Feb 4, 2026
Most people discover stem cell peptides the wrong way. They read a headline about a celebrity flying to Panama for a $50,000 stem cell injection. They see a clinic promising miracle results with mesenchymal stem cell IV drips. They assume regeneration requires either extreme wealth or extreme risk. Here is what almost nobody talks about: your body already contains the machinery for regeneration. Stem cells sit in your bone marrow, your adipose tissue, your skin, your gut lining, and dozens of other niches throughout your body.
The problem is not a shortage of stem cells. The problem is that the signals telling those cells to activate, migrate, and repair become weaker with age. Peptides change that equation entirely. A small but growing body of research shows that specific peptide sequences can wake up dormant progenitor cells, accelerate their migration to damaged tissue, amplify the growth factors they need to do their work, and even protect the telomeres that determine how many times they can divide. GHK-Cu modulates over 4,000 human genes. TB-500 increases cell migration speed by two to three times. BPC-157 activates VEGF signaling cascades that build new blood vessels. Epitalon extends telomere length through telomerase activation. These are not hypothetical mechanisms.
They are published findings from peer-reviewed laboratories around the world. This guide covers every stem cell peptide with meaningful research behind it, the molecular pathways they engage, how researchers stack them for different goals, and the practical details of preparation and administration that most sources leave out.
Whether you are recovering from an injury, pursuing longevity, or simply trying to understand what peptide-driven regeneration actually means at the cellular level, this is the resource you have been looking for.
What stem cell peptides actually are (and why they matter)
The term "stem cell peptides" refers to short-chain amino acid sequences that interact with stem cells and progenitor cells in measurable ways. Some activate dormant stem cell populations. Others enhance migration, proliferation, or differentiation. A few protect stem cells from the DNA damage and telomere shortening that limit their regenerative capacity over time.
This is not the same thing as stem cell therapy.
Stem cell therapy involves harvesting cells from one location, often bone marrow or adipose tissue, processing them in a laboratory, and injecting them back into the body at a target site. It is expensive. It carries contamination risks. And the survival rate of transplanted cells remains a significant challenge in the field. Stem cell peptides take a fundamentally different approach. Rather than introducing external cells, they optimize the performance of the stem cells you already have. Think of it this way: your body is a factory with thousands of workers sitting idle because the foreman stopped giving instructions. Peptides are not new workers. They are the instructions. They are the chemical signals that tell existing stem cells where to go, what to become, and when to start working. The distinction matters because it changes everything about accessibility, cost, safety profile, and practical application.
Peptides themselves are simply short chains of amino acids, typically between 2 and 50 amino acids in length. They occur naturally throughout the human body as signaling molecules, hormones, and regulatory factors. The ones classified as stem cell peptides share a common trait: they have demonstrated the ability to influence stem cell behavior in laboratory or clinical settings. That influence can take many forms. GHK-Cu upregulates genes associated with stem cell maintenance. TB-500 physically mobilizes progenitor cells and guides them toward injury sites. Epitalon activates telomerase, the enzyme that rebuilds the protective caps on chromosomes, effectively extending the replicative lifespan of stem cells. Each peptide has its own mechanism. Each targets a different part of the regenerative cascade. And when combined strategically, they can address multiple bottlenecks in the regeneration process simultaneously.
Why does this matter right now? Because the science has reached a tipping point. Ten years ago, most of what we knew about peptides and stem cells came from isolated cell culture experiments. Researchers would drop GHK-Cu into a petri dish with mesenchymal stem cells and measure what happened. That work was valuable, but limited. Today, we have animal models showing systemic effects, human safety data from pilot studies, gene expression analyses covering thousands of genes simultaneously, and clinical trials for specific peptide-derived therapeutics. The gap between laboratory curiosity and practical application is closing rapidly. Understanding how peptides work at the stem cell level is no longer optional knowledge for anyone serious about regenerative health. It is foundational.
How peptides activate and support stem cells (the science)
Stem cell peptides do not all work the same way. That is actually one of their greatest strengths, because regeneration is not a single process. It is a cascade of interconnected events that begins with cellular signaling and ends with functional tissue repair. Different peptides intervene at different points in this cascade. Understanding those intervention points is essential for choosing the right peptides and combining them effectively. Four primary mechanisms drive most of the stem cell activity observed in peptide research.
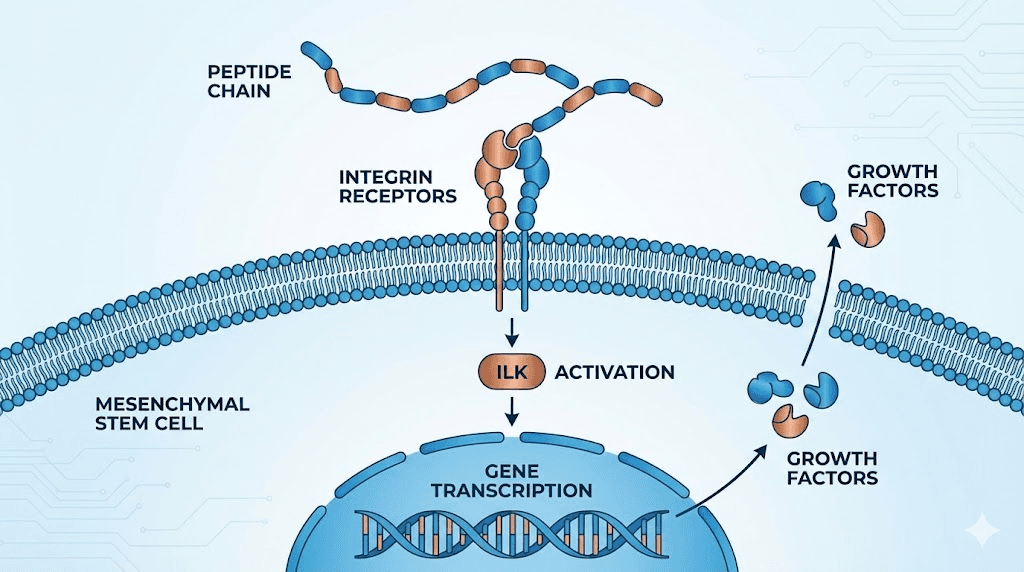
The integrin pathway
Integrins are transmembrane proteins that act as bridges between the outside environment and the inside of a cell. They are receptors. They are anchors. They are signal transducers. When a peptide binds to an integrin on the surface of a stem cell, it triggers a chain reaction that can alter gene expression, cell movement, and cell survival. GHK-Cu and TB-500 both use this pathway, though they engage it differently.
GHK-Cu binds to integrin-linked kinase on cell membranes. This activates ILK-related pathways that influence stem cell behavior downstream. Research has shown that at concentrations between 0.1 and 10 micromolar, GHK-Cu increases the expression of epidermal stem cell markers including integrins and p63, a transcription factor critical for maintaining the regenerative capacity of basal stem cells. Bock-Marquette and colleagues published a landmark paper in Nature in 2004 demonstrating that thymosin beta-4, the parent molecule of TB-500, also activates integrin-linked kinase. In that study, ILK activation promoted cardiac cell migration and survival, establishing a direct connection between peptide signaling, integrin engagement, and stem cell mobilization.
The practical implication is straightforward. Integrin-targeting peptides do not just tell stem cells to divide. They tell them to move. They tell them to survive hostile environments like inflamed or ischemic tissue. And they tell them to maintain their identity as stem cells rather than differentiating prematurely. For anyone interested in fast injury healing, this pathway is one of the most important mechanisms to understand.
Growth factor amplification
Growth factors are the heavy machinery of tissue repair. Vascular endothelial growth factor builds new blood vessels. Basic fibroblast growth factor drives cell proliferation. Transforming growth factor beta regulates extracellular matrix production. Without adequate growth factor signaling, stem cells that have been activated and mobilized cannot complete their mission because the tissue environment does not support their survival or differentiation.
Several stem cell peptides dramatically amplify growth factor production. Jose and colleagues found that adding GHK to mesenchymal stem cell cultures increased both VEGF and bFGF concentrations. BPC-157 promotes angiogenesis through the VEGFR2-Akt-eNOS signaling pathway, which is the primary route through which the body builds new blood supply to healing tissue. This is not a minor effect. Angiogenesis is often the rate-limiting step in tissue repair. Without new blood vessels, regenerating tissue starves. BPC-157 also activates ERK1/2 signaling and the FAK-paxillin pathway, both of which are critical for fibroblast migration into wound sites.
GHK-Cu takes growth factor amplification to another level entirely. Gene expression studies have shown that GHK-Cu modulates 31.2 percent of human genes, affecting 4,192 genes with expression changes of 50 percent or more. That number is staggering. It means GHK-Cu does not simply turn on one or two growth factors. It reshapes the entire gene expression landscape of a cell, pushing it toward a state that favors repair, regeneration, and stem cell maintenance. Decorin production increased by 302 percent in GHK-Cu treated cells. Decorin is a proteoglycan that regulates collagen fiber assembly and modulates growth factor activity. More decorin means better organized tissue repair and stronger signaling to nearby stem cells.
For researchers exploring tissue repair peptides, growth factor amplification represents perhaps the most immediately practical mechanism. It supports every phase of healing, from initial inflammation resolution through remodeling.
Epigenetic regulation
Epigenetics refers to changes in gene expression that do not involve alterations to the DNA sequence itself. Instead, chemical modifications to DNA or the histone proteins around which DNA wraps determine which genes are accessible for transcription and which remain silent. Stem cell fate, whether a cell remains a stem cell, differentiates into a specific tissue type, or enters senescence, is largely controlled by epigenetic programming.
Epitalon, the tetrapeptide with the sequence Ala-Glu-Asp-Gly, operates primarily through epigenetic mechanisms. Research has demonstrated that Epitalon interacts with histone proteins, influencing chromatin structure and gene accessibility. In studies on human gingival mesenchymal stem cells, Epitalon stimulated the expression of neurogenic differentiation markers including Nestin, GAP43, Beta Tubulin III, and Doublecortin. The mRNA expression of these markers increased by 1.6 to 1.8 times compared to untreated controls. This suggests that Epitalon does not just keep stem cells alive. It can influence the direction in which they differentiate, potentially guiding them toward neural lineages.
The bioregulator peptide family, of which Epitalon is perhaps the most well-known member, appears to exert many of its effects through this epigenetic channel. Khavinson peptides like Thymalin, Pinealon, and Cortagen all share short amino acid sequences that appear to interact with DNA and histones in tissue-specific ways. The emerging picture is one of precise epigenetic tuning, where specific peptide sequences can shift the gene expression profile of stem cells toward regenerative activity without permanently altering the genome.
Telomere maintenance
Every time a cell divides, its telomeres get shorter. Telomeres are the protective caps at the ends of chromosomes, often compared to the plastic tips on shoelaces. When telomeres become critically short, the cell enters senescence and stops dividing. For stem cells, telomere length directly determines regenerative capacity. A stem cell with long telomeres can divide many more times than one with short telomeres. This is one of the primary reasons why regenerative capacity declines with age.
Epitalon activates telomerase, the enzyme responsible for rebuilding telomere length. This is not speculation. Multiple studies have confirmed telomerase activation following Epitalon administration. The implications for stem cell function are profound. By maintaining telomere length, Epitalon effectively extends the working lifespan of stem cells, allowing them to continue dividing and repairing tissue long after they would normally have entered senescence. The Epitalon dosage protocols used in research typically involve cyclical administration, reflecting the idea that periodic telomerase activation may be more beneficial than continuous exposure.
One clinical case documented a biological age reduction of 7.9 years using combined therapy that included Epitalon. While a single case study cannot prove causation, it aligns with the mechanistic data showing telomere preservation and the broader body of longevity peptide research. Epitalon also increases BDNF (brain-derived neurotrophic factor) and CREB1, which connect its telomere-protective effects to neuroprotection, an area of growing interest for those researching peptides for brain repair.
The most researched stem cell peptides
Not all peptides marketed as "stem cell peptides" have meaningful evidence behind them. The ones that follow do. Each has been studied in multiple laboratories, through multiple experimental models, with findings published in indexed scientific journals. They are listed roughly in order of the breadth and depth of stem cell-related evidence available.
GHK-Cu (the stem cell activator)
GHK-Cu is a tripeptide, just three amino acids, glycine-histidine-lysine, bound to a copper ion. It occurs naturally in human plasma. At age 20, circulating levels average around 200 nanograms per milliliter. By age 60, that drops to approximately 80 nanograms per milliliter. This decline parallels the decline in regenerative capacity, wound healing speed, and skin quality that characterizes aging. The correlation is not coincidental.
The stem cell evidence for GHK-Cu is remarkably comprehensive. At the gene expression level, GHK-Cu modulates 4,192 human genes with expression changes exceeding 50 percent. That represents 31.2 percent of the entire human genome. No other peptide comes close to this breadth of gene modulation. Many of the affected genes are directly involved in stem cell maintenance, tissue repair, and anti-inflammatory signaling. GHK-Cu stimulates collagen synthesis at picomolar to nanomolar concentrations, meaning it works at extraordinarily low doses. It increased decorin production by 302 percent, which improves the structural organization of healing tissue and modulates the activity of TGF-beta, a key growth factor in stem cell differentiation.
The direct stem cell evidence is equally compelling. At concentrations between 0.1 and 10 micromolar, GHK-Cu increased the expression of epidermal stem cell markers including integrins and p63. Integrins anchor stem cells in their niches and mediate communication between cells and the extracellular matrix. P63 is a transcription factor essential for the self-renewal capacity of epithelial stem cells. Higher p63 expression means stem cells maintain their "stemness" rather than differentiating prematurely or entering senescence.
Jose and colleagues demonstrated that GHK added to mesenchymal stem cell cultures increased both VEGF and bFGF concentrations. VEGF drives angiogenesis. bFGF drives cell proliferation. Together, they create the conditions necessary for stem cells to actually do their repair work, building new blood supply and expanding the cell population at the repair site. Copper peptides also promote basal stem cell survival in skin, which has direct implications for wound healing, skin aging, and hair follicle regeneration.
For hair specifically, GHK-Cu for hair has gained significant research attention. The peptide appears to enlarge hair follicle size, stimulate hair growth, and support the stem cell populations within the follicle bulge region that are responsible for hair cycling. The connection between GHK-Cu and peptides for hair growth is one of the most practically relevant applications of stem cell peptide research.
Researchers interested in GHK-Cu protocols can find detailed information in the GHK-Cu dosage chart and injection guide. The 50mg GHK-Cu dosage guide covers the specific vial sizes commonly available from research suppliers.
Thymosin beta-4 / TB-500 (the cell mobilizer)
If GHK-Cu is the stem cell activator, TB-500 is the stem cell mobilizer. Its primary superpower is not just waking up progenitor cells, but physically moving them to where they are needed. Thymosin beta-4, the full-length protein, is a 43-amino acid naturally occurring peptide present in virtually all tissues and cell types. TB-500 is a synthetic fragment that retains the key active region responsible for cell migration, angiogenesis, and anti-inflammatory effects.
The cell migration data is striking. TB-500 increased the speed of cell migration by two to three times in multiple experimental models. In rat wound healing studies, re-epithelialization increased by 42 percent at day four and 61 percent at day seven. These are not marginal improvements. They represent a dramatic acceleration of the healing timeline. Wound closure rates up to 61 percent faster in rat models establish TB-500 as one of the most potent cell migration promoters identified in peptide research.
The cardiac regeneration research takes this further. Smart and colleagues published in Nature in 2007 that thymosin beta-4 induces adult epicardial progenitor mobilization and neovascularization. This is worth pausing on. The study showed that TB-4 could activate dormant cardiac progenitor cells in adult animals, progenitor cells that were thought to be largely inactive after development. Once activated, these cells contributed to the formation of new blood vessels in the heart. Subsequent research confirmed that TB-4 stimulates proliferation of adult rat cardiac progenitor cells, promoting their differentiation into vascular endothelial cells, coronary smooth muscle cells, and cardiomyocytes. TB-500 became the first known molecule to initiate simultaneous myocardial and vascular regeneration after systemic administration.
TB-500 also enhances proliferation of mesenchymal stem cells derived from adipose tissue. This is particularly relevant because adipose-derived MSCs are one of the most abundant and accessible stem cell populations in the adult body. By enhancing their proliferation, TB-500 expands the pool of available repair cells without requiring any external cell transplantation. For anyone researching the benefits of TB-500, the stem cell mobilization data represents perhaps its most fundamental mechanism of action. The TB-500 dosage calculator can help researchers determine appropriate amounts based on body weight and research goals.
The relationship between TB-500 and injury recovery makes it a cornerstone of many peptide stacking strategies. When combined with BPC-157, the two peptides address complementary aspects of the healing cascade, a combination so popular it has earned its own dedicated guide: the BPC-157 and TB-500 stacking guide. This stack is also central to what many researchers call the Wolverine stack, named for its association with accelerated healing.
BPC-157 (the growth factor amplifier)
BPC-157 stands for Body Protection Compound-157. It is a pentadecapeptide, fifteen amino acids, originally isolated from human gastric juice. While its direct stem cell effects are less dramatic than those of GHK-Cu or TB-500, BPC-157 plays a critical supporting role in the regenerative cascade by creating the tissue environment that stem cells need to thrive.
The VEGFR2-Akt-eNOS signaling pathway is central to how BPC-157 works. This pathway drives angiogenesis, the formation of new blood vessels. Without adequate blood supply, mobilized stem cells cannot survive at the repair site. They arrive, find no oxygen or nutrients, and die. BPC-157 prevents this by rapidly establishing new vasculature. It also activates ERK1/2 signaling, which promotes cell proliferation and survival, and engages the FAK-paxillin pathway, which is essential for fibroblast migration into wound sites. Fibroblasts are not stem cells, but they are critical partners in tissue repair, laying down the extracellular matrix scaffolding that guides stem cell differentiation.
BPC-157 also induces growth hormone receptor expression. This is a less discussed but potentially significant mechanism. By increasing the density of growth hormone receptors on cell surfaces, BPC-157 may sensitize tissues to the effects of circulating growth hormone and its downstream mediators, including IGF-1. The connection between BPC-157 and IGF peptides through this receptor upregulation represents an area of active research interest.
The most exciting recent development came from a pilot study showing that intravenous BPC-157 infusion at doses of 10mg and 20mg was well tolerated in human subjects. This was the first formal human safety data for systemic BPC-157 administration at therapeutic doses. While the study was designed to assess safety rather than efficacy, it opens the door to more rigorous clinical investigation. For those exploring BPC-157 at standard research doses, the BPC-157 5mg dosing guide and the BPC-157 dosage calculator provide detailed reference information. Standard research protocols typically involve 200 to 500 micrograms daily via subcutaneous injection.
BPC-157 has demonstrated dose-dependent migration induction in tendon fibroblasts, making it particularly relevant for tendon repair, joint pain, and shoulder injuries. The peptide interacts with leukocytes and stem cells in vivo, creating a coordinated immune and regenerative response at injury sites. Its gut-protective origins also make it a subject of intense interest in gut health peptide research, where the integrity of intestinal stem cell populations is essential for maintaining the gut lining.
Epitalon / AEDG (the telomere protector)
Epitalon is a tetrapeptide with the sequence Ala-Glu-Asp-Gly. It was developed by Professor Vladimir Khavinson at the Saint Petersburg Institute of Bioregulation and Gerontology, based on decades of research into pineal gland extracts. Its primary mechanism is telomerase activation, but its effects on stem cells extend well beyond telomere maintenance.
The telomerase data is well established. Epitalon increases telomere length via telomerase activation in multiple cell types. For stem cells specifically, this means extending their replicative lifespan, the number of times they can divide before entering senescence. A stem cell that can divide 50 more times has dramatically more regenerative potential than one approaching its Hayflick limit. This is why Epitalon is considered foundational in anti-aging peptide research.
But the neurogenic differentiation data adds another dimension. Studies on human gingival mesenchymal stem cells showed that Epitalon stimulated the expression of neurogenic differentiation markers, Nestin, GAP43, Beta Tubulin III, and Doublecortin, with mRNA expression increasing by 1.6 to 1.8 times. This suggests that Epitalon does not just extend stem cell lifespan. It may actively guide differentiation toward neural lineages. For researchers interested in brain function peptides, this finding positions Epitalon as more than a longevity peptide. It positions it as a potential neuroprotective and neuroregenerative agent.
Epitalon reduces DNA damage in neurons and increases both BDNF and CREB1. BDNF is the primary growth factor for neuronal survival and plasticity. CREB1 is a transcription factor involved in memory formation and neuronal health. The combination of telomere protection, neurogenic differentiation support, and neurotrophic factor upregulation makes Epitalon uniquely positioned at the intersection of stem cell science and brain health. The BDNF peptide guide explores this neurotrophic connection in greater detail.
The epigenetic dimension of Epitalon action involves histone interaction. By influencing chromatin structure, Epitalon can shift which genes are accessible for transcription. This is a fundamentally different mechanism than growth factor signaling or integrin activation. It represents a deeper level of cellular reprogramming, one that could theoretically influence stem cell fate decisions at the most fundamental level. The broader Khavinson peptide framework contextualizes Epitalon within a larger family of bioregulatory peptides, each targeting specific tissue types through similar epigenetic mechanisms.
PEDF-derived short peptide
Pigment epithelium-derived factor, or PEDF, is a 50-kilodalton glycoprotein with well-documented anti-angiogenic and neuroprotective properties. PEDF-derived short peptide, often abbreviated PDSP, is a smaller fragment that retains key stem cell-activating properties of the parent molecule.
PDSP promotes growth and expansion of limbal epithelial stem cells. These are the stem cells responsible for maintaining the corneal epithelium, the clear outer surface of the eye. Limbal stem cell deficiency causes progressive vision loss, making this a clinically relevant application. PDSP also promotes mesenchymal stem cell growth with potential for bone, cartilage, and muscle differentiation pathways. This broad mesenchymal activity distinguishes it from peptides like GHK-Cu or TB-500, which tend to have more specific tissue targets.
The clinical development of PDSP is further along than most stem cell peptides. It has been tested in two Phase II clinical studies involving over 300 patients. While detailed results from these trials are still emerging, the fact that PDSP has progressed to multi-hundred-patient Phase II trials places it among the most clinically validated stem cell peptide candidates. For those tracking the regulatory landscape of peptide therapeutics, the peptide regulation news section covers ongoing developments.
Other notable stem cell peptides
Beyond the primary four, several other peptides have demonstrated meaningful interactions with stem cell populations. MGF E Peptide, a fragment of Mechano Growth Factor, enhances bone marrow mesenchymal stem cell migration. This is relevant because bone marrow MSCs are among the most versatile stem cell populations in the adult body, capable of differentiating into bone, cartilage, fat, and potentially other tissue types. By enhancing their migration, MGF E Peptide could improve the delivery of these cells to distant repair sites. Researchers interested in the relationship between growth factors and peptides should review the IGF-1 LR3 guide, as IGF signaling is closely related to MGF activity.
Cerebrolysin is a neurotrophic peptide preparation containing low-molecular-weight peptides and free amino acids produced by enzymatic breakdown of purified brain proteins. It includes fragments with BDNF and NGF activity. While technically a peptide mixture rather than a single peptide, its ability to support neural stem cell survival and differentiation has been demonstrated in multiple clinical contexts, making it relevant to the stem cell peptide conversation.
Defensins, both alpha and beta variants, represent another fascinating connection between innate immunity and stem cell biology. Research has shown that defensins stimulate LGR6-positive stem cells in hair follicles, providing yet another mechanistic link between peptide signaling and tissue-specific stem cell activation. The hair loss peptide guide covers the broader landscape of peptides that interact with follicular stem cell populations.
The biomimetic peptides guide provides additional context on synthetic peptides designed to mimic the activity of natural growth factors and signaling molecules. Many biomimetic peptides are being developed specifically to target stem cell pathways, representing the next generation of stem cell peptide therapeutics. A comprehensive overview of all available peptides, including those with stem cell activity, can be found in the complete peptide list.
Stem cell peptides vs stem cell therapy
The comparison between peptide-based stem cell activation and traditional stem cell therapy is not about declaring a winner. Both approaches have legitimate applications. But understanding their differences helps researchers and clinicians make informed decisions about which approach, or combination of approaches, best serves a given goal.
Factor | Stem cell peptides | Stem cell therapy |
|---|---|---|
Mechanism | Activate, mobilize, and support endogenous stem cells | Transplant exogenous or autologous stem cells |
Cell source | Your own existing stem cell populations | Harvested from bone marrow, adipose tissue, cord blood, or donor |
Administration | Subcutaneous injection, topical, or oral (peptide-dependent) | IV infusion, direct injection at target site, or surgical implantation |
Cost per treatment | Low to moderate | High to very high ($5,000-$100,000+) |
Accessibility | Available through research suppliers | Requires specialized clinics and medical professionals |
Safety profile | Generally well tolerated in research settings | Contamination risk, immune rejection possible, tumor risk with some cell types |
Systemic vs local | Can achieve systemic effects through subcutaneous injection | Often requires targeted delivery to specific sites |
Cell survival | Not applicable, activates existing cells in their native environment | Transplanted cell survival rates can be low (10-25% in some studies) |
Repeatability | Easy to repeat and cycle | Each treatment requires new harvest or preparation |
Regulatory status | Research use, varies by jurisdiction | Heavily regulated, limited approved indications |
Combination potential | Multiple peptides can be stacked for synergistic effects | Can be combined with PRP, growth factors, or scaffolds |
Evidence base | Strong preclinical, growing clinical | Mixed clinical results depending on indication |
One of the most significant practical differences is the survival rate problem. When stem cells are harvested, processed in a laboratory, and reinjected into the body, a substantial percentage of those cells die before they can integrate into the target tissue. The environment they are injected into is often inflamed, ischemic, or otherwise hostile. Studies have reported transplanted cell survival rates as low as 10 to 25 percent in some contexts.
Stem cell peptides sidestep this problem entirely. They activate cells that are already in their native environment, already connected to local blood supply, already embedded in the appropriate extracellular matrix. There is no processing step where cells can be damaged. There is no injection into hostile territory. The cells are already there. The peptides simply tell them to start working. This is why many forward-thinking researchers view peptides not as a replacement for stem cell therapy, but as both a standalone approach and a potential adjunct. Administering stem cell peptides before, during, or after cell transplantation could theoretically improve transplanted cell survival, migration, and integration. The peptide safety guide provides important context for anyone evaluating the risk-benefit profile of peptide-based approaches.
The comparison between peptides and other research compounds is another important consideration. Unlike SARMs or anabolic agents, stem cell peptides work through signaling and gene expression modulation rather than receptor agonism. This fundamentally different mechanism explains their generally more favorable safety profile in research settings.
Stem cell peptide protocols and stacking strategies
Individual stem cell peptides are powerful. Combined strategically, they address multiple bottlenecks in the regenerative cascade simultaneously. Stacking is the practice of using two or more peptides together, either concurrently or sequentially, to achieve effects that exceed what any single peptide could produce alone. The peptide stack calculator can help researchers plan multi-peptide protocols based on their specific goals.
Understanding how many peptides you can take at once is a common starting question. The answer depends on the specific peptides involved, their mechanisms of action, and whether those mechanisms are complementary or redundant. The stacks outlined below represent commonly researched combinations based on the published mechanisms of each peptide.
Regeneration stack
This is the broadest stack, targeting the maximum number of regenerative pathways simultaneously.
GHK-Cu: Stem cell activation, gene modulation, growth factor amplification
TB-500: Cell mobilization, migration acceleration, anti-inflammatory
BPC-157: Angiogenesis, growth factor receptor upregulation, tissue protection
The logic is straightforward. GHK-Cu wakes up stem cells and shifts gene expression toward repair. TB-500 mobilizes those activated cells and moves them to injury sites. BPC-157 builds the blood vessel infrastructure they need to survive and provides growth factor support for their differentiation. Each peptide handles a different phase of the regenerative cascade. None is redundant.
Research protocols for this combination typically run for four to eight weeks. Some researchers cycle with two weeks on and one week off. The peptide cycle planning guide covers the rationale behind different cycling approaches. Dosage specifics for each peptide can be referenced through the peptide dosage chart, and the peptide calculator helps convert between different measurement units.
Longevity stack
This stack focuses less on acute repair and more on maintaining stem cell function over time.
Epitalon: Telomere maintenance, epigenetic regulation, BDNF upregulation
GHK-Cu: Gene expression optimization, stem cell marker maintenance
MOTS-c: Mitochondrial function, metabolic health, cellular energy
Epitalon protects telomere length so stem cells can continue dividing. GHK-Cu maintains the gene expression landscape that supports stem cell identity and function. MOTS-c, a mitochondrial-derived peptide, ensures that cells have the energy production capacity needed to support regenerative activity. The MOTS-c dosage chart provides specific protocol information.
This combination is often cycled over longer periods, with Epitalon administered in defined courses (commonly 10 to 20 days) separated by longer breaks, while GHK-Cu and MOTS-c may be used on different schedules. SS-31, another mitochondria-targeted peptide, is sometimes substituted for or added to MOTS-c in longevity-focused protocols. The Klotho peptide guide covers additional longevity-focused peptide options that may complement this stack.
Injury recovery stack
When the goal is recovering from a specific injury, the peptide selection and dosing strategy shifts toward acute repair.
BPC-157: Angiogenesis, growth factor signaling, tissue protection (primary)
TB-500: Cell mobilization, migration, wound healing acceleration
GHK-Cu: Local application for stem cell activation at injury site (optional)
BPC-157 and TB-500 form the core of this stack. Their mechanisms are highly complementary, which is why the BPC-157 vs TB-500 comparison is one of the most frequently searched peptide topics. The short answer is that you do not need to choose between them. They work better together. BPC-157 handles the vascular and growth factor dimension. TB-500 handles the cellular migration and mobilization dimension. GHK-Cu can be added topically for skin and soft tissue injuries or subcutaneously for deeper tissue support.
For specific injury types, additional context is available in the guides for bone healing, bone and cartilage repair, herniated disc recovery, and back pain. The Wolverine peptide concept is essentially a popularized version of this injury recovery stack, emphasizing the accelerated healing properties of the BPC-157 and TB-500 combination.
Recovery timelines vary significantly based on injury type and severity. The peptide timeline guide offers realistic expectations for when researchers typically observe measurable changes. Many researchers report noticeable improvement in healing markers within the first two weeks, though complete recovery from significant injuries may require longer protocols.
Neuroprotection stack
Neural stem cell support requires a specialized approach because the brain has unique regenerative constraints.
Epitalon: BDNF upregulation, CREB1 activation, neural stem cell differentiation support
Cerebrolysin: Neurotrophic peptide mixture with BDNF and NGF activity
Pinealon: Pineal bioregulator with neuroprotective effects
BPC-157: Neuroprotection, dopamine system regulation
Epitalon provides the differentiation support data from the gingival MSC studies, showing increased expression of Nestin, GAP43, Beta Tubulin III, and Doublecortin. Cerebrolysin delivers neurotrophic support directly. Pinealon targets the pineal region specifically. BPC-157 adds systemic neuroprotection and has demonstrated effects on the dopaminergic system. This stack is particularly relevant for researchers exploring neuropathy and depression and anxiety.
The broader landscape of organ-specific bioregulator peptides can complement any of these stacks. Cardiogen targets cardiac tissue. Chonluten targets respiratory mucosa. Livagen targets hepatic tissue. Vesugen targets vascular tissue. Ventfort provides additional vascular support. Bronchogen targets bronchial tissue. Vilon provides immune modulation. And Pancragen targets pancreatic function. Each of these can be layered onto a base stem cell stack to provide tissue-specific support.
How to prepare and administer stem cell peptides
The gap between understanding stem cell peptide science and actually working with these compounds is where many researchers get stuck. Preparation, storage, and administration all require attention to detail. Peptides are fragile molecules. Handle them incorrectly and you end up with expensive vials of degraded amino acids.
Reconstitution
Most stem cell peptides arrive as lyophilized powder, a freeze-dried form that remains stable for extended periods when stored properly. Before use, this powder must be reconstituted with an appropriate solvent, almost always bacteriostatic water.
The reconstitution process involves several critical steps. First, allow both the peptide vial and the bacteriostatic water to reach room temperature. Cold solvent added to cold powder can cause clumping and incomplete dissolution. Next, use a sterile syringe to draw the desired amount of bacteriostatic water. Insert the needle into the vial at an angle and let the water run down the side of the glass rather than spraying it directly onto the powder. Peptides can be damaged by the physical force of direct water impact, a phenomenon called shear degradation.
Once the water is added, do not shake the vial. Gentle swirling is acceptable. Some researchers simply place the vial in the refrigerator and allow it to dissolve over 30 to 60 minutes. The result should be a clear, colorless solution. If the solution is cloudy or contains visible particles, the peptide may have been damaged during reconstitution or may have been degraded before you received it.
The detailed process for each step is covered in the peptide reconstitution guide. The bacteriostatic water mixing guide addresses the specific technique for combining solvent and peptide. One of the most common questions is how much bacteriostatic water to add, and the answer depends on the peptide amount and the desired concentration per unit volume. The peptide reconstitution calculator removes the guesswork by computing exact volumes based on your specific vial size and target concentration.
Bacteriostatic water contains 0.9 percent benzyl alcohol, which acts as a preservative and prevents bacterial growth in the reconstituted solution. Using sterile water without this preservative is possible for single-use applications, but reconstituted peptides stored for multiple uses require bacteriostatic water. More information on the role of the solvent is available in the bacteriostatic water guide.
Injection technique
The most common administration route for stem cell peptides in research settings is subcutaneous injection. This involves injecting into the fat layer beneath the skin, typically in the abdominal area, thigh, or upper arm. Subcutaneous injection provides slower, more sustained absorption compared to intramuscular or intravenous routes, which is generally preferred for peptides that benefit from extended exposure.
The peptide injection overview covers the fundamentals, while the detailed peptide injections guide walks through site selection, needle gauge, angle, and technique step by step. Some researchers prefer peptide injection pens for more consistent dosing and easier administration.
Not all stem cell peptides require injection. The injectable vs oral peptide comparison outlines which delivery routes are appropriate for different peptide types. Some peptides, particularly smaller ones like the bioregulators, are available in capsule form. Others can be administered via nasal spray, which provides direct access to the central nervous system for neuroactive peptides. The choice of delivery route depends on the specific peptide, the target tissue, and the research protocol being followed.
Storage
Proper storage is essential for maintaining peptide integrity. Unreconstituted lyophilized peptides should be stored in a freezer, ideally at minus 20 degrees Celsius. At this temperature, most peptides remain stable for years. The powder form stability guide provides specific degradation data for different storage conditions.
Once reconstituted, peptides must be refrigerated at 2 to 8 degrees Celsius and used within a defined timeframe. Most reconstituted peptides remain viable for three to four weeks under proper refrigeration. Some degrade faster. The refrigerated peptide stability guide and the comprehensive peptide storage guide cover temperature requirements, light sensitivity, and other factors that affect stability. The question of how to store peptides after reconstitution is one of the most important practical considerations for anyone working with these compounds.
Avoid repeated freeze-thaw cycles with reconstituted peptides. Each cycle damages a percentage of the molecules through ice crystal formation and protein denaturation. If you need to store reconstituted peptide for extended periods, consider aliquoting the solution into single-use volumes before freezing. Also avoid exposing peptide solutions to direct light, especially GHK-Cu, where the copper ion can participate in photochemical reactions that degrade the peptide. Questions about peptide expiration are common, and the answer is yes, peptides do degrade over time, but proper storage dramatically extends their useful life.
The peptide cost calculator can help researchers plan budgets by estimating the total cost of a multi-peptide protocol over a defined period, accounting for vial sizes and dosing frequency.
Safety, side effects, and what the research actually shows
Stem cell peptides generally demonstrate favorable safety profiles in published research, but "generally favorable" is not the same as "zero risk." Honest evaluation of the evidence requires acknowledging both what we know and what we do not know.
GHK-Cu has an extensive safety record. It occurs naturally in human blood, so the body has existing mechanisms for metabolizing and clearing it. Topical GHK-Cu has been used in cosmetic products for decades without significant adverse event reports. Injected GHK-Cu is less extensively studied in humans, but the combination of its natural occurrence, its low effective concentration (picomolar to nanomolar), and its well-characterized metabolic pathway provides a reasonable basis for its safety profile. The copper component requires mention. Excessive copper intake can cause toxicity, including liver damage. However, the amounts of copper delivered by typical GHK-Cu research protocols are orders of magnitude below toxic thresholds.
TB-500 has been studied extensively in veterinary medicine, particularly in equine applications, and more recently in preclinical models. The wound healing and cardiac regeneration studies report no significant adverse effects at research doses. The peptide is well tolerated at the concentrations used in published protocols. However, its ability to promote cell proliferation and angiogenesis raises theoretical questions about whether it could promote the growth of existing tumors. No published evidence supports this concern, but it remains a theoretical consideration that researchers should acknowledge.
BPC-157 now has the most encouraging human safety data following the pilot study showing that IV infusion at 10mg and 20mg was well tolerated. Prior to this, BPC-157 safety data came primarily from animal models, where it has been studied at doses ranging from micrograms to milligrams per kilogram without reported toxicity. Common reported side effects in anecdotal research reports include injection site reactions, mild headache, and transient nausea, all typically mild and self-resolving.
Epitalon has been studied in Russian clinical settings for several decades, with safety data published primarily in Russian-language journals. The available data shows a favorable safety profile with no significant adverse effects reported at standard research doses. Its mechanism of telomerase activation raises the same theoretical cancer concern as TB-500, since telomerase is active in most cancer cells. However, it is important to note that telomerase activation in normal cells is not the same as the dysregulated telomerase activity seen in malignant cells. Epitalon increases telomerase activity in normal cells to maintain healthy telomere length, not to enable unlimited replication.
For a comprehensive overview of peptide safety considerations, the peptide safety and risks guide covers contraindications, interactions, and risk mitigation strategies. The question of whether peptides show up on drug tests is relevant for athletes and others subject to testing. The peptide legality guide addresses the regulatory framework in different jurisdictions.
Source quality matters enormously. Peptides from unreliable suppliers may contain impurities, incorrect sequences, or degradation products that can cause adverse reactions unrelated to the peptide itself. The peptide vendor guide and testing lab guide cover how to verify peptide purity and authenticity. The difference between research and pharmaceutical grade peptides is also worth understanding when evaluating safety. Third-party testing with certificates of analysis showing purity above 98 percent is the minimum standard serious researchers should require. Understanding the difference between lyophilized and liquid peptides also affects stability and safety considerations.
Who should consider stem cell peptides
Stem cell peptides are not for everyone. They are most relevant for specific populations with specific goals. Here is an honest assessment of who stands to benefit most from understanding and potentially researching these compounds.
Individuals recovering from injuries represent the most straightforward use case. When ligaments, tendons, muscles, or bones are damaged, the body repair process depends on stem cell activation, migration, and differentiation. Peptides that enhance these processes can theoretically accelerate healing timelines. The research on TB-500 wound healing (42 to 61 percent faster re-epithelialization) and BPC-157 angiogenesis suggests meaningful potential for injury recovery. Specific injury types covered in dedicated guides include tendon repair, joint pain, shoulder pain, back pain, and herniated disc recovery.
Adults over 40 experiencing age-related decline are another key demographic. The natural drop in GHK-Cu levels from 200 ng/ml at age 20 to 80 ng/ml by age 60 correlates with declining wound healing speed, skin quality, hair density, and overall regenerative capacity. Replenishing peptide signals that have diminished with age is a logical strategy for maintaining function. Women over 40 face additional considerations related to hormonal changes that affect stem cell function. The broader anti-aging peptide landscape addresses multiple aspects of age-related decline.
Athletes and active individuals who push their bodies hard create ongoing microdamage that requires efficient repair. Stem cell peptides may support the recovery process between training sessions, potentially reducing injury risk and improving adaptation. The connection between peptides and athletic performance extends beyond stem cell activation to include muscle growth, inflammation management, and recovery optimization.
Those interested in longevity and healthspan represent a growing demographic. Telomere shortening is one of the hallmarks of aging, and Epitalon telomerase activation directly addresses this mechanism. Combined with GHK-Cu gene modulation and mitochondria-targeted peptides like MOTS-c, a longevity-focused stem cell peptide protocol represents a multi-pathway approach to slowing biological aging. The longevity peptide guide provides the broader context.
People with autoimmune conditions may also find stem cell peptide research relevant, as several of these peptides demonstrate immunomodulatory properties alongside their regenerative effects. The autoimmune peptide guide and immune system peptide guide explore this dimension in detail. KPV, while not primarily classified as a stem cell peptide, adds powerful anti-inflammatory effects that can complement a stem cell-focused protocol, and the KPV inflammation guide covers this mechanism.
Gender-specific considerations exist. Safe peptides for women require attention to hormonal interactions and reproductive considerations. Peptides for men often focus on the intersection of stem cell activation with testosterone optimization and testosterone support. Hormone balance peptides address the broader endocrine context. Women navigating menopause may find stem cell peptides particularly relevant as declining estrogen levels affect stem cell function in multiple tissues, including bone, where osteoporosis risk increases significantly.
Who should NOT consider stem cell peptides? Anyone with an active cancer diagnosis should avoid peptides that promote cell proliferation, angiogenesis, or telomerase activation until more safety data is available for this population. Pregnant or breastfeeding individuals should avoid these compounds due to insufficient safety data. And anyone who is not willing to invest the time to learn proper preparation, storage, and administration should not work with injectable peptides. SeekPeptides provides the educational foundation that every responsible researcher needs before working with these compounds.
Building your knowledge foundation
The stem cell peptide field is moving quickly. New studies are published regularly, new peptides are identified, and the understanding of existing peptides deepens with each passing year. Staying current requires both foundational knowledge and ongoing learning.
For those just beginning their peptide education, the getting started with peptides guide provides a structured introduction. The what are peptides overview covers fundamental concepts. And the how peptides work guide explains the basic mechanisms that apply across all peptide classes.
The peptide dosing guide is essential reading for understanding how dosage relates to effect. The relationship between peptide cycling strategies and the results researchers observe is covered in its own dedicated guide. And for those who want to see what others have experienced, the peptides before and after compilation provides real-world context.
SeekPeptides members access detailed protocols, dosage references, stacking strategies, and safety information that goes well beyond what any single blog post can cover. The membership is designed for serious researchers who want comprehensive, evidence-based peptide education in one place. If stem cell peptides represent the future of accessible regenerative medicine, and the research increasingly suggests they do, then having a thorough understanding of the science is not optional. It is the price of admission.
Additional resources for specific areas of interest include skin tightening peptides, wrinkle reduction peptides, glow peptides, and the glow stack for skin-focused applications. The weight loss peptide guide, fat burning peptide guide, and fat loss resource page cover metabolic applications. And for those interested in muscle-specific stem cell activation, the best peptides for muscle growth and safest muscle growth peptides provide focused analysis. The ACE-031 myostatin inhibitor guide and Penta Deca Arginate guide cover additional peptides relevant to musculoskeletal regeneration.
Growth hormone secretagogue peptides like Ipamorelin, Sermorelin, CJC-1295, and GHRP-6 are not classified as stem cell peptides per se, but they increase growth hormone output, which in turn supports stem cell function through IGF-1 mediated pathways. The Ipamorelin vs CJC-1295 comparison, the CJC-1295 dosage calculator, the Semaglutide dosage calculator, and the HGH Fragment 176-191 calculator provide tools for researchers working with these related compound classes. The testosterone peptide guide connects growth hormone support with androgenic optimization.
The peptide vial research guide, the list of injectable peptides, and the Klow peptides overview round out the practical resources available on SeekPeptides.
Frequently asked questions
What are stem cell peptides and how do they work?
Stem cell peptides are short-chain amino acid sequences that interact with stem cells and progenitor cells through specific molecular mechanisms. They do not introduce new stem cells into the body. Instead, they activate, mobilize, and support the stem cells you already have. Different peptides work through different pathways. GHK-Cu modulates gene expression in over 4,000 genes and increases stem cell marker expression. TB-500 accelerates cell migration by two to three times and mobilizes cardiac progenitor cells. BPC-157 promotes angiogenesis through VEGFR2 signaling and creates the vascular environment stem cells need. Epitalon activates telomerase to extend stem cell replicative lifespan. When combined, these peptides address multiple bottlenecks in the regeneration process simultaneously.
Can peptides actually replace stem cell therapy?
Peptides and stem cell therapy address regeneration through fundamentally different mechanisms, and one does not necessarily replace the other. Stem cell therapy transplants cells from an external source. Peptides optimize the function of your existing stem cells. For many applications, peptides may be sufficient on their own, particularly for age-related regenerative decline, injury recovery, and general tissue maintenance. For severe conditions requiring large numbers of new cells, such as extensive tissue damage or degenerative diseases, stem cell therapy may still be necessary. The most promising approach may ultimately be combining both, using peptides to improve the survival, migration, and integration of transplanted stem cells.
Which stem cell peptide is the most effective?
No single stem cell peptide is universally "the most effective" because each targets different mechanisms. GHK-Cu has the broadest gene modulation profile, affecting 4,192 genes. TB-500 has the strongest cell migration data, increasing migration speed by two to three times. BPC-157 has the most robust angiogenesis evidence and the most encouraging human safety data. Epitalon has the unique ability to activate telomerase and extend stem cell replicative lifespan. The most effective approach for most research goals involves combining two or more of these peptides to address multiple regenerative pathways. The best single peptide depends entirely on the specific goal. For injury healing, TB-500 and BPC-157 are most directly relevant. For longevity, Epitalon and GHK-Cu. For comprehensive regeneration, a multi-peptide stack.
Are stem cell peptides safe?
Published research on the primary stem cell peptides (GHK-Cu, TB-500, BPC-157, Epitalon) generally reports favorable safety profiles at research doses. GHK-Cu occurs naturally in human blood, providing a baseline safety argument. BPC-157 has the most recent human safety data, with a pilot study demonstrating tolerability at IV doses of 10mg and 20mg. The main theoretical concern with stem cell-activating peptides is whether promoting cell proliferation, angiogenesis, or telomerase activation could promote tumor growth. No published evidence supports this concern, but it remains a theoretical consideration, particularly for individuals with active malignancies. Source quality is a significant practical safety factor. Impure peptides can contain contaminants that cause adverse reactions unrelated to the peptide itself. Third-party testing and certificates of analysis are essential.
How long do stem cell peptides take to show results?
Timelines vary by peptide, dosage, and research goal. GHK-Cu gene expression changes occur within hours at the cellular level, but visible tissue-level effects like improved skin quality or hair changes may take four to twelve weeks to become apparent. TB-500 wound healing acceleration was measurable within four days in rat models, with 42 percent faster re-epithelialization. BPC-157 angiogenesis can begin within days. Epitalon telomere effects develop over the course of a treatment cycle, typically 10 to 20 days, with cumulative benefits observed over multiple cycles. For injury recovery, many researchers report noticeable improvement within two to four weeks. For longevity goals, meaningful biomarker changes may require months of consistent use across multiple cycles.
Can you stack stem cell peptides with growth hormone peptides?
Yes, and many researchers do. Growth hormone secretagogues like Ipamorelin, CJC-1295, and Sermorelin increase circulating growth hormone levels, which in turn raises IGF-1. IGF-1 is itself a growth factor that supports stem cell proliferation and differentiation. Adding growth hormone peptides to a stem cell peptide stack can amplify the regenerative stimulus by increasing the systemic growth factor environment. The typical approach is to use growth hormone secretagogues as a base layer and add specific stem cell peptides on top for targeted regenerative support. The key consideration is managing the total number of peptides to avoid unnecessary complexity and ensure each component is serving a distinct purpose.
What is the best way to start with stem cell peptides?
Start with one peptide at a time. This allows you to establish a baseline, observe individual effects, and rule out adverse reactions before adding complexity. BPC-157 is often recommended as a starting point because it has the most human safety data, works through well-understood mechanisms, and provides broad tissue-protective effects. Once comfortable with one peptide, add a second with a complementary mechanism. TB-500 is the most common addition to BPC-157 because the two address different aspects of the healing cascade. GHK-Cu and Epitalon can be added later for longer-term stem cell support and longevity goals. The getting started guide provides a structured framework for beginning peptide research responsibly.
Do stem cell peptides work for older adults?
Older adults may actually be the population most likely to benefit from stem cell peptides. The decline in GHK-Cu levels from 200 ng/ml at age 20 to 80 ng/ml by age 60 demonstrates that the body natural peptide signaling diminishes with age. Stem cells themselves do not disappear entirely in older adults. They become less active, less mobile, and more prone to senescence, largely because the signals telling them to work have weakened. Replenishing those signals through exogenous peptide administration addresses the root cause of age-related regenerative decline rather than just treating symptoms. Epitalon telomerase activation is particularly relevant for older adults, as accumulated telomere shortening is one of the primary factors limiting stem cell function in aging. Combined with GHK-Cu gene modulation, an age-appropriate stem cell peptide protocol targets the molecular mechanisms of regenerative aging directly.
External resources
PubMed - National Library of Medicine - Search for peer-reviewed research on GHK-Cu, thymosin beta-4, BPC-157, and Epitalon using the MeSH database
Nature Journal - Home to the landmark Smart et al. (2007) and Bock-Marquette et al. (2004) studies on thymosin beta-4 cardiac regeneration
ClinicalTrials.gov - Search for active and completed clinical trials involving stem cell peptides, including PEDF-derived peptide Phase II studies
Stem cell peptides represent one of the most accessible entry points into regenerative medicine. The research is real. The mechanisms are well characterized. The practical application is within reach of any serious researcher willing to invest the time in understanding the science. But knowledge without structure is just noise. SeekPeptides membership gives you the structured protocols, dosage references, safety frameworks, and ongoing education that transform scattered research into actionable understanding. The peptides are the tools. The knowledge is what makes them work.



