Jan 31, 2026
At the moment a bone fractures, a cascade begins. Platelets rush to the fracture site within minutes, forming a hematoma that serves as a biological scaffold. Inflammatory cytokines flood the area. Tumor necrosis factor alpha signals the cleanup crews. Bone morphogenetic proteins begin recruiting mesenchymal stem cells from surrounding tissue. Vascular endothelial growth factor starts building new blood vessel networks to feed the repair zone. This is not a simple process. It is one of the most complex regenerative events in the human body, requiring precise coordination between immune cells, stem cells, blood vessels, and nerve endings across weeks and months of continuous biological activity.
And sometimes it fails.
Between five and ten percent of the roughly eighteen million bone fractures that occur annually in the United States result in delayed union or non-union, where the bone simply does not heal properly on its own. That leaves hundreds of thousands of people each year stuck in a cycle of pain, immobility, and frustration. Standard treatments work for most fractures, but when healing stalls, options become limited. This is precisely where peptides enter the conversation. Specific peptide compounds have demonstrated the ability to influence every single stage of bone repair, from the initial inflammatory response through final remodeling. Understanding how these peptides work at the molecular level reveals why researchers are increasingly interested in their potential for accelerating fracture recovery, supporting bone density, and addressing the stubborn problem of fractures that refuse to heal.
This guide covers the science behind bone healing, the specific peptides that influence each stage of recovery, detailed protocols used in research settings, practical handling and preparation guidance, and the nutritional strategies that support peptide-driven bone regeneration. Whether you are recovering from a fracture, dealing with a slow-healing break, or simply want to understand how modern peptide research applies to skeletal health, what follows is the most comprehensive resource available on this topic.
How bones heal after a fracture: the four stages
Bone is living tissue. That statement surprises people who think of their skeleton as a static framework, but bones are dynamic organs with blood supply, nerve innervation, and continuous cellular turnover. When a bone breaks, the body launches a sophisticated repair process that unfolds in four distinct but overlapping stages. Understanding these stages is essential because tissue repair peptides target specific phases of this process, and knowing where and how they intervene helps explain why certain compounds show promise for faster injury healing.
Stage one: the inflammatory phase
The moment a bone fractures, blood vessels within the bone and surrounding periosteum rupture. Blood floods the fracture gap and forms a hematoma, a clot that becomes the initial scaffold for everything that follows. This hematoma is not just a passive blood clot. It is a highly active biological environment.
Within hours, neutrophils arrive to clear debris and dead cells. Macrophages follow shortly after, and these immune cells are critical. They release a cocktail of signaling molecules including TNF-alpha, interleukin-1, interleukin-6, bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-beta), and vascular endothelial growth factor (VEGF). Each of these molecules plays a specific role in orchestrating the repair process. TNF-alpha recruits mesenchymal stem cells. BMPs direct those stem cells toward becoming bone-forming cells. VEGF initiates the formation of new blood vessels to supply the growing repair tissue.
This phase lasts roughly one week. It is painful, and the inflammation is necessary. Suppressing it too aggressively with anti-inflammatory medications can actually impair healing. Researchers studying bone and cartilage repair peptides pay close attention to this inflammatory balance.
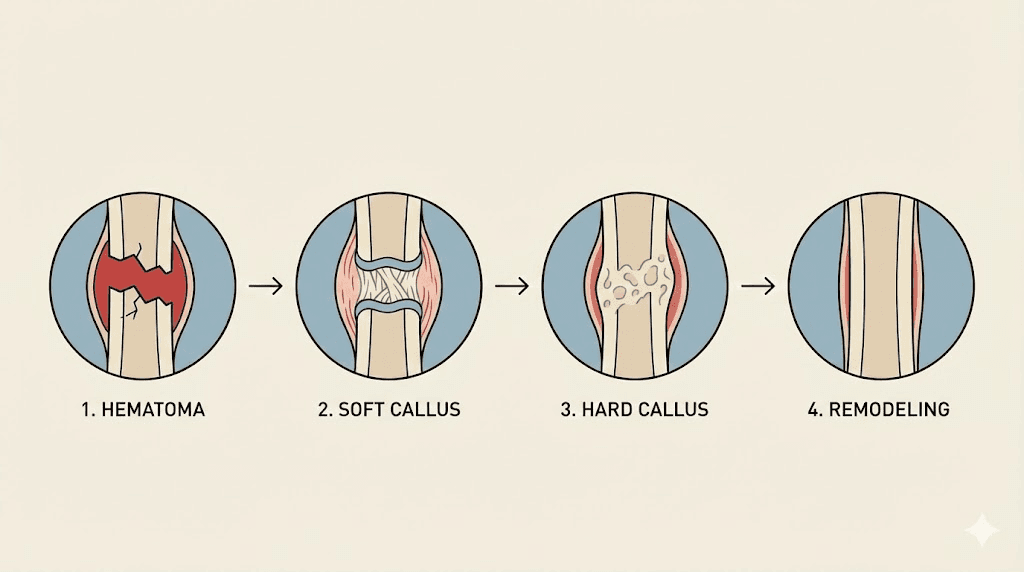
Stage two: soft callus formation
Between weeks one and four, the real construction begins. Mesenchymal stem cells that were recruited during the inflammatory phase differentiate into two critical cell types: chondroblasts and osteoblasts. Chondroblasts produce cartilage. Osteoblasts produce bone. Together, they build a fibrocartilaginous bridge across the fracture gap called a soft callus.
Think of the soft callus as a temporary splint made from the inside. It stabilizes the fracture fragments and provides a framework that will eventually be replaced by actual bone. During this phase, managing inflammation becomes important because excessive inflammatory signaling can disrupt the delicate process of stem cell differentiation. The soft callus is visible on X-rays as a cloudy mass surrounding the fracture line, and its size often correlates with eventual healing success.
Stage three: hard callus formation
From roughly week four through week twelve, endochondral ossification transforms the soft cartilage callus into hard, mineralized bone. This is the same process that builds bones during embryonic development. Chondrocytes within the soft callus undergo programmed cell death, leaving behind a calcified cartilage matrix. Osteoblasts then deposit new bone mineral onto this scaffold, gradually converting the soft callus into a rigid bony bridge.
Blood supply is absolutely critical during this phase. Without adequate vascularization, the calcification process stalls. This is one reason why peptides that promote both bone formation and angiogenesis are particularly interesting to researchers. They address two requirements simultaneously. The relationship between collagen-boosting peptides and connective tissue repair also becomes relevant during this phase, as collagen forms the organic scaffold for mineralization.
Stage four: bone remodeling
The final stage can last months to years. During remodeling, osteoclasts resorb the irregular woven bone of the hard callus while osteoblasts deposit organized lamellar bone in its place. The result is bone that gradually approaches the original structure and strength. This process is guided by mechanical forces, which is why appropriate loading and weight-bearing during recovery matters so much. Wolff law states that bone adapts to the loads placed upon it, and remodeling follows this principle precisely.
Each of these four stages represents an opportunity for intervention. And each stage has specific peptides that appear to support or accelerate the biological processes involved. The skin and tissue repair applications of many of these same peptides demonstrate that their regenerative effects extend across multiple tissue types, including bone.
Why some fractures fail to heal
Not every fracture follows the textbook healing timeline. When a bone has not healed after six months, clinicians classify it as a non-union. When healing is progressing but significantly slower than expected, it is termed delayed union. Both conditions affect a substantial number of people and create real consequences, from chronic pain and disability to multiple corrective surgeries.
The risk factors fall into several categories.
Biological factors include age, hormonal status, nutritional deficiencies (particularly vitamin D, calcium, and protein), smoking, diabetes, and certain medications. Corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and some anticonvulsants can all impair bone healing. Conditions like osteoporosis weaken bone structure before a fracture even occurs, making recovery more challenging. The anti-aging peptide research community has identified this connection between age-related peptide decline and skeletal fragility. Age-related peptide decline also plays a role, as levels of endogenous growth factors and repair peptides decrease with aging.
Mechanical factors include inadequate fracture stabilization, excessive gap size between bone fragments, and the location of the fracture itself. Fractures in areas with poor blood supply, like the scaphoid bone in the wrist or the femoral neck, are notoriously difficult to heal.
Vascular factors may be the most critical of all. Without sufficient blood supply, cells in the fracture zone do not receive the oxygen and nutrients they need to build new bone. High-energy injuries that damage surrounding soft tissue and blood vessels are particularly prone to healing complications. The importance of angiogenesis in fracture healing cannot be overstated, which is why peptides that stimulate new blood vessel formation have attracted significant research interest.
Infection represents another major obstacle. Open fractures that break through the skin carry a significant risk of bacterial contamination, and infected bone heals poorly or not at all. The immune system peptides that modulate inflammatory and antimicrobial responses may have relevance in these complicated scenarios.
Understanding why fractures fail provides the context for understanding why peptides are being studied. They do not replace proper medical treatment. They are being investigated as compounds that might address the specific biological bottlenecks that prevent normal healing. The muscle growth peptides that many people are familiar with share some of the same regenerative pathways that apply to bone, which is why cross-tissue peptide research continues to expand.
How peptides support each stage of bone healing
Peptides are short chains of amino acids, typically between two and fifty amino acids in length. They serve as signaling molecules throughout the body, directing cellular behavior, modulating inflammation, and triggering growth and repair processes. For a foundational understanding, the complete guide to peptides explains how these molecules function at the cellular level.
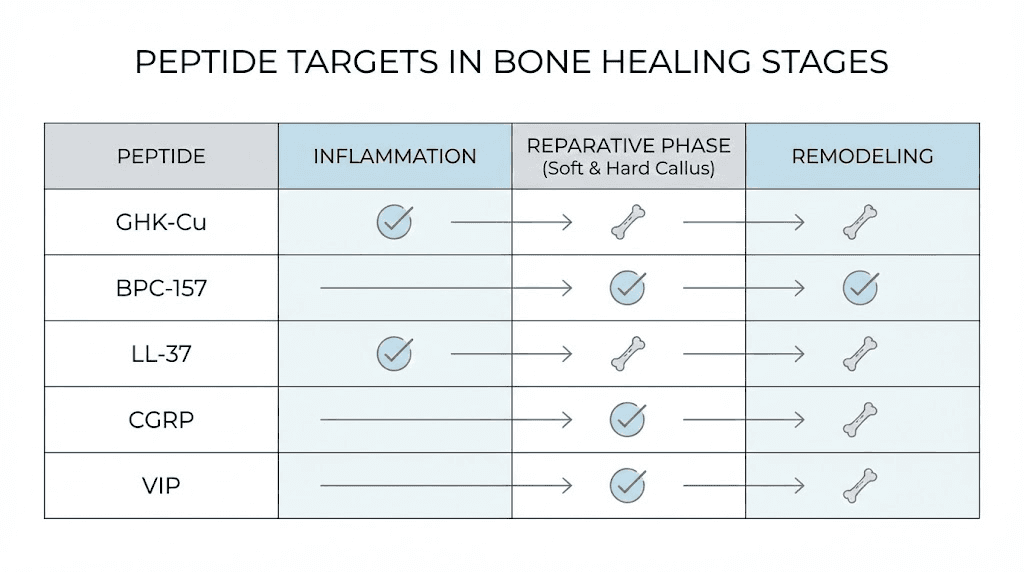
What makes certain peptides relevant to bone healing is their ability to influence the specific biological processes that drive fracture repair. Different peptides target different stages and mechanisms.
During the inflammatory phase, peptides like BPC-157 and TB-500 modulate the inflammatory response, ensuring it is robust enough to initiate repair but controlled enough to prevent tissue damage from excessive inflammation. Anti-inflammatory peptides work through multiple pathways to achieve this balance.
During soft and hard callus formation, peptides influence mesenchymal stem cell recruitment, osteoblast differentiation, chondrocyte activity, and angiogenesis. BPC-157 promotes blood vessel formation through VEGFR2 signaling. GHK-Cu stimulates osteoblast attachment and collagen synthesis. PTH-based peptides like teriparatide directly stimulate osteoblast activity and enhance endochondral ossification.
During remodeling, peptides that balance osteoblast and osteoclast activity help ensure the new bone is properly structured. CGRP, the calcitonin gene-related peptide, inhibits excessive osteoclast activity while promoting osteoblast function, supporting a remodeling process that favors net bone formation.
The latest peptide research continues to reveal new mechanisms and interactions. For a broad overview of every compound currently being studied, the complete peptide directory provides useful orientation. What follows is a detailed examination of each major peptide compound studied for bone healing applications.
BPC-157 and bone fracture recovery
BPC-157 stands for Body Protection Compound 157. It is a synthetic peptide consisting of fifteen amino acids, with the sequence Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val and a molecular weight of 1419 daltons. The peptide derives from a protein found naturally in human gastric juice, and it has been studied extensively for its protective and regenerative effects across multiple tissue types. For a thorough overview of this compound, the complete BPC-157 guide covers its mechanisms, applications, and research history.
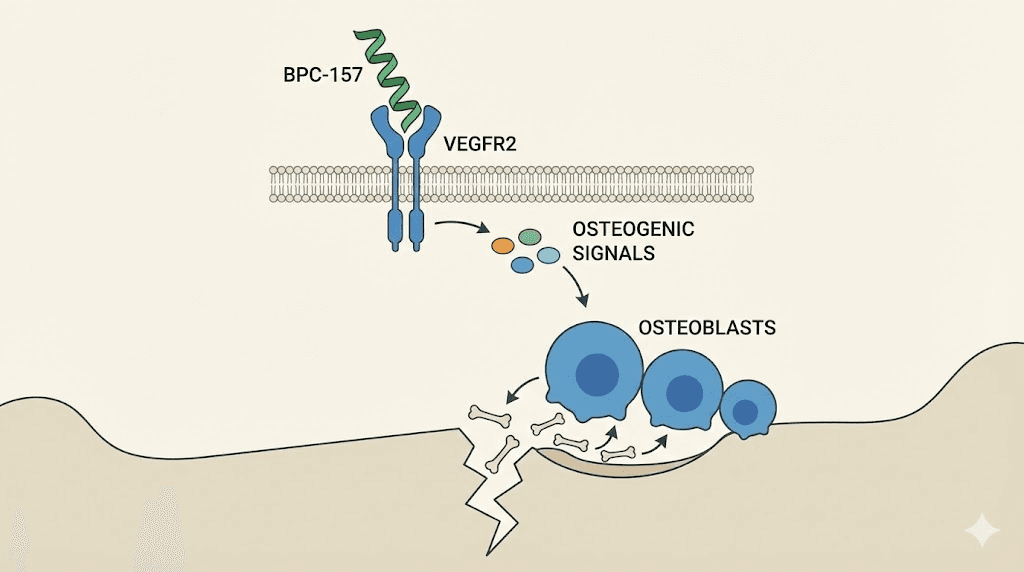
The mechanism: how BPC-157 promotes bone formation
BPC-157 promotes osteogenesis through several interconnected pathways. The primary mechanism involves VEGFR2-NO signaling. BPC-157 activates vascular endothelial growth factor receptor 2 (VEGFR2), which triggers nitric oxide synthesis through the Akt-eNOS axis. This promotes angiogenesis within the bone tissue, creating the blood vessel networks that are absolutely essential for delivering nutrients and oxygen to the fracture site.
But the story does not stop at blood vessels.
BPC-157 also activates the ERK1/2 (extracellular signal-regulated kinase) pathway, a key signaling cascade involved in cell growth, proliferation, and survival. Through this pathway, the peptide enhances osteoblast activity, the cells directly responsible for depositing new bone matrix. It also modulates inflammatory signaling, reducing excessive inflammation that can impair healing while maintaining the beneficial inflammatory signals needed to recruit stem cells and initiate repair.
The connection between gastric peptides and bone health has an interesting clinical basis. Gastrectomy, the surgical removal of part or all of the stomach, is known to increase osteoporosis risk and fracture rates. This bone loss does not respond to calcium supplementation alone and is not fully explained by vitamin D deficiency. Researchers hypothesized that a gastric peptide factor might be involved in maintaining bone health, which partly explains the rationale for studying BPC-157 in bone healing contexts. Understanding what BPC-157 is and where it originates helps frame these research directions.
The landmark rabbit study
The most significant preclinical evidence for BPC-157 in bone healing comes from a study by Sebecic and colleagues published in 1999. The researchers created segmental osteoperiosteal bone defects in rabbits, removing a 0.8 centimeter section from the middle of the left radius. This type of defect is particularly challenging because the gap is too large for the body to bridge through normal healing processes alone. All control animals in the study remained unhealed after six weeks.
The treated groups received BPC-157 at 10 micrograms per kilogram of body weight through three different administration protocols. One group received local injection directly into the bone defect. A second group received intermittent intramuscular injections on days 7, 9, 14, and 16. A third group received continuous daily intramuscular injections from day 7 through day 21.
The results were striking.
All BPC-157 treated groups showed significantly improved healing compared to controls. The surface area of callus formed within the bone defect after two weeks was twice as large in treated animals compared to controls. By six weeks, complete bony continuity across the defect site was achieved in the treated groups, while all control animals still had unhealed gaps. Radiographic assessment, callus surface measurement, microphotodensitometry, and quantitative histomorphometry all confirmed the effect.
Perhaps most remarkably, the healing achieved with BPC-157 was comparable to that seen with bone marrow grafts and autologous cortical bone implantation, which are considered gold standard treatments for segmental bone defects. For a peptide administered through simple injection to match the performance of surgical bone grafting is a significant finding, though it must be noted that this was an animal study and human confirmation is still needed. BPC-157 alternatives have been explored for similar applications, but none have matched the breadth of evidence for this specific compound in bone defect models.
Safety profile and dosing in research
Across preclinical studies, no toxic or lethal dose of BPC-157 has been identified across a remarkably wide dosing range, from 6 micrograms per kilogram to 20 milligrams per kilogram. The peptide is metabolized in the liver with a half-life of less than 30 minutes and is excreted in urine, where it remains detectable for up to four days by mass spectrometry. No acute adverse events were reported in animal models within six weeks of administration.
Research protocols for bone healing applications have typically used dosages in the range of 200 to 300 micrograms administered subcutaneously, though the specific BPC-157 dosing protocols vary depending on the study design and objectives. The BPC-157 dosage calculator can help researchers determine appropriate amounts based on vial concentration and body weight.
Human data remain extremely limited. A systematic review found only one human study examining BPC-157 for musculoskeletal conditions, in which seven out of twelve patients with chronic knee pain reported relief lasting more than six months after a single intra-articular injection. Until well-designed clinical trials are conducted, BPC-157 for bone healing should be considered investigational. For information on how researchers administer BPC-157, including routes of administration and timing protocols, several resources provide detailed guidance.
The World Anti-Doping Agency (WADA) banned the use of BPC-157 by athletes in 2022. Researchers should also be aware of the current regulatory status of BPC-157 and the legal landscape surrounding peptides in their jurisdiction.
TB-500 and thymosin beta-4 for bone repair
Thymosin beta-4 (abbreviated as Tb4) is a naturally occurring 43-amino acid peptide that plays fundamental roles in cellular migration, tissue regeneration, and wound healing throughout the body. TB-500 is the synthetic version commonly used in research settings. The compound works through a unique mechanism involving G-actin sequestration, which essentially maintains a reservoir of cellular building blocks ready for rapid deployment when tissue repair is needed. For comprehensive information, the complete TB-500 guide provides detailed coverage.
The actin connection to bone healing
Actin is a fundamental protein that forms the cytoskeleton of cells, allowing them to maintain shape and, critically, to move. Cell migration is essential for fracture healing because osteoblasts, mesenchymal stem cells, and endothelial cells all need to travel to the fracture site to participate in repair. By binding to actin monomers (G-actin), thymosin beta-4 maintains a readily available supply that can be rapidly assembled into filaments when cells need to migrate.
This makes thymosin beta-4 particularly relevant to fracture healing because it directly supports the cellular mobility required at every stage of bone repair.
The fracture healing study in mice
A significant preclinical study investigated the effects of thymosin beta-4 on fracture healing using a mouse model with bilateral fibular osteotomy. Mice received intraperitoneal injections of either thymosin beta-4 at 6 milligrams per kilogram or saline as a control.
The biomechanical results were compelling. Tb4-treated calluses showed a 41 percent increase in peak force to failure and were approximately 25 percent stiffer than saline-treated controls. These are not trivial improvements. A 41 percent increase in the force required to break the healing bone represents a substantial enhancement in structural integrity.
Micro-CT analysis at 21 days post-fracture revealed that the fractional volume of new mineralized tissue was 18 percent greater and new highly mineralized tissue was 26 percent greater in Tb4-treated mice compared to controls. Histomorphometry added an interesting detail: Tb4-treated calluses were 23 percent smaller in overall size but contained 47 percent less old cortical bone. This suggests more efficient remodeling, where the peptide did not just add more material but helped organize the healing response more effectively.
Osteoblast-specific effects
For researchers interested in the full spectrum of muscle and tissue growth peptides, the overlap between muscle repair and bone repair mechanisms is notable. Separate research examined how thymosin beta-4 affects osteoblasts directly. Studies on osteoblastic cell behavior showed that Tb4 promotes both adhesion and proliferation of osteoblasts through focal adhesion signaling and ERK1/2 activation. When Tb4 was knocked down using siRNA, osteoblast adhesion and proliferation decreased significantly. These findings indicate that thymosin beta-4 directly supports the bone-building cells responsible for fracture repair. The safest peptides for tissue growth often share these osteoblast-supporting mechanisms.
TB-500 also demonstrates anti-inflammatory properties and inhibits osteoclast differentiation, the cells responsible for bone resorption. By simultaneously promoting osteoblast activity and restraining osteoclast activity, thymosin beta-4 creates conditions that favor net bone formation at the fracture site. The full range of TB-500 benefits extends well beyond bone, but these skeletal effects are particularly relevant for fracture recovery.
Researchers exploring alternatives to TB-500 should note that no other peptide replicates its exact mechanism of G-actin sequestration and cell migration support. The TB-500 dosage calculator provides weight-based calculations for research applications. For broader context on the peptide categories and how tissue repair compounds fit within the larger peptide landscape, the category pages provide useful orientation.
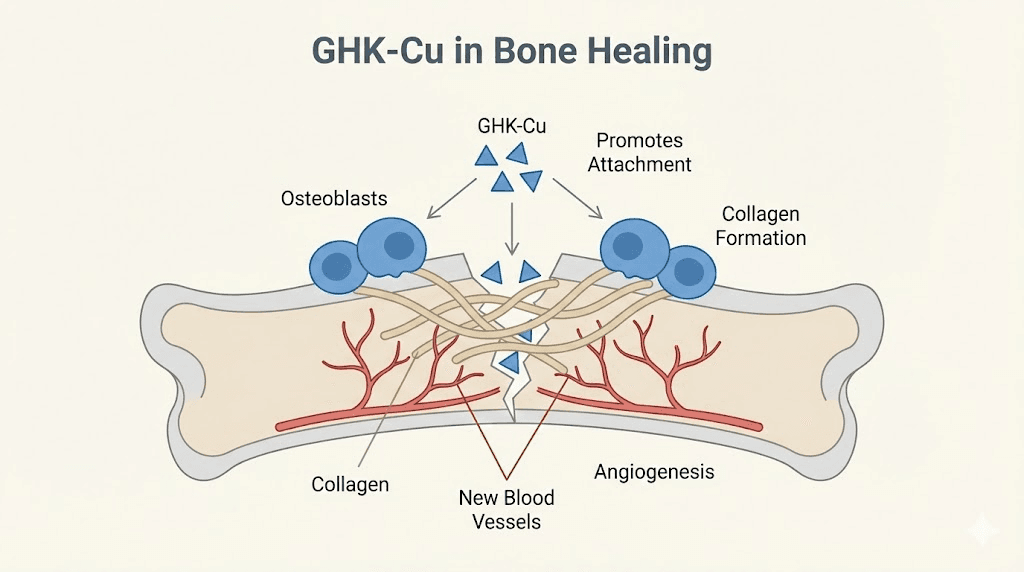
GHK-Cu and copper peptides for bone regeneration
GHK-Cu is a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine. First isolated from human plasma in 1973 by Loren Pickart, it was discovered as an activity in human albumin that caused old liver tissue to synthesize proteins like younger tissue. This tripeptide has a strong affinity for copper(II) ions, and the copper complex is essential for many of its biological activities. The comprehensive GHK-Cu guide explains the full scope of this peptide copper complex.
Age-related decline and bone health
In plasma, GHK levels sit at approximately 200 nanograms per milliliter at age 20. By age 60, levels have dropped to around 80 nanograms per milliliter. That is a 60 percent decline. This reduction coincides with a noticeable decrease in regenerative capacity across all tissues, including bone. The anti-aging peptide category exists largely because of these age-related declines in endogenous peptide levels. The connection between declining GHK-Cu levels and reduced bone healing capacity in older adults is an area of active research interest. For context on how age-related changes affect peptide function, the discussion of longevity peptides provides useful background.
How GHK-Cu supports bone formation
GHK-Cu influences bone regeneration through multiple mechanisms that work in concert.
Osteoblast attachment and differentiation. Research by Godet and Marie examined the effects of GHK-Cu on osteoblastic cell spreading, attachment, and phenotype. The peptide promotes the attachment of bone-forming cells to surfaces and enhances their differentiation into mature, active osteoblasts. This effect is particularly relevant in the early stages of hard callus formation when osteoblast recruitment and activity are critical.
Collagen synthesis. GHK-Cu can increase collagen production by up to 70 percent. Since collagen forms the organic matrix of bone, providing the framework onto which mineral crystals are deposited, enhanced collagen production directly supports bone formation. This collagen-boosting effect is well documented in the comparison between GHK-Cu and other copper peptides.
Angiogenesis. GHK-Cu stimulates the secretion of VEGF and fibroblast growth factor 2 (FGF2) from bone marrow stromal cells. These growth factors promote endothelial cell migration and new blood vessel formation, addressing the critical vascular requirement for bone healing. Without adequate blood supply, fracture healing fails.
Macrophage polarization. The peptide promotes M2 macrophage polarization, shifting the immune response from a pro-inflammatory to a pro-regenerative state. M2 macrophages secrete growth factors and anti-inflammatory cytokines that support tissue repair. This mechanism is relevant during the transition from the inflammatory phase to the repair phase of fracture healing.
Copper as a cofactor. Copper itself is essential for bone maturation because it serves as a cofactor for lysyl oxidase, an enzyme required for collagen and elastin cross-linking. Copper deficiency has been linked to osteoporosis and bone development abnormalities in low-birth-weight infants and young children. By delivering copper directly to cells in a bioavailable form, GHK-Cu ensures this essential mineral is available where it is needed.
Bone regeneration research with GHK-Cu
A study examining bone fracture healing in rats used a combination of small molecules including GHK at 0.5 micrograms per kilogram, combined with the peptide dalargin and the biological peptide thymogen. The total peptide dosage was approximately 2.2 micrograms per kilogram. Scaled for a human body, this would translate to roughly 140 micrograms per injection with treatments administered over 10 days.
More recently, researchers have incorporated GHK-Cu into three-dimensional silk-based scaffolds designed for vascularized bone regeneration. The released GHK-Cu promoted bone marrow stromal cell proliferation and influenced the paracrine effects of transplanted cells, indirectly inducing neovascularization. Another study demonstrated that GHK-Cu-containing nanocomposite hydrogels significantly enhanced maxillary sinus floor reconstruction in animal models.
These scaffold-based approaches highlight an important principle: GHK-Cu may be most effective for bone regeneration when delivered locally and in a sustained manner, rather than through systemic injection alone. The GHK-Cu dosage guide discusses various approaches, while the injection-specific dosage information covers administration protocols. For those exploring injection methods, the guide on how to use GHK-Cu injections provides step-by-step information.
One significant limitation of GHK-Cu is its short in vivo half-life of approximately 30 minutes in plasma, combined with the instability of the metal-peptide complex due to copper high reactivity. This rapid clearance after injection means that sustained delivery methods may be necessary for meaningful bone healing effects. Research into the duration and stability of GHK-Cu provides additional detail on this challenge. The 50mg vial dosing information and the dosage chart are useful references for research planning.
PTH-based peptides and bone density
Parathyroid hormone (PTH) based peptides represent the most clinically validated category of peptides for bone health. Unlike BPC-157, TB-500, and GHK-Cu, which remain investigational, teriparatide (PTH 1-34) and abaloparatide have received FDA approval for treating osteoporosis and are prescribed to patients worldwide.
Teriparatide: the first anabolic bone peptide
Teriparatide is a recombinant N-terminal peptide fragment consisting of the first 34 amino acids of human parathyroid hormone. It was approved by the FDA in 2002 for the treatment of postmenopausal women and men with osteoporosis who are at high risk of fracture. Unlike bisphosphonates, which work by slowing bone loss, teriparatide is a true anabolic agent. It stimulates new bone formation.
The mechanism depends entirely on the dosing pattern. This is a crucial distinction.
Intermittent exposure to PTH, such as a single daily subcutaneous injection, triggers bone formation that exceeds bone resorption. The net result is increased bone mass and density. Continuous exposure to PTH, however, produces the opposite effect. Sustained high levels of parathyroid hormone, as seen in hyperparathyroidism, stimulate osteoclasts and lead to net bone loss and hypercalcemia. This paradox is why teriparatide must be administered as a once-daily injection rather than through continuous delivery.
Clinical evidence for fracture healing
The evidence for teriparatide in established osteoporosis is robust. Clinical trials have demonstrated a 65 percent reduction in vertebral fracture risk and a 35 to 53 percent reduction in non-vertebral fracture risk after 20 months of treatment at 20 micrograms per day.
For active fracture healing, the picture is more nuanced but still promising. A randomized controlled trial found that teriparatide administered postoperatively accelerated the healing process, with bone union time averaging 3.12 weeks faster in the treatment group compared to placebo. Studies in both normal healing and delayed healing models have shown improvements in callus volume, mineralization, bone mineral content, rate of successful union, and mechanical strength at fracture sites.
The basic science data suggest teriparatide accelerates chondrocyte recruitment and differentiation during early endochondral ossification. This means it enhances the very process that converts the soft cartilage callus into hard bone. It increases both the cartilaginous and mineralized components of the fracture callus while improving the biomechanical properties of the healing tissue.
Clinical scenarios where teriparatide is most commonly considered include patients with severe osteoporosis who have been on bisphosphonates for years and sustain a fracture not expected to heal predictably, such as atypical femur fractures. It is also considered when an osteoporotic patient has failed fracture healing and faces potential surgical intervention for non-union.
Protocol and limitations
The standard approved protocol is 20 micrograms administered subcutaneously once daily. Bioavailability after subcutaneous injection is approximately 95 percent, with peak serum concentrations reached within 30 minutes. Treatment for fracture healing typically runs three to nine months, though the maximum approved treatment duration is 24 months cumulative lifetime use.
The treatment duration limit stems from animal safety data. Studies in rats showed an increased incidence of osteosarcoma with long-term PTH exposure, leading to an FDA boxed warning. However, this risk has not been demonstrated in humans at therapeutic doses, and the warning is being reevaluated based on post-marketing surveillance data.
Other limitations include high cost and storage requirements. Teriparatide must be refrigerated and administered daily by injection, which presents compliance challenges for some patients. Weekly dosing protocols are being investigated as alternatives. Research has shown that weekly injections at higher doses may promote fracture healing to a similar extent as daily lower-dose injections in rodent models, though human verification of weekly protocols is still in progress.
Abaloparatide: the next generation
Abaloparatide is a synthetic analog of parathyroid hormone-related protein (PTHrP) that received FDA approval in 2017. In clinical trials, it demonstrated an 86 percent reduction in vertebral fractures compared to placebo, with greater bone mineral density increases than teriparatide at all measured skeletal sites. Abaloparatide may cause less hypercalcemia than teriparatide, making it potentially better tolerated. Like teriparatide, it works through daily subcutaneous injection and is limited to a treatment duration of approximately two years.
For additional context, the HGH Fragment 176-191 calculator covers another PTH-adjacent growth peptide with bone density implications. Both PTH-based peptides highlight an important principle in peptide pharmacology: dosing pattern matters as much as the compound itself. The same molecule that builds bone when given intermittently destroys bone when given continuously. This principle of timing-dependent effects applies to many peptide dosing strategies and underscores the importance of understanding proper peptide dosing protocols.
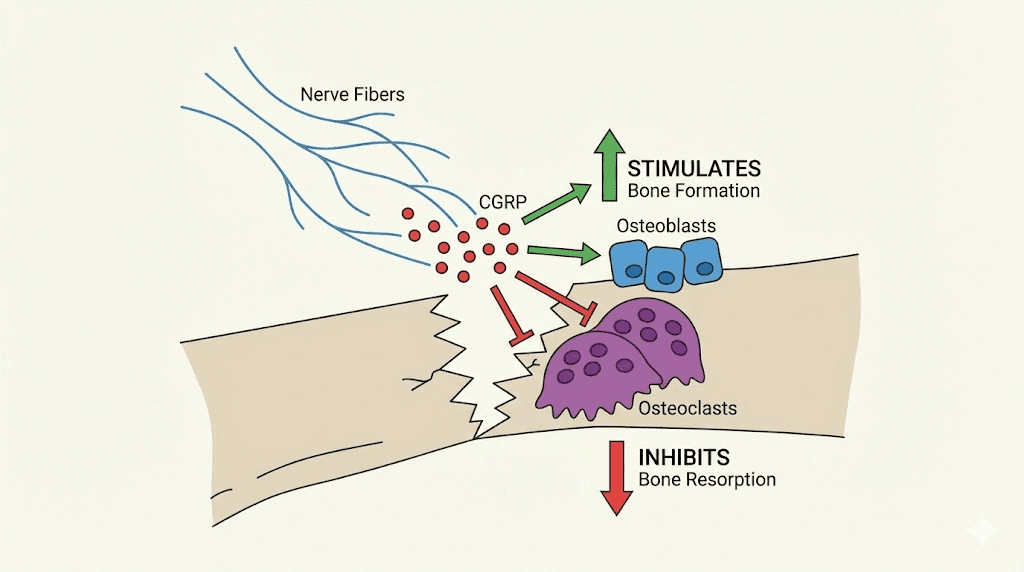
CGRP and the nerve-bone connection
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide that has fundamentally changed how researchers understand the relationship between the nervous system and bone health. Initially studied for its roles in vasodilation and pain signaling, CGRP has emerged as a critical mediator of bone repair. It is, in many ways, the bridge between the nervous system and the skeletal system.
Where CGRP lives in bone
CGRP-positive nerve fibers are widely distributed throughout the periosteum and bone marrow. These sensory neurons continuously release CGRP into bone tissue, creating a direct line of communication between the nervous system and the cells responsible for bone maintenance and repair. Blood vessels and nerve fibers are distributed throughout bone tissue, providing not just oxygen and nutrients but also signaling molecules that regulate bone growth, development, and healing.
The receptors for CGRP, specifically CLR and RAMP1, are expressed on macrophages, osteoblasts, and endothelial cells within bone tissue. This widespread receptor distribution means CGRP can influence immune cells, bone-forming cells, and blood vessels simultaneously.
How CGRP promotes bone repair
CGRP supports fracture healing through at least four distinct mechanisms.
Osteoblast stimulation. CGRP binds to CLR receptors on osteoblast cell surfaces, directly enhancing osteogenic gene expression. It stimulates the production of BMP-2, alkaline phosphatase (ALP), osteocalcin, and type I collagen alpha 1 (ColIa1), all of which are markers and drivers of active bone formation. This pro-osteogenic effect operates through the cAMP/CREB signaling pathway.
Osteoclast inhibition. CGRP inhibits the RANKL/RANK/NF-kB pathway by increasing osteoprotegerin (OPG) levels. This reduces osteoclast proliferation and activity, decreasing bone resorption at the fracture site. By simultaneously promoting bone formation and inhibiting bone breakdown, CGRP creates an environment strongly biased toward net bone building.
Angiogenesis. CGRP enhances new blood vessel formation by promoting the expression of VEGF and HIF (hypoxia-inducible factor) in endothelial cells. The accelerated vascular growth into the bone defect site occurs through the CGRP/FAK/VEGF signaling axis. This addresses the critical vascular supply requirement that determines whether a fracture heals or develops into a non-union.
Immune microenvironment regulation. CGRP enhances the transformation of M0 macrophages into M2 macrophages, the anti-inflammatory, pro-regenerative phenotype. This shifts the immune microenvironment at the fracture site from one dominated by inflammation to one that supports tissue repair and bone formation.
Evidence from knockout studies
Some of the most compelling evidence for CGRP importance in bone healing comes from genetic studies. Alpha-CGRP-deficient mice display profoundly impaired bone regeneration characterized by a striking reduction in the number of bone-forming osteoblasts and a high rate of incomplete callus bridging and non-union. Wild-type mice with femoral fractures show increased CGRP serum levels after injury, and alpha-CGRP mRNA expression is exclusively induced in callus tissue but not in other organs, suggesting a targeted skeletal response.
Conversely, transgenic mice with CGRP overexpression in osteoblasts showed higher trabecular bone density, confirming the positive relationship between CGRP signaling and bone mass.
Clinical implications and the migraine connection
An important clinical consideration has emerged from the migraine treatment field. Novel agents that block CGRP or its receptor (anti-CGRP monoclonal antibodies and gepants) are now widely prescribed for migraine prevention and treatment. Because CGRP is essential for bone healing, the potential negative impact of these medications on bone regeneration warrants clinical investigation. Patients taking CGRP-blocking medications who sustain a fracture may face increased risk of healing complications, though this has not yet been studied systematically.
Researchers have also explored methods to enhance CGRP signaling at fracture sites. Electrical stimulation of dorsal root ganglia promotes CGRP synthesis and release, and this approach has enhanced osteoporotic fracture healing in rat models. Magnesium-based implants have been shown to induce local neuronal production of CGRP, improving bone fracture healing in animal studies published in Nature Medicine. These findings suggest that future therapeutic strategies may focus on enhancing the nerve-bone communication pathway rather than administering CGRP directly.
Emerging peptides for bone healing
Beyond the well-studied compounds discussed above, several emerging peptides show promise for bone regeneration applications. The field is expanding rapidly, and the complete peptide list continues to grow as new compounds are identified and characterized.
Osteogenic growth peptide (OGP)
OGP is a naturally occurring 14-amino acid peptide that was identified in serum and is produced by osteogenic cells. It increases bone formation and overall bone mass while potentially slowing bone aging. OGP works by activating the MAPK signaling pathway in osteoblasts and promoting their proliferation and differentiation. Studies have shown that systemic OGP administration increases bone formation rate and trabecular bone volume in intact animals and accelerates fracture healing in models of skeletal injury.
What makes OGP particularly interesting is that its levels increase naturally after fracture, suggesting it is part of the body endogenous bone repair system. Supplementing this natural response with additional OGP may amplify the healing process. The peptide also promotes bone marrow stromal cell proliferation and osteogenic differentiation, providing more raw material for the bone-building process.
PEPITEM and immune-mediated bone repair
PEPITEM is a peptide derived from the adiponectin pathway that modulates immune cell trafficking. While primarily studied for its roles in autoimmune conditions, PEPITEM influence on immune cell behavior has implications for bone healing because the inflammatory phase of fracture repair depends heavily on coordinated immune cell activity. Researchers studying peptides for autoimmune conditions have noted potential crossover applications in tissue repair.
Fracture-targeted peptide delivery systems
One of the most exciting developments in the field is not a new peptide itself but rather new ways to deliver existing peptides directly to fracture sites. Researchers have developed peptide-based targeting systems that can bind specifically to hydroxyapatite, the mineral component of bone exposed at fracture surfaces. By conjugating therapeutic peptides to these bone-targeting sequences, they can achieve higher local concentrations at the exact site where healing is needed while minimizing systemic exposure.
This approach addresses one of the major challenges in peptide therapy for bone healing: getting enough of the active compound to the fracture site. Systemic administration distributes peptides throughout the entire body, with only a small fraction reaching the intended target. Fracture-targeted delivery could dramatically improve efficiency and reduce the total dose required. The broader landscape of bioregulator peptides includes several compounds with potential bone health applications. The cardiogen peptide, for instance, demonstrates tissue-specific regenerative effects that illustrate the diversity of bioregulatory peptide actions. Similarly, epitalon and NAD peptides target cellular aging pathways that may indirectly influence bone regenerative capacity.
Growth hormone secretagogues and bone
Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) are well-established mediators of bone growth and remodeling. Peptides that stimulate growth hormone release, such as ipamorelin and CJC-1295, may indirectly support bone healing by elevating systemic GH and IGF-1 levels. The benefits of ipamorelin include growth hormone stimulation without the cortisol and prolactin spikes associated with other secretagogues, making it a cleaner option for this application.
IGF-1 directly stimulates osteoblast proliferation and collagen synthesis, and it plays a critical role in the coupling of bone formation and resorption during remodeling. The IGF peptide guide explores these mechanisms in detail. Similarly, sermorelin stimulates natural growth hormone production and may support bone density maintenance through this pathway.
For researchers interested in comparing growth hormone releasing peptides, the ipamorelin versus CJC-1295 comparison details the differences between these two popular secretagogues. The CJC-1295 dosage calculator provides weight-based calculations for research planning.
Peptide stacking strategies for bone recovery
Because different peptides target different mechanisms and stages of bone healing, combining them in strategic stacks is a topic of considerable interest among researchers. The concept is straightforward: if BPC-157 primarily promotes angiogenesis and GHK-Cu primarily supports osteoblast attachment and collagen synthesis, combining them might address multiple aspects of bone healing simultaneously. The comprehensive peptide stacking guide covers general principles and considerations.
The BPC-157 and TB-500 combination
This is the most commonly discussed pairing for injury recovery. BPC-157 provides localized tissue repair through angiogenesis and growth factor modulation, while TB-500 offers systemic regenerative support through enhanced cell migration and anti-inflammatory effects. A retrospective study by Lee and Padgett compared BPC-157 alone to BPC-157 combined with thymosin beta-4 for knee injections, finding potential additive benefits from the combination.
The BPC-157 and TB-500 stacking guide provides detailed protocols for this combination. For a side-by-side analysis of these two peptides, the BPC-157 versus TB-500 comparison explains their distinct mechanisms and where they overlap. This combination is sometimes referred to as the wolverine stack in peptide communities due to its reputation for accelerating recovery, and the wolverine peptide combination has become one of the most discussed stacks for injury repair.
Adding GHK-Cu to a bone healing stack
GHK-Cu brings collagen synthesis, copper delivery, and osteoblast support to the combination. Since it works through mechanisms distinct from both BPC-157 and TB-500, it theoretically complements both without redundancy. The challenge is that GHK-Cu has a very short half-life and may be most effective when delivered locally to the fracture area. Researchers exploring multi-peptide approaches for bone healing often consider GHK-Cu as a third component specifically for its collagen and mineral support roles.
Growth hormone secretagogues as a foundation
Some researchers use growth hormone secretagogues like ipamorelin or CJC-1295 as a base layer, reasoning that elevated GH and IGF-1 levels create a more favorable systemic environment for bone healing. On top of this foundation, they add targeted peptides like BPC-157 or TB-500 for direct fracture repair support. The peptide stack calculator helps researchers plan multi-peptide protocols. The question of how many peptides can be taken simultaneously is an important practical consideration, as is understanding how to cycle different peptides for extended protocols.
Stacking considerations and cautions
While the theoretical rationale for stacking is sound, it is important to acknowledge that very few studies have examined specific multi-peptide combinations for bone healing. Most preclinical evidence comes from single-peptide studies. The interactions between different peptides at the cellular and molecular level are not fully characterized. What seems logical based on mechanism of action may not translate directly to additive or synergistic clinical effects.
Additionally, more is not always better. The common mistakes beginners make with peptides often involve overcomplicated protocols with too many compounds. For bone healing specifically, selecting two or three well-characterized peptides with complementary mechanisms is likely more prudent than attempting to use everything at once. Understanding peptide safety and risks is essential before designing any multi-compound protocol.
Practical protocols for bone healing support
The following protocols reflect approaches described in the research literature and discussed within the peptide research community. They are presented for educational purposes. All peptide use should be conducted under appropriate medical supervision, and individuals should understand the fundamentals of getting started with peptides before attempting any protocol.
Protocol one: BPC-157 focused approach for acute fracture support
Goal: Support natural fracture healing processes through angiogenesis and growth factor modulation.
Compound: BPC-157 at 200 to 300 micrograms per day, split into two administrations (morning and evening). Subcutaneous injection near the fracture site when anatomically feasible, or intramuscular injection otherwise.
Duration: Six to eight weeks, starting as soon after injury as possible.
Expected timeline based on research: Initial vascular effects may begin within the first week. Enhanced callus formation visible on imaging around weeks two to four. Continued support through the hard callus formation phase.
The timeline for peptide effects varies between individuals and depends on factors including fracture severity, location, overall health status, and age. The cycle planning guide helps structure protocols around healing timelines.
Protocol two: BPC-157 and TB-500 combination for complex fractures
Goal: Address both local tissue repair and systemic regenerative support for fractures with soft tissue involvement or delayed healing risk.
Compounds: BPC-157 at 250 micrograms twice daily, subcutaneous near the injury site. TB-500 at loading dose of approximately 2 to 2.5 milligrams twice weekly for the first two weeks, then maintenance at 2 milligrams once weekly for four to six additional weeks.
Duration: Eight to twelve weeks total.
Rationale: The BPC-157 provides localized angiogenic and growth factor support while TB-500 enhances systemic cell migration and anti-inflammatory effects. The loading phase of TB-500 builds tissue concentrations of the peptide before tapering to maintenance. For detailed information on each compound, refer to the best peptides for injury recovery resource.
Protocol three: GHK-Cu adjunct for collagen and mineral support
Goal: Enhance collagen synthesis and osteoblast activity during the callus formation phase.
Compound: GHK-Cu at 1 to 2 milligrams per day, subcutaneous injection, ideally near the fracture site. Based on the rat study data, much lower doses (scaling from 0.5 micrograms per kilogram) may also be effective.
Duration: Four to six weeks, beginning two to three weeks after fracture (during the transition from soft to hard callus formation).
Note: The short half-life of GHK-Cu means that twice-daily dosing or local delivery methods may be more effective than single daily injections. This peptide may work best as an adjunct to BPC-157 or TB-500 rather than as a standalone approach for bone healing.
Protocol four: growth hormone secretagogue foundation
Goal: Elevate endogenous GH and IGF-1 to create a favorable systemic environment for bone healing.
Compound: Ipamorelin at 200 to 300 micrograms, subcutaneous injection, administered before bed to synergize with natural nighttime GH pulses. Alternatively, CJC-1295 DAC at 2 milligrams once weekly for sustained GH elevation.
Duration: Eight to twelve weeks.
Rationale: Elevated GH and IGF-1 support osteoblast proliferation, collagen synthesis, and overall tissue repair capacity. This approach is sometimes used as a baseline strategy combined with one or more direct-acting peptides. The peptide strength protocol discusses how growth hormone secretagogues support various tissue repair goals.
All of these protocols require proper peptide preparation and handling. Using the peptide calculator ensures accurate dosing, while understanding proper injection techniques is essential for subcutaneous and intramuscular administration. Some researchers prefer using a peptide injection pen for convenience and accuracy.
How to handle, store, and prepare peptides for bone recovery
The effectiveness of any peptide protocol depends heavily on proper handling, storage, and preparation. A perfectly designed protocol becomes worthless if the peptide degrades before it ever reaches the fracture site. This section covers the practical knowledge needed to maintain peptide potency from vial to injection.
Reconstitution basics
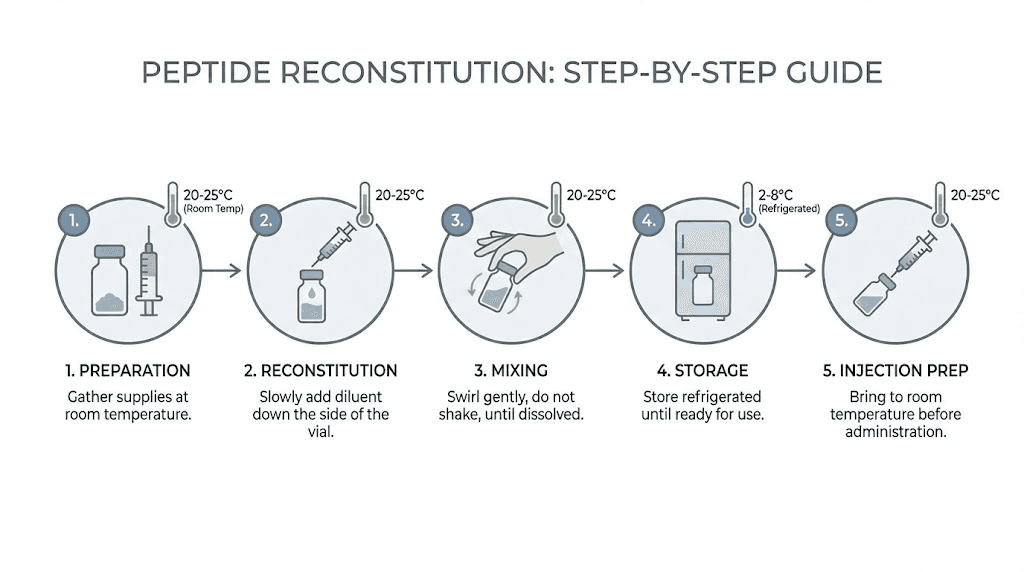
Most research peptides arrive as lyophilized (freeze-dried) powder that must be reconstituted with bacteriostatic water before use. The process is straightforward but requires attention to detail. The complete reconstitution guide walks through each step.
The key principles are gentle handling and sterile technique. Direct the stream of bacteriostatic water against the glass wall of the vial rather than blasting it directly onto the powder. Allow the solution to sit for a few minutes if the powder does not dissolve immediately. Never shake the vial vigorously, as this can damage the peptide molecular structure. Gentle swirling is sufficient.
For calculating exact volumes and concentrations, the peptide reconstitution calculator eliminates guesswork. Understanding which water to use for peptide reconstitution is also important, and the guide on water for mixing peptides explains the differences between bacteriostatic water, sterile water, and other options. The step-by-step mixing guide provides a visual walkthrough of the entire process. Some researchers may prefer peptide capsules or nasal spray formulations for certain compounds, though injectable peptides remain the standard for most bone healing applications.
Storage requirements
Peptide stability varies significantly depending on form and storage conditions. Lyophilized (powder) peptides are the most stable and can last for extended periods when stored properly. Once reconstituted, the clock starts ticking. The comprehensive peptide storage guide covers all scenarios.
Unreconstituted lyophilized peptides should be stored in a freezer or refrigerator, protected from light. The stability of peptides in powder form depends on the specific compound but is generally measured in months to years at proper temperatures.
Reconstituted peptides must be refrigerated at 2 to 8 degrees Celsius (standard refrigerator temperature). The shelf life of reconstituted peptides in the fridge varies by compound but typically ranges from two to four weeks with bacteriostatic water. Information about exactly how long reconstituted peptides remain viable helps researchers plan their protocols without waste.
Temperature excursions are the primary enemy of peptide stability. The tolerance of peptides at room temperature is limited, and prolonged exposure to warmth accelerates degradation. Never leave reconstituted peptides at room temperature for extended periods. Understanding whether and when peptides expire helps prevent the use of degraded compounds that will not deliver expected results.
Quality verification
The peptide market includes both reputable suppliers and unreliable sources. For bone healing applications where results genuinely matter, using verified, high-purity peptides is not optional. The peptide testing laboratory guide explains how third-party testing works and what to look for in certificates of analysis. The best peptide vendor comparison helps researchers identify reliable sources.
Key quality indicators include HPLC purity (should be 98 percent or higher for research use), mass spectrometry confirmation of molecular identity, and proper packaging in sealed, light-protected vials. The distinction between research-grade and pharmaceutical-grade peptides matters, as does understanding the difference between lyophilized and liquid peptide formulations. Cost is also a factor in sustained protocols, and the peptide cost calculator helps estimate expenses for different protocol durations. The broader discussion of peptide costs and the full therapy cost guide provide additional context. Choosing between gut health peptides and bone healing peptides is not always necessary, as some compounds like BPC-157 serve both purposes given its origin as a gastric peptide.
Injectable versus oral considerations for bone healing
For bone healing applications, subcutaneous injection remains the preferred administration route for most peptides because it provides reliable bioavailability and the option for localized delivery near the fracture site. The injectable versus oral peptide comparison explains why oral bioavailability is generally poor for most peptides due to gastrointestinal degradation. BPC-157 is notable for demonstrating some oral efficacy in certain contexts, but for bone healing specifically, injectable administration has been the standard in research. The free reconstitution calculator simplifies the math for any peptide preparation.
Nutrition, lifestyle, and adjunct strategies that support peptide-driven bone healing
Peptides do not work in a vacuum. Even the most promising peptide protocol will produce suboptimal results if the biological foundation is not in place. Bone healing requires raw materials, energy, and favorable systemic conditions. This section covers the factors that support or undermine peptide-driven bone recovery.
Protein and amino acid requirements
Bone is roughly 35 percent organic matrix by weight, and that organic matrix is predominantly collagen. Building new bone requires substantial protein intake. Research suggests that fracture patients need 1.5 to 2.0 grams of protein per kilogram of body weight per day during active healing, significantly more than the general recommendation of 0.8 grams per kilogram. Insufficient protein intake is one of the most common and most preventable causes of impaired fracture healing.
Specific amino acids matter too. Proline, glycine, and hydroxyproline are the primary building blocks of collagen. Lysine is required for collagen cross-linking. Arginine supports nitric oxide production, which is relevant because BPC-157 works partly through nitric oxide signaling pathways. Ensuring adequate intake of these amino acids supports both natural healing and the mechanisms through which peptides exert their effects. Understanding the peptide formula and amino acid basics provides useful background.
Calcium and vitamin D
These are the obvious ones, but they bear repeating because deficiencies are extremely common. Calcium provides the mineral component of bone. Vitamin D is required for calcium absorption from the gut and for proper osteoblast function. During fracture healing, calcium requirements increase to approximately 1200 milligrams per day, and vitamin D levels should be maintained at 30 to 50 nanograms per milliliter (serum 25-hydroxyvitamin D).
Testing vitamin D levels before and during fracture healing is worthwhile because deficiency is widespread and easily correctable. Supplementation with vitamin D3 at 2000 to 5000 IU per day is commonly recommended during healing, though individual needs vary based on baseline levels, sun exposure, and body composition.
Trace minerals and micronutrients
Beyond calcium, several trace minerals play essential roles in bone healing. Copper is a cofactor for lysyl oxidase and is directly relevant to GHK-Cu mechanisms. Zinc supports osteoblast function and is required for alkaline phosphatase activity. Magnesium is involved in hundreds of enzymatic reactions including those that regulate calcium metabolism and bone mineral density. Silicon stimulates collagen synthesis and may enhance bone mineralization.
Vitamin C is required for collagen synthesis (specifically for the hydroxylation of proline and lysine residues). Vitamin K2 directs calcium into bones and teeth rather than soft tissues. Boron influences calcium and magnesium metabolism and may enhance the effects of vitamin D.
What to avoid during bone healing
Certain substances actively impair fracture healing and can undermine peptide protocols.
Smoking is the single greatest modifiable risk factor for impaired fracture healing. Nicotine constricts blood vessels, reducing blood flow to the fracture site. Carbon monoxide from cigarette smoke impairs oxygen delivery. Smoking also impairs osteoblast function directly and increases the risk of non-union by as much as four to six times.
NSAIDs (ibuprofen, naproxen, and similar drugs) inhibit cyclooxygenase-2 (COX-2), an enzyme involved in the inflammatory phase of fracture healing. While short-term, low-dose use may be acceptable for pain management, prolonged NSAID use during the early weeks of fracture healing has been associated with delayed union in some studies. Acetaminophen is generally considered a safer pain management option during fracture healing.
Excessive alcohol impairs osteoblast function, reduces bone mineral density, and increases fall risk. Even moderate alcohol consumption during active fracture healing may slow the process.
Corticosteroids suppress bone formation and promote bone resorption. Patients who require corticosteroids for other medical conditions face additional challenges in fracture healing, and this is one scenario where teriparatide may be particularly beneficial.
Mechanical loading and rehabilitation
Appropriate mechanical loading is essential for optimal bone healing. Wolff law dictates that bone adapts to the forces placed upon it, and this applies during fracture repair as well. Complete immobilization beyond what is necessary for fracture stability can actually impair healing by depriving the callus of the mechanical signals that guide remodeling.
Progressive weight-bearing, as directed by an orthopedic provider, supports bone healing by stimulating osteoblast activity and guiding callus organization. Physical therapy and rehabilitation complement peptide protocols by providing the mechanical environment that peptides alone cannot create. The peptides for athletic performance resource discusses how peptide use integrates with physical activity and training programs.
Sleep and recovery
Growth hormone secretion peaks during deep sleep, and GH is a critical mediator of bone formation and repair. Poor sleep directly impairs fracture healing by reducing GH pulses and elevating cortisol, a stress hormone that inhibits osteoblast function. Individuals dealing with anxiety during recovery may also experience elevated cortisol that compounds the problem. For those using growth hormone secretagogues like ipamorelin as part of a bone healing protocol, timing the dose before bed and ensuring seven to nine hours of quality sleep maximizes the synergy between the peptide and the body natural repair processes. The peptides for energy optimization may also interest those managing fatigue during recovery.
SeekPeptides provides members with detailed nutritional protocols tailored to specific recovery goals, helping researchers and individuals optimize the biological environment that supports peptide-driven bone healing. Getting the foundations right, protein, minerals, sleep, and avoiding harmful substances, creates the conditions where peptides can work most effectively.
The role of inflammation management in bone healing
Inflammation is both necessary and potentially harmful during bone healing. This paradox makes it one of the trickiest aspects of fracture recovery to manage. Too little inflammation and the repair process never properly initiates. Too much inflammation and the repair process stalls or produces fibrous tissue instead of bone.
The initial inflammatory phase is essential. TNF-alpha, interleukin-1, and interleukin-6 all play critical roles in recruiting mesenchymal stem cells and initiating the healing cascade. Studies in which these cytokines were blocked or absent showed significantly impaired fracture healing. The inflammatory response is part of the solution, not merely a symptom to be suppressed.
However, chronic or excessive inflammation shifts the balance toward tissue destruction. Elevated inflammatory markers beyond the first week or two suggest a healing environment that has become counterproductive. This is where inflammation-modulating peptides become relevant. Rather than broadly suppressing inflammation like NSAIDs do, peptides like BPC-157 and TB-500 appear to modulate the inflammatory response, supporting the beneficial early phase while promoting resolution and transition to the repair phase.
KPV, an alpha-MSH derivative, is another peptide with anti-inflammatory properties that has been studied for various inflammatory conditions. While not specifically studied for bone healing, its mechanism of reducing NF-kB activation could theoretically support the transition from inflammatory to regenerative phases. The KPV peptide benefits profile discusses its anti-inflammatory mechanisms in detail.
For individuals dealing with chronic pain during fracture recovery, understanding the best peptides for pain management and the back pain peptide options may provide relevant context. Those dealing with associated joint pain or tendon involvement around fracture sites can find targeted information on peptide approaches for those specific tissues. The broader discussion of peptides for chronic pain conditions may also be relevant for individuals managing complex pain presentations during fracture recovery.
Comparing peptide approaches to bone healing
With multiple peptides showing potential for bone healing, choosing the right approach requires understanding how they compare across key factors. The peptides versus SARMs comparison is also relevant here because some individuals consider SARMs for musculoskeletal support, and the differences in mechanism and safety profile are important to understand. Similarly, comparing peptides versus steroids helps frame peptides within the broader landscape of compounds used for tissue repair and performance.
BPC-157 stands out for its direct bone healing evidence in animal models, its dual angiogenic and osteogenic effects, and its favorable safety profile across a wide dosing range. Its main limitation is the lack of human clinical data specifically for bone healing.
TB-500 offers strong biomechanical evidence, with treated fractures showing 41 percent greater force to failure and 25 percent greater stiffness. Its systemic mechanism of enhancing cell migration addresses a fundamental requirement of fracture repair. Like BPC-157, it lacks human bone healing data.
GHK-Cu brings unique value through copper delivery and collagen synthesis support, but its short half-life limits practical application through injection alone. Scaffold-based delivery methods may unlock its full potential for bone regeneration in future clinical applications.
Teriparatide has the strongest clinical evidence by far, with FDA approval and robust trial data showing fracture risk reduction and accelerated healing. However, it requires daily injection, is expensive, and carries a boxed warning regarding long-term use.
CGRP represents the most intriguing biological mechanism, linking the nervous system to bone repair, but direct therapeutic application is complicated by its role in pain signaling and the current clinical use of CGRP-blocking agents for migraine treatment.
The peptide before and after results resource provides real-world context for what researchers and individuals experience across various peptide applications. The peptide transformation documentation also offers insight into typical timelines and outcomes.
Who should consider peptides for bone healing
Not every fracture requires peptide intervention. Most simple fractures in healthy individuals heal perfectly well with standard medical care, appropriate immobilization, good nutrition, and time. Peptide research for bone healing is most relevant to specific populations and scenarios.
Individuals with risk factors for delayed healing represent the primary group of interest. This includes older adults with age-related declines in growth factors and bone density, smokers (though quitting is more impactful than any supplement), diabetic patients whose healing capacity is compromised by metabolic dysfunction, and those with nutritional deficiencies.
Complex fracture patterns with significant soft tissue damage, large fracture gaps, or poor vascular supply are more likely to benefit from angiogenic peptides like BPC-157 that address the blood supply limitation directly.
Non-unions and delayed unions where conventional healing has stalled represent perhaps the most compelling scenario for peptide investigation. Teriparatide is already used clinically in some of these cases, and the preclinical evidence for BPC-157 specifically addressed bone defects that failed to heal in control animals.
Athletes and active individuals who need to return to activity as quickly as safely possible are interested in peptides for their potential to accelerate the healing timeline. The drug testing implications of peptide use are an important consideration for competitive athletes, given that WADA has banned several peptides including BPC-157. The resources covering peptides for men and peptides for women discuss gender-specific considerations that may affect bone healing approaches.
Those dealing with age-related bone density loss may find relevant information in the peptides for menopause discussion, as postmenopausal bone loss is a significant risk factor for fractures and impaired healing. The SS-31 peptide, which targets mitochondrial function, may have indirect relevance to bone healing through its effects on cellular energy production.
For any individual considering peptides for bone healing, consulting with a healthcare provider who understands both fracture management and peptide pharmacology is strongly recommended. The guide to finding peptide therapy providers and the online peptide therapy options can help connect individuals with qualified practitioners.
Understanding the research limitations
Transparency about what we know and what we do not know is essential in peptide science. The excitement around peptides for bone healing is warranted by the preclinical evidence, but several important limitations must be acknowledged.
Most bone healing evidence for BPC-157, TB-500, and GHK-Cu comes from animal studies, primarily in rabbits, rats, and mice. Animal models are valuable for identifying mechanisms and generating hypotheses, but they do not always translate directly to human outcomes. Bone healing in rabbits, for instance, occurs faster than in humans, and the biomechanical demands are different. The current state of peptide research helps contextualize where the evidence stands.
Human clinical data for investigational peptides in bone healing is essentially nonexistent. The one human study on BPC-157 for musculoskeletal applications involved chronic knee pain, not fracture healing, and included only twelve patients. Teriparatide has robust human data but is a prescription medication with specific indications and limitations.
Optimal dosing, timing, route of administration, and duration of treatment for human bone healing have not been established for investigational peptides. The protocols described in this guide are based on preclinical data and community experience, not on controlled human trials. SeekPeptides encourages evidence-based decision-making and provides members with regularly updated research summaries as new data becomes available.
None of this means that peptides do not work for bone healing. It means that the evidence is promising but incomplete, and individuals should make informed decisions with appropriate medical guidance rather than treating preclinical results as established clinical fact. Understanding what peptides are actually used for across different contexts helps maintain realistic expectations.
Frequently asked questions
Which peptide is best for bone healing?
Based on current evidence, no single peptide has been proven definitively best for bone healing in humans. Teriparatide (PTH 1-34) has the strongest clinical evidence and is FDA-approved for osteoporosis. For investigational peptides, BPC-157 has the most direct preclinical evidence specifically for bone defect healing, with the Sebecic rabbit study showing complete bony continuity in treated animals while controls remained unhealed. TB-500 demonstrated the strongest biomechanical improvements with 41 percent greater force to failure. The injury recovery peptide guide compares these compounds in more detail.
Can BPC-157 help a broken bone heal faster?
Preclinical evidence in rabbits showed that BPC-157 significantly accelerated healing of segmental bone defects, with callus formation twice the size of controls and complete bony bridging achieved within six weeks. The peptide promotes angiogenesis and osteoblast activity through VEGFR2-NO signaling. However, no human clinical trials have confirmed these effects in fracture patients. The complete BPC-157 guide covers the full spectrum of evidence for this compound.
How long do peptides take to show effects on bone healing?
Based on animal study timelines, vascular and cellular effects may begin within the first week of treatment. Visible callus enhancement on imaging typically appears between weeks two and four. Meaningful structural improvements in bone continuity and strength have been observed between weeks four and eight in animal models. Human healing timelines are generally longer than animal models. The peptide timeline guide provides context for expected timeframes across different applications.
Can I stack multiple peptides for bone healing?
Combining peptides with complementary mechanisms is a common approach in research settings. The BPC-157 plus TB-500 combination is the most widely discussed stack for tissue repair, offering localized and systemic benefits. Adding GHK-Cu for collagen support or a growth hormone secretagogue for IGF-1 elevation are additional options. However, multi-peptide interactions have not been systematically studied for bone healing. The stacking guide and the stack calculator help researchers plan combination protocols.
Are peptides safe for bone healing?
Safety profiles vary by compound. BPC-157 showed no toxic dose in animal studies across a wide dosing range. Teriparatide has a known safety profile from clinical use but carries a boxed warning regarding osteosarcoma risk observed in rats. TB-500 and GHK-Cu have favorable preclinical safety data but lack human safety studies. All peptide use should be conducted under medical supervision. The comprehensive safety guide discusses risk factors and monitoring recommendations.
Do I need a prescription for bone healing peptides?
Teriparatide and abaloparatide are prescription medications that require a physician order. BPC-157, TB-500, and GHK-Cu are available as research compounds but are not FDA-approved for any therapeutic indication. The legal status of peptides varies by jurisdiction and compound, and researchers should understand the regulatory landscape in their area.
Can peptides replace surgery for non-union fractures?
Current evidence does not support using peptides as a replacement for surgical intervention when surgery is indicated for non-union fractures. Peptides may serve as adjuncts to surgical treatment or may be investigated for cases where surgery is not feasible or has failed. The peptide therapy guide discusses the appropriate role of peptides within a comprehensive treatment plan.
How do I calculate the right dose for bone healing peptides?
Dosing depends on the specific peptide, the vial concentration, body weight, and the protocol being followed. The peptide calculator handles the math for any compound, while peptide-specific calculators like the BPC-157 calculator and the TB-500 calculator provide targeted calculations for those compounds.
For those serious about optimizing their understanding and approach to peptide-supported bone healing, SeekPeptides offers the most comprehensive resource available, with evidence-based guides, detailed protocols, dosing calculators, and a community of researchers who have navigated these exact questions. Membership provides access to regularly updated research summaries as new bone healing data becomes available.
The future of peptide-assisted bone regeneration
The field of peptide research for bone healing is still in its early stages relative to its potential. Several developments are likely to shape the future landscape.
Targeted delivery systems that concentrate peptides at fracture sites will likely improve efficiency and reduce the need for systemic exposure. Hydroxyapatite-binding peptide sequences, injectable hydrogels, and bioactive scaffolds are all being actively investigated. These technologies could transform compounds like GHK-Cu, which are effective locally but limited by short systemic half-lives, into practical therapeutic tools.
Combination therapies that pair peptides with other regenerative approaches, such as platelet-rich plasma, mesenchymal stem cell injections, or low-intensity pulsed ultrasound, may produce effects greater than any single intervention. The integration of peptide therapy with conventional orthopedic rehabilitation is another area where coordinated approaches could accelerate timelines.
Personalized protocols based on individual biology are becoming increasingly feasible. The semaglutide dosage calculator and similar tools demonstrate how personalized dosing is already being applied to peptide therapy in other contexts. Genetic variations in growth factor expression, receptor sensitivity, and metabolic capacity all influence how individuals respond to peptide interventions. As our understanding of these individual differences grows, peptide protocols for bone healing will become more targeted and effective.
Most importantly, human clinical trials are needed. The preclinical foundation is strong, particularly for BPC-157 and thymosin beta-4, but the gap between animal evidence and clinical application can only be bridged through rigorous controlled studies in human fracture patients. Until then, the evidence remains promising but preliminary.
SeekPeptides members stay informed as this field evolves, with access to research updates, protocol refinements, and community discussions that track the latest developments in peptide-assisted bone regeneration. Getting the right information at the right time can make the difference between an optimized recovery and a frustrating plateau.
External resources
PubMed: Osteogenic effect of BPC-157 on healing of segmental bone defect in rabbits
PMC: Research progress in calcitonin gene-related peptide and bone repair
In case I do not see you, good afternoon, good evening, and good night. May your fractures heal strong, your calluses mineralize fully, and your bones emerge more resilient than before.



