Jan 20, 2026

You've purchased peptides from what seemed like a reputable vendor. The website looked professional. The prices seemed reasonable. The product arrived with a certificate of analysis showing 99% purity. Everything appears legitimate.
But here's what you don't know.
That certificate could be fake. The purity claim could be fabricated. The peptide in your vial might not even be the compound you ordered. Without independent verification, you're trusting marketing claims over scientific evidence. You're assuming quality rather than confirming it. And in the peptide world, that assumption can lead to wasted money, failed research, and potentially serious safety concerns.
This is why peptide testing labs exist. These independent facilities provide the analytical verification that separates legitimate products from counterfeits, accurate concentrations from diluted compounds, and pure peptides from contaminated ones. Understanding how these labs work, what tests they perform, and when to use them transforms you from a hopeful buyer into an informed researcher.
The stakes are real. Research-grade peptides aren't regulated by the FDA. Quality varies dramatically between vendors. And the only way to know what you're actually getting is through proper analytical testing. Understanding what peptides are is foundational knowledge, but understanding how to verify their quality is what separates successful researchers from those who struggle with inconsistent results.
This comprehensive guide covers everything you need to know about peptide testing labs. You'll learn which testing methods reveal what information, how to interpret certificates of analysis, which labs provide reliable independent verification, what tests actually cost, and when you should absolutely invest in third-party testing. Whether you're verifying a new vendor or troubleshooting unexpected results, this resource gives you the knowledge to make informed decisions about peptide quality.
Why peptide testing matters more than you think
The peptide market operates largely without regulatory oversight. Unlike pharmaceutical medications that undergo rigorous testing before reaching consumers, research peptides exist in a gray area where quality depends entirely on vendor integrity. Some vendors maintain excellent standards. Others cut corners. And without testing, you cannot distinguish between them.
The consequences of poor quality peptides extend beyond wasted money.
Contaminated products can cause adverse reactions. Underdosed compounds produce suboptimal results that lead researchers to abandon effective protocols prematurely. Mislabeled peptides introduce variables that confound research outcomes. And counterfeit products, containing something entirely different than claimed, create unpredictable and potentially dangerous situations.
Consider the research implications. You're following a peptide dosing protocol precisely. You've calculated your doses using a peptide calculator. You've followed proper reconstitution procedures. But your results don't match what others report. The problem might not be your protocol. It might be your peptide.
The vendor verification problem
Most peptide vendors provide certificates of analysis. These documents supposedly verify purity, identity, and other quality metrics. But these COAs often come from the vendor's own testing, creating an obvious conflict of interest. Even when vendors claim third-party testing, verifying those claims requires effort most buyers don't make.
The best peptide vendors invest in legitimate quality control. They test every batch. They use accredited laboratories. They provide verifiable documentation. But distinguishing these vendors from those who cut corners requires understanding what proper testing looks like.
The counterfeit problem
Counterfeit peptides represent a growing concern. Some vendors sell products containing different compounds than advertised. Others dilute peptides significantly while claiming full potency. A few sell completely inert substances, capitalizing on the difficulty of detection without proper testing.
These counterfeits aren't always obvious. The packaging looks professional. The powder appears normal. Only analytical testing reveals the deception. One documented case involved a bodybuilder who injected what was labeled as Follistatin. The actual contents were a GHRP analog with potent cardiovascular effects. Hours later, he experienced chest pain and severe anxiety. The mislabeling could have been fatal.
Understanding peptide safety and risks includes understanding that product quality directly affects safety outcomes.

The essential peptide testing methods explained
Different analytical methods reveal different information about peptide quality. Understanding what each test measures helps you know which verification approaches matter for your specific needs.
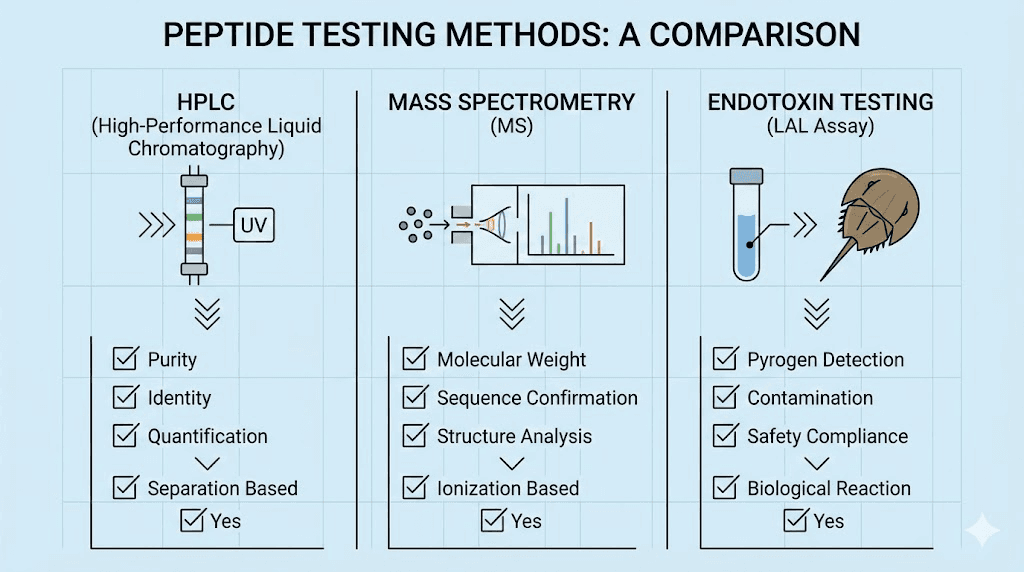
High-Performance Liquid Chromatography (HPLC)
HPLC is the gold standard for assessing peptide purity. This technique separates peptide mixtures and quantifies the relative amounts of different components. When a COA states "99% purity by HPLC," this means the analysis showed 99% of the detected material was the target peptide, with only 1% being impurities.
The process works by passing the peptide solution through a column packed with specialized material. Different compounds interact with this material differently, causing them to emerge from the column at different times. A detector measures what comes out, producing a chromatogram showing peaks for each compound present.
The main peak represents your target peptide. Smaller peaks indicate impurities, which might include truncated sequences, deletion sequences, or degradation products. The ratio of main peak to total peaks determines the reported purity percentage.
Important limitations exist. HPLC measures relative purity among detected compounds. It cannot confirm identity. A peptide missing one amino acid might appear 99% pure on HPLC because the truncated version is still the dominant species. This is why HPLC alone isn't sufficient for complete verification.
For researchers following specific peptide dosage charts, HPLC purity directly affects actual dosing. A peptide labeled 10mg but only 90% pure contains only 9mg of active compound.
Mass spectrometry for identity confirmation
Mass spectrometry (MS) confirms peptide identity by measuring molecular weight. The technique determines the exact mass of the peptide, which serves as a molecular fingerprint. If the measured mass matches the expected mass for your peptide sequence, you have confirmation that the correct compound is present.
Two main MS approaches are used for peptides:
MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight) is faster and simpler. It produces predominantly single-charge ions, making spectra easier to interpret. MALDI-TOF is often preferred for routine identity confirmation because of its speed and tolerance for sample impurities.
ESI-MS (Electrospray Ionization Mass Spectrometry) produces multiply-charged ions, which enables analysis of larger peptides and provides higher sensitivity. ESI is often coupled with liquid chromatography (LC-MS), combining separation and identification in a single analysis.
Mass spectrometry answers the fundamental question: Is this actually the peptide I ordered? HPLC tells you how pure it is. MS tells you what it is. Both matter.
For researchers working with specific compounds like BPC-157 or TB-500, mass spectrometry confirmation ensures you're working with the actual compound rather than something else entirely.
Net peptide content analysis
Here's a detail many researchers miss: purity and net peptide content are different things.
Purity measures peptide versus peptide impurities. Net peptide content measures peptide versus everything else, including water, salts, and counter-ions. A peptide can be 99% pure yet have only 70-80% net peptide content.
This matters enormously for dosing accuracy. If you assume 100% content when the actual content is 75%, your calculated doses are 25% lower than intended. This explains why some researchers report weaker effects than expected despite following published protocols.
Amino acid analysis determines net peptide content by breaking down the peptide and measuring the actual amino acid content. This provides the most accurate basis for dosing calculations.
When using the peptide reconstitution calculator or planning doses, net peptide content provides more accurate calculations than assuming full content.
Endotoxin testing
Endotoxins are lipopolysaccharides from gram-negative bacteria. Even trace amounts can cause pyrogenic (fever-inducing) responses in humans. Endotoxins are extremely heat-stable, so normal sterilization cannot destroy them. They also adhere well to laboratory equipment, allowing contamination to spread easily.
Endotoxin testing follows USP <85> methodology, typically using the Limulus Amebocyte Lysate (LAL) assay. Results are reported in Endotoxin Units (EU) per milligram. The FDA standard for injectable medications suggests less than 10 EU/mg, though research applications may tolerate slightly higher levels.
When is endotoxin testing necessary? If you're using injectable peptides, endotoxin testing provides important safety information. This is particularly relevant for injectable peptides where the administration route bypasses normal protective barriers.
Heavy metals screening
Heavy metals testing screens for arsenic, cadmium, chromium, mercury, and lead. These contaminants aren't typically present in properly synthesized peptides, but occasional checks confirm manufacturing quality.
Testing uses Inductively Coupled Plasma Mass Spectrometry (ICP-MS), which can detect even trace levels of metals. Heavy metals accumulate in the body over time, potentially causing neurological damage, kidney dysfunction, and other chronic problems.
Heavy metals testing is less critical for routine verification than purity and identity testing. However, when evaluating a new vendor or investigating unexpected adverse effects, metals screening can rule out or identify contamination issues.
Sterility testing
Sterility testing verifies the absence of viable microorganisms. Two main protocols exist: USP <61> for nonsterile microbial enumeration and USP <71> for complete sterility testing.
For most research applications, sterility testing can be unnecessary if you use proper technique: reconstituting with bacteriostatic water, filtering through 0.22μm PES filters, maintaining sterile technique, and storing properly.
The peptide storage guide covers proper handling that maintains sterility without requiring formal testing.
Understanding certificates of analysis
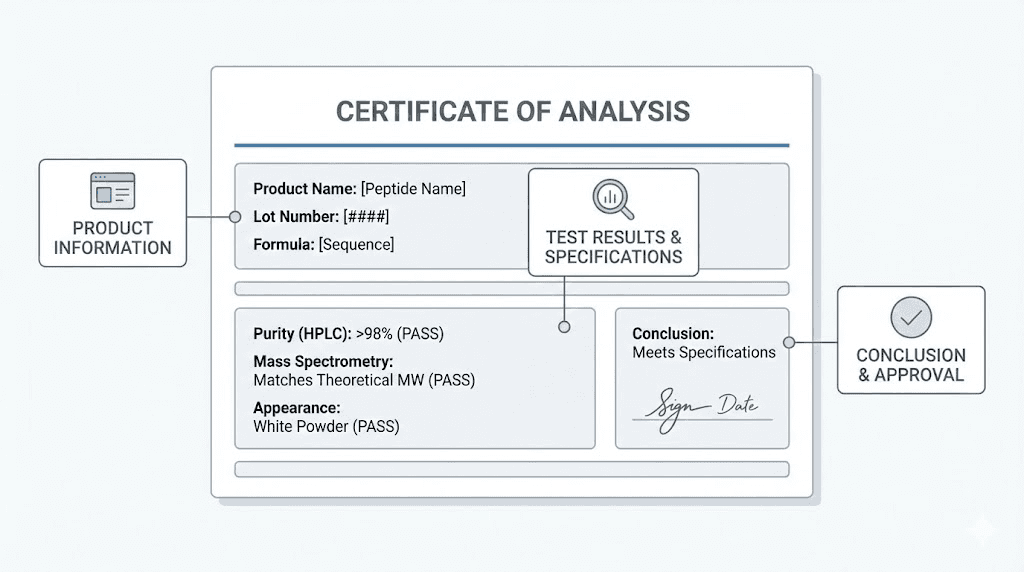
A Certificate of Analysis (COA) documents the analytical testing performed on a specific peptide batch. Learning to read and verify COAs is essential for any serious researcher.
Essential COA elements
A legitimate COA should include:
Peptide identification: The peptide name, sequence (if applicable), and molecular weight. This basic information establishes what compound was tested.
Batch or lot number: This unique identifier links the COA to a specific production batch. Every vial from that batch should reference the same lot number. If your product's lot number doesn't match the COA, the document doesn't verify your specific product.
Test date: When was the testing performed? COAs should be recent, typically within 12 months. Older documents may not reflect current product quality, especially if storage conditions varied.
Testing methods: What analytical techniques were used? Look for specific method descriptions, not just results. "HPLC purity: 99%" tells you less than "RP-HPLC, C18 column, water/acetonitrile gradient, UV detection at 220nm, purity: 99.2%."
Purity results: The percentage purity, ideally with a chromatogram showing the actual data. Numbers without supporting graphs are less trustworthy.
Mass spectrometry data: The observed molecular weight compared to theoretical weight. A match confirms identity. Any discrepancy requires explanation.
Laboratory information: Who performed the testing? Internal QC or third-party lab? If third-party, what lab? Can you verify the lab exists?
Authorized signature: A dated signature from quality assurance personnel.
Purity thresholds and what they mean
Not all purity levels serve all purposes equally.
≥98% purity: Excellent quality. The US Pharmacopeia considers 98% the minimum acceptable standard. This level suits quantitative assays, structural biology, binding studies, and sensitive cell work.
95-97% purity: Standard research-use-only (RUO) grade. Suitable for most discovery-phase research where absolute precision isn't critical.
<95% purity: Potentially problematic for precision applications. Lower purity means more impurities, which might interfere with results or introduce variability.
For most research applications following how peptides work at the cellular level, higher purity provides more reliable outcomes.
Reading chromatograms
A chromatogram shows peaks representing different compounds detected during HPLC analysis. The main peak, usually the largest, represents your target peptide. Its area relative to total peak area determines the reported purity percentage.
What to look for:
A dominant main peak: The target peptide should be clearly the major component, with the main peak much larger than any others.
Clean baseline: The space between peaks should be relatively flat, indicating good separation and low background noise.
Minimal side peaks: Small peaks near the main peak typically represent synthesis-related impurities like deletion sequences. Their size indicates impurity levels.
Retention time: The time at which the main peak appears should match expected values for that peptide under the stated conditions.
A purity percentage without a chromatogram is scientifically insufficient. The chromatogram provides visual confirmation of the claimed purity.
Interpreting mass spectrometry data
Mass spectrometry results typically show:
Theoretical (expected) mass: The calculated molecular weight based on the peptide sequence.
Observed (measured) mass: The actual molecular weight detected in the sample.
These values should match within acceptable tolerance, typically ±0.1% or ±1 Da for smaller peptides. Any significant discrepancy suggests the sample isn't the claimed peptide.
For peptides with modifications (acetylation, amidation), the theoretical mass should account for these modifications. Unmodified versus modified versions have different masses.
How to spot fake or misleading COAs
Counterfeit certificates of analysis exist. Some vendors fabricate documents to appear legitimate. Learning to identify red flags protects you from deception.
Visual warning signs
Altered text or graphics: Look for misaligned text, inconsistent fonts, different text sizes within sections, or graphics that appear cut and pasted. Fabricators often use screenshot tools to modify legitimate documents, leaving visible artifacts.
Missing chromatograms: A purity claim without the actual chromatogram data is a significant red flag. Legitimate labs provide the raw data, not just a number.
Generic templates: Be suspicious of COAs that look identical across different batches or even different vendors. Legitimate labs use their own formats.
Scanned or photographed documents: Reputable organizations provide clean PDF documents, not scans or photos of paper documents.
Content red flags
Missing or vague method descriptions: "Tested by HPLC" tells you almost nothing. Legitimate COAs describe specific methods: column type, mobile phase, detection method, and conditions.
No mass spectrometry data: HPLC alone cannot confirm identity. A COA with only HPLC purity and no MS confirmation is incomplete.
Unrealistic results: 100.0% purity is essentially impossible. Values like 99.99% should raise eyebrows. Real analytical results show realistic numbers with appropriate precision.
Mismatched lot numbers: If the lot number on your product doesn't match the COA, the document doesn't verify your specific batch.
Outdated testing: COAs from years ago don't reflect current product quality. Demand recent testing for current batches.
Verification steps
Check for QR codes: Many legitimate labs include QR codes linking to online verification. Scan the code and confirm the linked data matches the paper document. QR codes are difficult to fake because altering the paper version doesn't change the linked online data.
Verify the lab exists: Search for the testing laboratory online. Does it have a legitimate website? Contact information? Physical address? Can you find independent references to it?
Request lot-specific COAs: Ask vendors for COAs matching your specific lot number before purchasing. Legitimate vendors provide this within 24 hours. Excuses for not providing documentation are themselves red flags.
Cross-reference testing dates: The testing date should precede your purchase date and correspond logically to the lot number. A COA dated after you received the product is obviously problematic.
The recurring batch problem
A common deceptive practice: vendors test one batch, then use that same COA for subsequent batches as if they're covered by the original testing. While this might seem efficient, it provides no assurance of batch-to-batch consistency.
Each batch should have its own testing. At minimum, identity and purity should be verified per batch. Vendors who provide the same COA for months of production are not demonstrating adequate quality control.
Major independent peptide testing labs
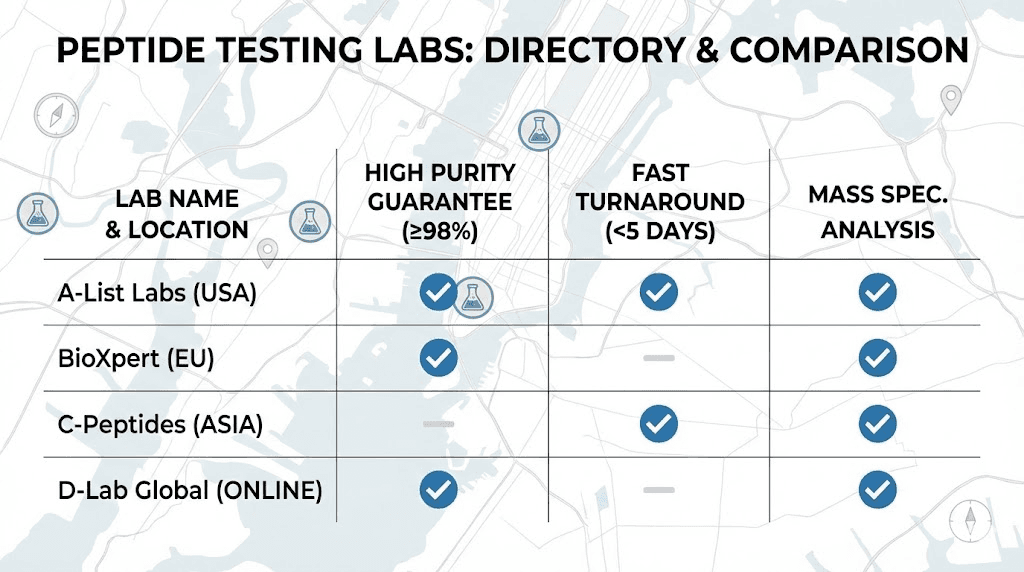
Several independent laboratories provide peptide testing services. Understanding their strengths, limitations, and reputations helps you choose appropriate verification resources.
Janoshik Analytical
Janoshik has established itself as a respected name in independent peptide testing, often described as the "gold standard" within the research community.
The lab provides HPLC purity analysis and mass spectrometry identity confirmation.
COAs from Janoshik include QR codes for online verification, allowing you to scan and confirm results against the lab's database. This verification feature significantly increases trust in the documentation.
The lab's reputation comes from consistent, reliable results and independence from any vendor. Researchers frequently reference Janoshik testing when evaluating new vendors or verifying claims.
Finnrick Analytics
Finnrick provides an independent testing platform specifically designed for the peptide market. The organization has tested over 4,500 samples from more than 169 vendors, creating a substantial database of comparative quality information.
Notably, Finnrick offers free testing for samples shipped to their Texas facility. They request HPLC as the primary method, measuring both identity and purity. Results are published on their platform, creating transparency about vendor quality over time.
The vendor rating system aggregates testing results, allowing researchers to compare quality trends across different suppliers. This comparative data provides context beyond individual test results.
Finnrick accepts most common single peptides in lyophilized powder form, though they currently don't accept peptide blends and only process shipments from within the USA.
Peptide Test (US-based)
Peptide Test is a US-based laboratory offering HPLC and UV analysis for peptide verification. The lab emphasizes efficient sample processing and accuracy in peptide analysis.
For researchers prioritizing domestic testing with quick turnaround, Peptide Test represents a viable option. The US location simplifies shipping logistics for American researchers.
MZ Biolabs
MZ Biolabs provides third-party testing services referenced by various peptide vendors. The lab offers standard analytical services including HPLC and mass spectrometry analysis.
Like Janoshik, MZ Biolabs COAs can be verified through their platform, providing an additional layer of authenticity confirmation.
Chromate
Chromate offers analytical testing with verifiable online results. QR codes on their COAs link to verification pages where researchers can confirm document authenticity.
The lab is frequently mentioned alongside Janoshik and MZ Biolabs as a trusted third-party verification resource.
Commercial analytical laboratories
Beyond peptide-focused testing services, general analytical laboratories offer peptide testing:
Creative Proteomics provides peptide purity analysis using analytical reversed-phase HPLC combined with mass spectrometry. They offer MALDI-TOF MS and other mass spectrometry options routinely.
Eurofins BioPharma offers comprehensive cGMP testing supporting drug substance and drug product programs, from raw materials release through sterile fill-finish services.
GenScript provides quality control testing including mass spectrometry and analytical HPLC throughout peptide synthesis and purification processes.
These commercial labs typically serve pharmaceutical and biotech clients but may accept individual research samples at appropriate price points.
Choosing a testing lab
Consider these factors when selecting a testing lab:
Independence: The lab should have no financial relationship with peptide vendors. True independence ensures unbiased results.
Verification capability: Can you verify results through the lab's website? QR codes or online lookup features add authenticity confirmation.
Reputation: What do other researchers say about the lab? Community feedback provides real-world quality assessment.
Methods offered: Does the lab provide both HPLC and MS analysis? Complete verification requires both purity and identity confirmation.
Turnaround time: How quickly will you receive results? Urgent needs may require labs with faster processing.
Cost: Testing costs vary significantly. Balance cost against the value of the information received.
What peptide testing actually costs
Testing costs vary based on analysis type, laboratory, sample complexity, and turnaround requirements. Understanding typical pricing helps budget for appropriate verification.
Standard pricing ranges
Basic HPLC purity analysis: $100-200 per sample. This provides purity percentage and typically includes a chromatogram.
Mass spectrometry identity confirmation: $150-250 per sample when performed alone. Often bundled with HPLC at reduced combined cost.
Combined HPLC + MS analysis: $200-350 per sample. This comprehensive testing provides both purity and identity verification.
Net peptide content (amino acid analysis): $120-150 per sample. This specialized analysis determines actual peptide content versus total mass.
Endotoxin testing: $75-150 per sample. Turnaround is typically 3-5 business days.
Heavy metals panel: $100-200 per sample. Screens for arsenic, cadmium, chromium, lead, and mercury.
Factors affecting cost
Sample complexity: Short synthetic peptides (5-10 residues) are faster and cheaper to analyze. Longer peptides or those with modifications (PEGylation, cyclization) increase analysis time and cost.
Sample quality: Low-quality or contaminated samples may require additional preparation, increasing labor costs.
Turnaround time: Rush processing typically adds 50-100% to base pricing. Standard turnaround is usually 5-7 business days.
Method development: Novel peptides requiring custom analytical methods incur additional development charges.
GMP compliance: Testing meeting GMP regulatory standards costs significantly more due to documentation requirements and qualified personnel.
Free testing options
Finnrick offers free peptide testing for samples shipped to their facility. This remarkable resource allows researchers to verify products without cost, though shipping expenses and sample preparation remain your responsibility.
Some vendors offering quality products encourage third-party testing and may reimburse testing costs if results confirm their claims. This confidence indicator suggests the vendor trusts their product quality.
Cost-benefit analysis
When is testing worth the cost?
High-value purchases: If you're buying expensive peptides, testing costs represent a small percentage of total investment. Verifying a $500 purchase with $150 of testing adds 30% but provides certainty.
New vendor evaluation: Before committing to ongoing purchases from a new vendor, testing their product once provides information that guides future decisions.
Inconsistent results: If your research produces inconsistent outcomes, testing rules out product quality as the variable.
Safety-critical applications: When using injectable peptides, verification provides confidence that directly affects safety decisions.
For researchers following protocols from resources like the BPC-157 and TB-500 stacking guide, verified product quality ensures the protocol receives a fair evaluation.
When you should absolutely get third-party testing
Not every purchase requires independent verification. Testing everything would be impractical and expensive. But certain situations strongly warrant the investment.
Evaluating a new vendor
Your first purchase from any vendor should be considered a test case. Before committing to larger orders or ongoing purchases, verify that initial products meet claimed specifications.
This evaluation purchase approach limits risk. If testing reveals problems, you've lost only a small investment rather than a large one. If testing confirms quality, you've gained confidence for future orders.
When researching the best peptide vendors, independent testing of actual products provides more reliable information than reviews or marketing claims.
Unexpected results from research
When protocols that should work don't produce expected outcomes, product quality becomes a suspect variable. Before abandoning a protocol or blaming individual response variation, verify that you're actually using what you think you're using.
Testing can reveal underdosed products, degraded peptides, or outright counterfeits that explain failed research. This information either confirms product issues requiring vendor change or rules out product quality, directing troubleshooting elsewhere.
Understanding how long peptides take to work helps set realistic expectations, but verified product quality ensures the timeline assessment is valid.
High-stakes applications
Some research carries higher stakes than others. Competitive athletes considering peptides face severe consequences if products contain banned substances not listed on labels. Health-focused applications involving injection create direct safety implications from product quality.
For these high-stakes situations, the cost of testing is trivial compared to potential consequences of using unverified products.
Bulk purchases
Before committing significant money to large quantity purchases, testing a sample provides assurance that the bulk order will meet expectations. The percentage cost of testing decreases as order size increases, making verification increasingly affordable on a per-unit basis.
Signs of potential problems
Certain observations suggest testing might reveal issues:
Unusual appearance: Peptides that look different than expected, unusual powder consistency, or unexpected coloration might indicate problems worth investigating.
Vendor red flags: New vendors, vendors with mixed reviews, unusually low prices, or vendors who avoid providing COAs warrant verification.
Changed supplier behavior: If a previously reliable vendor changes ownership, location, or manufacturing processes, quality might change. Testing confirms whether previous quality standards continue.
Community reports: If other researchers report quality issues with a vendor, testing your specific products either confirms or contradicts those reports for your batch.
How to submit samples for testing
The testing process involves specific steps to ensure accurate results and proper sample handling.
Sample preparation
Keep samples sealed: Unopened vials provide the cleanest samples. If you must test reconstituted peptides, follow lab-specific instructions for liquid sample handling.
Maintain cold chain: Most peptides should remain cold during shipping. Use ice packs and insulated packaging to prevent degradation during transit. Understanding proper peptide storage applies to shipping as well as long-term keeping.
Provide adequate quantity: Labs typically need 1-5mg minimum for testing. Check specific requirements before shipping, as insufficient sample prevents complete analysis.
Label clearly: Include the peptide name, your contact information, and any relevant reference numbers. Clear labeling prevents sample confusion.
Documentation to include
What you believe the sample contains: The expected peptide identity helps labs confirm or contradict your expectation.
Requested tests: Specify exactly what analysis you want: HPLC purity, MS identity, endotoxin, or other specific tests.
Any relevant history: If you're testing because of suspected problems, describe your concerns. This context helps labs interpret results.
Shipping considerations
Use appropriate carriers: Some carriers handle biological materials better than others. Follow lab recommendations for preferred shipping methods.
Insure valuable shipments: If the sample represents significant value, shipping insurance provides protection against loss.
Time shipping appropriately: Avoid shipping before weekends or holidays when packages might sit unattended. Aim for delivery on business days when labs can process incoming samples promptly.
Understanding turnaround times
Standard testing typically takes 5-7 business days from sample receipt. Rush processing can reduce this to 2-3 days at additional cost. Plan your research timeline to accommodate testing delays.
For researchers planning peptide cycles, building testing time into planning ensures verified products before beginning protocols.

Interpreting your test results
Receiving test results is just the beginning. Understanding what the data means guides your decisions about the tested product.
Purity assessment
≥98% purity: Excellent. This meets pharmacopeial standards and suits virtually any research application. Products at this level reflect quality manufacturing and handling.
95-97% purity: Acceptable for most research purposes. Minor impurities present but unlikely to significantly affect typical applications. Consider whether your specific use case requires higher purity.
90-94% purity: Below typical standards. Significant impurities present. Evaluate whether the impurity level affects your intended use. May be acceptable for preliminary experiments but questionable for quantitative work.
<90% purity: Problematic. Substantial impurities that likely affect research quality. Unless specifically purchased as lower-grade material, this result suggests vendor quality issues.
Identity confirmation
Mass match within tolerance: The observed mass matching expected mass confirms identity. The peptide is what it claims to be.
Mass mismatch: Discrepancy between observed and expected mass indicates a problem. The sample might be a different peptide, a modified version, or contaminated. Significant mismatches warrant vendor contact and likely product rejection.
Multiple masses detected: If MS shows multiple distinct masses, the sample may contain mixture of different peptides or significant degradation products. This complexity requires evaluation based on your specific needs.
What to do with results
Results confirm quality: Proceed with confidence. Document the verification for future reference. Consider this vendor validated for future purchases.
Results show minor issues: Evaluate whether the issues affect your intended use. Minor purity reduction might be acceptable. Identity confirmation with slight purity reduction is better than high claimed purity without identity verification.
Results reveal major problems: Contact the vendor with your results. Legitimate vendors respond constructively to quality concerns, offering replacement or refund. Vendors who dismiss independent testing results warrant reconsideration for future purchases.
Counterfeit detection: If testing reveals the product is entirely different than claimed, document everything and avoid that vendor permanently. Consider sharing results with community resources to warn others.
Building quality assurance into your research
Beyond individual testing decisions, systematic approaches to quality assurance improve overall research reliability.
Vendor qualification process
Develop a systematic approach to evaluating new vendors:
Research vendor reputation through community resources and reviews
Request COAs before purchasing and evaluate their completeness
Make a small initial purchase for testing
Submit for independent verification
Based on results, decide whether to continue with larger orders
This process creates a qualified vendor list you can trust for ongoing purchases. Resources like SeekPeptides provide guidance for vendor evaluation alongside other research support.
Batch documentation
Maintain records connecting specific products to their verification status:
Lot numbers: Record the lot number from every product you use.
COA archives: Save COAs for products you use, creating a reference library.
Testing results: Document any independent testing performed, including results and testing dates.
Research correlation: Note which products were used in which research, enabling correlation between product quality and outcomes.
This documentation supports troubleshooting if problems arise and demonstrates diligence in quality-focused research.
Periodic re-verification
Even trusted vendors warrant occasional verification:
Annual testing: Consider testing at least one product annually from regular vendors to confirm ongoing quality.
After vendor changes: If a vendor announces ownership changes, manufacturing updates, or significant operational shifts, verify that quality remains consistent.
Random checks: Occasional unannounced testing of regular purchases maintains confidence and catches any quality decline early.
Community contribution
Sharing testing results with appropriate community resources benefits everyone:
Finnrick submissions: Contributing test results to Finnrick's database helps build comprehensive vendor quality data.
Forum discussions: Sharing verified quality information in peptide forums helps other researchers make informed decisions.
Warning about problems: If testing reveals significant issues, alerting the community prevents others from making the same mistake.
Collective vigilance improves quality standards across the market by rewarding quality vendors and exposing problematic ones.
Special considerations for specific peptide types
Different peptide categories may warrant specific testing considerations.
Healing peptides
Peptides like BPC-157 and TB-500 used for injury recovery applications should receive full verification. Identity confirmation is critical since substitution with different compounds would fundamentally change the research approach.
The comparison between BPC-157 and TB-500 relies on both compounds being accurately identified. Testing confirms you're comparing what you intend to compare.
Weight management peptides
GLP-1 agonists and related compounds for weight loss applications carry specific concerns. These peptides work through specific receptor mechanisms, making identity verification particularly important. A compound with similar but different activity could produce unexpected effects.
Given the popularity of compounds discussed in resources like the semaglutide versus tirzepatide comparison, verification ensures research with the intended compound.
Growth hormone secretagogues
Peptides like ipamorelin and sermorelin stimulate natural hormone production. Purity matters because impurities might have their own biological activity, confounding results. Identity verification confirms the specific secretagogue being studied.
Cognitive peptides
Nootropic peptides like Semax and Selank affect central nervous system function. The stakes of using contaminated or misidentified products in cognitive applications warrant verification investment.
The best nootropic peptides provide cognitive benefits only when properly manufactured and verified.
Skin and cosmetic peptides
Peptides like GHK-Cu used for skin applications might be applied topically rather than injected. While injection-grade standards may be less critical for topical use, identity verification remains important to ensure the intended compound is being applied.
For those following peptides for skin tightening protocols, verified products ensure the protocol receives fair evaluation.
Frequently asked questions
How much does it cost to get a peptide tested?
Basic HPLC purity testing typically costs $100-200 per sample. Combined HPLC and mass spectrometry analysis runs $200-350. Specialized tests like endotoxin or heavy metals add $75-200 each. Finnrick offers free testing for samples shipped to their facility, making verification accessible without cost for US-based researchers.
Which peptide testing lab is most reliable?
Janoshik is often considered the gold standard in the peptide research community, with verifiable COAs and established reputation. Finnrick provides extensive comparative data across vendors. For US-based researchers, domestic labs like Peptide Test offer convenience. The most reliable choice depends on your specific needs, location, and the tests required.
Can I verify a peptide COA is legitimate?
Yes, through several methods. Legitimate COAs from reputable labs often include QR codes linking to online verification. You can contact the listed laboratory directly to confirm they performed the testing. Look for complete documentation including chromatograms, not just numbers. Compare lot numbers on your product to those on the COA.
Missing verification features suggest potential problems.
What purity level should peptides have?
The US Pharmacopeia considers 98% the minimum acceptable standard. Peptides at 95-97% purity suit most research applications. Below 95% suggests quality issues that may affect results. For precision applications, structural biology, or safety-critical uses, prioritize 98%+ purity. The peptide dosage chart assumes reasonable purity levels for accurate dosing.
How long does peptide testing take?
Standard turnaround is typically 5-7 business days from sample receipt. Rush processing can reduce this to 2-3 days at additional cost. Endotoxin testing specifically takes 3-5 days. Plan research timelines to accommodate testing delays, especially for time-sensitive applications.
Do I need to test every peptide purchase?
Not necessarily. Testing makes most sense for new vendor evaluation, expensive purchases, safety-critical applications, or when troubleshooting unexpected results. Once you've verified a vendor's quality through testing, ongoing purchases may not require individual verification unless circumstances change. Periodic spot-checks maintain confidence over time.
What's the difference between vendor COA and third-party testing?
Vendor COAs come from the seller, creating potential conflict of interest. Third-party testing uses independent laboratories with no financial stake in the results. Third-party results are inherently more trustworthy because the testing lab has no incentive to misrepresent findings. Independent verification confirms or contradicts vendor claims.
Can peptides degrade after testing but before use?
Yes. Testing reflects quality at a specific moment. Subsequent degradation from improper storage, temperature exposure, or contamination during handling can reduce quality. The peptide storage guide covers proper handling that maintains quality between testing and use. Questions like how long peptides last become relevant after verification.
External resources
Finnrick Analytics - Independent peptide testing platform with vendor ratings and free testing options
United States Pharmacopeia - Standards organization defining pharmaceutical quality benchmarks
U.S. Food and Drug Administration - Regulatory guidance on pharmaceutical quality standards
ISO 17025 - International standard for testing laboratory competence
For researchers committed to quality-verified peptide research, SeekPeptides provides comprehensive guidance on protocols, dosing, and vendor evaluation. The combination of evidence-based educational resources and practical tools helps researchers make informed decisions throughout their peptide journey, from initial vendor selection through protocol optimization.
In case I don't see you, good afternoon, good evening, and good night. May your tests stay accurate, your peptides stay pure, and your research stay verified.