthen.
PE-22-28 activates TrkB receptors similar to natural BDNF. By mimicking BDNF and stimulating TrkB, PE-22-28 may improve neuroplasticity, help the brain adapt to stress, and enhance emotional resilience. This dual mechanism, combining ion channel effects with growth factor signaling, potentially amplifies the neurogenic response.
Why BDNF signaling matters for mood
BDNF levels are reduced in depressed patients. Successful antidepressant treatment typically restores BDNF levels. This correlation led to the neurotrophin hypothesis of depression, suggesting that reduced trophic support for neurons contributes to the disorder.
Direct BDNF administration as a therapeutic is impractical. The protein is large, unstable, and does not cross the blood-brain barrier efficiently. Various strategies have been explored to circumvent these limitations, including small molecules that increase BDNF expression and peptides that mimic BDNF effects.
PE-22-28 may represent an effective BDNF mimetic approach. By activating TrkB receptors directly, it bypasses the need to increase BDNF production. The signaling pathways downstream of TrkB get activated regardless of BDNF levels. This could be particularly valuable in conditions where BDNF production capacity is compromised.
Implications for neuroregeneration
The combination of TREK-1 inhibition and TrkB activation positions PE-22-28 at an interesting intersection. One mechanism increases neuronal excitability and serotonin transmission. The other promotes cell survival and growth. Together they create conditions favoring both new neuron production and survival of those new neurons.
This has potential relevance beyond depression. Neurodegenerative conditions involve progressive neuron loss. Traumatic brain injury damages existing neural networks. Age-related cognitive decline correlates with reduced synaptic density and neurogenesis. Interventions that support neurogenesis and synaptogenesis could theoretically help in multiple contexts.
The research applications extend to inflammation research, as neuroinflammation contributes to many brain disorders. PE-22-28 effects on hippocampal function connect to memory studies. The mood-regulating properties relate to anxiety research and stress biology.
Comparison with pharmaceutical approaches
PE-22-28 represents a different paradigm from conventional antidepressants. Understanding these differences helps contextualize its potential role in research and future therapeutic development.
SSRIs work by blocking serotonin reuptake transporters. This increases serotonin availability in synapses but does not change how much serotonin neurons produce. Effects require weeks to manifest fully as the brain adapts to altered serotonin dynamics. Side effects include sexual dysfunction, emotional blunting, and discontinuation symptoms.
SNRIs add norepinephrine reuptake inhibition to serotonin effects. This may help certain symptom profiles but adds blood pressure and heart rate effects to the side effect burden.
Tricyclic antidepressants affect multiple neurotransmitter systems simultaneously. Their lack of selectivity produces anticholinergic effects like dry mouth, constipation, and cognitive impairment. Overdose danger limits their use.
Ketamine represents the recent breakthrough in fast-acting antidepressants. It works through NMDA receptor blockade and produces rapid effects within hours. But it requires supervised administration due to dissociative effects and has abuse potential.
Speed of effect as a differentiator
The delay between starting antidepressant treatment and experiencing benefit represents a significant clinical problem. Patients in crisis need help quickly. Three to four weeks of worsening before improvement is suboptimal at best, dangerous at worst.
PE-22-28 effects on neurogenesis appear within four days. Behavioral effects in animal models emerge with similar speed. If these rapid effects translate to humans, it could address a major unmet need in depression treatment.
The comparison with ketamine is instructive. Ketamine works within hours but requires medical supervision due to acute effects. PE-22-28 appears to work within days but without the psychoactive effects requiring monitoring. Different speed profiles suit different situations.
Mechanistic advantages of ion channel targeting
Most antidepressants target neurotransmitter handling, whether reuptake, release, or receptor sensitivity. PE-22-28 targets fundamental neuronal excitability through ion channels. This is arguably more foundational.
Ion channel modulation changes how neurons respond to all inputs, not just specific neurotransmitter signals. The effects may be more pervasive and harder to compensate against through feedback mechanisms. The rapid neurogenic response may reflect this more fundamental intervention point.
Other ion channels are being explored as antidepressant targets. But TREK-1 offers particular advantages in terms of brain distribution, specificity for mood-relevant circuits, and the availability of selective blockers like PE-22-28.
Applications in stress research
Chronic stress damages the brain in measurable ways. Hippocampal volume shrinks. Dendritic complexity decreases. Neurogenesis slows. Synaptic connections are pruned. These changes underlie the cognitive and emotional symptoms that accompany prolonged stress exposure.
TREK-1 expression increases under chronic stress conditions. More TREK-1 means more neuronal braking, less firing, less activity-dependent plasticity. The brain becomes less flexible, less able to adapt, more locked into maladaptive patterns.
Interventions that block TREK-1 may counteract these stress effects. By restoring normal neuronal excitability, they may enable the plasticity needed to form new patterns and escape maladaptive ones. The neurogenic effects provide raw material for circuit remodeling.
Early life stress and TREK-1
Adverse early life experiences predispose individuals to depression and anxiety later in life. These experiences produce lasting changes in brain structure and function, including alterations in TREK-1 expression.
Research has shown that rats exposed to early life stress exhibit higher TREK-1 levels in the prelimbic cortex. This brain region is important for emotional regulation and decision making. Elevated TREK-1 may contribute to the emotional dysregulation seen in individuals with adverse childhood experiences.
TREK-1 inhibition stimulates synaptic plasticity in the prelimbic cortex. This effect is more pronounced in animals subjected to early life stress during postnatal development. The damaged brain may be more responsive to intervention than the healthy brain.
This observation has important implications for therapeutic approaches. Interventions might be most valuable precisely in the populations most damaged by early adversity. The greater TREK-1 elevation provides more target for inhibition, potentially yielding more pronounced effects.
Stress resilience versus stress recovery
Two related but distinct concepts apply here. Stress resilience refers to the ability to maintain function despite stress exposure. Stress recovery refers to the ability to restore function after stress-induced damage.
TREK-1 knockout mice display stress resilience. They do not develop depression-like behavior when exposed to stress paradigms that depress normal mice. The absence of the channel protects against stress effects.
PE-22-28 treatment may promote stress recovery. By blocking TREK-1 in animals already showing stress-induced changes, it may restore normal function and reverse accumulated damage. The neurogenic effects support this interpretation.
Both applications are relevant. Preventing stress damage is valuable. Reversing existing damage is also valuable. Different research protocols test different aspects of PE-22-28 activity in these related but distinct domains.
Pain modulation research
TREK-1 channels participate in pain signaling. Their presence in sensory neurons contributes to pain perception. This raises questions about how TREK-1 inhibitors like PE-22-28 affect pain processing.
Studies with spadin specifically assessed pain-related functions. At doses producing antidepressant effects, spadin did not alter pain thresholds or responses. The channel still performed its pain-related functions normally. Only the depression-relevant signaling appeared affected.
This selectivity is important because chronic pain and depression commonly co-occur. An intervention that worsened pain while treating depression would have limited utility. Conversely, an intervention that treated depression without affecting or potentially helping pain would be valuable.
Some users of peptides for mood support report incidental pain improvements. Whether this reflects direct PE-22-28 effects or secondary effects of mood improvement remains unclear. The best peptides for pain may work through different mechanisms, but the absence of pain worsening with PE-22-28 is reassuring.
Central versus peripheral pain effects
TREK-1 exists in both central nervous system neurons and peripheral sensory neurons. Central effects relate to pain processing in the brain and spinal cord. Peripheral effects relate to pain signal generation at injury sites.
The binding characteristics of PE-22-28 may favor central effects. Access to peripheral neurons requires crossing biological barriers that central penetration does not. Dosing studies might show different thresholds for central versus peripheral effects.
For researchers studying pain, this selectivity matters. A compound that affects one component of pain processing without affecting others can be a valuable tool for dissecting mechanisms. PE-22-28 might selectively probe central pain processing while leaving peripheral generation intact.
Neuroprotection applications
TREK-1 channels play roles in neuronal survival during ischemia. When blood flow to brain tissue is interrupted, potassium channel activity can influence whether neurons survive the insult. TREK-1 opening may actually protect neurons during ischemia by reducing their activity and metabolic demands.
This creates an apparent paradox. Blocking TREK-1 helps with depression but might that blocking worsen ischemic outcomes? Studies with spadin specifically addressed this concern. No interference with ischemia-related TREK-1 functions was observed at antidepressant doses.
The resolution may lie in context-dependent channel activity.
TREK-1 responds to multiple signals. Ischemia activates the channel through specific mechanisms, acidosis and metabolic changes, that differ from the arachidonic acid pathway PE-22-28 antagonizes. Blocking one mode of activation may leave others intact.
Seizure threshold considerations
Similarly, TREK-1 contributes to seizure threshold. The channel activity may help prevent runaway neuronal excitation that leads to seizures. Could TREK-1 inhibitors lower seizure threshold and increase seizure risk?
Again, spadin studies found no effect on seizure threshold at antidepressant doses. The channel continued to perform its seizure-protective function despite inhibition of its depression-relevant activity. The specificity of spadin-type compounds for particular channel states may explain this selectivity.
For researchers working with seizure-prone models or subjects, this data provides reassurance. But it also emphasizes the importance of using appropriate doses and recognizing that higher doses might have different effects than therapeutic levels.
Broader neuroprotective implications
The BDNF-mimetic activity of PE-22-28 may confer general neuroprotective benefits separate from TREK-1 effects. BDNF signaling promotes neuronal survival, encourages neurite outgrowth, and supports mitochondrial function. These effects could protect neurons from various insults.
Combining immediate effects on excitability with longer-term effects on neuronal health creates a potentially valuable profile. Acute protection might prevent initial damage. Sustained trophic support might promote recovery. The neurogenic effects might replace lost cells.
This positions PE-22-28 as potentially relevant to neuroinflammatory conditions, traumatic brain injury research, and age-related cognitive decline studies. These represent active areas of peptide research where SeekPeptides continues to compile emerging evidence.
Practical research considerations
Researchers approaching PE-22-28 studies face several practical decisions. Route of administration, dosing schedules, and outcome measures all require careful consideration.
Intraperitoneal injection provides reliable absorption for initial studies. This route is standard for rodent experiments and allows precise dose delivery. Subcutaneous administration may be equally effective with a simpler technique.
Oral administration, while less common for peptides, appears feasible with PE-22-28 at higher doses. This route opens possibilities for chronic studies where repeated injection causes stress that confounds behavioral outcomes.
Intranasal delivery has been explored for other brain-targeting peptides. This route can bypass the blood-brain barrier to some extent, potentially improving brain concentrations. Whether PE-22-28 is suitable for intranasal delivery requires investigation, but its small size is favorable.
Treatment duration for different endpoints
Different endpoints require different treatment durations. Acute behavioral effects can be assessed after single doses. Neurogenesis requires at least four days of treatment to detect significant changes. Synaptogenesis markers change within 36 hours.
Researchers should match treatment duration to their primary outcome measure. Acute dosing suffices for immediate behavioral testing. Sub-chronic dosing is appropriate for neurogenic endpoints. Chronic dosing would be needed to assess long-term neuroplastic changes.
The extended duration of PE-22-28 effects simplifies chronic dosing protocols. Once-daily administration appears sufficient based on the roughly 24-hour effect duration. This reduces handling stress compared to compounds requiring multiple daily doses.
Controls and comparators
Appropriate controls are essential for valid interpretation. Vehicle controls establish baseline behavior. Active comparators provide context for effect sizes.
Dose-response curves characterize the therapeutic window.
For antidepressant studies, fluoxetine or other SSRIs provide standard active comparators. These established drugs allow researchers to benchmark PE-22-28 effects against known quantities. The speed advantage should be apparent within the typical SSRI onset period.
For neurogenesis studies, positive controls that stimulate neurogenesis through other mechanisms help validate assay sensitivity. Running wheels, enriched environments, and certain growth factors are established neurogenic stimuli.
Current limitations and research gaps
Despite promising preclinical data, significant gaps remain in PE-22-28 characterization. Acknowledging these limitations is essential for researchers planning studies and for anyone evaluating the peptide potential.
Human data is essentially absent. No clinical trials have been published. The translation from mouse models to human therapy involves many uncertainties. Pharmacokinetics, effective doses, and safety profiles all require human investigation before any therapeutic claims can be made.
Long-term effects remain uncharacterized. Most studies lasted days to weeks. What happens with months or years of exposure is unknown. Whether beneficial effects persist after cessation is unclear. Tolerance, dependence, and rebound phenomena have not been systematically assessed.
Mechanistic questions
Several mechanistic questions remain open. The relative contribution of TREK-1 inhibition versus TrkB activation to overall effects is uncertain. Whether these mechanisms are additive, synergistic, or partially redundant requires investigation.
The selectivity for TREK-1 versus other two-pore domain potassium channels needs further characterization. While spadin appears relatively selective, the full selectivity profile of PE-22-28 against all K2P family members has not been published.
Brain penetration and regional distribution data would help understand where PE-22-28 acts. Current data demonstrates functional effects but does not map precisely where the peptide concentrates in brain tissue.
Comparative studies needed
Direct comparisons with other cognitive and mood-affecting peptides would help position PE-22-28 appropriately. Head-to-head studies with Semax, Selank, Dihexa, and other nootropic peptides using standardized protocols would clarify relative advantages.
Combination studies exploring potential interactions with other peptides or conventional therapeutics represent another gap.
Can PE-22-28 augment antidepressant effects of SSRIs? Does it interact with other neurotrophin-targeting approaches? These questions await investigation.
Regulatory and access considerations
PE-22-28 exists in a regulatory gray area common to research peptides. It is not FDA approved for any therapeutic indication. It is not illegal to possess for research purposes in most jurisdictions. It cannot legally be marketed for human consumption or treatment of any condition.
Researchers working with PE-22-28 should ensure compliance with their institutional and jurisdictional requirements. Animal research requires appropriate approvals and oversight. Human research would require investigational new drug applications and extensive regulatory pathway navigation.
The legal status of peptides varies by jurisdiction and changes over time. What is permissible for research today may be restricted tomorrow. Staying current with peptide regulation news helps researchers maintain compliance.
Quality and purity concerns
Research peptides come from various sources with varying quality standards. Purity affects experimental reproducibility. Contaminants can produce confounding effects. Verification of peptide identity and purity should precede important studies.
Peptide testing laboratories can verify identity through mass spectrometry and assess purity through HPLC. Certificate of analysis documents from reputable suppliers provide starting assurance but independent verification adds confidence.
Researchers should select vendors carefully. The peptide vendor landscape includes reputable suppliers and questionable sources. Quality matters enormously when experimental conclusions depend on having the intended compound at stated purity.
Integration with other peptide research
PE-22-28 does not exist in isolation. It joins a growing toolkit of research peptides with neurological applications. Understanding how it fits with other compounds helps researchers design comprehensive studies.
Nootropic peptides as a category share some features while differing in specifics. PE-22-28 offers neurogenic effects that complement the more purely cognitive effects of compounds like Semax. Combining them might produce additive benefits though this requires investigation.
Bioregulator peptides like Pinealon target brain function through different mechanisms. Pinealon works at the gene expression level, potentially affecting long-term cellular programs. This complements the more immediate receptor-level effects of PE-22-28.
Healing-focused peptides like BPC-157 and TB-500 primarily target tissue repair. Their effects on nerve tissue overlap somewhat with PE-22-28 neuroprotective properties. Researchers studying nerve injury or regeneration might consider combinations.
Building research programs around cognitive peptides
For laboratories interested in cognitive peptide research, PE-22-28 offers a valuable addition to the experimental toolkit. Its rapid neurogenic effects allow time-efficient studies. Its well-characterized mechanism provides a foundation for hypothesis-driven research.
Starting with peptide fundamentals helps researchers new to the field. Understanding how peptides work generally provides context for specific compounds. Learning common mistakes helps avoid pitfalls.
Peptide stacking involves combining compounds for potentially enhanced effects. This approach is common in performance and wellness contexts. Research applications require more rigorous design but the principle of combining complementary mechanisms applies.
Frequently asked questions
What is PE-22-28 peptide?
PE-22-28 is a synthetic seven amino acid peptide derived from spadin, a naturally occurring compound. It blocks TREK-1 potassium channels with approximately 300 times greater affinity than spadin, producing neurogenic effects and potential mood benefits in research settings.
How does PE-22-28 differ from spadin?
PE-22-28 is smaller (7 amino acids versus 17), more potent (IC50 of 0.12 nM versus 40-60 nM), and longer lasting (effect duration of approximately 24 hours versus 7 hours). These improvements make it more practical for research applications.
What is the TREK-1 channel?
TREK-1 is a two-pore domain potassium channel that regulates neuronal excitability. It concentrates in brain regions involved in mood and memory. Blocking this channel increases neuronal firing and serotonin transmission while stimulating neurogenesis.
How quickly does PE-22-28 produce neurogenic effects?
Research shows doubled BrdU-positive cells in the hippocampus after just four days of treatment at 3.0-4.0 micrograms per kilogram per day. This is substantially faster than the three to four weeks required for traditional neurogenic approaches.
Is PE-22-28 approved for human use?
No. PE-22-28 is a research compound only. It has not received FDA approval or similar regulatory clearance for any therapeutic indication. Human consumption for treatment purposes is not approved and falls outside current legal frameworks.
How does PE-22-28 compare to Semax and Selank?
Semax primarily targets cognitive enhancement through BDNF modulation. Selank primarily targets anxiety reduction through GABA effects. PE-22-28 combines TREK-1 inhibition with BDNF-mimetic activity, emphasizing neurogenesis and mood regulation over pure cognition or anxiolysis.
What are the potential side effects of PE-22-28?
Preclinical data shows a favorable safety profile with no effects on cardiac channels, blood pressure, or pain perception at therapeutic doses. Limited observations report occasional mild gastrointestinal effects and injection site reactions. Human safety data remains insufficient for definitive conclusions.
Can PE-22-28 be taken orally?
Research studies have demonstrated oral activity at doses of 1 mg/kg in animal models. This is unusual for peptides which typically degrade in the gastrointestinal tract. However, oral dosing requirements exceed those for injection administration.
For researchers serious about advancing their understanding of cognitive peptides, SeekPeptides offers the most comprehensive resources available. Access detailed protocol guidance, evidence-based analysis, and a community of researchers working at the forefront of this field.



