Feb 3, 2026
Somewhere inside a damaged neuron, a cascade of molecular signals is failing. Calcium floods through ruptured membranes. Glutamate surges to toxic levels. Mitochondria sputter and stall, starving the cell of the energy it needs to survive. Within minutes, inflammatory cytokines begin recruiting immune cells that, in their urgency to help, often cause more destruction than the original injury. This is what brain damage looks like at the cellular level. It is chaotic. It is relentless. And for decades, neuroscience offered precious few tools to intervene once this cascade began.
That is changing.
A growing body of research points to peptides, short chains of amino acids with highly specific biological activity, as some of the most promising molecules for supporting brain repair. These are not broad-spectrum drugs that blunt entire systems. They are precise. They operate at the level of individual receptors, growth factors, and gene expression pathways. Some stimulate brain-derived neurotrophic factor (BDNF), the protein most essential for neuronal survival and new connection formation. Others reduce neuroinflammation by suppressing the exact cytokines that drive secondary damage. A few cross the blood-brain barrier with remarkable efficiency, delivering their signals directly to injured tissue. The result is a new frontier in peptide research that spans traumatic brain injury, stroke recovery, neurodegenerative disease, and the broader pursuit of cognitive resilience. What makes peptides uniquely suited to neurological repair is their ability to work with the brain inherent mechanisms, amplifying the signals the body already uses to heal rather than overriding them. This guide covers the most researched peptides for brain repair, the mechanisms that make them effective, the clinical and preclinical evidence behind each one, and the practical considerations that matter for anyone exploring this rapidly evolving field.
How peptides support brain repair at the cellular level
Understanding why peptides are effective for brain repair requires understanding how the brain actually heals. Or more accurately, how it tries to heal and where it falls short. The brain is not like skin or bone. It does not simply regenerate lost tissue in a straightforward way. Instead, it relies on a complex interplay of neurotrophic factors, immune modulation, vascular remodeling, and synaptic reorganization. Peptides work by intervening at specific points in this process, amplifying what works and suppressing what causes further damage. SeekPeptides covers these mechanisms extensively because understanding the biology behind brain repair is the foundation for evaluating any peptide protocol, whether the goal is recovery from injury, protection against degeneration, or the maintenance of cognitive sharpness over a lifetime.
BDNF and neurotrophic factor stimulation
Brain-derived neurotrophic factor is arguably the single most important protein for neuronal health. It promotes neuron survival. It drives the formation of new synapses. It supports long-term potentiation, the cellular mechanism behind learning and memory. When the brain suffers injury, BDNF levels in the affected region typically drop, leaving neurons vulnerable to apoptosis.
Several brain-targeting peptides work primarily by elevating BDNF. Semax rapidly increases BDNF and its receptor TrkB in the hippocampus, the brain region most critical for memory formation. PE-22-28 is a fragment derived from BDNF itself, offering a more stable and smaller alternative to the native protein. BDNF-related peptides as a category represent one of the most active areas of neuroscience research, precisely because this single growth factor sits at the intersection of so many repair pathways. Even Selank, primarily known as an anxiolytic, increases hippocampal BDNF as part of its mechanism of action.
The importance of BDNF cannot be overstated. Without adequate BDNF signaling, new synapses do not form. Damaged neurons do not recover. Neuroplasticity, the brain ability to rewire around injuries, stalls.
Neurogenesis and new neuron formation
For most of the twentieth century, neuroscience held a core belief: the adult brain does not produce new neurons. That belief has been overturned. The dentate gyrus of the hippocampus and the subventricular zone are now confirmed sites of adult neurogenesis. The problem is that the rate of new neuron production is slow, and it declines sharply with age, injury, and chronic stress.
This is where certain peptides show remarkable potential. P21, a peptide derived from ciliary neurotrophic factor (CNTF), increased new neurons in the dentate gyrus by 80% in research models. PE-22-28 doubled the number of BrdU-positive cells, a direct marker of neurogenesis. Epithalon, better known for its telomere and longevity effects, has also demonstrated the ability to increase neurogenesis rates and accelerate neuron differentiation. These are not marginal improvements. An 80% increase in new neuron production in the hippocampus represents a fundamental shift in the brain capacity to repair and reorganize itself after damage.
Neuroinflammation reduction
Inflammation after brain injury is a double-edged sword. The initial inflammatory response is necessary. It clears debris, signals for repair, and isolates damaged tissue. But when inflammation becomes chronic, lasting weeks or months instead of days, it destroys healthy neurons, disrupts synaptic connections, and creates a toxic microenvironment that prevents recovery.
The key inflammatory markers in brain injury include interleukin-1 beta (IL-1beta), tumor necrosis factor alpha (TNF-alpha), and interleukin-6 (IL-6). Multiple anti-inflammatory peptides target these specific cytokines. Dihexa reduces pro-inflammatory IL-1beta and TNF-alpha while increasing the anti-inflammatory cytokine IL-10. Selank suppresses IL-6 and TNF-alpha. Cerebrolysin reduces neuroinflammation broadly across multiple pathways. BPC-157 counteracts brain edema and hemorrhagic damage following traumatic brain injury. The precision of these anti-inflammatory effects is what separates peptides from conventional anti-inflammatory drugs, which tend to suppress the entire inflammatory response indiscriminately rather than modulating specific pathways.
Synaptogenesis and neural connection rebuilding
Forming new neurons matters. But those neurons are useless without connections. Synaptogenesis, the formation of new synaptic junctions between neurons, is what actually restores function after brain injury. It is the process that allows a stroke patient to regain speech, a TBI survivor to recover working memory, or an aging brain to maintain cognitive sharpness despite accumulating damage.
Dihexa stands out in this category with extraordinary potency. Research demonstrates that it promotes synapse formation at approximately 10 million times the potency of BDNF itself, working through the hepatocyte growth factor (HGF) and c-Met receptor pathway. That number is not a typographical error. The HGF/c-Met system is a powerful driver of neural connectivity, and Dihexa activates it with an efficiency that no other known compound matches. P21 has similarly shown the ability to reverse dendritic and synaptic density loss caused by traumatic brain injury, restoring the physical architecture of neural connections that had been damaged.
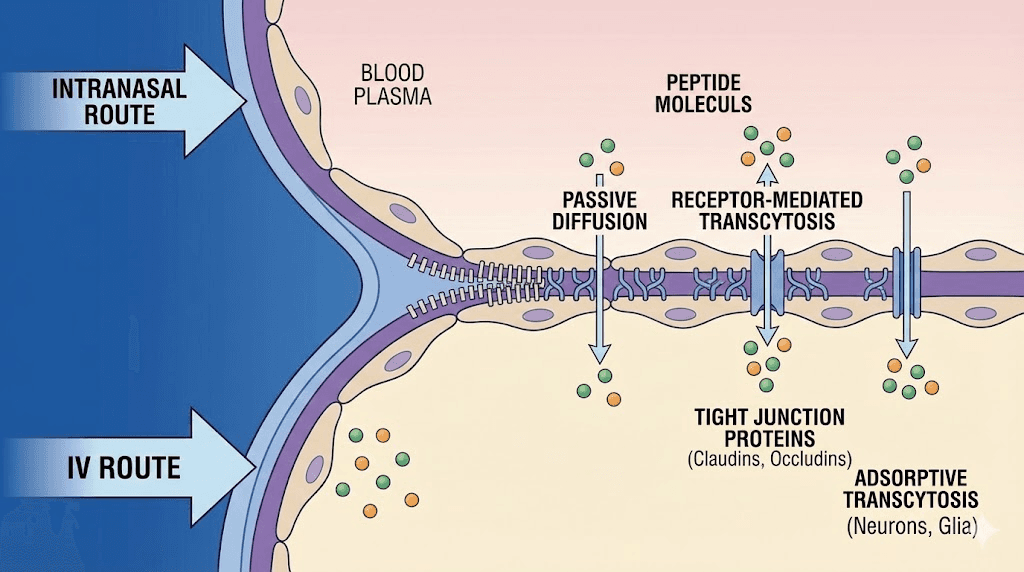
Blood-brain barrier considerations
The blood-brain barrier (BBB) is both the brain greatest protector and the greatest obstacle to treating it. Most molecules in the bloodstream cannot cross this barrier, which is why so many promising neurological drugs fail in clinical trials. They simply cannot reach the brain in therapeutic concentrations.
Peptides vary widely in their BBB permeability. Some cross efficiently. Dihexa is orally bioavailable and crosses the BBB, a rare combination. Cerebrolysin crosses the barrier effectively when administered intravenously. Semax reaches the brain rapidly through intranasal administration, bypassing the BBB entirely through the olfactory pathway. P21 is BBB permeable with a plasma half-life exceeding six hours, giving it sustained access to brain tissue. Understanding which peptides cross the BBB, and by which route, is essential for anyone evaluating peptide dosing protocols for neurological applications.
A newly identified peptide called CAQK takes a different approach entirely. This four-amino-acid sequence, administered intravenously, actually homes to injured brain tissue specifically. It seeks out damage. This targeted delivery mechanism could revolutionize how neuroprotective compounds reach the exact cells that need them most.
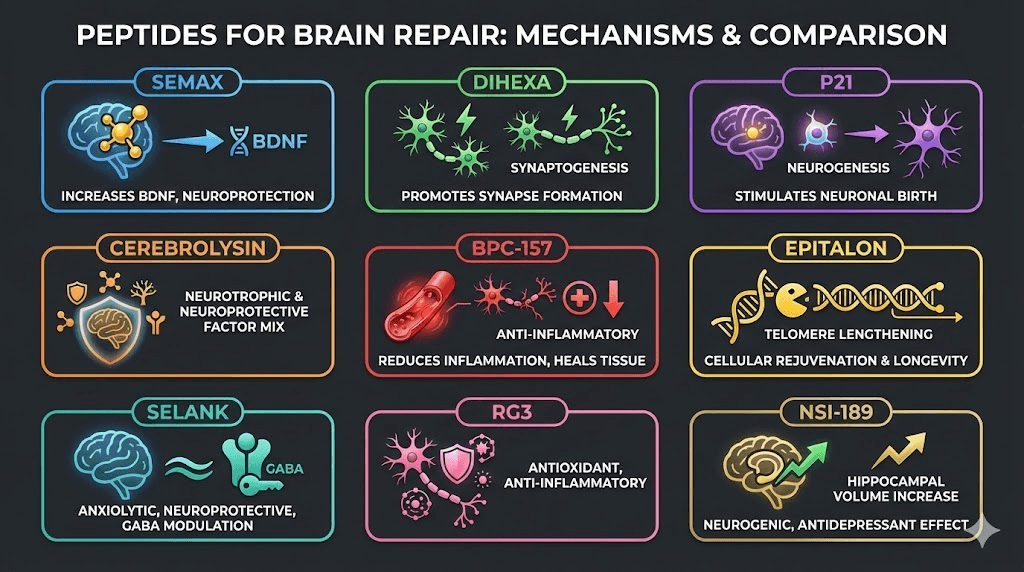
The most researched peptides for brain repair
Not all peptides are created equal when it comes to neurological applications. Some have decades of clinical use behind them. Others are early in preclinical research but show extraordinary promise. The following section examines each of the most significant peptides in the current research landscape for brain repair, covering their mechanisms, evidence base, and practical considerations.
Cerebrolysin
Cerebrolysin is not a single peptide. It is a complex mixture of neuropeptides and free amino acids derived from purified porcine brain proteins. This makes it unique among the compounds discussed here, because it delivers a broad spectrum of neurotrophic factors rather than a single targeted signal. Think of it as a biological cocktail that mirrors, in many ways, the natural growth factor environment of a healthy brain.
The clinical evidence behind Cerebrolysin is substantial. The CAPTAIN I and CAPTAIN II trials examined its effects on moderate to severe traumatic brain injury, defined as Glasgow Coma Scale scores between 7 and 12. Patients received 50 mL per day for 10 days, followed by maintenance cycles of 10 mL per day for 10-day periods. The results showed meaningful improvements in functional outcomes compared to standard care alone. A separate cohort study from the Philippines demonstrated even more striking results: patients receiving 30 mL per day for 14 days achieved favorable outcomes in 87% of cases, compared to just 50% in the control group. A meta-analysis encompassing 8,749 patients across multiple studies confirms these findings are not isolated.
Cerebrolysin crosses the blood-brain barrier effectively. It stimulates BDNF production. It reduces neuroinflammation across multiple cytokine pathways simultaneously. Administration is typically intravenous or intramuscular, which limits its accessibility compared to orally available or injectable peptides, but the clinical evidence supporting its use in serious neurological injury is stronger than that for almost any other peptide in this category. For tissue repair applications involving the brain, Cerebrolysin remains the most clinically validated option available.
Semax
Semax is a synthetic analog of ACTH(4-10), a fragment of adrenocorticotropic hormone. It was developed in Russia, where it holds pharmaceutical approval for the treatment of stroke and cognitive disorders. This is not a fringe compound. It has been used in clinical neurology for years, with an established safety profile and well-documented mechanisms of action.
The primary mechanism of Semax centers on rapid BDNF elevation. Within hours of administration, BDNF and its receptor TrkB increase in the hippocampus. But Semax does much more than boost a single growth factor. It modulates both dopaminergic and serotonergic neurotransmitter systems. It suppresses inflammatory gene expression while simultaneously activating genes involved in neurotransmission, effectively shifting the brain transcriptional profile away from damage and toward repair. Research following ischemic events shows this dual action clearly: inflammatory pathways quiet down while plasticity pathways ramp up.
Clinical stroke protocols use 6,000 mcg per day for 10 days. Standard cognitive support protocols use much lower doses, typically 100 to 200 mcg administered intranasally once or twice daily. The intranasal route is particularly effective because it delivers the peptide directly to the brain through the olfactory mucosa, bypassing first-pass metabolism and the blood-brain barrier entirely. This makes Semax one of the most accessible nootropic peptides available. For those new to getting started with peptides, Semax intranasal administration also avoids the complexity of reconstitution that injectable peptides require.
Dihexa
Dihexa demands attention for a single reason: potency. This angiotensin IV derivative promotes synapse formation at approximately 10 million times the potency of BDNF through the HGF/c-Met receptor pathway. That level of activity is almost unprecedented in neuropharmacology.
The research evidence, while preclinical, is compelling. At a dose of 0.5 mg/kg, Dihexa completely reversed scopolamine-induced cognitive deficits in rat models. In APP/PS1 transgenic mice, the standard animal model for Alzheimer disease pathology, Dihexa restored spatial learning to levels indistinguishable from healthy controls. It accomplished this while simultaneously reducing pro-inflammatory cytokines IL-1beta and TNF-alpha and increasing the anti-inflammatory cytokine IL-10. Two actions at once: rebuilding connections and calming inflammation.
What makes Dihexa particularly notable is its pharmacokinetic profile. It is orally bioavailable. It crosses the blood-brain barrier. These two properties together are exceedingly rare in neuroprotective compounds and open the door to practical administration protocols that do not require injections or intranasal delivery. Researchers interested in peptides for memory consistently identify Dihexa as one of the most promising candidates under investigation. The peptide capsule format may eventually make this compound even more accessible, though current research relies primarily on solution-based administration.
A word of caution is appropriate here. Dihexa is powerful. Its effects on the HGF/c-Met pathway, while beneficial for neural repair, require careful consideration regarding long-term use. The same pathway that promotes synaptogenesis also plays roles in cell proliferation more broadly. This is a compound that demands respect, thorough understanding, and careful attention to peptide safety and risks.
BPC-157
BPC-157 is a 15-amino-acid peptide originally isolated from human gastric juice. It is best known for its remarkable injury healing properties in tendons, ligaments, and the gut. But its effects on the brain are increasingly well-documented and deserve serious attention in any discussion of neurological repair.
The mechanisms are multifaceted. BPC-157 modulates both serotonergic and dopaminergic neurotransmitter systems. It counteracts the consequences of traumatic brain injury directly, reducing brain edema, hemorrhagic lacerations, and subarachnoid bleeding in experimental models. When administered just 30 seconds after a 20-minute ischemic event, BPC-157 significantly reduced neuronal damage. That is a remarkably narrow therapeutic window executed successfully, suggesting rapid and potent neuroprotective action.
Perhaps most impressive is BPC-157 activity against MPTP, a neurotoxin that selectively destroys dopaminergic neurons and is used to model Parkinson disease in research. BPC-157 counteracted MPTP-induced damage, suggesting potential relevance for dopaminergic neurodegenerative conditions. It also promotes nerve regeneration after physical transection, meaning it does not just protect neurons from dying but actively supports the regrowth of damaged nerve fibers.
The administration of BPC-157 is typically subcutaneous, and dosage calculators can help determine appropriate amounts based on bodyweight. The standard 5mg vial dosing guide provides detailed protocols. For brain applications specifically, the gut-brain axis connection is significant. BPC-157 influences brain function partly through its effects on the gastrointestinal system, and the relationship between gut health and neurological function is now one of the most active areas in neuroscience. Some researchers are exploring whether BPC-157 combined with TB-500 might offer complementary neuroprotective and neuroregenerative effects, given TB-500 benefits in tissue repair and inflammation reduction.
Selank
Selank is a tuftsin analog, a heptapeptide that modifies the natural immune-modulating peptide tuftsin with a stabilizing proline-glycine-proline sequence. Like Semax, it was developed in Russia and has been used clinically, primarily for anxiety and cognitive enhancement. Its relevance to brain repair, however, extends well beyond anxiolysis.
The BDNF increase in the hippocampus is one component. Selank also protects against ethanol-induced memory impairment, a model that tests the ability to preserve cognitive function under toxic conditions. Its anti-inflammatory profile is substantial, suppressing IL-6 and TNF-alpha, two of the cytokines most damaging during chronic neuroinflammation. The anxiolytic effects are comparable to benzodiazepines, but without the addiction potential, sedation, or cognitive blunting that makes benzodiazepines problematic for long-term use.
Why does anxiety reduction matter for brain repair? Because chronic anxiety and stress hormones actively impair neurogenesis and synaptic plasticity. Cortisol, the primary stress hormone, literally shrinks the hippocampus over time. By reducing anxiety without the cognitive costs of traditional anxiolytics, Selank creates a neurochemical environment more conducive to repair. Administration is typically intranasal at 300 to 600 mcg once or twice daily, or subcutaneous at 300 to 500 mcg daily. Cycles typically run 4 to 6 weeks. For those dealing with anxiety alongside neurological recovery, Selank represents a dual-purpose option that addresses both conditions through overlapping mechanisms.
PE-22-28
PE-22-28 is a seven-amino-acid fragment derived from BDNF itself. If BDNF is the master regulator of neuronal health, PE-22-28 is a distilled, optimized signal extracted from that regulator. It works through inhibition of the TREK-1 potassium channel, a mechanism that enhances neuronal excitability and promotes serotonergic transmission.
The neurogenesis data is striking. In mouse models, PE-22-28 doubled the number of BrdU-positive cells, which are newly born neurons actively incorporating DNA during division. Doubled. That level of neurogenesis enhancement is significant for any brain repair application, because the limiting factor in many neurological recovery scenarios is simply not having enough new neurons to replace those lost to injury or disease.
PE-22-28 offers practical advantages over native BDNF. It is smaller, which improves tissue penetration. It is more stable, which extends its effective window of activity. And because it is derived from a naturally occurring protein, its mechanism of action works with existing biological pathways rather than introducing foreign signaling. This peptide is still in preclinical stages, but the neurogenesis and serotonergic data position it as one of the most promising compounds for future brain function and focus applications.
P21 (Peptide 6)
P21, also known as Peptide 6, is derived from ciliary neurotrophic factor (CNTF). Its primary effect is driving neurogenesis in the dentate gyrus, the hippocampal subregion most associated with memory formation and spatial navigation.
The numbers are worth sitting with. P21 increased new neurons in the dentate gyrus by 80%. It reversed TBI-induced loss of dendritic density and synaptic connections. It did both of these things while crossing the blood-brain barrier and maintaining a plasma half-life exceeding 6 hours, which means sustained brain exposure from a single administration. Long-term safety studies extending up to 12 months showed no adverse effects, which is rare for a compound with such potent biological activity.
For traumatic brain injury specifically, P21 addresses what is perhaps the most frustrating aspect of recovery: the loss of neural architecture. TBI does not just kill neurons. It strips away the dendritic branches and synaptic connections that surviving neurons need to communicate. P21 reverses this architectural damage while simultaneously generating new neurons to replace those lost entirely. This dual action, both structural repair and cell replacement, makes P21 one of the most complete neuroregenerative peptides currently under investigation.
Pinealon (EDR)
Pinealon is a tripeptide, just three amino acids: glutamic acid, aspartic acid, and arginine. It belongs to the bioregulator peptide class developed by Vladimir Khavinson at the Saint Petersburg Institute of Bioregulation and Gerontology. Despite its tiny size, Pinealon operates through a mechanism fundamentally different from most peptides discussed here.
It does not bind to surface receptors. It penetrates cell membranes and interacts directly with DNA. This is epigenetic modulation, the regulation of gene expression without altering the genetic code itself. Pinealon influences which genes are active and which are silent, and the genes it upregulates are those involved in neuronal protection and survival.
The neuroprotective data shows protection against oxidative stress and hypoxia, two of the primary killers of neurons following injury. Pinealon also reduces NMDA excitotoxicity, which is the overactivation of glutamate receptors that leads to calcium overload and cell death. This is one of the earliest and most destructive events in both TBI and stroke, making Pinealon potentially valuable in the acute phase of brain injury. Standard protocols use 100 to 300 mcg subcutaneously daily for periods of 10 to 20 days.
Cortagen
Cortagen is another Khavinson bioregulator, this one derived from cerebral cortex tissue. Like Pinealon, it works at the epigenetic level rather than through receptor binding. Its specific target is the regeneration of brain cells in the cerebral cortex, the outer layer of the brain responsible for higher cognitive functions including reasoning, language, planning, and sensory processing.
An important characteristic of Cortagen is its bell-curve dose-response relationship. More is not better. There is an optimal dosing range below which effects are minimal and above which effects actually diminish. This is unusual in pharmacology but common among bioregulator peptides, and it underscores the importance of precise peptide dosing when working with these compounds. The peptide calculator can help determine starting points, but the bell-curve nature of Cortagen response means that individual titration is essential.
Peptides for traumatic brain injury recovery
Traumatic brain injury is not a single event. It is an event followed by a cascade. The initial mechanical damage, the primary injury, is followed by hours, days, and weeks of secondary injury processes that often cause more functional loss than the impact itself. Neuroinflammation escalates. Cerebral edema compresses healthy tissue.
Free radicals accumulate. The blood-brain barrier breaks down, allowing toxic blood components to enter the brain parenchyma. Mitochondrial dysfunction spreads from the impact zone outward. Even mild TBI, often dismissed as "just a concussion," initiates these secondary processes at a reduced but still significant scale.
The statistics underscore the urgency. Millions of people worldwide sustain traumatic brain injuries each year. The majority are classified as mild. But even mild injuries can produce lasting cognitive deficits, particularly when they accumulate over time. Moderate and severe TBI carries substantially higher risks of long-term disability, including impaired memory, executive function deficits, emotional dysregulation, and increased vulnerability to neurodegenerative disease later in life. Current standard-of-care treatments focus primarily on preventing secondary damage through surgical intervention and supportive care. There are remarkably few pharmacological interventions that actively promote brain repair after the acute phase stabilizes.
This is precisely why peptides are so relevant to TBI recovery. Each secondary injury process represents a potential intervention point, and different peptides target different points in the cascade.
Cerebrolysin has the strongest clinical evidence in TBI specifically. The CAPTAIN trials demonstrated improved outcomes in moderate to severe injuries, the kind that leave patients unconscious or in altered states of consciousness. The Philippine cohort study using 30 mL daily for 14 days showed an outcome disparity that is difficult to ignore: 87% favorable outcomes versus 50% in controls. These are large effect sizes for a condition where neurology has historically had few effective interventions. For pain management following TBI, the anti-inflammatory effects of these peptides provide secondary benefits beyond direct neuroprotection.
BPC-157 addresses the acute phase with particular effectiveness. Its ability to reduce brain edema, hemorrhagic lacerations, and subarachnoid bleeding directly counters the most dangerous secondary injury processes. The fact that it showed significant neuroprotection when administered just 30 seconds after ischemia suggests a rapid mechanism of action, which is critical in the narrow therapeutic windows that characterize acute brain injury.
P21 addresses the chronic phase. After the acute danger passes and the brain stabilizes, the challenge shifts to rebuilding what was lost. The 80% increase in dentate gyrus neurogenesis and the reversal of dendritic and synaptic density loss make P21 particularly suited to the recovery phase, where the goal is not preventing further damage but restoring function. Its 12-month safety profile in long-term studies adds confidence for the extended treatment periods that TBI recovery demands.
The CAQK peptide represents a newer approach entirely. Rather than relying on systemic distribution to deliver neuroprotective compounds to the brain, CAQK homes specifically to injured brain tissue when administered intravenously. In animal models of TBI, including both mice and larger pig models, CAQK reduced inflammation and cell death while improving memory and motor function. Aivocode, the company developing CAQK, is currently seeking FDA authorization for Phase I clinical trials. If successful, CAQK could serve as a delivery vehicle for other therapeutic peptides, concentrating their effects exactly where damage has occurred.
The concept of peptide stacking becomes particularly relevant in TBI because the injury involves so many different pathological processes simultaneously. A stack combining acute neuroprotection (BPC-157), broad neurotrophic support (Cerebrolysin), and long-term neurogenesis (P21) could theoretically address the full timeline of TBI recovery. Understanding how many peptides can be combined safely is essential before attempting any such protocol.
The Wolverine stack, which typically combines BPC-157 and TB-500 for accelerated injury recovery, has gained attention in the peptide community for its tissue repair effects. While originally designed for musculoskeletal healing, the neuroprotective properties of BPC-157 within this stack make it potentially relevant for TBI recovery as well. The combination addresses both systemic inflammation and localized neural damage through complementary mechanisms. Researchers investigating musculoskeletal pain, tendon repair, and bone healing have noted the overlapping benefits that extend to neural tissue, since the inflammatory and regenerative pathways share common molecular ground.
Peptides for stroke recovery and neuroplasticity
Stroke kills neurons through oxygen deprivation. The ischemic core, the region directly deprived of blood flow, suffers irreversible damage within minutes. But surrounding this core is the ischemic penumbra, a larger region of at-risk tissue that is damaged but not yet dead. The penumbra is where intervention matters most, because neurons in this zone can be saved if the right signals arrive in time.
Semax holds pharmaceutical approval in Russia specifically for stroke recovery, a regulatory status backed by clinical evidence. The stroke protocol calls for 6,000 mcg per day for 10 days, a dose substantially higher than standard nootropic use. At this dose, Semax produces a dramatic shift in gene expression: inflammatory genes are suppressed while neurotransmission and plasticity genes are activated. This dual action directly addresses the two biggest challenges in stroke recovery. First, stopping the inflammatory cascade that expands damage from the ischemic core into the penumbra. Second, promoting the neuroplastic reorganization that allows surviving brain regions to assume functions previously handled by damaged tissue.
BPC-157 demonstrated neuroprotection when given 30 seconds after ischemia onset. While clinical stroke treatment obviously cannot achieve that speed, the finding demonstrates that BPC-157 neuroprotective mechanisms engage rapidly and effectively against ischemic damage. The reduction in subarachnoid bleeding is also relevant for hemorrhagic stroke, where bleeding into the space surrounding the brain causes both direct pressure damage and inflammatory toxicity.
Dihexa, with its extraordinary synaptogenic potency, is theoretically ideal for the post-stroke recovery phase when the goal shifts from neuroprotection to functional reorganization. The brain compensates for stroke damage through neuroplasticity, forming new connections that bypass the damaged area. Synaptogenesis is the cellular basis of this compensation, and no known compound drives synaptogenesis as powerfully as Dihexa. Its ability to restore spatial learning in Alzheimer model mice suggests robust pro-cognitive effects that could translate to memory recovery after stroke.
Pinealon protection against NMDA excitotoxicity is particularly relevant to stroke. During ischemia, dying neurons release massive amounts of glutamate, which overstimulates NMDA receptors on neighboring neurons, causing calcium influx and further cell death. This excitotoxic cascade is one of the primary mechanisms by which the ischemic penumbra is recruited into the infarct core. By reducing NMDA excitotoxicity, Pinealon could help preserve penumbral tissue during the critical hours following stroke onset.
Neuroplasticity after stroke is not automatic. It requires neurotrophic support, reduced inflammation, and sufficient neural substrate, meaning enough surviving and newly generated neurons to form the new pathways needed for functional recovery. Peptides that support brain function through these mechanisms provide the biological foundation that rehabilitation therapy then builds upon. The combination of pharmacological neuroplasticity enhancement with targeted physical and cognitive rehabilitation represents what many neuroscientists consider the most promising approach to stroke recovery currently available.
The relationship between stroke recovery and hormonal balance also deserves mention. Stroke disrupts the hypothalamic-pituitary axis, often producing hormonal imbalances that further impair recovery. Growth hormone deficiency, thyroid dysfunction, and cortisol dysregulation are common after stroke and each independently impairs neuroplasticity. Peptides like Ipamorelin, while not directly neuroprotective, support growth hormone secretion that influences brain repair indirectly. The comparison between Ipamorelin and CJC-1295 is relevant here, as both growth hormone secretagogues have different pharmacokinetic profiles that affect their suitability for sustained versus pulsatile growth hormone elevation. The CJC-1295 dosage calculator helps researchers determine appropriate protocols for these complementary compounds. Whether growth hormone optimization should be considered part of a comprehensive brain repair strategy is an active area of investigation, and the intersection of energy-supporting peptides with neuroprotective ones represents a frontier that few researchers have fully explored.
Peptides for neurodegenerative conditions
Neurodegenerative diseases, Alzheimer, Parkinson, multiple sclerosis, ALS, share a common thread despite their different presentations. They all involve the progressive loss of neurons and neural connections. The specific neurons targeted differ. The underlying pathologies differ. But the functional result is the same: a brain losing capacity over time, with current medicine offering few effective interventions to slow or reverse the process.
Peptides enter this space not as cures but as potential tools for slowing degeneration, supporting remaining neurons, and in some cases, promoting the replacement of lost cells.
For Alzheimer disease research, Dihexa has shown the most dramatic results. The restoration of spatial learning in APP/PS1 transgenic mice, which develop amyloid plaques and cognitive deficits analogous to human Alzheimer pathology, suggests that massive synaptogenic stimulation can override the connectivity losses caused by amyloid accumulation. This does not mean Dihexa treats Alzheimer disease. But it suggests that the cognitive deficits associated with early and moderate Alzheimer pathology may be partly compensated through aggressive synapse formation. The simultaneous reduction in pro-inflammatory cytokines and increase in anti-inflammatory IL-10 also addresses the neuroinflammatory component that many researchers now believe drives Alzheimer progression as much as amyloid itself.
For Parkinson disease research, BPC-157 counteraction of MPTP neurotoxicity is directly relevant. MPTP selectively destroys dopaminergic neurons in the substantia nigra, producing a pattern of damage that closely mirrors Parkinson pathology. BPC-157 ability to protect against this specific toxic insult suggests potential relevance for dopaminergic neuroprotection. Its additional effects on the dopaminergic system through neurotransmitter modulation add further interest.
Multiple sclerosis involves autoimmune-mediated destruction of myelin, the insulating sheath around nerve fibers. Peptides for autoimmune conditions that reduce neuroinflammation and support neural repair could theoretically address both the immune dysregulation and the resulting neural damage. Selank anti-inflammatory profile, suppressing IL-6 and TNF-alpha without broad immunosuppression, is particularly interesting for autoimmune neurological conditions where the goal is to modulate rather than eliminate immune function. The immune-modulating properties of several brain-active peptides make them relevant to conditions where the immune system itself is causing the neurological damage.
The longevity peptide category overlaps significantly with neuroprotection. Epithalon, primarily known for its telomere-lengthening effects, also increases neurogenesis rates and accelerates neuron differentiation. The Epithalon dosage guide covers the protocols used in research, and the neurogenesis data adds a compelling reason to consider this peptide beyond its better-known anti-aging applications. SS-31, a mitochondria-targeting peptide, addresses the energy deficit that underlies much neurodegenerative pathology. When mitochondria fail, neurons die. It is that simple. And mitochondrial dysfunction is a hallmark of virtually every neurodegenerative disease. Supporting mitochondrial function through peptides like SS-31 while simultaneously promoting neurogenesis and reducing inflammation through other peptides represents a multi-target approach to conditions that have resisted single-target therapies for decades. The growing interest in peptides for hair loss has inadvertently contributed to neuroprotection research, because some of the growth factor pathways involved in hair follicle cycling overlap with those governing neuronal survival and regeneration.
Anti-aging peptide research and neurodegeneration research are converging. The mechanisms that keep the brain young, adequate BDNF, active neurogenesis, controlled inflammation, healthy mitochondria, are the same mechanisms that degenerate in disease. Supporting these mechanisms through peptides may not cure neurodegenerative conditions, but the research increasingly suggests they could significantly alter the trajectory of decline.
The concept of neuroprotection as a proactive strategy rather than a reactive one is gaining traction. Rather than waiting for symptoms of cognitive decline to appear and then attempting to reverse established damage, some researchers advocate for early intervention with neuroprotective peptides in at-risk populations. Individuals with genetic predispositions to Alzheimer disease, those with a history of repeated mild TBI, or people experiencing early subjective cognitive decline might benefit from peptide protocols aimed at maintaining neuronal health before significant degeneration occurs. This preventive approach aligns with the broader shift in medicine toward early intervention, and peptides with established safety profiles like Semax and Selank are natural candidates for such strategies. SeekPeptides provides resources for understanding both the therapeutic and preventive applications of these compounds, recognizing that the line between cognitive optimization and neuroprotection is increasingly blurred.
The role of NAD-related compounds in neuroprotection adds another dimension. While NAD itself is not a peptide, the NAD peptide guide explores how these molecules interact with peptide-based neuroprotective strategies. Mitochondrial function, which NAD precursors support, is fundamental to neuronal survival. Combining mitochondrial support with the neurotrophic and anti-inflammatory effects of brain-active peptides creates a multi-layered approach to neurodegeneration that no single compound can achieve alone. The peptide formula guide helps researchers understand how different compounds interact at the molecular level.
Research protocols and practical considerations
Understanding the science behind each peptide is one thing. Knowing how to approach them practically is another entirely. Dosing, administration routes, timing, cycling, and storage all affect outcomes in ways that the research literature does not always make explicit. This section covers the practical details that matter.
Dosing protocols from the research literature
Each peptide has its own dosing range established through research. These are not recommendations for clinical use but rather data points from published studies and clinical protocols.
Cerebrolysin: Clinical TBI protocols range from 10 mL to 50 mL daily, administered intravenously or intramuscularly. The CAPTAIN trials used 50 mL daily for acute treatment and 10 mL daily for maintenance. The Philippine cohort used 30 mL daily. Lower cognitive enhancement doses are not well-established in published research.
Semax: The clinical stroke protocol is 6,000 mcg daily for 10 days. Standard cognitive protocols use 100 to 200 mcg intranasally one to two times daily. The Semax dosage guide provides more detailed information on these protocols.
Dihexa: Research dosing in animal models used 0.5 mg/kg. Human equivalent dosing has not been established through clinical trials. The oral bioavailability means that subcutaneous injection is not the only viable route.
BPC-157: Standard research dosing is approximately 250 to 500 mcg subcutaneously, one to two times daily. The BPC-157 dosage calculator adjusts for bodyweight. The 5mg vial dosing guide covers practical preparation.
Selank: Intranasal administration at 300 to 600 mcg one to two times daily. Subcutaneous administration at 300 to 500 mcg daily. The Selank injection dosage guide provides comprehensive protocol details. Cycles run 4 to 6 weeks.
PE-22-28: Research dosing is not yet standardized for human use. Preclinical data is based on animal models.
P21: Like PE-22-28, human dosing protocols are not established through clinical trials. The 12-month animal safety data is encouraging but does not directly translate to human dosing.
Pinealon: 100 to 300 mcg subcutaneously daily for 10 to 20 days. As a bioregulator, the cycle length and rest periods follow the Khavinson protocol framework.
Cortagen: Similar to Pinealon in cycle structure. The bell-curve dose-response means starting low and titrating to the individual response is more important than with other peptides.
Administration routes and their implications
The route by which a peptide enters the body significantly affects how much reaches the brain and how quickly it gets there.
Intranasal: Delivers peptides directly to the brain through the olfactory mucosa. Bypasses the blood-brain barrier and first-pass liver metabolism. Most relevant for Semax and Selank. The nasal spray peptides guide covers technique and equipment.
Subcutaneous injection: The most common route for most peptides. Provides systemic distribution with relatively consistent absorption. Requires reconstitution with bacteriostatic water. The peptide injection basics and injection technique guide cover the practical details. The reconstitution calculator ensures accurate preparation. Those wondering how much bacteriostatic water to add will find specific ratios there.
Intravenous: Required for Cerebrolysin at clinical doses. Provides immediate and complete bioavailability. Not practical for most individual research use.
Oral: Rare for peptides due to gastrointestinal degradation. Dihexa is notable for its oral bioavailability. The peptide capsule guide discusses the current state of oral peptide delivery.
The choice of administration route affects not just bioavailability but also the practical logistics of daily use. Intranasal administration is quick and painless. Subcutaneous injection requires bacteriostatic water, proper mixing technique, and either syringes or an injection pen. Understanding these practical requirements is part of getting started with peptides effectively.
Storage and stability
Peptides are proteins. They degrade. Proper storage is not optional, it is essential for maintaining biological activity, especially for compounds intended for neurological applications where potency directly affects outcomes.
Lyophilized (freeze-dried) peptides are the most stable form. The shelf life of lyophilized peptides is considerably longer than reconstituted solutions. Understanding the differences between lyophilized and liquid peptides helps inform purchasing and storage decisions.
Once reconstituted, peptides require refrigeration. The peptide storage guide covers temperature requirements, light sensitivity, and contamination prevention. Refrigerated peptide stability varies by compound but generally ranges from days to weeks. Post-reconstitution storage protocols should be followed precisely. The question of whether peptides expire has a clear answer: yes, and using degraded peptides for neurological applications is not just wasteful but potentially counterproductive.
How to evaluate peptide quality for neurological research
When peptides are intended for neurological applications, quality is not a minor consideration. It is the consideration. A peptide that is 85% pure might be acceptable for some research contexts. For brain-targeting compounds, where the goal is modulating delicate neurotransmitter systems and growth factor pathways, impurities are not just unwanted, they are potentially dangerous.
Purity matters more for brain applications than perhaps any other peptide use case. The blood-brain barrier, when intact, filters most contaminants. But many brain-active peptides are specifically designed to cross that barrier, and any contaminants that co-cross with them have direct access to neural tissue. Third-party peptide testing through independent labs should confirm identity, purity (minimum 98% for neurological applications), and the absence of endotoxins, heavy metals, and residual solvents.
The distinction between research-grade and pharmaceutical-grade peptides becomes particularly significant here. Pharmaceutical-grade peptides undergo more stringent manufacturing and quality control processes. For compounds targeting the brain, this additional quality assurance is worth the investment. Vendor selection should prioritize those who provide certificates of analysis from accredited testing laboratories, not just in-house quality checks.
Understanding the legal landscape is also necessary. Peptide regulation varies by jurisdiction and is evolving rapidly. Some peptides discussed in this guide are approved pharmaceuticals in certain countries. Others are available only for research purposes. The question of whether doctors can prescribe research-grade peptides depends on location and regulatory framework. Online peptide therapy platforms are expanding access but also raise quality and oversight questions that users should evaluate carefully.
Stability testing is another dimension of quality that affects neurological peptides specifically. Some brain-active peptides are more fragile than their musculoskeletal counterparts. Cerebrolysin, as a complex mixture, has specific handling requirements that differ from single-peptide compounds. Bioregulator peptides like Pinealon and Cortagen, while small and relatively stable, still require proper cold chain management. Every step in the supply chain, from manufacturing to shipping to storage, represents a potential point of degradation. The refrigerated stability timelines for each compound should guide how quickly reconstituted vials are used, and understanding whether a peptide has been compromised before use is critical for brain applications where there is no room for ineffective dosing.
Cost is a practical reality. The cost of peptides varies dramatically by compound, purity level, and source. Comprehensive cost analysis should factor in not just the peptide itself but also bacteriostatic water, syringes or injection pens, and testing costs. The peptide cost calculator helps estimate total expenses for different protocols. For brain repair applications where multiple peptides may be used across extended timelines, understanding the total financial commitment upfront prevents the common mistake of starting a protocol that cannot be sustained long enough to produce meaningful results. The bone and cartilage repair community learned this lesson early: incomplete protocols often produce no measurable benefit, and the same principle applies with even greater force to neurological applications where the biological timelines are longer.
Combining peptides for brain repair
The brain does not heal through a single mechanism. Neuroinflammation, oxidative stress, excitotoxicity, growth factor deficiency, mitochondrial dysfunction, and synaptic loss all occur simultaneously. It follows logically that addressing multiple mechanisms simultaneously through carefully selected peptide combinations could produce outcomes exceeding what any single peptide achieves alone.
This is the rationale behind peptide stacking for neurological applications. But stacking for brain repair requires more caution and more knowledge than stacking for, say, muscle growth or fat loss. The brain is orders of magnitude more complex than muscle tissue, and the interactions between multiple neuroactive compounds are not always predictable.
Complementary mechanism stacking
The most rational approach to brain repair stacking pairs peptides that target different mechanisms with minimal overlap. Consider a stack designed for post-TBI recovery.
BPC-157 addresses acute neuroprotection, reducing edema, bleeding, and direct neuronal damage. Semax elevates BDNF and promotes a pro-repair gene expression profile. P21 drives neurogenesis to replace lost neurons. Each peptide targets a different phase and mechanism of recovery. The peptide stack calculator can help organize the timing and dosing of multi-peptide protocols.
Another rational combination pairs Selank with Semax. Both are intranasal peptides with established safety profiles. Selank provides anxiolysis and IL-6/TNF-alpha suppression. Semax provides BDNF elevation and pro-plasticity gene activation. Together, they address the emotional and cognitive dimensions of brain recovery simultaneously. This particular combination has a long history of concurrent use in Russian clinical practice.
For neurodegenerative applications, a combination of Dihexa (synaptogenesis), Cerebrolysin (broad neurotrophic support), and Pinealon (epigenetic neuroprotection) addresses connectivity loss, growth factor deficiency, and oxidative vulnerability in parallel. Whether combining these specific compounds produces additive or synergistic effects has not been formally studied, but the non-overlapping mechanisms provide a theoretical rationale.
Timing and sequencing considerations
Not all peptides in a stack need to be administered simultaneously. In fact, sequencing may be more effective in some scenarios.
Acute phase peptides (BPC-157, Pinealon) that address immediate neuroprotection and excitotoxicity could be started first. Neurotrophic peptides (Semax, Cerebrolysin) that create the biological conditions for repair could follow once the acute danger has passed. Neurogenesis-promoting peptides (P21, PE-22-28) that drive new cell production could be introduced once the inflammatory environment has been controlled and neurotrophic support is established.
This phased approach mirrors how the brain itself heals: first survive, then stabilize, then rebuild. Understanding how to cycle different peptides and how many can be used concurrently is essential for designing any multi-peptide protocol. The common mistakes beginners make often include combining too many compounds without understanding their interactions or starting everything simultaneously rather than sequencing intelligently.
What not to combine
Some combinations warrant caution. Multiple peptides that strongly stimulate the same pathway could cause overstimulation. Stacking Semax, PE-22-28, and Selank simultaneously would drive BDNF elevation through three different mechanisms, and there is such a thing as too much BDNF. Excessive BDNF signaling has been associated with epileptiform activity in some research models. The dose-response curve for neurotrophic factors is not linear, and more is not always better.
Dihexa extreme potency at the HGF/c-Met receptor means it should probably not be combined with other compounds that activate the same pathway. The long-term implications of sustained, high-intensity c-Met activation are not fully characterized.
The principle is straightforward: combine peptides that complement each other across different mechanisms rather than stacking multiple peptides that do the same thing at higher intensity. For a comprehensive understanding of how peptide combinations should be approached, the BPC-157 and TB-500 stacking guide provides a well-studied example of complementary mechanism stacking, and the principles it covers apply to neurological stacking as well.
Timeline expectations and realistic outcomes
One of the most common questions about peptides for brain repair is also the most difficult to answer precisely: how long does it take? The honest answer depends on what kind of brain damage is being addressed, which peptides are being used, the severity and age of the injury, and individual biological factors that vary from person to person.
But research does provide some guideposts.
Acute neuroprotection (hours to days)
Peptides that protect neurons from ongoing damage, like BPC-157 and Pinealon, begin working within hours. BPC-157 neuroprotective effects were demonstrated within 30 seconds of administration in the ischemia model. Pinealon reduction of NMDA excitotoxicity similarly operates on an acute timescale. These peptides are not rebuilding anything. They are preventing further loss. The benefits are immediate but invisible, measured not in what improves but in what damage does not occur.
Neurotrophic and anti-inflammatory effects (days to weeks)
Semax elevates BDNF within hours, but the downstream effects of that elevation, including new synaptic connections, improved neurotransmitter signaling, and reduced inflammatory gene expression, unfold over days and weeks. The clinical stroke protocol of 10 days reflects this timeline. Cerebrolysin clinical protocols similarly span 10 to 14 days for acute treatment, with maintenance cycles extending over longer periods.
Selank anxiolytic effects may be noticed within the first week, with full anti-inflammatory benefits developing over the 4-to-6-week cycle period. How long peptides take to work varies by compound and mechanism, and setting appropriate expectations is important for maintaining commitment to protocols that require weeks to show measurable results.
Neurogenesis and structural repair (weeks to months)
New neurons do not appear overnight. The neurogenesis process, from neural stem cell division to migration, differentiation, and functional integration into existing circuits, takes weeks to months. P21 80% increase in new dentate gyrus neurons is measured after sustained treatment periods. PE-22-28 doubling of BrdU-positive cells similarly reflects a cumulative process.
Structural repair, the rebuilding of dendritic arbors and synaptic connections, is even slower. P21 reversal of TBI-induced dendritic and synaptic density loss occurred over treatment periods measured in weeks to months. Dihexa synaptogenic effects, while potent at the molecular level, still require time for the physical structures of new synapses to form, mature, and stabilize.
Functional recovery (months to years)
The ultimate measure of brain repair is functional recovery: the ability to remember, think, move, speak, and navigate the world. This depends not just on biological healing but on rehabilitation, environmental enrichment, and the integration of newly formed neural connections into useful circuits. Research consistently shows that the biological window for neuroplasticity can be extended by peptide interventions, but translating biological potential into functional recovery still requires active engagement with the tasks and skills being recovered.
Patience is not optional. The brain heals slowly. Even with the most effective peptide support, meaningful neurological recovery typically unfolds over months, not days. Understanding and accepting this timeline is essential for anyone exploring peptides for brain repair, whether personally or as a research interest.
Factors that influence recovery speed
Several variables affect how quickly peptide-supported brain repair progresses. Age is significant. Younger brains have higher baseline neurogenesis rates and more robust plasticity mechanisms, which peptides can amplify more effectively. The severity and type of injury matters enormously. A mild concussion with intact blood-brain barrier presents a very different repair challenge than a severe TBI with diffuse axonal injury and widespread inflammation.
Nutritional status plays a role that is often underestimated. The brain requires specific building blocks to construct new neurons and synapses: omega-3 fatty acids for cell membranes, B vitamins for myelination, zinc and magnesium for enzymatic processes. Peptides provide the signals for repair, but the raw materials must also be available. Sleep quality directly affects neurogenesis and synaptic consolidation. Growth hormone, released primarily during deep sleep, is a key driver of neural repair. Physical exercise increases BDNF production independently of any peptide intervention, creating a synergistic effect when combined with BDNF-elevating peptides like Semax or PE-22-28.
Concurrent health conditions can accelerate or impair recovery. Chronic inflammation from any source, whether autoimmune, metabolic, or infectious, competes with and often overwhelms the anti-inflammatory effects of neuroprotective peptides. Addressing systemic inflammation through dietary changes, sleep optimization, and stress management creates a more favorable environment for peptide-mediated brain repair. SeekPeptides members often find that optimizing these foundational factors dramatically improves the effectiveness of any peptide protocol, not just those targeting the brain. The interaction between anti-aging strategies and brain repair is bidirectional: a healthier body supports a healthier brain, and vice versa.
Frequently asked questions
What is the most researched peptide for brain repair?
Cerebrolysin has the largest clinical evidence base, including randomized controlled trials (CAPTAIN I and II), cohort studies, and a meta-analysis spanning 8,749 patients. It holds regulatory approval in multiple countries for neurological conditions. Semax is the second most clinically validated, with pharmaceutical approval in Russia for stroke recovery. Both have decades of clinical use behind them, which provides a level of evidence that newer peptides like Dihexa and P21 have not yet achieved despite their promising preclinical results.
Can peptides cross the blood-brain barrier?
Some can and some cannot. Dihexa crosses the BBB and is orally bioavailable. P21 crosses the BBB with a plasma half-life exceeding six hours. Cerebrolysin crosses effectively when administered intravenously. Semax and Selank bypass the BBB entirely through intranasal administration, reaching the brain via the olfactory pathway. Larger peptides and those without specific transport mechanisms may have limited BBB penetration, which is why administration route matters so much for neurological applications. Understanding injectable versus oral peptide bioavailability is particularly important for brain-targeted protocols.
Are peptides for brain repair safe?
Safety profiles vary by compound and are better established for some peptides than others. Cerebrolysin and Semax have extensive clinical safety data spanning decades. P21 showed no adverse effects in studies lasting up to 12 months. Selank demonstrated no addiction potential despite its anxiolytic effects. However, newer compounds like Dihexa and PE-22-28 have limited human safety data. The peptide safety and risks guide provides a thorough overview of what is known and where knowledge gaps remain. Quality and purity of the peptide source significantly affect the safety equation.
Can BPC-157 help with brain injuries?
Research evidence supports BPC-157 effectiveness for brain-related applications. It reduces brain edema, hemorrhagic lacerations, and subarachnoid bleeding in TBI models. It demonstrated significant neuroprotection when given 30 seconds after ischemia onset. It counteracted MPTP neurotoxin damage to dopaminergic neurons and promoted nerve regeneration after transection. Its action through the gut-brain axis also suggests that its gut health benefits may indirectly support neurological function. The BPC-157 administration guide covers dosing and delivery methods.
What is the difference between peptides and traditional nootropics?
Traditional nootropics like racetams, modafinil, or caffeine primarily modulate existing neurotransmitter activity, essentially turning up the volume on signals already present. Nootropic peptides do something fundamentally different: they drive structural changes in the brain, including neurogenesis, synaptogenesis, and neurotrophic factor elevation. The distinction is between temporarily boosting performance on existing hardware versus actually building new hardware. This makes peptides more relevant for genuine brain repair rather than just cognitive enhancement, though many energy and focus peptides provide both benefits. Peptides differ from SARMs and other performance compounds in their mechanism and safety profiles as well.
How should peptides for brain repair be stored?
Proper storage is critical for maintaining peptide potency, especially for neuroactive compounds where degradation directly reduces effectiveness. Lyophilized peptides should be stored at minus 20 degrees Celsius for long-term preservation. Reconstituted peptides require refrigeration at 2 to 8 degrees Celsius and should be used within their stability window. The comprehensive storage guide covers all specifics. Avoid repeated freeze-thaw cycles, protect from light, and use bacteriostatic water rather than sterile water for reconstitution to prevent microbial contamination during multi-dose use.
Do peptides for brain repair require a prescription?
This depends entirely on jurisdiction and the specific peptide. Cerebrolysin is a prescription pharmaceutical in many countries. Semax and Selank are approved pharmaceuticals in Russia but available as research compounds elsewhere. Most other peptides discussed in this guide are available for research purposes. The peptide legality guide covers the regulatory landscape, and regulation news tracks ongoing changes. Working with a healthcare provider familiar with peptide protocols is advisable for anyone considering peptides for neurological applications, and online peptide therapy platforms are expanding access to qualified guidance.
What role does BDNF play in brain repair?
BDNF, brain-derived neurotrophic factor, is the central signaling molecule in neuronal survival, synaptic plasticity, and neurogenesis. It promotes neuron survival by activating anti-apoptotic pathways through its TrkB receptor. It drives synapse formation and strengthening, which is the cellular basis of learning and memory recovery. It supports long-term potentiation, the electrophysiological process underlying memory consolidation. Multiple peptides exert their brain repair effects primarily through BDNF elevation, including Semax, Selank, and PE-22-28. The BDNF peptide guide provides an in-depth look at this critical growth factor and the peptides that modulate it.
External resources
PubMed - The primary database for searching published research on all peptides discussed in this guide, including Cerebrolysin clinical trials and preclinical studies on newer compounds.
ClinicalTrials.gov - Search for ongoing and completed clinical trials involving neuropeptides, Cerebrolysin, and novel brain-targeting peptide therapies.
National Institute of Neurological Disorders and Stroke (NINDS) - Authoritative resource on traumatic brain injury, stroke, neurodegenerative diseases, and the neuroscience of brain repair mechanisms.
Nature Neuroscience - Leading journal publishing cutting-edge research on neuroplasticity, neurogenesis, and the molecular mechanisms underlying brain repair.
Brain Trauma Foundation - Evidence-based guidelines and research on traumatic brain injury treatment, recovery protocols, and emerging therapies.
The brain was never supposed to be a dead end. For decades, neuroscience treated it as one, a fixed organ that could only lose capacity, never regain it. That story is being rewritten. Not by grand pronouncements or miracle cures, but by small molecules doing precise work at the cellular level. BDNF elevated in the hippocampus. New neurons born in the dentate gyrus. Synapses forming at rates that were previously unimaginable. Inflammation modulated rather than merely suppressed.
The peptides covered in this guide, Cerebrolysin, Semax, Dihexa, BPC-157, Selank, PE-22-28, P21, Pinealon, Cortagen, and the emerging CAQK, represent different approaches to the same fundamental goal: giving the brain the molecular tools it needs to repair itself. Some are clinically validated with thousands of patients in the evidence base. Others are early in research but backed by data that demands attention. All of them work with the brain own repair mechanisms rather than against them. The diversity of mechanisms is a strength, not a complication. It means there are multiple entry points for supporting brain repair, and the right approach depends on the specific situation, whether acute injury, chronic degeneration, or proactive neuroprotection.
This field is moving fast. New studies publish monthly. Regulatory landscapes are shifting. Compounds that were obscure a few years ago are entering clinical trials. SeekPeptides tracks these developments closely, providing the tools, calculators, and educational resources that make this complex landscape navigable. From the peptide calculator for dosing to the research and studies archive, the goal is simple: make reliable information accessible to those who need it. SeekPeptides members gain access to detailed protocols, comprehensive guides, and the latest research summaries that would otherwise require hours of literature searching to compile. For a field moving this rapidly, having a trusted source that synthesizes new findings into practical, actionable knowledge is not a luxury. It is a necessity.
The brain can heal. Not perfectly. Not always completely. But far more than anyone believed possible even a decade ago. The research is clear, the mechanisms are understood, and the tools are available. What remains is the work of applying them wisely, patiently, and with the rigor that the most complex organ in the known universe deserves.
In case I do not see you, good afternoon, good evening, and good night.
May your neurons stay resilient, your synapses stay strong, and your recovery stay steady. Join us here.



