Feb 1, 2026
Understanding eczema at the molecular level
Your skin is at war. Not with the outside world, not with some random allergen, but with itself. The itch comes first. Then the redness. Then the cracking, the flaking, the raw patches that burn when you sweat or shower. You have tried corticosteroids. They helped, until they thinned your skin and stopped working. You have tried moisturizers. They soothe for an hour. Maybe two. And then the cycle starts again.
This is eczema. And if you are reading this, you already know the frustration.
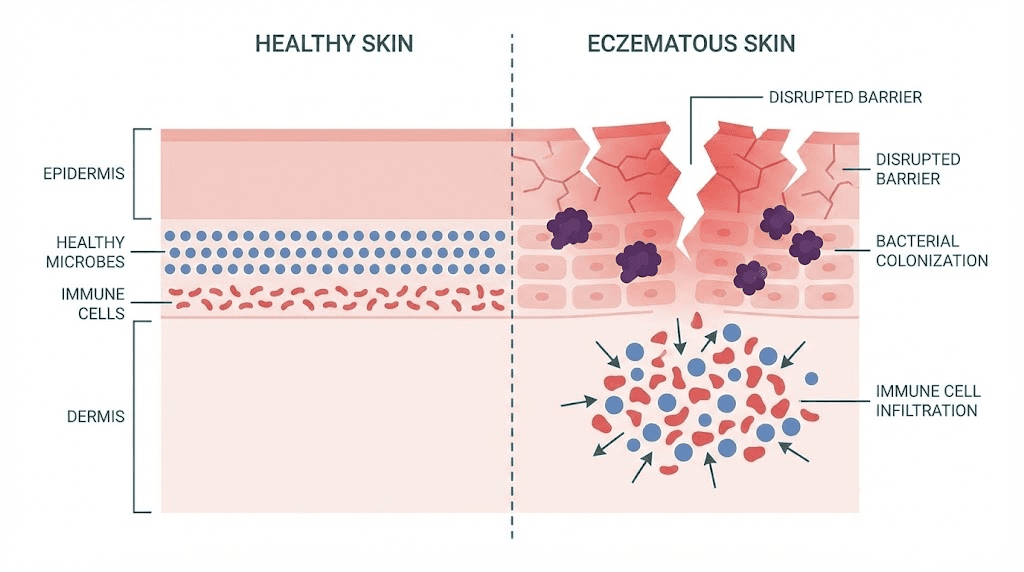
But here is what most guides will not tell you. Eczema is not just a skin problem. It is an immune system malfunction, a barrier defect, and a microbial imbalance happening simultaneously. The Th2-dominant immune response suppresses your skin natural antimicrobial defenses. The barrier proteins that should seal moisture in and keep irritants out, like filaggrin and loricrin, get downregulated. And Staphylococcus aureus, a bacterium that thrives on compromised skin, colonizes up to 90% of eczema lesions.
That is three problems converging into one miserable condition.
Peptides offer something different. Not a single-target drug that hammers one pathway and creates side effects elsewhere, but multi-mechanism molecules that can address inflammation, barrier repair, and microbial defense at the same time. Research from the New England Journal of Medicine showed that eczema patients have significantly reduced levels of key antimicrobial peptides compared to those with psoriasis. A study published in PLOS ONE demonstrated that topical application of a tetravalent peptide resolved eczema in mouse models within 14 days, restoring normal epidermal morphology. And at Vanderbilt University Medical Center, a topical peptide drug that controls 15 inflammatory genes responsible for atopic dermatitis has advanced to multicenter clinical trials.
This guide covers everything researchers need to know about peptides for eczema. From the anti-inflammatory tripeptide KPV to barrier-rebuilding GHK-Cu, from tissue-repairing BPC-157 to the body natural antimicrobial peptide defenses that eczema disrupts, we will examine the mechanisms, the research, and the practical considerations that matter. SeekPeptides has compiled the most current evidence available, so you can understand exactly how these molecules interact with eczematous skin and what the science actually supports.
The immunology of eczema and why it matters for peptide therapy
Before you can understand how peptides help eczema, you need to understand what is actually going wrong immunologically. This is not optional background information. It is the foundation that determines which peptides might work, why they work, and when they would be most useful.
The Th2-dominant immune shift
Healthy skin maintains a balance between different T-helper cell populations. Th1 cells fight intracellular pathogens and drive cellular immunity. Th2 cells handle parasitic infections and allergic responses. In eczema, this balance collapses. The immune system shifts dramatically toward Th2 dominance, flooding the skin with interleukin-4 (IL-4), interleukin-13 (IL-13), and interleukin-31 (IL-31). These cytokines do not just cause inflammation. They actively sabotage the skin barrier.
IL-4 and IL-13 suppress the production of filaggrin, a structural protein critical for maintaining the stratum corneum. Without adequate filaggrin, the outermost skin layer becomes porous. Moisture escapes. Allergens and bacteria penetrate freely. The Th2 cytokines also downregulate loricrin and involucrin, two additional barrier proteins that normally create a sealed, protective envelope around skin cells.
The result is a vicious cycle. Barrier breakdown allows more allergens in, which triggers more Th2 activation, which further weakens the barrier. Research published in the Journal of Allergy and Clinical Immunology demonstrated that this is not just a Th2 problem. Chronic eczema involves intensification of Th2, Th1, and Th17 responses simultaneously. The progression from acute to chronic disease involves quantitative escalation across multiple immune pathways, not a simple switch from one pathway to another.
This complexity is precisely why peptides are interesting. Single-mechanism drugs target one cytokine or one receptor. Peptides, particularly immune-modulating peptides, can influence multiple pathways simultaneously. Understanding the underlying research helps clarify which peptides target which aspects of eczema pathology.
Antimicrobial peptide deficiency in eczema
This is one of the most important discoveries in eczema research, and most people with the condition have never heard about it.
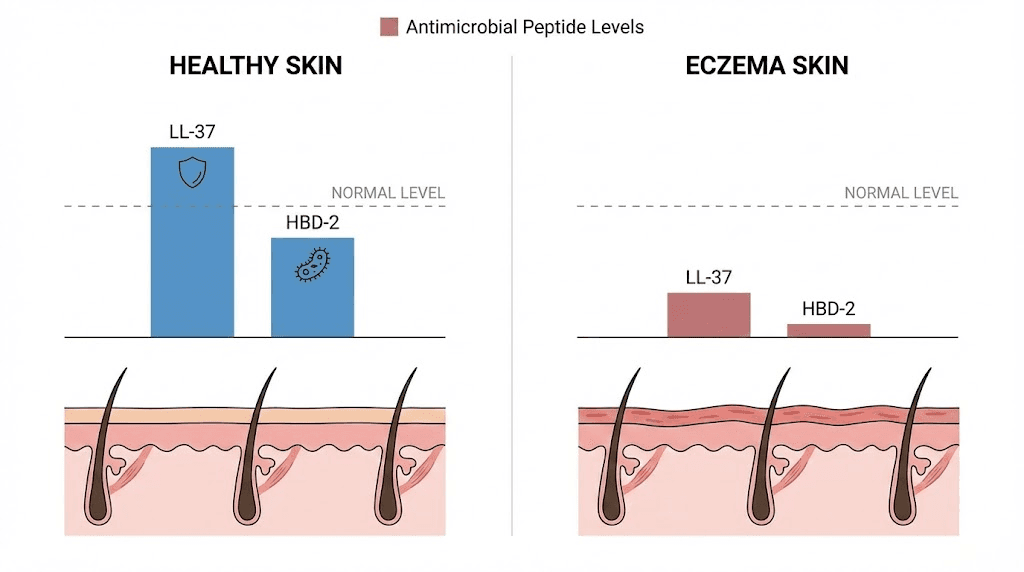
In a landmark study published in the New England Journal of Medicine, researchers compared antimicrobial peptide expression between eczema and psoriasis patients. Both conditions involve inflamed skin. Both cause visible lesions. But the antimicrobial peptide profiles are dramatically different. Psoriasis patients showed abundant LL-37 (cathelicidin) and human beta-defensin 2 (HBD-2) in the superficial epidermis. Eczema patients showed significantly decreased levels of both peptides, with P-values of 0.006 and 0.03 respectively.
Why does this matter? Because LL-37 and HBD-2 are the skin primary defense against bacterial, fungal, and viral pathogens. They are produced by keratinocytes in the epidermis and work by disrupting microbial membranes. When their levels drop, the skin becomes vulnerable. And in eczema, levels drop significantly because those same Th2 cytokines, IL-4 and IL-13, actively suppress antimicrobial peptide production.
This creates a perfect storm for Staphylococcus aureus colonization. The bacterium is found on the skin of up to 90% of eczema patients, compared to roughly 5% of healthy individuals. S. aureus worsens eczema through multiple mechanisms: it produces superantigens that amplify the immune response, it damages the skin barrier directly, and it has evolved sophisticated resistance mechanisms against the few antimicrobial peptides that remain. The S. aureus metalloproteinase aureolysin cleaves LL-37, while staphylokinase inhibits the bactericidal effect of defensins.
A 2023 study published in Cell Reports added another layer. During Th2 inflammation, the antimicrobial peptides that are still produced preferentially kill beneficial commensal bacteria rather than S. aureus. The antibiotic-producing strains of coagulase-negative staphylococci (CoNS), the beneficial bacteria that normally keep S. aureus in check, are more sensitive to the remaining AMPs than S. aureus itself. This means the weakened antimicrobial defense actually makes the microbial imbalance worse, not better.
The skin barrier defect
Filaggrin mutations affect up to 50% of moderate-to-severe eczema patients. Even in patients without genetic mutations, the Th2 inflammatory environment suppresses filaggrin expression epigenetically. The loss of this single protein has cascading effects. Filaggrin breaks down into natural moisturizing factor (NMF) components that maintain skin hydration. Without NMF, the skin dries out. Without filaggrin proper function in cross-linking keratin filaments, the stratum corneum becomes structurally compromised.
Transepidermal water loss (TEWL) increases. Skin pH rises, creating conditions that favor S. aureus growth. Allergens that would normally bounce off intact skin penetrate through the gaps and activate the immune response beneath.
The peptide approach to eczema recognizes that you cannot solve this problem by addressing only one of these three interconnected failures. You need to modulate the immune response, rebuild the barrier, and restore antimicrobial defense. Different peptides target different aspects of this triad, and understanding which does what is essential for designing effective peptide approaches.
KPV: the anti-inflammatory peptide with direct dermatitis evidence
If one peptide stands out for eczema specifically, it is KPV.
KPV is a tripeptide, just three amino acids: lysine, proline, and valine. It is the C-terminal fragment of alpha-melanocyte-stimulating hormone (alpha-MSH), stripped down to its essential anti-inflammatory core. And it retains the full anti-inflammatory activity of the parent hormone without the broader melanogenic and hormonal effects.
How KPV works against eczema inflammation
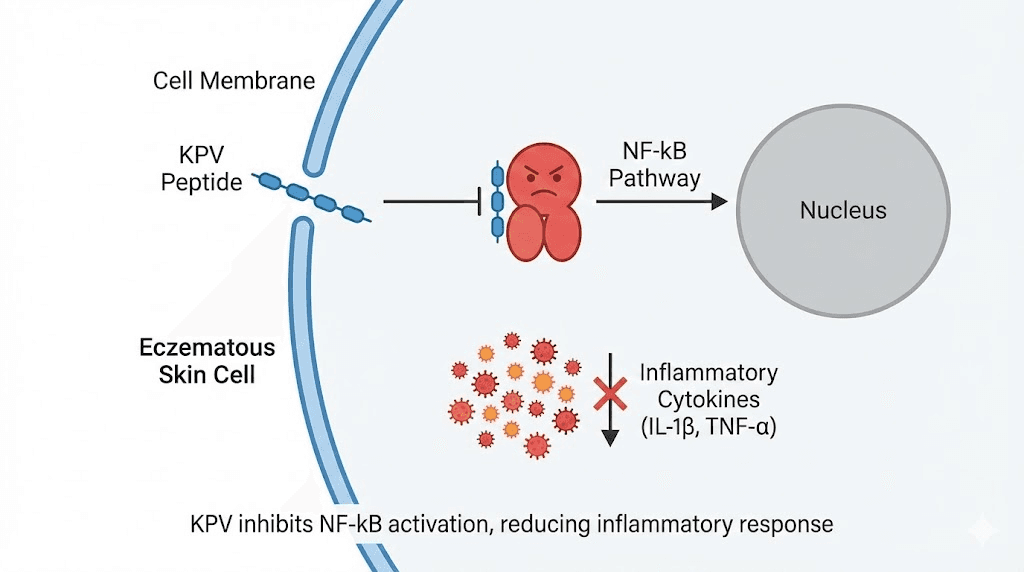
KPV reduces inflammation by binding to melanocortin receptors, specifically MC1R and MC3R. This binding blocks the NF-kB signaling pathway, the master switch for inflammatory gene expression. When NF-kB is suppressed, the production of TNF-alpha, IL-1-beta, and IL-6 drops. IL-10, an anti-inflammatory cytokine, increases. Neutrophil infiltration decreases. The inflammatory cascade that drives eczema flares gets interrupted at a fundamental level.
But KPV does something else that matters specifically for eczema. Research on alpha-MSH peptides, including KPV, demonstrated suppression of contact dermatitis reactions and induction of hapten-specific tolerance. In human studies, alpha-MSH applied topically in a cream reduced nickel-induced contact eczema. This is direct evidence of anti-eczema activity, not extrapolated from general anti-inflammatory data. The KPV mechanism targets the specific inflammatory pathways that drive atopic skin disease.
There is also a patent (US20020183255A1) specifically covering the use of KPV for dermatological disorders, including eczematous dermatitis. The patent documentation describes formulations designed for topical delivery to affected skin areas.
KPV antimicrobial properties relevant to eczema
KPV does not just fight inflammation. Alpha-MSH and its C-terminal tripeptide KPV exhibit antimicrobial activity against Staphylococcus aureus and Candida albicans, two pathogens directly implicated in eczema severity. This dual action, anti-inflammatory plus antimicrobial, addresses two of the three core eczema problems simultaneously.
For researchers exploring KPV dosing protocols, the evidence suggests topical application is the most relevant delivery method for skin conditions. KPV can also support gut inflammation reduction, which is relevant because the gut-skin axis plays a documented role in atopic conditions. Many eczema patients have concurrent gut inflammation, and addressing both may produce better outcomes than targeting skin alone.
Practical considerations for KPV and eczema
KPV is highly hydrophilic, which limits skin penetration in standard formulations. Research has explored iontophoresis, microneedle delivery, and specialized cream formulations to improve transdermal delivery. For eczema specifically, the disrupted skin barrier actually allows better peptide penetration than intact skin would, which is an ironic advantage of the condition.
Researchers report that skin conditions like eczema may show improvement in inflammation and clarity within 3 to 6 weeks of consistent application. This timeline aligns with the natural turnover cycle of epidermal cells, suggesting KPV needs time to influence the new cell populations as they differentiate and migrate to the surface.
The safety profile is favorable. Because KPV is a small, naturally occurring tripeptide fragment, it is generally well tolerated. Unlike immunosuppressive drugs, KPV reduces inflammation without compromising immune function broadly, making it a more targeted approach.
GHK-Cu: rebuilding the eczema skin barrier
While KPV addresses the inflammatory component, GHK-Cu targets something equally critical: the structural integrity of the skin itself. Copper peptide GHK-Cu (glycyl-L-histidyl-L-lysine complexed with copper) is one of the most thoroughly studied peptides in dermatology, and its mechanisms align directly with what eczema-damaged skin needs most.
Collagen and extracellular matrix restoration
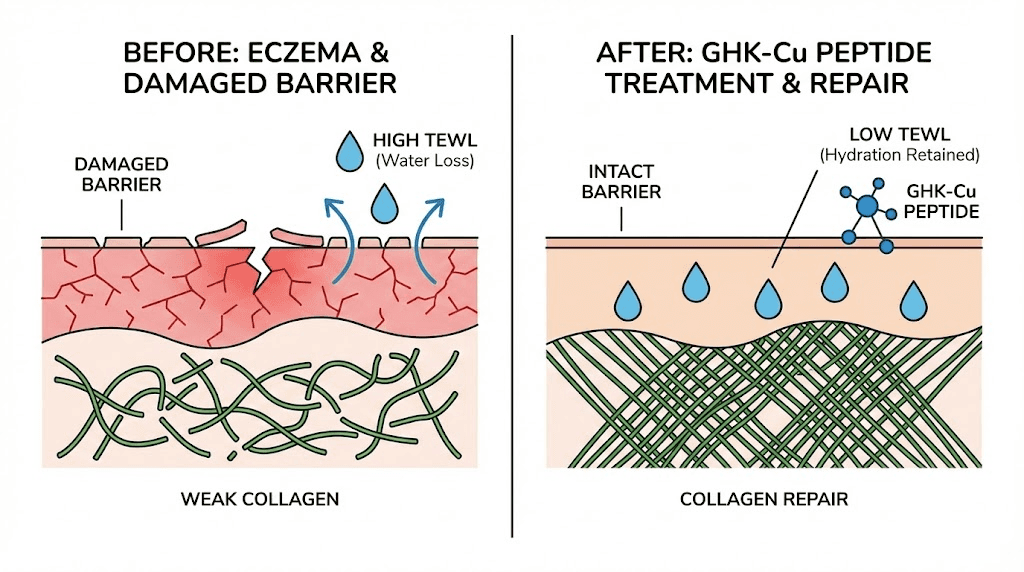
GHK-Cu stimulates collagen synthesis, elastin production, and glycosaminoglycan formation. In a 12-week trial, GHK-Cu applied to thigh skin improved collagen production in 70% of treated women, compared to 50% for vitamin C cream and 40% for retinoic acid. This is not minor improvement. It outperformed two of the most well-established dermatological actives available.
For eczema, collagen restoration matters because chronic scratching and inflammation degrade the dermal matrix. The skin becomes thinner, more fragile, more prone to cracking. Rebuilding the structural scaffold helps the skin recover its mechanical resilience. GHK-Cu also stimulates the production of decorin, a small proteoglycan that regulates collagen fibril formation and maintains tissue architecture.
Anti-inflammatory effects of GHK-Cu
GHK-Cu downregulates pro-inflammatory cytokines at the gene level. Broad gene expression studies show it acts as an "on/off switch" that inhibits inflammation while promoting cell-extracellular matrix interactions. For eczema patients dealing with chronic skin irritation, this combination of structural repair and inflammation control is particularly valuable.
The peptide also has documented anxiolytic effects. This might sound irrelevant to a skin condition, but research shows that psychological stress directly worsens psoriasis and atopic dermatitis. The itch-scratch-stress cycle is a well-documented phenomenon in dermatology. Anything that reduces the stress component can help break the cycle. Understanding the proper approach to GHK-Cu helps researchers optimize these effects.
Barrier reinforcement
GHK-Cu supports the production of proteins and lipids that reinforce the skin barrier. It reduces transepidermal water loss (TEWL), the hallmark measurement of barrier dysfunction in eczema. By enhancing the skin natural protective envelope, GHK-Cu helps prevent the cycle of moisture loss, allergen penetration, and immune activation that perpetuates the disease.
People with inflammatory skin conditions such as rosacea and eczema can benefit from adding copper peptide products to their routine. The peptide helps calm inflammation while simultaneously supporting barrier function, addressing both sides of the eczema equation. For those interested in topical application methods, concentrations and formulation details matter significantly for outcomes.
One important caveat: while GHK-Cu shows promise, direct clinical trials on copper peptides specifically for atopic dermatitis remain limited. Most evidence comes from wound healing studies, general dermatological research, and mechanistic data extrapolated to eczema pathology. The scientific rationale is strong, but large-scale randomized controlled trials for eczema specifically have not yet been completed.
GHK-Cu delivery for eczema-prone skin
Topical application is the primary delivery method for skin barrier repair. Concentration matters, as too little provides insufficient active ingredient and too much can cause irritation in already sensitized eczema skin. Research suggests starting with lower concentrations and increasing gradually, monitoring for any signs of irritation.
The relationship between copper peptides and vitamin C deserves mention. Some sources recommend avoiding concurrent use due to potential oxidation reactions, though the clinical significance of this interaction in eczema patients has not been specifically studied. Those using copper peptides alongside retinol should also exercise caution, as retinol can further compromise an already damaged eczema barrier. For sensitive eczema-prone skin, combining peptides with retinol requires careful sequencing and monitoring.
BPC-157: tissue repair from the inside out
BPC-157 is a 15-amino-acid peptide derived from human gastric juice. Its primary research applications have focused on gut protection and musculoskeletal healing, but its mechanisms have direct relevance to eczema-damaged skin.
Why BPC-157 matters for eczema
Unlike corticosteroids that suppress inflammation at the cost of skin thinning, BPC-157 actively promotes tissue repair and regeneration. It modulates angiogenesis through VEGF pathways, increasing blood flow to damaged tissue. It stimulates collagen synthesis. It regulates inflammatory pathways without the immunosuppressive side effects that make long-term steroid use problematic for eczema patients.
This distinction matters enormously. Topical steroids remain the first-line treatment for eczema flares, but they cannot be used long-term. Skin thinning, stretch marks, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression are all documented consequences of prolonged steroid use. BPC-157 does not cause skin thinning. It does the opposite, promoting structural repair that steroids actively undermine.
For researchers interested in BPC-157 dosing, topical application is the most logical approach for localized eczema. The peptide can be prepared in creams, gels, or solutions designed to penetrate the skin. Formulation factors including pH, penetration enhancers, and stability protection are important considerations. The various administration methods each have advantages depending on whether the eczema is localized or widespread.
The gut-skin axis connection
BPC-157 was originally studied for its gastrointestinal protective properties, and this matters for eczema through the gut-skin axis. Research consistently shows connections between gut health and atopic conditions. Many eczema patients have concurrent gut inflammation, food sensitivities, or altered gut microbiome composition. By supporting gut lining integrity, BPC-157 may address a root contributor to eczema rather than just treating skin symptoms. The gut healing properties of BPC-157 have been documented across multiple studies.
Anecdotal reports from researchers using BPC-157 for injury healing frequently mention improved skin appearance and decreased inflammation as secondary observations. However, rigorous clinical trials specifically testing BPC-157 for eczema in humans are not yet available. The evidence remains primarily preclinical and anecdotal at this stage.
BPC-157 and steroid withdrawal support
One potential application deserves specific attention. Topical steroid withdrawal (TSW) is a recognized complication where eczema patients who discontinue long-term steroid use experience severe rebound inflammation. The skin, dependent on external corticosteroids, loses its ability to regulate inflammation independently. BPC-157 ability to promote tissue repair without immunosuppression makes it theoretically interesting for supporting skin recovery during TSW, though this application has not been formally studied.
Researchers exploring alternatives to BPC-157 or interested in combination approaches with TB-500 should consider that multi-peptide protocols may address eczema more comprehensively than single-peptide approaches. The synergistic potential of combining anti-inflammatory, barrier-repairing, and tissue-regenerating peptides mirrors the multi-mechanism nature of eczema itself.
TB-500: systemic healing and skin regeneration
Thymosin beta-4, sold as TB-500, is a 43-amino-acid protein found throughout human tissues. It is especially concentrated in white blood cells and platelets, which release it at sites of injury to initiate the repair cascade. For eczema, its relevance centers on two key properties: accelerated dermal healing and anti-inflammatory activity.
Dermal healing evidence
The evidence for TB-500 in skin repair is more robust than for many other peptides. Phase 2 clinical trials on stasis and pressure ulcers demonstrated that thymosin beta-4 accelerated healing by almost a month compared to placebo. Animal studies showed accelerated dermal healing in normal rats, steroid-treated rats, diabetic mice, and aged mice, all populations with compromised healing capacity similar to chronic eczema patients.
The mechanism involves actin binding. Thymosin beta-4 sequesters actin monomers, freeing cells to migrate more effectively. Cells at wound edges can crawl and cover injured areas, enhancing re-epithelialization. For eczema, where scratching creates repeated micro-wounds across large skin areas, this accelerated healing capacity is directly relevant.
TB-500 also promotes stem cell mobilization and differentiation while inhibiting inflammation, apoptosis, and infection. This multi-pronged approach to tissue repair makes it valuable for conditions like eczema where damage occurs across multiple tissue layers and through multiple mechanisms. Understanding the full range of TB-500 benefits helps researchers assess its relevance to their specific situation.
Systemic versus topical approach
Unlike KPV and GHK-Cu, which are primarily studied as topical applications for skin conditions, TB-500 works systemically. This is actually advantageous for widespread eczema, where applying topical treatments to large body surface areas is impractical and compliance-limited. A systemic peptide that promotes healing throughout the body can address eczema across all affected areas simultaneously.
For researchers considering BPC-157 versus TB-500, the two peptides work through complementary mechanisms. BPC-157 acts primarily at the local tissue level, stimulating repair through VEGF and collagen pathways. TB-500 works systemically through actin binding and stem cell mobilization. Together, they address different aspects of the healing process. The Wolverine stack concept combining these healing peptides has gained attention in the research community for precisely this reason.
Thymosin alpha-1: immune rebalancing for atopic conditions
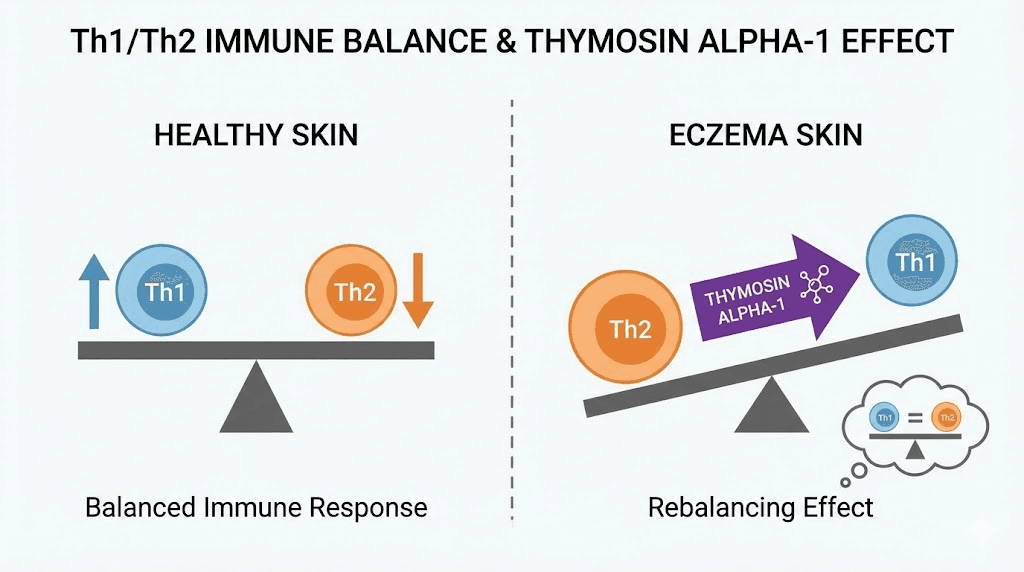
If eczema is fundamentally an immune disorder driven by Th2 dominance, then a peptide that restores Th1/Th2 balance should theoretically address the root cause rather than just the symptoms.
Thymosin alpha-1 (Ta1) is a 28-amino-acid peptide originally isolated from the thymus gland. It has been studied extensively for immune modulation across various conditions, from hepatitis B and C to cancer immunotherapy to vaccine enhancement in elderly populations. Its mechanism of action involves Toll-like receptor signaling in dendritic cells, which leads to enhanced Th1 and regulatory T-cell (Treg) responses.
Relevance to eczema immunology
The Th1-promoting activity of thymosin alpha-1 is precisely what the Th2-skewed eczema immune environment needs. By priming dendritic cells and enhancing Th1 and Treg responses, Ta1 could theoretically rebalance the immune dysregulation that drives atopic disease. Treg enhancement is particularly important because Treg cells suppress excessive immune responses and maintain tolerance, both of which are deficient in eczema.
Ta1 also stimulates IL-2 receptor expression and enhances lymphokine-activated killer cell activity. For eczema patients with suppressed cellular immunity, as evidenced by their susceptibility to viral skin infections like eczema herpeticum, restoring these immune functions could provide meaningful clinical benefit.
However, it is critical to note that no clinical trials have specifically tested thymosin alpha-1 for eczema or atopic dermatitis. The theoretical rationale is strong, supported by decades of immunology research demonstrating its Th1/Treg-promoting effects and the well-established Th2 dominance of eczema. But theory and practice do not always align. The context-dependent nature of Ta1 effects means it could behave differently in atopic tissue than predicted from other clinical settings.
Researchers interested in immune-modulating peptides should review the comprehensive literature on Ta1 while recognizing the distinction between demonstrated anti-viral and anti-cancer immune effects and hypothetical anti-atopic effects. The thymalin peptide, a related thymic peptide, operates through similar but distinct mechanisms that may also have relevance to immune dysregulation conditions.
Novel peptide therapies in clinical development
Beyond the established peptides, several novel peptide-based treatments are advancing through preclinical and clinical pipelines specifically for eczema. These represent the next generation of targeted therapies and signal where the field is heading.
NTCI cSN50.1 peptide (Vanderbilt University)
Researchers at Vanderbilt University Medical Center developed a topical peptide drug that controls 15 genes responsible for the painful inflammation in atopic dermatitis. The peptide, designated NTCI cSN50.1, suppresses the gene encoding thymic stromal lymphopoietin (TSLP), a cytokine considered the master switch of eczema inflammation. TSLP activates dendritic cells, which then drive the Th2 response that characterizes atopic disease.
Study results showed the NTCI peptide drastically reduced itching, inflammation, and open skin lesions without apparent toxicity. This is significant because itching is often the most debilitating symptom for eczema patients, driving the scratch-damage cycle that perpetuates the disease. The peptide has advanced to multicenter clinical trials, making it one of the most advanced peptide-based eczema therapies currently in development.
Multivalent peptide svL4
A study published in the journal Biomedical Journal of Scientific and Technical Research examined the tetravalent peptide svL4 for resolving eczema in mouse models. Eczema was induced using lipopolysaccharide or a mixture of Staphylococcal enterotoxin B and house dust mite extract. Within 14 days of topical treatment with 1 micromolar svL4, neutrophils were absent from the dermis and the epidermis returned to normal morphology.
The proposed mechanism is remarkable. The researchers believe svL4 serves as a substrate for transglutaminases (TGMs), enabling cross-linking of structural proteins to reconstruct a functional stratum corneum. Essentially, the peptide provides raw material for the skin to rebuild its own barrier. This is fundamentally different from anti-inflammatory approaches. Instead of suppressing the immune response, svL4 repairs the structural defect that allows the immune response to perpetuate.
Notably, dexamethasone, a potent corticosteroid, was ineffective in restoring normal epidermal morphology in the same study. The peptide succeeded where the steroid failed. This underscores the importance of barrier repair as a therapeutic strategy independent of anti-inflammatory effects.
AES16-2M wound healing peptide
Researchers at Korea University identified the peptide AES16-2M (amino acid sequence REGRT) as a candidate for atopic dermatitis treatment. In atopic dermatitis mouse models, AES16-2M administration downregulated disease scores, ear thickness, serum IgE (a key marker of allergic disease), and TSLP. The peptide also reduced IL-4, IL-13, and IL-17 producing CD4 T cells, essentially dialing down the specific immune cell populations that drive eczema.
In human keratinocyte studies, AES16-2M significantly reduced TSLP expression. Given that TSLP is considered the upstream initiator of the Th2 cascade in eczema, a peptide that specifically reduces its expression could interrupt the disease at its origin point rather than managing downstream consequences.
JEL3108 peptide (Carocell Bio)
Carocell Bio developed JEL3108, a peptide that inhibits intracellular mitogen-activated protein kinase (MAPK/p38), a signaling pathway central to inflammatory cytokine production. In human cellular models, JEL3108 significantly reduced TNF-alpha, IL-1-beta, and IL-6 markers. Human atopic dermatitis biopsy studies showed effects similar to or better than comparative steroids.
Histological studies after three treatments demonstrated significant improvement in skin architecture superior to comparative steroids. The peptide is being developed for topical application, and one additional benefit is that as a small protein, it will be safely broken down into naturally occurring amino acids after exerting its therapeutic effects. This contrasts with synthetic drugs that may accumulate or produce active metabolites.
Brain natriuretic peptide (BNP) research
Researchers at North Carolina State University identified brain natriuretic peptide (BNP) as a key activator of atopic dermatitis. BNP is elevated in AD patients and is expressed in sensory neurons that convey sensation to the brain via the spinal cord. Previous work showed BNP helps translate the itch sensation from skin to brain. This research does not provide a peptide treatment but rather identifies a peptide pathway that could be targeted to reduce eczema-associated itch, potentially through BNP receptor antagonists.
Understanding the biology of BNP provides context for why eczema itch is so persistent and difficult to treat with conventional antihistamines. The itch in eczema is not primarily histamine-mediated, which is why antihistamines generally provide poor relief. Peptide-mediated itch pathways represent a fundamentally different mechanism.
Antimicrobial peptides: restoring the skin natural defense
The third pillar of eczema pathology, after immune dysregulation and barrier defects, is antimicrobial defense failure. And this is where the body own peptides become the story.
LL-37 and human beta-defensins
LL-37 is the only human cathelicidin, produced by keratinocytes, neutrophils, and mast cells in the skin. It has broad-spectrum antimicrobial activity against gram-positive and gram-negative bacteria, viruses, and fungi. Human beta-defensin 2 (HBD-2) provides complementary antimicrobial coverage. Together, they form the front line of skin innate immunity.
In combination, LL-37 and HBD-2 show synergistic killing of S. aureus. At concentrations where neither peptide alone is fully effective, the combination kills significantly more bacteria than either individual peptide. This synergy is important because it means boosting either peptide might partially compensate for deficiency in the other.
LL-37 also demonstrates anti-biofilm activity against S. aureus at low microgram-per-milliliter concentrations. Biofilms are structured bacterial communities that are far more resistant to antibiotics than free-floating bacteria, and S. aureus biofilms on eczema skin contribute to treatment resistance and chronic colonization.
Strategies to boost endogenous antimicrobial peptides
Rather than applying antimicrobial peptides topically (which faces formulation challenges), some research focuses on boosting the body own AMP production. Vitamin D is the most studied approach. Oral vitamin D supplementation induces cathelicidin production in the skin of atopic dermatitis patients. Topical vitamin D application similarly increases cathelicidin immunoreactivity. Even sunlight exposure, through the vitamin D pathway, stimulates antimicrobial peptide production.
This has practical implications. Eczema patients often avoid sun exposure because heat and sweat trigger flares. But carefully managed, moderate sun exposure might actually help by boosting antimicrobial peptide levels. The key is finding the balance between beneficial vitamin D synthesis and triggering heat-related flares. For those interested in vitamin and peptide interactions, understanding how micronutrients influence endogenous peptide production adds another dimension to eczema management.
Engineered antimicrobial peptides for eczema
Given the potential to manage the skin microbiome, fight infections, and modulate local immune responses simultaneously, antimicrobial peptides from diverse origins have become one of the most promising alternative solutions for atopic dermatitis management. Some are already being used with preliminary success.
The advantage of engineered AMPs over conventional antibiotics is specificity. While broad-spectrum antibiotics kill beneficial commensal bacteria alongside pathogens, AMPs can potentially be designed to target S. aureus preferentially while preserving the beneficial microbiome. This is critical because studies show that disruption of the commensal bacterial community actually worsens eczema outcomes.
For researchers interested in the latest peptide research, antimicrobial peptide development for atopic dermatitis represents one of the most active and promising areas of investigation. The approach addresses a root cause, microbial imbalance, rather than just suppressing symptoms.
Peptide stacking strategies for comprehensive eczema management
Because eczema involves three simultaneous problems, the most logical peptide approach addresses all three. No single peptide does everything. But strategic combinations can cover the inflammatory, barrier, and antimicrobial aspects of the disease.
The eczema-focused peptide framework
Based on the research reviewed in this guide, here is how different peptides map to different aspects of eczema pathology:
Peptide | Primary eczema target | Mechanism | Evidence level | Delivery |
|---|---|---|---|---|
KPV | Inflammation + antimicrobial | NF-kB suppression, MC1R/MC3R binding | Direct dermatitis evidence | Topical, subcutaneous |
GHK-Cu | Barrier repair + inflammation | Collagen synthesis, TEWL reduction | Strong dermatological evidence | Topical |
BPC-157 | Tissue repair + gut-skin axis | VEGF, collagen, anti-inflammatory | Preclinical, anecdotal | Topical, subcutaneous, oral |
TB-500 | Systemic healing | Actin binding, cell migration, stem cells | Phase 2 wound healing data | Subcutaneous |
Thymosin alpha-1 | Immune rebalancing | Th1/Treg enhancement via TLR signaling | Theoretical for eczema | Subcutaneous |
The general principles of peptide stacking apply here. Combining peptides that work through different mechanisms can produce additive or synergistic effects.
The key is selecting combinations that address your specific eczema presentation. Someone with primarily inflammatory eczema might prioritize KPV. Someone with severe barrier dysfunction might lead with GHK-Cu. Someone with widespread disease and slow healing might benefit most from systemic TB-500.
Layered approach example
Topical layer: GHK-Cu for barrier repair plus KPV for localized anti-inflammatory and antimicrobial effects. Apply to affected areas following copper peptide skincare protocols.
Systemic layer: TB-500 or BPC-157 for overall tissue repair support. Those exploring peptide injection methods should familiarize themselves with proper reconstitution techniques and bacteriostatic water preparation.
Immune layer: Thymosin alpha-1 for those with documented Th2-dominant immune profiles seeking to restore balance. This is the most theoretical application and should be approached with appropriate caution.
Supportive: Vitamin D supplementation to boost endogenous antimicrobial peptide production. This has the strongest direct evidence and the simplest implementation.
What the Klow peptide blend offers
The Klow peptide blend combines BPC-157, TB-500, GHK-Cu, and KPV in a single formulation. This combination was not designed specifically for eczema, but it addresses the key mechanisms relevant to the condition: BPC-157 for microvascular repair, TB-500 for systemic healing, GHK-Cu for matrix restoration and fibrosis reduction, and KPV for local immune modulation. The Klow dosage guide provides specific information on the blend ratios and protocols used in research settings.
For researchers exploring peptide cycling strategies, eczema management may require longer duration protocols than acute injury healing. Chronic conditions typically demand sustained intervention, and the cycle planning guide can help structure appropriate timelines.
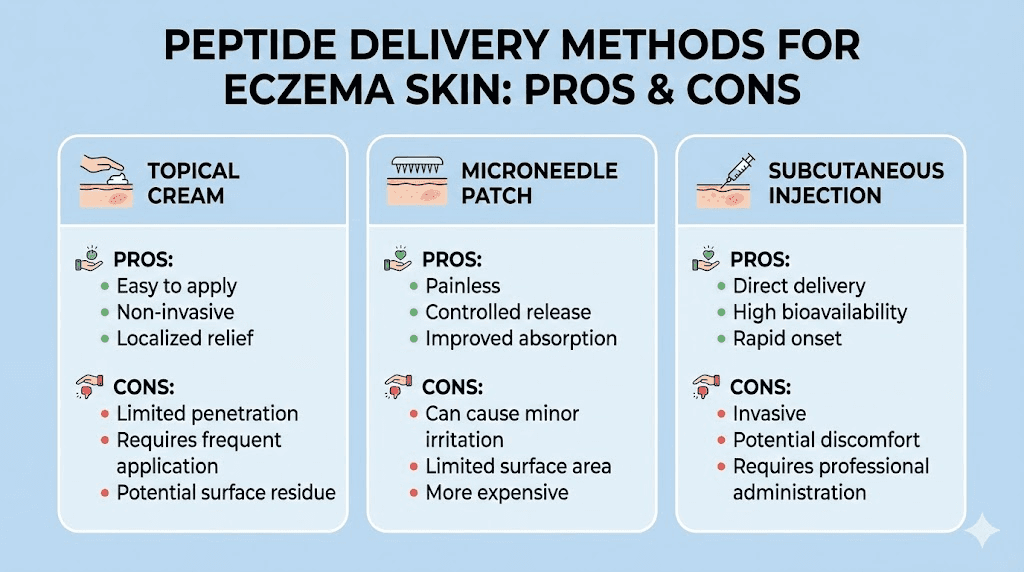
Topical peptide formulations for eczema-prone skin
Delivery matters. A potent peptide in the wrong formulation will not reach its target. And for eczema-prone skin, formulation is particularly tricky because the skin is both compromised and sensitized.
Formulation challenges
Eczema skin presents a paradox for topical delivery. The disrupted barrier means peptides penetrate more easily than through healthy skin, which is beneficial for absorption. But the same disruption means the skin is more reactive to potential irritants in the formulation, including preservatives, fragrances, surfactants, and even some peptide stabilizers.
pH matters enormously. Healthy skin has a pH around 4.5 to 5.5. Eczema skin often shifts to a higher pH, which favors S. aureus growth and impairs barrier recovery. Formulations that maintain acidic pH can both improve peptide stability and support the skin natural acid mantle.
Cream and gel formulations
For most topical peptide applications to eczema skin, cream or gel formulations with minimal inactive ingredients are preferred. Common ingredients to avoid in eczema-prone skin include sodium lauryl sulfate, fragrances, certain alcohols, and propylene glycol at high concentrations. Research-grade formulations typically use simple bases like petroleum jelly, ceramide-containing moisturizers, or hyaluronic acid gels as carriers.
The oral capsule route is also worth considering for peptides like BPC-157 that show oral bioavailability. For patients who cannot tolerate topical applications during severe flares, oral peptide delivery circumvents the damaged skin entirely while still providing systemic anti-inflammatory effects.
Microneedle and iontophoresis delivery
Advanced delivery systems are being developed to overcome the penetration challenges of topical peptide application. Microneedle patches create microscopic channels through the stratum corneum, allowing direct peptide delivery to the viable epidermis. Research on KPV delivery using iontophoresis and microneedles demonstrated improved transdermal delivery across human skin.
For eczema patients, microneedle peptide patches could provide controlled, sustained release directly to affected areas. This technology is still emerging but represents a potential advance over conventional topical application.
Storage and stability considerations
Peptide stability is always a concern, and it becomes more important when dealing with a condition that requires consistent long-term treatment. Most reconstituted peptides should be stored refrigerated. Proper peptide storage prevents degradation that reduces effectiveness. For those wondering about refrigerated shelf life or powder stability, maintaining cold chain integrity is essential for consistent results.
Using the peptide reconstitution calculator ensures accurate preparation every time. Inconsistent reconstitution leads to variable dosing, which makes it impossible to assess whether a peptide is actually working for your eczema or not.
The gut-skin axis and peptide interventions
You cannot fully address eczema without considering the gut. The relationship between intestinal health and skin conditions is one of the most well-documented connections in dermatology, even if it remains underappreciated in conventional treatment approaches.
How gut health influences eczema
The gut-skin axis operates through several mechanisms. First, intestinal permeability. When the gut barrier is compromised, a condition often called "leaky gut," partially digested food proteins and bacterial endotoxins enter the bloodstream. These trigger systemic immune activation that manifests in the skin. Second, the gut microbiome influences systemic immune balance. Dysbiosis in the gut, particularly reduced microbial diversity, correlates with increased eczema severity in both children and adults.
Third, the gut produces regulatory T cells that circulate systemically and influence immune responses throughout the body, including the skin. Gut-derived Tregs help maintain immune tolerance. When gut health is compromised, Treg production may decrease, contributing to the excessive immune reactivity that characterizes eczema.
Peptides that target the gut-skin connection
BPC-157 is the most studied peptide for gut barrier protection. Its original discovery and primary research focus centers on gastric and intestinal tissue repair. For eczema patients with concurrent gut inflammation, BPC-157 may address both conditions simultaneously. The peptide promotes tight junction integrity in intestinal epithelial cells, the same tight junction proteins that are compromised in eczema skin.
KPV also shows significant gut relevance. It has been studied extensively in colitis models, where it reduces intestinal inflammation through the same NF-kB suppression mechanism that benefits skin inflammation. For eczema patients whose skin condition worsens with gut flares, KPV dual gut and skin anti-inflammatory effects make it a particularly appealing option.
The peptides for gut health resource provides additional context on how intestinal peptide therapy can influence systemic health outcomes. For those managing eczema alongside anxiety or other stress-related conditions, the gut-brain-skin axis represents another relevant dimension. Stress increases intestinal permeability, which increases systemic inflammation, which worsens eczema. Peptides that address the gut component can help interrupt this cycle.
Comparing peptide approaches to conventional eczema treatments
How do peptides fit within the broader landscape of eczema therapy? Understanding the advantages and limitations of each approach helps researchers and patients make informed decisions.
Conventional treatments and their limitations
Treatment | Mechanism | Effectiveness | Limitations |
|---|---|---|---|
Topical corticosteroids | Broad immunosuppression | High for flares | Skin thinning, rebound, HPA suppression with long-term use |
Calcineurin inhibitors | T-cell suppression | Moderate | Burning sensation, cancer concerns (debated), cost |
Dupilumab | IL-4/IL-13 receptor blocker | High for moderate-severe | Injection site reactions, conjunctivitis, very expensive |
JAK inhibitors | Intracellular signaling block | High | Infection risk, cardiovascular warnings, liver monitoring required |
Moisturizers/emollients | Barrier support | Low-moderate alone | Symptomatic only, does not address underlying cause |
Where peptides offer advantages
Peptides occupy a unique space. They are more targeted than corticosteroids, which suppress immune function broadly. They are potentially safer for long-term use because they work through natural biological mechanisms rather than forcing pathways shut. They can address multiple eczema mechanisms simultaneously, unlike most conventional drugs that target single pathways.
BPC-157 promotes tissue repair without causing skin thinning. GHK-Cu rebuilds structural proteins rather than just suppressing inflammation. KPV offers anti-inflammatory effects without immunosuppression. TB-500 accelerates healing without the risks of systemic steroids. These are meaningful distinctions for patients managing a lifelong chronic condition.
Where peptides fall short
Honesty matters here. The evidence base for peptides in eczema is substantially thinner than for conventional treatments. Dupilumab has been tested in large randomized controlled trials involving thousands of patients. Most peptide evidence comes from animal models, small studies, and mechanistic extrapolation. Using peptides for eczema means accepting a higher degree of uncertainty about outcomes.
Formulation and delivery remain challenges. Most peptides are not available in pharmacy-grade eczema formulations. Researchers need to source, reconstitute, and formulate peptides themselves, introducing variability that standardized pharmaceutical products avoid. The importance of third-party testing cannot be overstated when sourcing peptides for any application, especially one involving compromised skin. Quality and purity verification protect against contamination that could worsen eczema.
Regulatory status is also a consideration. Peptides like BPC-157, TB-500, and KPV are classified as research chemicals in most jurisdictions. They are not FDA-approved for eczema or any other condition. This means they lack the quality controls, dosing standardization, and safety monitoring that approved drugs provide. Researchers exploring these options do so at their own informed discretion. Checking current peptide legality in your jurisdiction is essential before beginning any protocol.
Practical protocols and considerations
For researchers who have weighed the evidence and decided to explore peptide approaches for eczema, practical details matter. How you prepare, apply, and monitor peptides determines whether you get meaningful results or waste time and money.
Starting point selection
Not everyone should start with the same peptide. Your specific eczema presentation should guide your selection.
If your primary problem is inflammation and itching: KPV has the most direct evidence for dermatitis specifically. Start here if flares and itch dominate your experience.
If your primary problem is dry, cracked, structurally compromised skin: GHK-Cu addresses the barrier and collagen deficits. Start here if your skin feels thin, fragile, and unable to hold moisture regardless of how much moisturizer you apply.
If your eczema correlates with gut problems: BPC-157 addresses both gut barrier and skin repair. Start here if you notice eczema flares correlate with digestive symptoms, food reactions, or gut inflammation.
If your eczema is widespread and healing slowly: TB-500 provides systemic healing support. Consider this if eczema covers large body surface areas and individual lesions take weeks to heal even with treatment.
If you suspect immune imbalance is the root: Thymosin alpha-1 targets Th1/Th2 rebalancing. This is the most theoretical application and should generally not be the starting point.
Monitoring and assessment
Eczema severity can be assessed using validated tools like the SCORAD index or the Patient-Oriented Eczema Measure (POEM). Before starting any peptide protocol, document your baseline. Photograph affected areas in consistent lighting. Record your daily itch severity on a 0-10 scale. Note how many nights your sleep is disrupted by itching. Track moisturizer consumption as a proxy for barrier function.
The before and after documentation approach used for other peptide applications works equally well for eczema. Without baseline data, it is impossible to determine whether a peptide is actually helping or whether natural disease fluctuation is responsible for any changes you observe.
Minimum assessment period for most peptides is 4 to 8 weeks. Eczema is a chronic condition with natural fluctuations, and shorter periods cannot distinguish treatment effects from random variation. For barrier repair with GHK-Cu, expect visible changes over 8 to 12 weeks. For inflammatory reduction with KPV, improvements may appear within 3 to 6 weeks.
What to avoid during peptide protocols
Several common eczema triggers can confound peptide assessment. During your evaluation period, try to maintain stable environmental conditions. Avoid introducing new skincare products, changing detergents, or significantly altering your diet. Document any unavoidable changes such as seasonal transitions or stress events. The goal is to isolate the peptide effect from background variability.
Do not discontinue prescribed treatments abruptly to "test" peptides. Particularly for those on topical steroids, abrupt cessation can trigger severe rebound flares that make peptide assessment impossible. Any changes to conventional treatment should be gradual and ideally supervised by a healthcare provider familiar with your eczema history.
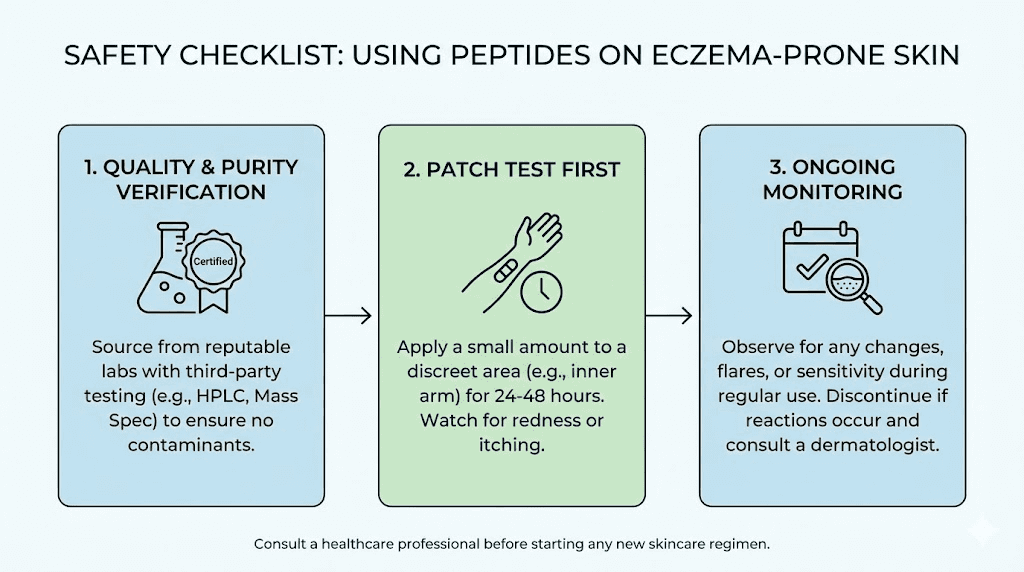
Peptide quality and sourcing for eczema applications
Skin integrity is already compromised in eczema. Applying contaminated or degraded peptides to broken skin is not just ineffective. It is potentially harmful. Quality assurance is not a suggestion for eczema applications. It is a requirement.
Third-party testing requirements
Every peptide used on eczema-affected skin should have a certificate of analysis from an independent laboratory. Minimum requirements include purity assessment (HPLC), identity confirmation (mass spectrometry), and endotoxin testing (LAL test). Endotoxin testing is particularly critical because lipopolysaccharides, the endotoxins produced by gram-negative bacteria, are potent inflammatory triggers. In eczema skin, even trace endotoxin contamination could trigger flares.
The peptide testing lab guide identifies laboratories that provide comprehensive analysis. For topical applications, you might also want sterility testing, especially if the peptide will be applied to open or fissured skin. Using the peptide calculator helps ensure accurate concentration preparation.
Reconstitution for topical use
Most peptides arrive as lyophilized powder that requires reconstitution. For subcutaneous injection protocols, standard bacteriostatic water reconstitution applies. For topical application, the reconstitution medium may need to be different. Some researchers mix reconstituted peptide solutions with simple, fragrance-free moisturizers to create topical formulations. Others use specialized bases like Vanicream or CeraVe as carriers.
The reconstitution calculator helps ensure precise concentration regardless of final formulation. Store reconstituted peptide solutions according to recommended storage guidelines and discard after the appropriate period. Fresh preparation is essential, especially for topical applications where degradation products could irritate sensitized skin.
Vendor selection
Not all peptide vendors maintain the quality standards needed for dermatological application. The vendor comparison guide evaluates suppliers on purity, testing, and reliability. For eczema applications specifically, prioritize vendors that provide batch-specific certificates of analysis, maintain proper cold chain shipping, and use pharmaceutical-grade synthesis processes.
SeekPeptides members access detailed vendor reviews, quality comparison databases, and community-sourced feedback on specific suppliers. When your skin barrier is already compromised, there is no room for guessing about peptide quality. Making informed cost and quality decisions requires reliable data.
Lifestyle factors that enhance peptide effectiveness for eczema
Peptides do not work in isolation. Their effectiveness is influenced by the overall environment you create for your skin and immune system. Certain lifestyle modifications can enhance peptide outcomes, while others can undermine them entirely.
Vitamin D optimization
As discussed earlier, vitamin D directly stimulates production of cathelicidin (LL-37) and other antimicrobial peptides in the skin. For eczema patients, who often have both low vitamin D levels and low antimicrobial peptide expression, supplementation represents one of the most evidence-based adjunct strategies available. Studies show oral vitamin D supplementation induces cathelicidin production in the skin of atopic dermatitis patients specifically. This is not a general wellness recommendation. It is a targeted intervention supported by direct clinical evidence in the eczema population.
Microbiome support
The skin microbiome and gut microbiome both influence eczema severity. Strategies that support microbial diversity, including probiotic supplementation (particularly Lactobacillus and Bifidobacterium strains studied in atopic conditions), prebiotic fiber intake, and avoidance of unnecessary antibiotics, create a more favorable environment for peptide interventions. When the microbial environment improves, the inflammatory burden decreases, and peptides working to repair the barrier face less opposition.
Stress management
Cortisol and other stress hormones directly impair skin barrier function and promote Th2 immune deviation. Chronic stress worsens eczema through measurable biological mechanisms, not just subjective symptom perception. GHK-Cu has documented anxiolytic effects, but relying on a peptide for stress management is insufficient. Structured stress reduction practices complement peptide protocols by reducing the inflammatory and barrier-disrupting effects of chronic psychological stress.
The relationship between peptides and anxiety management is increasingly recognized in research. For eczema patients caught in the itch-scratch-stress cycle, addressing the stress component alongside direct skin treatment produces better outcomes than either approach alone.
Environmental control
Even the best peptide protocol will struggle against constant environmental triggers. Dust mite allergen reduction (encasing mattresses and pillows, washing bedding in hot water), humidity control (keeping indoor humidity between 40-60%), temperature management (avoiding overheating, which triggers itch and sweating), and irritant avoidance (fragrance-free products, gentle cleansers) all create conditions where peptides can work more effectively.
Think of it this way. Peptides repair and protect. Environmental control reduces the ongoing damage they need to overcome. Combining both gives the best chance of breaking the chronic eczema cycle.
Eczema subtypes and peptide selection
Not all eczema is the same. Different subtypes involve different dominant mechanisms, and peptide selection should reflect these differences.
Atopic dermatitis (classic eczema)
This is the most common form, characterized by Th2-dominant inflammation, barrier defects, and S. aureus colonization. The full spectrum of peptides discussed in this guide is relevant. KPV for inflammation, GHK-Cu for barrier repair, and antimicrobial peptide support for microbial balance represent the comprehensive approach. The skin-supporting peptides that enhance structural integrity are particularly relevant for the barrier dysfunction component.
Contact dermatitis
Triggered by specific allergens or irritants. KPV has direct evidence for suppressing contact dermatitis reactions and inducing hapten-specific tolerance. For contact dermatitis that becomes chronic and resembles atopic disease, GHK-Cu for barrier repair becomes relevant.
Nummular eczema
Characterized by coin-shaped patches, often with significant barrier disruption and secondary infection. BPC-157 and TB-500 tissue repair properties may be particularly relevant for the localized tissue damage. Tissue repair peptides address the wound-like nature of nummular lesions.
Dyshidrotic eczema
Affecting hands and feet with deep-seated blisters. The unique location and morphology suggest barrier repair peptides (GHK-Cu) and anti-inflammatory peptides (KPV) applied to specific areas. The thicker skin of palms and soles presents additional delivery challenges that may require enhanced penetration strategies.
Seborrheic dermatitis
Overlapping with eczema but involving different pathogenic yeast (Malassezia). KPV antimicrobial properties and its activity against fungal organisms may be relevant here. However, seborrheic dermatitis is sufficiently different from atopic eczema that treatment approaches should be tailored accordingly.
What the research says about combining peptides with conventional treatments
Most eczema patients will not abandon conventional treatments entirely in favor of peptides. The more practical question is whether peptides can complement existing therapies and potentially allow reduction of treatments that cause long-term side effects.
Peptides alongside topical steroids
GHK-Cu barrier repair properties could theoretically counteract steroid-induced skin thinning. Corticosteroids degrade collagen and thin the dermis with prolonged use. GHK-Cu promotes collagen synthesis and dermal thickening. Using GHK-Cu during or after steroid courses might protect or restore skin thickness, though this specific combination has not been formally studied.
KPV anti-inflammatory effects might also allow steroid dose reduction in patients who can achieve adequate inflammation control with peptide supplementation. This is a common strategy in medicine, using a safer agent to reduce dependence on a riskier one, but it requires careful monitoring to ensure disease control is maintained.
Peptides alongside biologics
For patients on dupilumab (Dupixent) or other biologics, peptides could address aspects of eczema that biologics do not target. Dupilumab blocks IL-4/IL-13 signaling but does not directly repair the skin barrier or restore antimicrobial peptide levels. GHK-Cu for barrier repair and vitamin D supplementation for antimicrobial peptide production could complement biologic therapy.
Peptides during treatment gaps
Many eczema patients cycle between active treatment and periods without treatment. Peptides could potentially maintain disease control during treatment gaps, reducing the frequency and severity of flares that necessitate returning to stronger medications. This maintenance role, where peptides keep the skin stable between conventional treatment courses, may be the most practical near-term application.
Safety considerations specific to eczema
Applying any substance to eczema-affected skin carries inherent risks that do not apply to intact skin. Researchers need to be aware of these eczema-specific safety considerations.
Sensitization risk
Eczema patients have overactive immune systems in the skin. They are more prone to developing new allergies, including contact allergies to topical agents. While peptides are generally well tolerated because they break down into naturally occurring amino acids, the potential for sensitization exists with any foreign substance applied to immunologically active eczema skin. Patch testing a small area before widespread application is prudent.
Infection risk
Open fissures and excoriated skin create direct entry points for pathogens. Any topical preparation must be sterile or at minimum prepared under clean conditions. Reconstituted peptide solutions that are contaminated with bacteria can cause skin infections that dramatically worsen eczema. This is why peptide safety protocols are particularly important for dermatological applications.
Interaction with eczema medications
The interactions between peptides and common eczema medications are largely unstudied. Theoretical concerns include: GHK-Cu angiogenic properties potentially conflicting with calcineurin inhibitor mechanisms; thymosin alpha-1 immune enhancement potentially conflicting with immunosuppressive therapies; BPC-157 effects on nitric oxide pathways potentially interacting with topical PDE4 inhibitors. These are theoretical concerns, not documented interactions, but they warrant awareness and caution. The common mistakes guide covers general safety principles that apply to any peptide protocol.
Pediatric considerations
Eczema is extremely common in children, affecting up to 20% of children in some countries. However, peptide research is conducted almost exclusively in adults. Applying peptide protocols to children with eczema would be off-label to an extreme degree, and the developing immune system and skin barrier present additional unknowns. Pediatric application should not be assumed safe based on adult data.
Frequently asked questions
Can peptides cure eczema permanently?
No. Eczema is a chronic condition influenced by genetics, immune programming, and environmental factors. Peptides can potentially improve symptoms, strengthen the skin barrier, and reduce inflammation, but they do not alter the underlying genetic predisposition. Think of peptides as tools for managing the condition more effectively, not as a cure. For comprehensive management strategies, explore the getting started guide and work with qualified healthcare providers.
Which peptide should I start with for eczema?
KPV has the most direct evidence for dermatitis and addresses both inflammation and microbial defense. GHK-Cu is the best starting point if barrier dysfunction dominates your presentation. BPC-157 is most appropriate if gut health is a contributing factor. Consult the peptide dosing guide for general dosing principles before beginning any protocol.
Are peptides safe to use on broken eczema skin?
Peptides themselves are generally well tolerated, but applying any substance to open fissures or excoriated skin carries infection risk. Ensure sterile preparation, start with small test areas, and avoid application to actively infected or weeping lesions. The injection safety guide covers sterile technique principles applicable to topical preparation as well.
How long do peptides take to work for eczema?
Anti-inflammatory effects from KPV may be noticeable within 3 to 6 weeks. Barrier repair from GHK-Cu typically requires 8 to 12 weeks to show visible improvement. Systemic healing from TB-500 follows a similar timeline. Eczema fluctuates naturally, so minimum 4 to 8 week assessment periods are needed to distinguish real improvement from disease variation. Read about typical peptide timelines for additional context.
Can I use peptides while on dupilumab or other biologics?
There are no documented interactions between the peptides discussed in this guide and biologic therapies like dupilumab. However, this absence of data means safety has not been established, not that it has been proven. GHK-Cu for barrier repair and vitamin D for antimicrobial peptide support are the most conservative additions to biologic therapy. Always inform your prescribing physician about any peptide use.
Do peptides help with eczema-related itch specifically?
KPV reduces itch indirectly by suppressing the inflammatory mediators that cause pruritus. The NTCI cSN50.1 peptide currently in clinical trials specifically demonstrated itch reduction. BNP research at NC State identified a peptide pathway directly involved in eczema itch signaling, though therapeutic peptides targeting this pathway are still in development.
What about collagen peptides for eczema?
Oral collagen peptides provide amino acid building blocks for skin repair but do not have the targeted mechanisms of therapeutic peptides like KPV or GHK-Cu. They may support general skin health but should not be considered primary treatment for eczema. Their role is supplementary, providing raw materials rather than signaling molecules that direct repair processes. The question of whether collagen peptides count as protein is relevant for those managing dietary aspects of their eczema care.
Is topical or injectable peptide delivery better for eczema?
For localized eczema, topical delivery targets affected areas directly with minimal systemic exposure. For widespread eczema covering large body surface areas, systemic delivery (subcutaneous injection) ensures all affected areas receive benefit without the impracticality of applying topical preparations everywhere. Many researchers use both: topical for targeted flare management and systemic for baseline support. The injectable versus oral comparison provides additional context on delivery method selection.
External resources
Resolution of Eczema with Multivalent Peptides (PLOS ONE, PMC)
Endogenous Antimicrobial Peptides and Skin Infections in Atopic Dermatitis (NEJM)
Cathelicidin LL-37: An Antimicrobial Peptide with a Role in Inflammatory Skin Disease (PMC)
For researchers serious about managing eczema with an evidence-based, multi-mechanism approach, SeekPeptides provides the most comprehensive resource available. Members access detailed protocols, quality vendor databases, community experiences, and expert guidance for navigating peptide research safely and effectively. When you are dealing with a condition as complex and frustrating as eczema, having reliable information and a knowledgeable community makes all the difference between guessing and making informed decisions.
In case I do not see you, good afternoon, good evening, and good night. May your skin barrier stay strong, your inflammation stay quiet, and your nights stay itch-free.



