Jan 9, 2026

The regulatory landscape surrounding peptides has undergone significant transformation as these compounds transition from obscure research tools to mainstream health and performance interest. Understanding peptide regulation news requires recognizing the complex interplay between FDA enforcement actions, DEA scheduling decisions, state-level variations, and international differences that collectively shape what researchers can legally access and how vendors operate within compliance boundaries.
The FDA has increasingly scrutinized peptide products marketed with therapeutic claims, issuing warning letters to companies selling BPC-157, TB-500, and various growth hormone secretagogues while simultaneously approving pharmaceutical peptides like semaglutide and tirzepatide for specific medical conditions.
This regulatory dichotomy creates confusion for researchers navigating between approved medications, research chemicals, and prohibited substances.
And, this comprehensive guide covers current peptide legality, recent FDA actions, WADA prohibited lists, state-by-state variations, international regulations, and how SeekPeptides helps you stay informed about the evolving peptide regulatory environment.
Current FDA stance on peptides
The Food and Drug Administration occupies the central regulatory position for peptides in the United States. Understanding the FDA's current approach requires distinguishing between different categories of peptide products and how each faces distinct regulatory treatment.
FDA-approved peptide medications
Several peptides have achieved full FDA approval for specific medical indications. These pharmaceutical-grade peptides undergo rigorous clinical trials demonstrating safety and efficacy before receiving marketing authorization.
GLP-1 receptor agonists: Semaglutide (Ozempic, Wegovy, Rybelsus) and tirzepatide (Mounjaro, Zepbound) represent the most prominent FDA-approved peptide medications. These drugs received approval for type 2 diabetes management and, in some formulations, chronic weight management.
Growth hormone: Recombinant human growth hormone has FDA approval for specific conditions including growth hormone deficiency in children and adults, Turner syndrome, and HIV-associated wasting.
Other approved peptides: Bremelanotide (Vyleesi) received approval for hypoactive sexual desire disorder in premenopausal women. Tesamorelin gained approval for HIV-associated lipodystrophy. These targeted approvals demonstrate the FDA's willingness to approve peptide medications when adequate evidence supports safety and efficacy.
The tirzepatide dosing guide and resources on Ozempic alternatives cover these approved medications in detail.
Unapproved peptides and enforcement
Most peptides discussed in research communities lack FDA approval. This includes popular compounds like BPC-157, TB-500, Ipamorelin, and numerous others.
The FDA's position on unapproved peptides involves several key principles.
New drug status: Peptides intended for therapeutic use in humans are considered "new drugs" requiring FDA approval before marketing. Selling unapproved peptides with claims about treating diseases violates federal law.
Research chemical exception: Peptides sold explicitly for research purposes, not for human consumption, occupy a regulatory gray area. The FDA generally does not prioritize enforcement against research chemical sales when no therapeutic claims are made.
Warning letters: The FDA regularly issues warning letters to companies marketing peptides with health claims. These letters demand cessation of violating activities and can precede more serious enforcement actions.
Import seizures: Customs and Border Protection, working with FDA, can seize imported peptide products, particularly those appearing intended for human use.
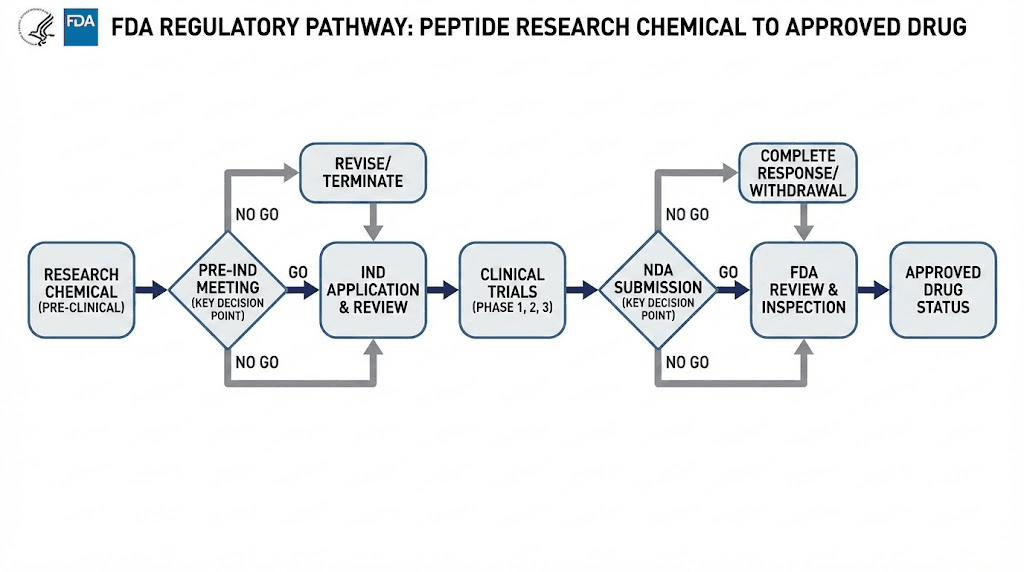
Recent FDA enforcement actions
FDA enforcement activity regarding peptides has intensified in recent years. Several notable actions illustrate current regulatory priorities.
Compounding pharmacy restrictions: The FDA has taken action against compounding pharmacies producing peptides like semaglutide without proper authorization. The agency maintains that compounded versions of commercially available drugs violate federal regulations.
Telehealth prescribing concerns: The rise of telehealth peptide clinics has attracted FDA attention. The agency has expressed concerns about appropriate prescribing practices and patient safety in remote consultation models.
Marketing claim crackdowns: Companies making specific therapeutic claims about unapproved peptides face increased scrutiny. Claims about treating diseases, improving specific health conditions, or providing medical benefits trigger enforcement responses.
The peptide therapy clinics guide discusses legitimate clinical access routes that operate within regulatory boundaries.
DEA and controlled substance considerations
The Drug Enforcement Administration plays a separate but related regulatory role for certain peptides. Understanding DEA involvement helps clarify which peptides face additional restrictions beyond FDA oversight.
Currently scheduled peptides
Few peptides currently appear on DEA controlled substance schedules. However, some related compounds face scheduling.
Human growth hormone: HGH distribution without a prescription is a federal crime, though HGH itself is not a traditional controlled substance. The Anti-Drug Abuse Act of 1988 created specific criminal penalties for HGH distribution.
Anabolic steroids: While not peptides, anabolic steroids' Schedule III status creates context for understanding how performance-enhancing compounds can face scheduling. The peptides vs steroids comparison explains these distinctions.
SARMs: Selective androgen receptor modulators face evolving regulatory pressure. The peptides vs SARMs comparison covers current legal status differences.
Scheduling considerations for peptides
Several factors influence whether peptides might face future DEA scheduling.
Abuse potential: Scheduling decisions consider whether substances have abuse potential. Most research peptides lack the psychoactive effects typically associated with scheduled substances.
Medical use: Substances with accepted medical use may receive less restrictive scheduling. The growing body of peptide research could influence these determinations.
Public health concerns: Widespread misuse or health emergencies could trigger scheduling discussions. So far, peptides have not generated the public health crises that precipitated scheduling of other substance categories.
Political pressure: Congressional action could mandate scheduling regardless of traditional criteria. Sports doping concerns or high-profile adverse events could generate such pressure.
WADA and sports regulations
The World Anti-Doping Agency maintains its own prohibited list affecting competitive athletes. WADA regulations matter for anyone involved in tested sports, regardless of FDA or DEA status.
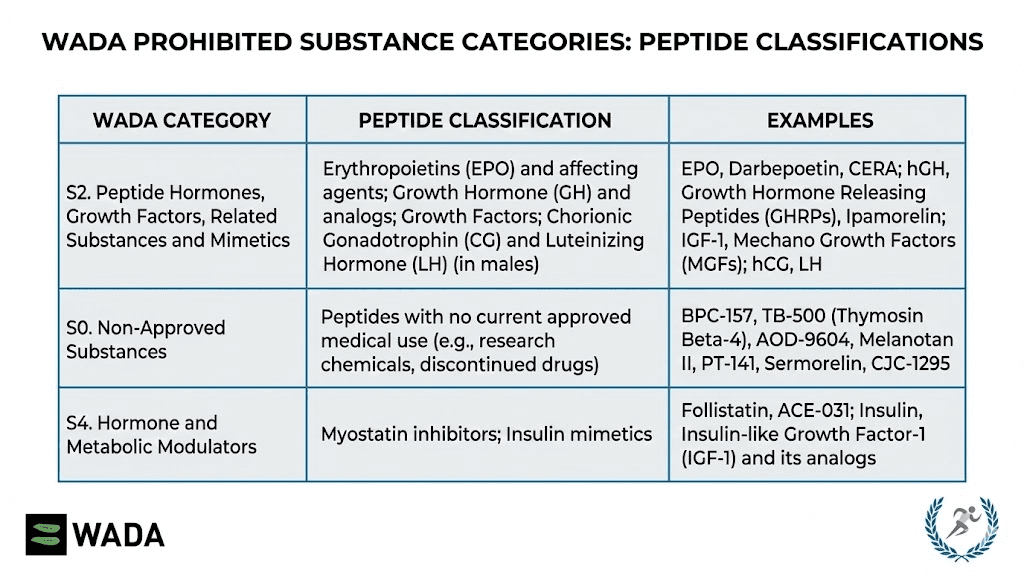
Prohibited peptides
WADA's prohibited list includes numerous peptide categories.
Growth hormone and releasing factors: All GH secretagogues including Ipamorelin, CJC-1295, GHRP-2, GHRP-6, and similar compounds are prohibited.
Peptide hormones: Erythropoietin (EPO), human growth hormone, and related peptides face prohibition.
Growth factors: Various growth factors including IGF-1, MGF, and others are prohibited.
Metabolic modulators: GLP-1 agonists like semaglutide recently joined the prohibited list, reflecting concerns about performance-enhancing potential.
Healing peptides: BPC-157 and TB-500 appear on WADA's prohibited list, preventing use by competitive athletes subject to testing.
Detection methods and testing
Anti-doping testing continues advancing peptide detection capabilities.
Mass spectrometry: Modern testing can detect many peptides at very low concentrations. Detection windows vary by compound and administration route.
Biomarker testing: Rather than detecting peptides directly, some tests measure biological effects indicating peptide use. Growth hormone testing often uses biomarker approaches.
Athlete biological passport: Longitudinal monitoring of athlete biomarkers can reveal peptide use even without direct detection.
Athletes considering any peptide use should verify current prohibited status with their sport's governing body. Lists update regularly, and what's permitted today may be prohibited tomorrow.
State-level peptide regulations
Beyond federal oversight, individual states may impose additional peptide restrictions. This patchwork creates compliance complexity for vendors and researchers.
States with specific peptide laws
Several states have enacted legislation specifically addressing peptides or related compounds.
Prescription requirements: Some states require prescriptions for certain peptides regardless of federal classification. These requirements exceed federal minimums.
Practice restrictions: State medical boards may limit which practitioners can prescribe or administer peptides. Naturopaths, nurse practitioners, and other non-physician providers face varying scope-of-practice rules.
Compounding regulations: State pharmacy boards regulate compounding pharmacies producing peptides. Requirements vary significantly between states.
State enforcement variations
Enforcement intensity varies dramatically by state.
Active enforcement states: Some states actively pursue peptide-related enforcement actions against vendors, clinics, or individuals.
Passive enforcement states: Other states focus resources elsewhere, resulting in minimal peptide-specific enforcement despite laws on the books.
Medical board actions: State medical boards may discipline practitioners for inappropriate peptide prescribing even when no criminal prosecution occurs.
The peptide therapy near me guide helps locate providers operating within their state's regulatory framework. Regional guides for Houston, Austin, Atlanta, Scottsdale, Las Vegas, and Miami Beach cover local regulatory environments.
International peptide regulations
Peptide regulations vary significantly across countries, affecting international shipping, travel with peptides, and research conducted across borders.
Major market regulations
European Union: EU regulations generally mirror FDA approaches, with approved pharmaceutical peptides and restrictions on unapproved therapeutic claims. Individual member states may impose additional requirements.
United Kingdom: Post-Brexit UK maintains its own regulatory framework through the MHRA. Research chemical sales face similar gray-area status as in the US.
Canada: Health Canada regulates peptides similarly to the FDA. Some peptides available as research chemicals in the US face stricter Canadian restrictions.
Australia: The TGA maintains strict pharmaceutical regulations. Many peptides popular elsewhere face significant access restrictions in Australia.
China: As a major peptide manufacturing hub, Chinese regulations affect global supply. Export restrictions and quality requirements impact international markets.
Import and export considerations
International peptide commerce faces numerous regulatory hurdles.
Customs enforcement: Border agencies may seize peptide shipments, particularly those appearing intended for human use.
Documentation requirements: Some countries require import permits or end-user certificates for peptide purchases.
Personal importation: Rules vary regarding individuals importing peptides for personal use. Some countries allow limited personal importation while others prohibit it entirely.
Traveling with peptides: Carrying peptides across international borders raises additional concerns. Prescription documentation may be required, and some countries prohibit certain peptides regardless of prescription status.
Research chemical market dynamics
The research chemical market represents where most peptide commerce occurs outside pharmaceutical channels. Understanding this market's regulatory position helps contextualize peptide availability and compliance considerations.
The research exception
Research chemicals sold for laboratory use occupy a legal gray area based on several factors.
Intended use disclaimer: Products labeled "not for human consumption" or "for research purposes only" avoid some regulatory triggers. However, these disclaimers don't provide absolute protection.
No therapeutic claims: Avoiding health claims is essential. Statements about treating diseases, improving health conditions, or providing medical benefits violate regulations regardless of product labeling.
Legitimate research market: Genuine research demand exists for peptides. Academic institutions, pharmaceutical companies, and independent researchers have legitimate needs that the research chemical market serves.
Vendor compliance challenges
Peptide vendors navigate complex compliance requirements.
Marketing restrictions: Vendors must avoid therapeutic claims while still communicating product information. This balance proves challenging in practice.
Customer screening: Some vendors implement procedures to ensure customers have legitimate research purposes. Effectiveness varies.
Payment processing: Financial institutions may restrict or terminate services to peptide vendors, creating operational challenges regardless of legal status.
Shipping restrictions: Carriers may refuse peptide shipments, and international shipping faces additional scrutiny.
The research vs pharmaceutical peptides guide explains quality and regulatory differences across market segments.
Recent regulatory developments
The peptide regulatory landscape continues evolving. Several recent developments indicate future directions.
GLP-1 agonist shortage responses
Shortages of approved weight loss peptides like semaglutide triggered regulatory responses.
Compounding permissions: During shortages, the FDA temporarily permitted compounding of drugs on the shortage list. This created temporary legal access routes for compounded semaglutide.
Shortage resolution: As manufacturers increased production, the FDA began restricting compounding permissions. Compounding pharmacies faced new compliance requirements.
Telehealth scrutiny: The proliferation of telehealth services prescribing weight loss peptides attracted regulatory attention. Questions arose about appropriate patient evaluation and ongoing monitoring.
The best peptides for weight loss guide covers both approved and research options in this category.
BPC-157 and TB-500 attention
Healing peptides have received increased regulatory attention as their popularity grew.
WADA prohibition: Both BPC-157 and TB-500 now appear on WADA's prohibited list, affecting competitive athletes.
FDA warning letters: Companies marketing these peptides with therapeutic claims have received FDA warning letters demanding cessation of violating activities.
Research interest: Despite regulatory pressure, legitimate research interest continues. The gap between anecdotal reports and clinical evidence keeps these peptides in a research-chemical limbo.
The BPC-157 vs TB-500 comparison covers current knowledge about these popular healing peptides.
Cosmetic peptide regulations
Peptides in skincare products face different regulatory treatment than those intended for injection.
Cosmetic classification: Topical peptides marketed for cosmetic purposes (reducing wrinkles, improving skin appearance) face less stringent regulation than those claiming drug-like effects.
Drug-cosmetic boundary: Claims about treating skin conditions, healing damage, or providing therapeutic benefits may trigger drug classification requirements.
GHK-Cu example: GHK-Cu (copper peptide) appears in both cosmetic products and research chemical markets. Regulatory treatment depends on product claims and intended use.
Resources on peptides for skin tightening, peptides for wrinkles, and natural peptides for skin cover cosmetic applications.
Understanding peptide legality
Peptide legality involves nuanced analysis that depends on specific compounds, intended use, and jurisdiction. Simple yes-or-no answers often miss important distinctions.
Legal for research
Most peptides can be legally purchased for legitimate research purposes. Key requirements include purchasing from vendors not making therapeutic claims, personal use must be for research and not human consumption, and compliance with any applicable state or local regulations.
This research exception enables the peptide market to function despite lack of FDA approval for most compounds.
Prescription required for medical use
Using peptides for medical purposes generally requires working within the healthcare system.
Physician prescribing: Doctors can prescribe both approved peptides and, in some cases, compounded or off-label peptides based on clinical judgment.
Clinic administration: Peptide therapy clinics provide medical supervision for peptide protocols under physician oversight.
Compounding pharmacies: These pharmacies can prepare peptides not commercially available when prescribed by a physician for a specific patient.
Prohibited for athletes
Competitive athletes face additional restrictions through sports governing bodies.
WADA prohibited list: Most performance-relevant peptides appear on this list, including healing peptides, growth hormone secretagogues, and metabolic modulators.
Sport-specific rules: Individual sports may have additional restrictions beyond WADA requirements.
Testing protocols: Athletes subject to testing must avoid prohibited peptides entirely, as therapeutic use exemptions are rarely granted for peptides.
Navigating regulatory uncertainty
Given regulatory complexity, researchers and users benefit from strategies for navigating uncertainty while minimizing risk.
Working with healthcare providers
Medical supervision provides the clearest legal path for peptide use.
Established patient relationship: Working with a physician who can prescribe and monitor peptide protocols provides legal protection and medical oversight.
Legitimate clinics: Finding peptide therapy providers operating within state and federal regulations offers structured access.
Documentation: Maintaining records of prescriptions, medical consultations, and purchase receipts creates audit trail if questions arise.
Understanding vendor positioning
Vendor practices affect buyer risk profiles.
Research-only positioning: Vendors clearly marketing products for research purposes only, without therapeutic claims, operate more defensibly.
Quality verification: Reputable vendors provide third-party testing and certificates of analysis. This documentation also indicates professional operations more likely to maintain compliance.
Red flags: Vendors making health claims, guaranteeing results, or operating unprofessionally raise both regulatory and quality concerns.
Staying informed
Regulatory changes can occur rapidly. Staying informed helps avoid inadvertent violations.
FDA announcements: Monitoring FDA news releases and warning letters reveals enforcement priorities and potential future actions.
Industry news: Peptide industry publications and communities often discuss regulatory developments affecting the market.
Legal consultation: For significant operations or concerns, consulting attorneys familiar with FDA and DEA regulations provides authoritative guidance.
SeekPeptides helps researchers stay informed about regulatory developments affecting peptide access and compliance.
Future regulatory directions
Predicting specific regulatory changes proves difficult, but several trends suggest possible future directions.
Increased FDA attention
Growing mainstream peptide interest will likely attract more FDA attention.
Market growth: As the peptide market expands, regulatory resources devoted to oversight will likely increase proportionally.
Safety signals: Any serious adverse events associated with peptide use could trigger accelerated regulatory response.
Political pressure: Media attention or congressional interest could prompt FDA action regardless of agency priorities.
Potential approval pathways
Some currently unapproved peptides might eventually gain approval.
Clinical trials: If pharmaceutical companies invest in clinical trials, popular peptides like BPC-157 could potentially gain approval for specific indications.
Accelerated pathways: FDA programs for breakthrough therapies or regenerative medicine could expedite approval for peptides showing exceptional promise.
Compounding standardization: Regulatory frameworks for compounding pharmacies might evolve to provide clearer pathways for peptide access.
International harmonization
Global regulatory alignment could affect peptide markets worldwide.
Mutual recognition: Agreements between regulatory agencies could streamline approval across multiple markets.
Standards convergence: International quality standards might become more uniform, affecting manufacturing and testing requirements.
Enforcement cooperation: Cross-border enforcement collaboration could complicate international peptide commerce.
Peptide categories and their regulatory status
Different peptide categories face distinct regulatory treatment. Understanding these distinctions helps navigate the landscape.
Growth hormone peptides
GH secretagogues and related peptides face significant regulatory attention.
Ipamorelin: Ipamorelin remains unapproved but available as a research chemical. WADA prohibits it for competitive athletes.
CJC-1295: The CJC-1295 compound faces similar status, available for research but prohibited in sport.
HGH: Human growth hormone itself has FDA approval for specific conditions but faces strict distribution controls and sports prohibition.
The HGH alternatives guide covers secretagogue options with different regulatory profiles.
Healing peptides
Tissue repair peptides occupy a distinctive regulatory niche.
BPC-157: BPC-157 lacks FDA approval but remains available for research. WADA prohibition affects athletes. The BPC-157 dosage calculator and 5mg dosing guide cover research protocols.
TB-500: TB-500 faces identical regulatory status to BPC-157. The TB-500 dosage calculator assists with research dosing.
GHK-Cu: Copper peptide has wider acceptance in cosmetic applications, creating different regulatory treatment for topical versus injectable forms.
Guides on fast injury healing, injury recovery peptides, and tendon repair cover healing peptide applications.
Weight loss peptides
Weight loss peptides span from fully approved to research-only status.
Semaglutide: FDA approved for diabetes and weight management. The semaglutide calculator covers dosing for approved formulations.
Tirzepatide: Tirzepatide has FDA approval, representing the dual-agonist approach.
Retatrutide: Retatrutide and retatrutide dosing guides cover this triple-agonist compound still in trials.
AOD-9604: The AOD-9604 peptide remains unapproved in most markets despite extensive research.
Cognitive and mood peptides
Nootropic peptides face varied regulatory treatment.
Semax: The Semax dosage guide covers this cognitive-enhancing peptide available in some countries but not FDA approved.
Selank: Selank has approval in Russia but remains unapproved in Western markets.
Nootropic peptides: The best nootropic peptides guide covers cognitive-enhancing options with varying regulatory status.
Guides on peptides for anxiety, memory peptides, and brain function cover mental performance applications.
Practical compliance considerations
Understanding regulatory theory matters, but practical compliance requires specific actions.
Documentation practices
Maintaining appropriate records supports compliance and protects researchers.
Purchase records: Retain invoices and receipts documenting peptide purchases from research chemical vendors.
Research notes: Document research purposes and activities using peptides. This establishes legitimate research intent.
Medical records: For peptides obtained through medical channels, maintain prescription records and clinical documentation.
Storage and handling
Proper peptide storage demonstrates professional research practices.
Secure storage: Keep peptides secured appropriately, particularly if regulations require controlled access.
Labeling: Maintain clear labeling identifying contents and research-only status.
Disposal: Follow appropriate procedures for disposing of unused or expired peptides.
The refrigerated peptide storage and peptide expiration guides cover proper handling.
Vendor selection
Choosing reputable vendors reduces regulatory and quality risks.
Third-party testing: Vendors providing certificates of analysis from independent laboratories demonstrate quality commitment.
Professional presentation: Professional website, clear policies, and responsive customer service indicate legitimate operations.
Marketing compliance: Vendors avoiding therapeutic claims operate more defensibly within regulations.
Reviews of vendors like Prime Peptides, Integrity Research Peptides, Profound Peptides, NextGen Peptides, and Elite Research Peptides help identify reliable sources.
Common questions about peptide regulations
Can I legally buy peptides online?
Purchasing peptides for research purposes from vendors not making therapeutic claims generally falls within legal gray areas. The key factors involve intended use (research, not human consumption), vendor marketing (no health claims), and compliance with any state-specific requirements. Medical use requires working with healthcare providers.
Are peptides illegal without a prescription?
Most peptides are not "illegal" in the sense of controlled substances. Possessing peptides for research is generally permissible. However, using peptides for self-treatment without medical supervision exists outside the regulated healthcare system. Competitive athletes face prohibition through sports regulations regardless of prescription status.
Will peptides become scheduled substances?
No immediate scheduling appears imminent for most research peptides. However, regulatory landscapes can change rapidly based on safety signals, political pressure, or enforcement priorities. Staying informed about regulatory developments helps anticipate changes.
Can my doctor prescribe any peptide?
Physicians have broad prescribing authority and can prescribe both approved and off-label medications based on clinical judgment. However, peptides not available through legitimate pharmaceutical channels present practical challenges. Compounding pharmacies can prepare some peptides when prescribed by physicians.
What happens if peptides are seized at customs?
Customs seizures of peptides do occur. Outcomes vary from simple confiscation to notification letters to, rarely, further investigation. Personal-use quantities typically result in confiscation only. Repeated importation or large quantities may attract more attention.
How do I know if a peptide is legal in my state?
State-level peptide regulations require individual research. Consulting with local attorneys familiar with pharmaceutical regulations provides authoritative guidance. Peptide therapy clinics operating in your state understand local regulatory requirements.
How SeekPeptides supports regulatory awareness
SeekPeptides provides resources helping researchers navigate the complex peptide regulatory landscape.
Educational content covers peptide legality, safety considerations, and regulatory updates affecting peptide access. Understanding the rules helps researchers operate within appropriate boundaries.
The getting started with peptides guide introduces newcomers to regulatory considerations alongside practical information. The common peptide mistakes resource helps avoid compliance errors.
Vendor analysis helps identify sources operating professionally within regulatory constraints. Reviews and comparisons provide context for vendor selection decisions that affect both quality and compliance.
Calculator tools including the peptide calculator, reconstitution calculator, and compound-specific calculators support research-quality protocols demonstrating professional practices.
SeekPeptides remains committed to providing accurate, up-to-date information as the regulatory landscape evolves.
Helpful resources
In case I don't see you, good afternoon, good evening, and good night. May your research stay compliant, your peptides stay legal, and your regulatory knowledge stay current. Join SeekPeptides for comprehensive peptide guidance and regulatory updates.