Feb 1, 2026
What is cerebrolysin and why does it matter for brain health?
Most neuroprotective compounds target one pathway. One receptor. One mechanism. Cerebrolysin targets dozens simultaneously. That distinction matters more than most researchers realize, and it explains why this porcine-derived peptide complex has accumulated over 200 clinical trials across more than 50 countries since its introduction in the 1970s.
Cerebrolysin is not a single peptide. It is a complex mixture of low-molecular-weight neuropeptides and free amino acids, enzymatically prepared from purified pig brain proteins. The peptide fragments are all below 10 kilodaltons in size, which allows them to cross the blood-brain barrier, a feat that most neurotrophic factors cannot accomplish on their own. This is the core advantage. Understanding how peptides work at the molecular level helps explain why size and bioavailability matter so much in neurological applications.
The composition includes fragments that mimic the activity of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CNTF). These are not trace amounts. The peptide mixture actively stimulates the production of endogenous neurotrophic factors while also providing direct neurotrophic support. Think of it as both supplying growth factors and telling the brain to make more of its own.
Why does this matter? Because neurological conditions rarely involve a single broken mechanism. Stroke damages through excitotoxicity, oxidative stress, inflammation, and apoptosis simultaneously. Traumatic brain injury triggers cascading failures across multiple cellular systems. Peptides that support brain function need to address this complexity, and cerebrolysin does exactly that through what researchers call a pleiotropic mechanism of action.
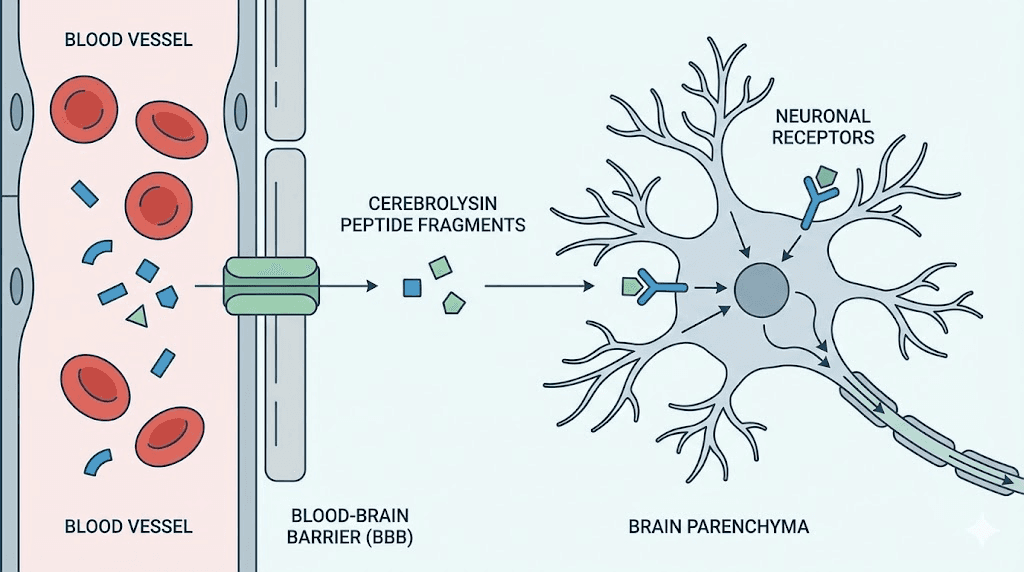
Approved in over 45 countries for conditions including ischemic stroke, hemorrhagic stroke, traumatic brain injury, and various forms of dementia, cerebrolysin remains one of the most extensively researched neuropeptide preparations in clinical medicine.
It is not, however, approved by the FDA in the United States. That regulatory distinction is important context for anyone evaluating this compound for research purposes. The clinical evidence base is substantial, with trials involving more than 15,000 patients across multiple neurological conditions.
For researchers exploring nootropic peptides, cerebrolysin occupies a unique position. It is neither a simple cognitive enhancer nor a narrow-spectrum therapeutic. It sits at the intersection of neuroprotection, neurorecovery, and cognitive enhancement, with data supporting applications across that entire spectrum. The breadth of research, combined with its multimodal mechanism, makes it one of the most compelling neuropeptide preparations available for study.
The neurotrophic factor cascade: how cerebrolysin works at the cellular level
Single-target drugs hit one lock with one key. Cerebrolysin brings an entire keyring.
The mechanism of action centers on modulating multiple neurotrophic factors simultaneously. This is not a theoretical advantage. It is a measurable, documented effect that distinguishes cerebrolysin from virtually every other neuroprotective compound studied in clinical trials. Researchers investigating peptide research and clinical studies consistently find that multimodal compounds outperform single-target interventions in complex neurological conditions.
BDNF upregulation and neuronal survival
Brain-derived neurotrophic factor is arguably the most important growth factor for neuronal health. It promotes neuronal survival, encourages the growth of new neurons and synapses, and supports learning and memory formation. Low BDNF levels are associated with depression, cognitive decline, Alzheimer disease, and numerous other neurological conditions.
Cerebrolysin increases BDNF through two distinct pathways. First, it increases the concentration of furin, a protease enzyme that processes pro-BDNF into its mature, active form. More furin means more functional BDNF available for neuronal signaling. Second, cerebrolysin inhibits glycogen synthase kinase-3 beta (GSK3beta), an enzyme whose overactivity suppresses BDNF production and promotes tau phosphorylation, a hallmark of Alzheimer pathology.
The BDNF increase is not just a laboratory finding. In clinical studies of Alzheimer patients, cerebrolysin treatment significantly increased serum BDNF levels by week 16. When combined with donepezil, the BDNF increase was even more pronounced and sustained through the end of the study period. Notably, patients carrying the apolipoprotein E epsilon-4 allele, the strongest genetic risk factor for Alzheimer disease, showed the highest BDNF responses to cerebrolysin treatment.
Elevated BDNF activates the TrkB receptor pathway, which triggers anti-apoptotic signaling cascades. In simpler terms, it tells neurons to survive rather than self-destruct. This is particularly relevant after tissue injury, when damaged neurons are deciding between repair and programmed cell death. The researchers studying BDNF peptide mechanisms have found this survival signaling to be one of the most clinically relevant effects of neurotrophic factor modulation.
NGF system modulation and aging brains
Nerve growth factor plays a critical role in maintaining cholinergic neurons, the same neurons that deteriorate in Alzheimer disease and contribute to age-related cognitive decline. Cerebrolysin modulates the NGF system in ways that directly counteract the neurochemical changes associated with aging.
In aging rat brain studies, cerebrolysin restored the balance of NGF receptors in the neocortex. Specifically, it normalized TrkA receptor expression (the "good" NGF receptor that promotes neuronal survival) and p75NTR receptor expression (which can promote either survival or death depending on context). It also decreased levels of proNGF, the precursor form that can trigger apoptosis when it binds to p75NTR without adequate TrkA signaling.
The practical significance: aging brains accumulate proNGF while losing TrkA receptors, creating a biochemical environment that favors neuronal death over survival. Cerebrolysin reverses this shift. The researchers described these effects as "pro-neuroplastic" and "anti-aging" in their impact on neocortical function.
Beyond BDNF and NGF: the full neurotrophic profile
The multimodal nature extends beyond these two factors. Cerebrolysin simultaneously upregulates vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1), both essential for anti-aging and neuroplasticity. VEGF promotes angiogenesis, the formation of new blood vessels, which is critical for delivering oxygen and nutrients to recovering brain tissue. IGF-1 supports neuronal growth and myelination.
At the same time, cerebrolysin downregulates tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory cytokine that drives neuroinflammation. This dual action, boosting growth factors while suppressing inflammatory signals, creates a neurochemical environment that strongly favors repair over damage.
Perhaps most remarkably, cerebrolysin shifts microglial phenotype from the pro-inflammatory M1 state to the anti-inflammatory M2 state. Microglia are the brain immune cells, and their activation state determines whether they contribute to damage or facilitate repair. M1 microglia release inflammatory mediators that can worsen neuronal injury. M2 microglia promote extracellular matrix deposition and angiogenesis, both essential for tissue repair. This microglial shift represents one of the most therapeutically relevant mechanisms in cerebrolysin research.
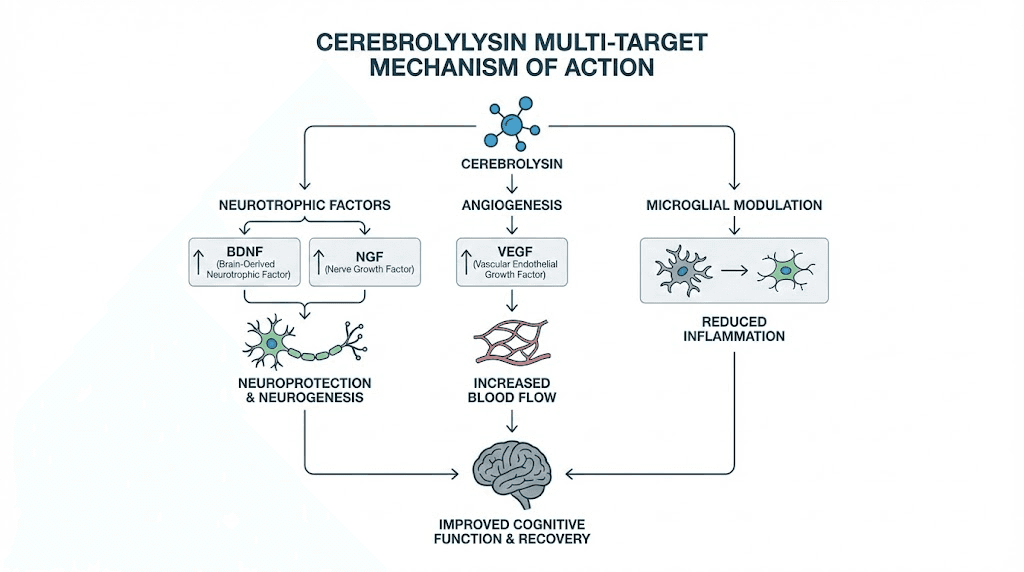
The following table summarizes the key molecular targets:
Target | Direction | Effect | Clinical relevance |
|---|---|---|---|
BDNF | Upregulated | Neuronal survival, synaptic plasticity | Memory, cognition, neuroprotection |
NGF | Modulated | Cholinergic neuron maintenance | Alzheimer disease, age-related decline |
VEGF | Upregulated | Angiogenesis | Post-stroke recovery, tissue repair |
IGF-1 | Upregulated | Neuronal growth, myelination | Neuroplasticity, recovery |
TNF-alpha | Downregulated | Reduced neuroinflammation | Neuroprotection, damage limitation |
GSK3beta | Inhibited | Reduced tau phosphorylation | Alzheimer pathology reduction |
Microglia | M1 to M2 shift | Anti-inflammatory phenotype | Brain tissue repair facilitation |
This simultaneous multi-target activity is why researchers studying longevity peptides and anti-inflammatory peptides frequently reference cerebrolysin as a benchmark compound. No single-target neuroprotective agent has demonstrated comparable breadth of mechanism.
Cerebrolysin for cognitive enhancement and memory
The brain does not decline in one way. It declines in many ways simultaneously. Memory falters. Processing speed drops. Attention fragments. Executive function erodes. Any compound that claims to support cognition needs to address this multifaceted reality, not just boost one measure while ignoring others.
Clinical trials of cerebrolysin in cognitive enhancement reveal a nuanced picture. The effects are real. They are statistically significant. They are also modest compared to what some promotional materials suggest.
Evidence from Alzheimer disease trials
A meta-analysis of randomized controlled clinical trials in mild-to-moderate Alzheimer disease found that cerebrolysin was significantly more effective than placebo across multiple outcome measures. At 4 weeks, cognitive function scores improved with a standardized mean difference of -0.40 points (p = 0.0031). Global clinical change showed improvement at both 4 weeks (odds ratio 3.32, p = 0.0212) and 6 months (odds ratio 4.98, p = 0.0150).
What do these numbers mean in practice? The cognitive improvements are real but modest. The Alzheimer Drug Discovery Foundation rated cerebrolysin as potentially useful, noting that while results from several clinical trials suggest small improvements to symptoms of Alzheimer disease and vascular dementia, the effects are generally lower than those of currently approved drugs like donepezil or memantine.
However, the combination data tells a more compelling story. When cerebrolysin (10 ml) was combined with donepezil (10 mg) in a randomized, double-blind trial, cognitive performance improved across all groups, but the combination therapy produced the best scores at every study visit. The mean ADAS-cog improvement was -2.3 points for combination therapy versus -1.7 for cerebrolysin alone and -1.2 for donepezil alone. Cerebrolysin was as effective as donepezil as a monotherapy, and the combination was safe and well-tolerated.
A comprehensive 30-year review published in Medicinal Research Reviews confirmed that cerebrolysin is safe and efficacious in Alzheimer treatment, with potential to enhance and prolong the efficacy of cholinergic drugs, particularly in moderate to advanced cases. This synergistic potential with existing medications represents one of cerebrolysin most practical clinical applications.
Vascular dementia results
A 24-week randomized, double-blind, placebo-controlled trial evaluated cerebrolysin in patients with vascular dementia. The cerebrolysin group showed statistically significant improvements across multiple measures: the Sandoz Clinical Assessment Geriatric scale, self-assessment scores, cognitive performance measures, and concentration testing for elderly patients.
Vascular dementia involves reduced blood flow to the brain, causing progressive cognitive decline. The fact that cerebrolysin, which promotes angiogenesis through VEGF upregulation, shows benefit in this specific condition aligns with its known mechanisms. Researchers investigating anti-aging peptide applications have noted that vascular health is often the bottleneck for brain health in aging populations.
Cognitive enhancement in healthy aging
Here is an honest assessment of the evidence gap: most cerebrolysin cognitive trials have been conducted in dementia populations, not healthy adults. The Alzheimer Drug Discovery Foundation notes that no studies have tested whether cerebrolysin can prevent dementia in healthy individuals, and limited evidence exists for cognitive enhancement in people without existing neurological conditions.
This matters because many people interested in cerebrolysin are seeking nootropic benefits without having a diagnosed condition. The mechanism of action, particularly BDNF upregulation, neuroplasticity enhancement, and microglial optimization, is theoretically supportive of cognitive enhancement in healthy brains. But theoretical mechanisms and clinical proof are different things.
What the existing research does suggest: cerebrolysin enhances cognitive processing speed, improves memory formation, and increases neuronal survival across multiple pathological conditions.
Whether these benefits translate proportionally to healthy brains remains an active research question.
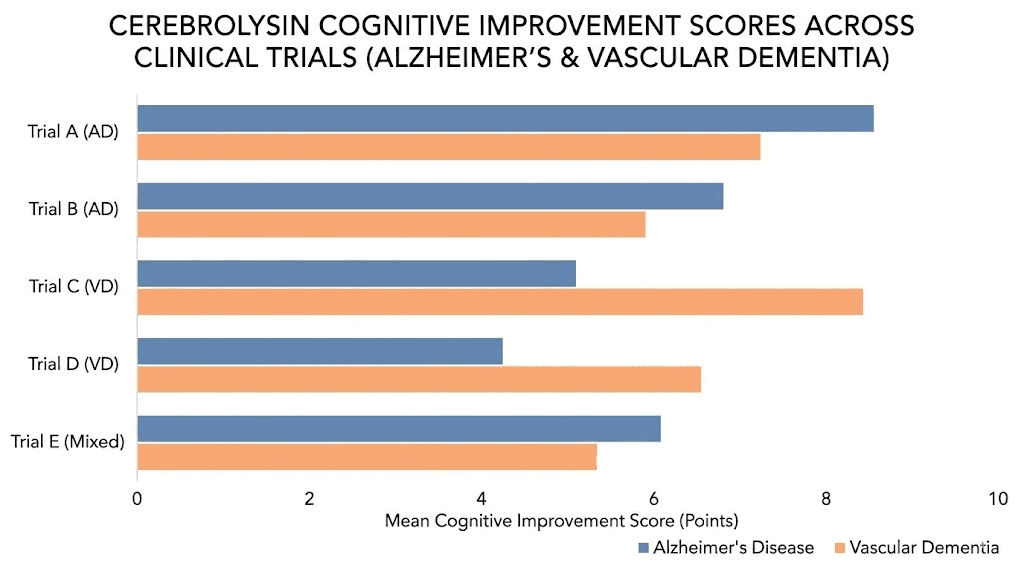
Neuroprotective benefits: stroke, TBI, and brain injury recovery
If cognitive enhancement is where cerebrolysin interest starts for many researchers, neuroprotection is where the strongest clinical evidence exists. The compound has been studied extensively in stroke recovery and traumatic brain injury, with results that range from encouraging to genuinely impressive depending on the specific condition and severity.
Stroke recovery: what the clinical trials actually show
The stroke data is complicated. It is important to present it honestly.
The largest stroke trial, CASTA (Cerebrolysin in Acute Stroke in Asia), enrolled 1,070 patients with acute ischemic hemispheric stroke. Patients received either 30 ml cerebrolysin daily or placebo (saline) as intravenous infusion for 10 days, in addition to aspirin. The primary endpoint showed no significant difference between cerebrolysin and placebo groups for the overall population.
That sounds like a failure. It is not the full picture.
The subgroup analysis revealed a noteworthy advantage for patients in the cerebrolysin group who had severe strokes (baseline NIHSS score above 12 points). This finding suggests that cerebrolysin may provide meaningful benefit in more severe cases where the brain has greater repair needs, precisely the situation where multimodal neurotrophic support would be most valuable.
The CARS trial (Cerebrolysin and Recovery After Stroke) painted a more positive picture. This prospective, randomized, double-blind, placebo-controlled, multicenter study treated patients with 30 ml cerebrolysin daily for 21 days, beginning 24 to 72 hours after stroke onset, alongside standardized rehabilitation. The results showed large superiority of cerebrolysin on the Action Research Arm Test score at day 90 (Mann-Whitney estimator 0.71, p less than 0.0001), indicating meaningful improvement in motor recovery.
A dose comparison study found that 50 ml daily for 10 days produced better 30-day neurological scores than 10 ml daily, suggesting a dose-response relationship. Animal studies support this, with effective doses in rats being considerably higher on a weight basis than typical human doses, raising the possibility that some clinical trials may have used subtherapeutic dosing.
For researchers studying tissue repair peptides and injury healing protocols, the stroke data illustrates a recurring theme in neuroprotection research: the brain injured state that benefits most from intervention is often the most severe, where the largest gap exists between damaged tissue and repair capacity.
Traumatic brain injury: the strongest clinical evidence
TBI is where cerebrolysin clinical data is most consistently positive.
A systematic review and meta-analysis of cerebrolysin in TBI patients found that intravenous administration after injury was associated with improved functional recovery across mild, moderate, and severe TBI grades. The functional outcomes measured included the Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS), both standard measures of neurological recovery.
In severe TBI, a multi-center retrospective study of 87 patients found that cerebrolysin-treated patients showed faster recovery rates, more significant improvement in GCS/GOS scores, and shorter hospital stays compared to standard treatment alone. The dosing protocol used 30 ml per day for 14 days, followed by 10 ml per day for another 14 days. All cerebrolysin-treated patients with cerebral contusion and diffuse axonal injury showed favorable GCS at day 21.
For mild TBI, a double-blinded, placebo-controlled randomized phase II study found that the cerebrolysin group showed significantly greater cognitive improvement than placebo by week 12. The CASI score (Cognitive Abilities Screening Instrument) difference was 21.0 points for cerebrolysin versus 7.6 points for placebo. Drawing function and long-term memory showed the most significant improvements.
A cohort study of moderate and severe head injury patients found that 67% of the cerebrolysin group achieved good outcomes (GOS 3-5) at 6 months, compared favorably to historical controls matched for age, sex, and initial severity.
The mechanisms behind these TBI benefits are well-characterized in animal studies. Cerebrolysin reduces brain edema by alleviating blood-brain barrier permeability and upregulating tight junction protein (ZO-1) levels. It decreases inflammatory cytokines TNF-alpha, IL-1beta, IL-6, and NF-kappaB. It inhibits neuroinflammation through TLR signaling pathway modulation and prevents apoptotic cell death.
The evidence has been strong enough to earn cerebrolysin recognition in evidence-based TBI treatment guidelines, including the INCOG guidelines for attention deficits. According to the manufacturer, cerebrolysin is described as the sole pharmacological agent supported by evidence for brain trauma recovery in certain guidelines.
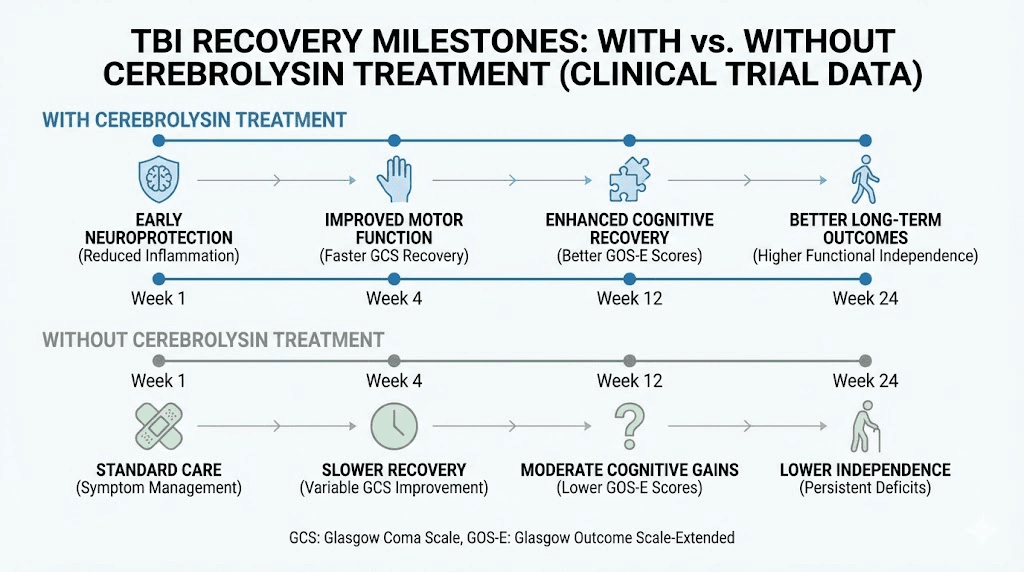
Other neurological applications
Preliminary research has explored cerebrolysin in several additional neurological conditions:
Subarachnoid hemorrhage: A pilot study of 50 patients receiving 30 ml per day for 14 days showed promising signals for improved neurological outcomes, though larger trials are needed.
Cerebral palsy in infants: A randomized controlled trial of 158 infants (6-21 months) with perinatal brain insult tested twice-weekly injections at 0.1 ml per kg body weight for 5 weeks. The cerebrolysin group showed improvement in communication deficits.
Multiple sclerosis, spinal cord injury, schizophrenia: Early studies have suggested potential applications across these conditions, though research remains preliminary. The use of peptides for autoimmune conditions is an expanding area of investigation where cerebrolysin multimodal anti-inflammatory properties may prove relevant.
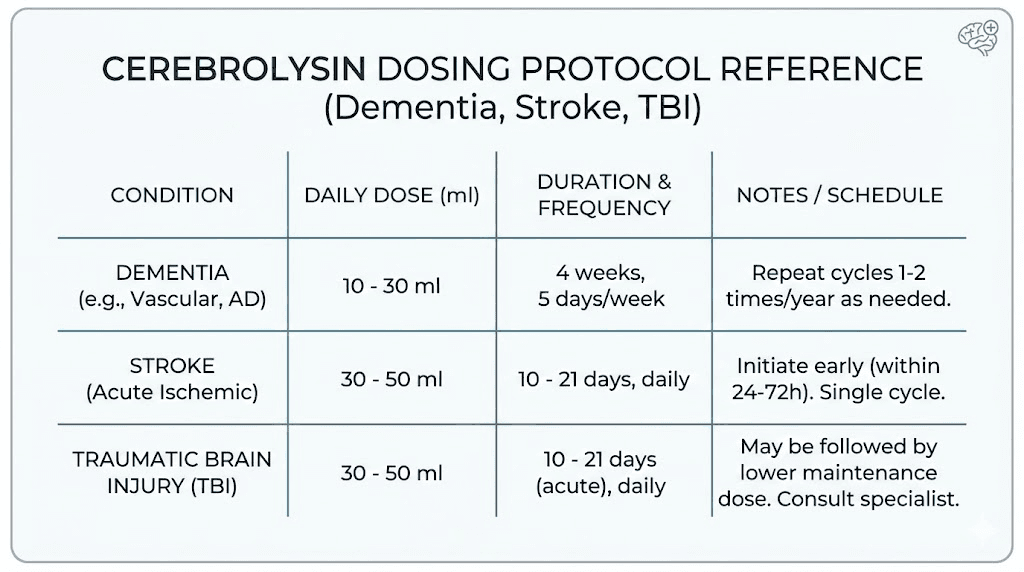
Dosage protocols and administration methods
Dosing cerebrolysin is not as straightforward as most single-peptide compounds. The therapeutic range spans from 1 ml to 60 ml per day depending on the condition, severity, and treatment goals. Getting it wrong, whether too low for effectiveness or too fast for safety, can meaningfully impact outcomes.
Clinical dosing ranges by condition
The following ranges come from published clinical trials and clinical practice guidelines in countries where cerebrolysin is approved:
Condition | Daily dose | Duration | Administration |
|---|---|---|---|
Mild cognitive complaints | 1-5 ml | 10-20 days | IM injection |
Mild to moderate dementia | 5-30 ml | 4-6 weeks, 5 days/week | IV infusion |
Acute ischemic stroke | 30-50 ml | 10-21 days | IV infusion |
Severe TBI | 30-50 ml initially, then 10 ml | 14 days + 14 days | IV infusion |
Hemorrhagic stroke | 10-60 ml | 10-20 days | IV infusion |
Alzheimer disease (maintenance) | 10-30 ml | 4 weeks on, 8 weeks off, repeat | IV infusion |
Several key points about dosing deserve emphasis. For Alzheimer disease, the most successful trial protocol used 30 ml five days per week for 4 weeks, with a repeat course after a 2-month therapy-free interval. The improvement was maintained until week 28, three months after treatment ended. This sustained effect, outlasting the treatment period, suggests genuine neuroplastic changes rather than mere symptomatic relief.
For TBI, the step-down protocol from 30 ml to 10 ml per day after the initial 14-day intensive period reflects the transition from acute neuroprotection to sustained recovery support. Researchers working with peptide dosing protocols recognize this pattern: higher initial doses for acute intervention followed by maintenance dosing for continued benefit.
An important finding from animal studies: the minimally effective weight-based dosages determined in rats (2.5-5 ml per kg) are considerably higher than typical human clinical doses. Some researchers have speculated that patients in certain clinical trials, particularly the CASTA stroke trial, may have received inadequate doses, potentially explaining the mixed results in the overall population analysis.
Administration routes and practical considerations
Cerebrolysin is administered via injection. The specific route depends on the dose:
Intramuscular injection: For doses up to 5 ml. Suitable for mild cases and maintenance protocols. Standard injection technique applies. The compound can be used with bacteriostatic water protocols similar to other injectable peptides, though cerebrolysin is typically supplied as a ready-to-use solution.
Intravenous injection (slow): For doses up to 10 ml. Must be injected slowly to avoid cardiovascular effects including increased heart rate, blood pressure changes, and arrhythmia related to administration speed.
Intravenous infusion: For doses above 10 ml, diluted in standard infusion solutions. The infusion approach allows larger doses to be administered safely over 15-60 minutes, significantly reducing administration-related side effects.
Speed of injection matters. Clinical experience consistently emphasizes injecting cerebrolysin slowly, regardless of the chosen route. Rapid administration is the most common cause of cardiovascular side effects, and these effects are almost entirely avoidable with appropriate injection speed.
For researchers familiar with peptide injection protocols and injection administration basics, cerebrolysin handling follows many standard principles but requires the additional consideration of larger volumes and intravenous administration for therapeutic doses.
Cycling and treatment schedules
Cerebrolysin is not typically used continuously. The clinical trial protocols that showed the best results used treatment cycles with rest periods in between.
The most validated cycling protocol for cognitive conditions: 4 weeks of treatment (5 days per week), followed by 8 weeks off, then repeat. This schedule allows the brain to consolidate the neuroplastic changes stimulated during treatment while avoiding potential downregulation of neurotrophic factor receptors from constant stimulation.
For acute conditions like stroke or TBI, treatment is typically a single intensive course of 10-21 days, sometimes followed by a second course after a rest period if recovery warrants continued treatment. The decision to repeat treatment depends on individual response and ongoing clinical assessment. Those interested in peptide cycling strategies will recognize this pattern of therapeutic windows followed by consolidation periods.
Cerebrolysin compared to other nootropic peptides
Researchers exploring neuroprotective peptides face a genuine dilemma. Multiple compounds show promise. The mechanisms overlap partially but not completely. Understanding how cerebrolysin compares to alternatives helps inform research protocol decisions.
Cerebrolysin versus Semax
Semax is a synthetic peptide derived from a fragment of ACTH (adrenocorticotropic hormone). Its primary mechanism involves increasing BDNF levels and receptor sensitivity. Users and researchers report improvements in focus, memory recall, and analytical clarity.
The key differences:
Factor | Cerebrolysin | Semax |
|---|---|---|
Composition | Complex mixture (100+ peptide fragments) | Single synthetic heptapeptide |
Mechanism breadth | Multi-target (BDNF, NGF, VEGF, IGF-1, microglial) | Primarily BDNF-focused |
Administration | Injection (IM or IV) | Intranasal spray |
Clinical evidence | 200+ trials, 15,000+ patients | Moderate evidence base, primarily Russian studies |
Best for | Neurological recovery, broad neuroprotection | Acute cognitive enhancement, focus |
Onset | Gradual (days to weeks) | Rapid (minutes to hours) |
Convenience | Lower (injection required) | Higher (nasal spray) |
Semax is often described as the non-invasive alternative to cerebrolysin. While this comparison has merit in terms of administration convenience, it oversimplifies the pharmacological differences. Cerebrolysin multi-target mechanism produces broader effects, particularly for neurological recovery. Semax produces more immediate, focused cognitive enhancement through its BDNF-centric mechanism.
Cerebrolysin versus Selank
Selank is structurally related to tuftsin, a natural immunomodulatory peptide. Its primary effects are anxiolytic and mood-stabilizing, working through serotonin and dopamine modulation and GABA receptor enhancement.
The comparison is less direct because the target applications differ. Selank excels at reducing anxiety and stress without sedation. Cerebrolysin excels at neuroprotection and neurological recovery. They occupy different niches within the peptide anxiety and cognition landscape.
Where they overlap: both modulate neuroinflammation and support neuroplasticity, but through different mechanisms. Selank provides calmer baseline brain function. Cerebrolysin provides more robust structural and functional repair.
The stacking perspective
Some researchers and clinicians combine all three: Semax for acute cognitive enhancement, Selank for emotional stability, and cerebrolysin for neurostructural repair. The rationale is pharmacologically sound. Semax signals the brain to produce more growth factors. Cerebrolysin provides the peptide building blocks and supportive environment for those factors to promote growth and repair. Selank optimizes the brain emotional state so other interventions can function at full potential.
Whether stacking provides genuinely additive or synergistic benefits versus using any single compound remains insufficiently studied. The theoretical mechanisms are complementary, but controlled trials of specific peptide stacking combinations are limited. Researchers interested in combining different peptides should approach stacking with awareness that the evidence base for combinations is substantially thinner than for individual compounds.
Comparison with other neuroprotective peptides
Beyond the Semax and Selank comparison, cerebrolysin can be evaluated against other compounds in the neuroprotective space:
Dihexa: A synthetic peptide with extremely potent BDNF-potentiating effects. Dihexa works through hepatocyte growth factor/MET receptor activation and is millions of times more potent than BDNF per weight. However, its clinical evidence base is minimal compared to cerebrolysin, consisting primarily of animal studies.
PE-22-28: A small peptide fragment that enhances cognitive function through modulation of the phosphodiesterase enzyme system. More narrowly focused than cerebrolysin but with potentially fewer side effects due to its targeted mechanism.
SS-31 (Elamipretide): A mitochondria-targeted peptide that reduces oxidative stress. While not directly comparable in mechanism, mitochondrial dysfunction is a major contributor to neurodegeneration, making SS-31 a complementary approach to cerebrolysin neurotrophic mechanisms.
Epitalon: A telomerase-activating peptide with potential longevity applications. Different mechanism entirely, but relevant for researchers interested in the intersection of aging and neuroprotection.
The honest assessment: cerebrolysin has the strongest clinical evidence base among neuroprotective peptides. Its 200+ trials dwarf the evidence for any alternative. However, its requirement for injection, larger volumes, and porcine sourcing create practical barriers that simpler compounds do not have.
Safety profile, side effects, and contraindications
Good safety data requires large patient populations and long follow-up periods. Cerebrolysin has both, with decades of clinical use in over 45 countries and systematic safety analyses from multiple randomized controlled trials.
Overall safety assessment
The consensus from meta-analyses and clinical experience: cerebrolysin is safe for use up to three years with few adverse effects that are usually temporary. In controlled clinical trials, the incidence of adverse events was similar in cerebrolysin-treated and placebo-treated groups. The general safety principles for peptide research apply, but cerebrolysin has an unusually robust safety record for a bioactive compound.
Analysis of safety parameters showed that treatment with up to 50 ml per day did not increase the incidence of treatment-emergent adverse events compared to placebo. Laboratory parameters, vital signs, and ECG examinations provided no evidence for any substance-specific toxic effect.
Common side effects
The most frequently reported side effects are mild and transient:
Dizziness (most common, usually related to injection speed)
Headache (typically resolves within 24 hours)
Skin flushing (related to vasodilation effects)
Nausea (more common at higher doses)
Fatigue or insomnia (varies by individual)
Agitation or anxiety (infrequent)
Weight loss (reported in some longer-term studies)
Feeling hot (transient, related to administration)
Most of these side effects correlate with administration speed rather than the compound itself. Slow injection or infusion significantly reduces their incidence. Clinical practice guidelines consistently emphasize this point: inject slowly, and most side effects disappear.
Cardiovascular effects and administration speed
Increased heart rate, blood pressure changes, and arrhythmia have been reported, but these are related to the speed of administration rather than being inherent pharmacological effects. They are very common with rapid IV bolus administration and rare with properly paced infusion. This is one of the most practical safety considerations for cerebrolysin use.
Drug interactions
Special attention is needed when using cerebrolysin alongside:
Antidepressants: Potential additive effects on serotonergic and noradrenergic systems. The recommendation is to reduce the antidepressant dose when combining with cerebrolysin.
MAO inhibitors: High doses of MAO inhibitors combined with higher cerebrolysin dosages (30 ml or above) have been reported to increase blood pressure. This interaction requires careful monitoring.
For researchers who use other peptides for mood and anxiety conditions, awareness of potential interactions with concurrent medications is essential.
Contraindications
Three established contraindications:
Hypersensitivity: To the protein lysate or excipients. Because cerebrolysin is derived from porcine brain tissue, individuals with pork allergies should not use it. A documented case of life-threatening anaphylaxis after intravenous administration has been reported, though this is extremely rare.
Epilepsy, especially grand mal convulsions: Cerebrolysin treatment may increase seizure frequency. This is one of the most clinically significant contraindications and should be taken seriously. It is worth noting that seizure activity was also reported as a side effect in some TBI studies.
Severe or acute kidney failure: The compound should not be used in patients with significant renal impairment.
Contamination considerations
Because cerebrolysin is purified from animal tissue (porcine brain), there is an inherent risk of bacterial, viral, or fungal contamination. This risk is managed through the manufacturing process but cannot be entirely eliminated. Researchers should source cerebrolysin from reputable manufacturers with documented quality control procedures. The principles of peptide quality testing and proper vial handling are especially important for animal-derived compounds.
Long-term safety
Clinical data supports safety for use up to three years. Beyond that timeframe, evidence is limited. Postmarketing surveillance in countries where cerebrolysin has been used for decades has not revealed unexpected long-term safety signals, but formal long-term controlled studies are lacking.
The rates of adverse effects in clinical trials were consistently comparable between people receiving cerebrolysin and those receiving placebo, providing strong evidence that the compound does not introduce meaningful additional risk when used within established dosing parameters.
Practical research considerations and sourcing
Knowledge of cerebrolysin pharmacology means little without understanding the practical realities of working with this compound. Sourcing, storage, preparation, and quality verification all present unique challenges compared to simpler peptide compounds.
Sourcing and quality
Cerebrolysin is manufactured by EVER Neuro Pharma (formerly Ebewe Pharmaceutica), an Austrian pharmaceutical company. The original pharmaceutical product goes through standardized manufacturing and quality control processes. However, the compound is not available through standard pharmaceutical channels in the United States, creating a complex sourcing landscape.
Researchers should be aware that cerebrolysin quality can vary depending on the source. Because it is a biological product derived from animal tissue, the concentration and composition of bioactive peptides can differ between preparations and manufacturers. The standardized pharmaceutical product from EVER Neuro Pharma has the most consistent composition and the strongest connection to the clinical trial data.
For researchers accustomed to working with research-grade peptide vials, cerebrolysin presents a different quality paradigm. Synthetic peptides can be verified through mass spectrometry and HPLC with clear purity percentages. Cerebrolysin, as a complex biological mixture, requires different analytical approaches to verify quality and composition.
Storage requirements
Cerebrolysin should be stored according to manufacturer specifications, typically at room temperature (below 25 degrees Celsius), protected from light. Unlike many reconstituted peptides, cerebrolysin comes as a ready-to-use solution and does not require reconstitution.
The shelf life varies by product formulation, but standard ampoules typically maintain stability for 3-5 years when stored properly. Once opened, single-use ampoules should be used immediately and not stored. Multi-dose vials, where available, should follow standard peptide storage protocols for opened containers, including refrigeration and use within a defined timeframe.
Do not freeze cerebrolysin. Freezing can denature the peptide fragments and reduce biological activity. For those familiar with general peptide stability at room temperature guidelines, cerebrolysin is relatively stable compared to many synthetic peptides, but temperature extremes should still be avoided.
Preparation and administration
Cerebrolysin is supplied as a clear, amber-colored solution ready for injection. No mixing or dilution is needed for IM injections of 5 ml or less. For IV infusions of larger doses, the solution is diluted in standard IV fluids (normal saline or dextrose solutions) and administered over 15-60 minutes.
Standard injection hygiene applies. Use sterile technique and appropriate needle gauge for the chosen route. IM injections use standard sites (deltoid, gluteal, vastus lateralis) and standard technique as described in peptide injection guides.
The solution should be inspected visually before each use. Do not use if the solution is cloudy, contains particles, or has changed color from its normal amber appearance.
Regulatory status
Cerebrolysin is approved for clinical use in over 45 countries, primarily in Europe and Asia. It is not FDA-approved in the United States. This does not mean it is illegal to possess or use for research purposes, but it cannot be marketed or sold as a treatment within the US. Researchers interested in the legal status of peptides should understand that regulatory approval status varies significantly by country and compound.
What the research community gets wrong about cerebrolysin
Every compound with a substantial evidence base accumulates myths alongside facts. Cerebrolysin is no exception. Addressing these misconceptions directly helps researchers evaluate the compound accurately.
Misconception 1: cerebrolysin is a miracle cognitive enhancer
It is not. The cognitive benefits are real but modest in healthy or mildly impaired populations. The strongest effects are seen in damaged brains recovering from injury or disease. Researchers seeking dramatic nootropic effects in healthy brains should calibrate expectations. Cerebrolysin shines in repair and recovery, not as a limitless pill for already-functional brains.
Misconception 2: oral cerebrolysin works
Peptides taken orally are broken down by digestive enzymes before reaching the bloodstream, let alone the brain. Although some products are marketed as oral cerebrolysin alternatives, the peptide fragments that make cerebrolysin effective are degraded in the gut. The compound requires injection to maintain bioactivity. This is a fundamental injectable versus oral peptide distinction that applies broadly across peptide therapeutics.
Misconception 3: all cerebrolysin products are equivalent
They are not. Because cerebrolysin is a complex biological mixture derived from animal tissue, the composition can vary between manufacturers and even between batches. The clinical trial data is based primarily on the standardized pharmaceutical product. Extrapolating those results to all cerebrolysin-like preparations is scientifically unjustified.
Misconception 4: the CASTA trial proved cerebrolysin does not work for stroke
The CASTA trial showed no overall benefit in the primary endpoint for the full population, but did show benefit in severe strokes. Subsequent trials like CARS showed significant motor recovery benefits. The evidence is mixed and condition-specific, not uniformly negative. Dismissing cerebrolysin for stroke based solely on CASTA ignores the broader evidence context.
Misconception 5: cerebrolysin is dangerous because it comes from pig brains
The manufacturing process involves enzymatic hydrolysis that breaks down large proteins into small peptide fragments below 10 kDa. This process eliminates prion risk and most contamination concerns. The safety record across decades of clinical use and millions of administered doses does not support the characterization of cerebrolysin as dangerous. Standard pharmaceutical manufacturing precautions are applied.
That said, sourcing from reputable manufacturers and following standard peptide safety protocols remains essential for any biological product.
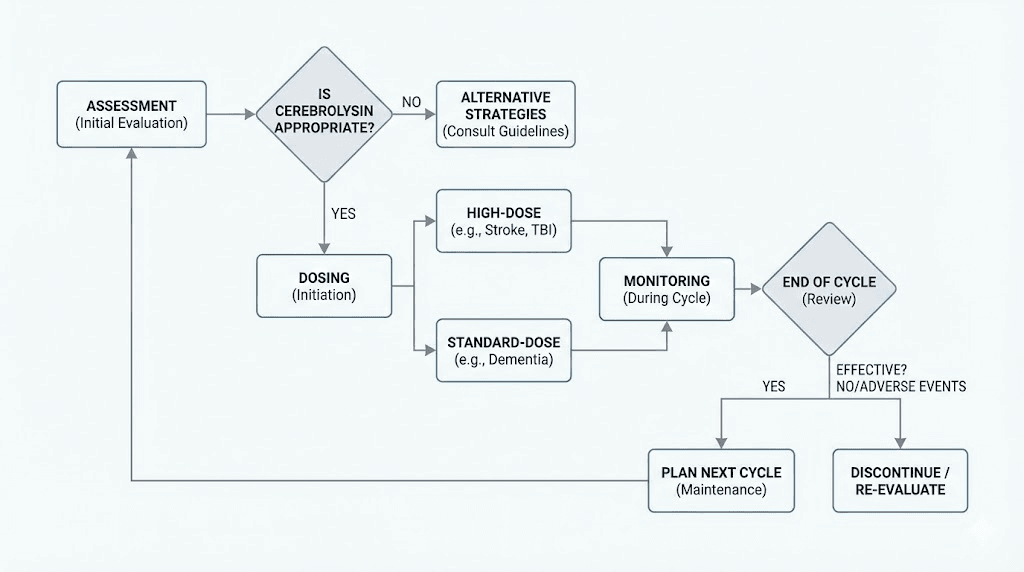
Building a cerebrolysin research protocol
For researchers who have evaluated the evidence and decided to include cerebrolysin in their study of neuroprotective compounds, the following framework draws from the most successful clinical trial protocols.
Protocol 1: cognitive support (mild impairment)
Goal: Support cognitive function in individuals with mild cognitive complaints or age-related decline.
Protocol details:
Dose: 5-10 ml per day
Route: Intramuscular or slow IV injection
Frequency: 5 days per week
Duration: 4 weeks per cycle
Rest period: 8 weeks between cycles
Cycles: 2-4 per year based on response
Expected timeline:
Week 1-2: Subtle changes in mental clarity reported by some individuals
Week 3-4: Measurable cognitive improvements on standardized tests
Week 4-12: Sustained improvements maintained through rest period
Month 6+: Cumulative benefits with repeated cycles
This protocol mirrors the Alzheimer disease trials that showed maintained improvement at week 28, three months after a 4-week treatment course. The sustained benefit suggests that cerebrolysin triggers neuroplastic changes that persist beyond the treatment window.
Protocol 2: neuroprotection and recovery support
Goal: Support neurological recovery after injury or provide intensive neuroprotective intervention.
Protocol details:
Initial phase dose: 20-30 ml per day
Initial phase route: IV infusion (diluted in 100-250 ml saline)
Initial duration: 14-21 days
Maintenance dose: 10 ml per day
Maintenance duration: 14 additional days
Infusion time: 30-60 minutes (never rapid bolus at these doses)
Expected timeline based on TBI trial data:
Day 7-10: Improved neurological scores on GCS
Day 21: Favorable GCS in treated patients with contusion and diffuse axonal injury
Week 12: Significant cognitive improvement (CASI scores) in mild TBI
Month 6: 67% achieved good outcomes (GOS 3-5) in moderate to severe cases
Protocol 3: combination approach with donepezil
Goal: Maximize cognitive benefit in established dementia through complementary mechanisms.
Protocol details:
Cerebrolysin: 10 ml IV, 5 days per week for 4 weeks
Donepezil: 10 mg daily (or current prescribed dose)
Assessment: ADAS-cog and CIBIC+ at baseline, week 4, and week 12
Repeat: Cerebrolysin courses every 3 months while maintaining daily donepezil
The clinical trial supporting this combination found that the dual approach produced the best cognitive scores at every study visit compared to either monotherapy. The neurotrophic (cerebrolysin) and cholinergic (donepezil) mechanisms are complementary, not redundant.
Researchers should use the SeekPeptides peptide calculator for dosing calculations on simpler peptide compounds and reference published clinical protocols specifically for cerebrolysin, given its unique dosing paradigm.
Monitoring and assessment
Regardless of protocol, regular monitoring should include:
Cognitive testing at baseline and regular intervals (MMSE, MoCA, or ADAS-cog)
Vital signs during and after each administration session
Hepatic and renal function panels (baseline and periodic)
Neurological examination for seizure risk assessment
Self-reported side effects diary
Track progress using standardized scales. Subjective improvement without objective measurement is insufficient for evaluating response. SeekPeptides provides tracking tools and protocol resources for members working with various peptide compounds, supporting organized documentation of research outcomes.
The disease-modifying question: does cerebrolysin slow neurodegeneration?
This is the most important question in cerebrolysin research. Symptomatic improvement, making someone feel and function better temporarily, is valuable but limited. Disease modification, actually slowing the underlying degenerative process, would be transformative.
The evidence is suggestive but not conclusive.
Several observations support the disease-modifying hypothesis. First, cerebrolysin inhibits GSK3beta, directly reducing tau phosphorylation, one of the two hallmark pathologies of Alzheimer disease (alongside amyloid-beta accumulation). Second, the sustained cognitive improvements that persist months after treatment cessation suggest structural brain changes, not merely temporary pharmacological effects. Third, the neurotrophic factor modulation, particularly the shift from pro-death to pro-survival signaling at the molecular level, is precisely the type of intervention that would slow progressive neuronal loss.
The evidence against a strong disease-modifying claim: the cognitive improvements, while sustained, are modest in magnitude. Truly disease-modifying treatments would be expected to produce increasingly divergent outcomes between treated and untreated patients over years, and this has not been clearly demonstrated in clinical trials.
A 2021 review covering 30 years of clinical data described cerebrolysin as demonstrating "a disease-modifying effect by slowing down the progression" of Alzheimer disease, but this language outpaces what the clinical trial designs can definitively prove. Most trials were too short and too small to demonstrate disease modification with statistical rigor.
For researchers exploring longevity and anti-aging peptides, the disease modification question is central. If cerebrolysin genuinely slows neurodegeneration, it would represent one of the most significant neuroprotective tools available. If it only provides symptomatic benefit, it remains useful but in a different category of clinical importance.
The question remains open. And that openness, combined with the strong mechanistic rationale and suggestive clinical data, is precisely what makes cerebrolysin one of the most actively researched neuropeptide preparations in the world.
Combining cerebrolysin with lifestyle and other interventions
No peptide works in isolation. The brain environment in which cerebrolysin operates determines how effectively its neurotrophic signals translate into actual neurological improvement. Several evidence-based interventions synergize with cerebrolysin mechanisms.
Exercise and BDNF amplification
Aerobic exercise is the most potent natural BDNF booster known. Combining exercise with cerebrolysin BDNF-upregulating effects creates a potentially synergistic environment for neuroplasticity. The CARS stroke trial, which showed cerebrolysin strongest results, included a standardized rehabilitation program alongside the peptide treatment. The combination of biological support (cerebrolysin) and physical rehabilitation (exercise) may have contributed to the impressive motor recovery outcomes.
Researchers interested in peptides for athletic performance and performance optimization should note this exercise synergy. The brain adaptations to physical training share molecular pathways with cerebrolysin mechanism.
Sleep optimization
The brain performs critical repair and consolidation processes during deep sleep. Growth hormone release, glymphatic clearance of metabolic waste, memory consolidation, and synaptic pruning all occur primarily during sleep. Disrupted sleep undermines the neuroplastic changes that cerebrolysin promotes. Researchers exploring peptides that support sleep quality and DSIP for sleep regulation may find these compounds relevant alongside cerebrolysin protocols.
Nutritional support for neurotrophin production
Several nutrients directly support the neurotrophic pathways that cerebrolysin activates. Omega-3 fatty acids (particularly DHA) are structural components of neuronal membranes and support BDNF expression. Magnesium is required for NMDA receptor function and synaptic plasticity. B vitamins, particularly B12 and folate, support methylation pathways critical for neurotransmitter synthesis. Vitamin D receptors are expressed throughout the brain, and deficiency is associated with accelerated cognitive decline.
None of these replace cerebrolysin. But they create the biological substrate on which cerebrolysin neurotrophic signals can most effectively operate.
Cognitive training and neuroplasticity
Cerebrolysin enhances neuroplasticity, the brain ability to form new connections and reorganize existing ones. This enhanced plasticity window is more useful if the brain is actively challenged. Cognitive training, learning new skills, language study, and novel problem-solving all stimulate the formation of new neural pathways. Performing these activities during and after cerebrolysin treatment may amplify the neuroplastic benefits.
The parallel to physical rehabilitation after stroke is direct: the peptide creates biological readiness for improvement, but active engagement is needed to direct that improvement toward meaningful functional gains.
Who should and should not consider cerebrolysin research
Not every researcher or every research question is well-served by cerebrolysin. Matching the compound strengths to appropriate applications, and recognizing its limitations, prevents wasted resources and disappointing results.
Potentially appropriate applications
Post-TBI cognitive recovery research: The strongest clinical evidence supports this application. Researchers studying recovery from mild, moderate, or severe traumatic brain injury have the most robust data to guide their protocols.
Alzheimer disease and vascular dementia studies: Multiple randomized controlled trials provide dosing guidance and outcome expectations. The combination with donepezil is particularly well-documented.
Stroke recovery research: Despite mixed overall results, specific populations (severe stroke, motor recovery focus) show meaningful benefit. Protocol design should account for the subgroup findings from major trials.
Neurotrophic factor modulation studies: For researchers studying how multi-target neurotrophic factor modulation affects brain function, cerebrolysin provides a unique tool, essentially a "neurotrophic cocktail" in a single preparation.
Aging and neuroplasticity research: The documented effects on NGF receptor balance, BDNF upregulation, and microglial phenotype shifting are directly relevant to age-related brain changes.
Less appropriate applications
Acute cognitive enhancement in healthy adults: Limited evidence for this application. Researchers seeking nootropic effects in healthy brains have better-studied alternatives. Compounds like Semax and other nootropic peptides have more relevant evidence for this use case.
Oral supplementation research: The peptide fragments are degraded by digestive enzymes. Oral cerebrolysin research is unlikely to produce meaningful results.
Individuals with epilepsy: The contraindication for seizure disorders means this population should not receive cerebrolysin.
Budget-constrained research: Cerebrolysin, particularly the pharmaceutical-grade product, is expensive relative to synthetic peptides. The volumes required (5-30 ml per day) create substantial material costs over treatment courses.
For researchers evaluating their options across the full spectrum of available peptides, cerebrolysin represents a high-evidence, high-complexity option. It is not the simplest or most accessible neuroprotective compound, but for the right applications, it has the strongest clinical data supporting its use.
SeekPeptides members access comprehensive protocol databases, expert guidance, and a community of researchers who have practical experience with neuroprotective peptides including cerebrolysin. The platform provides the context and support that transforms research knowledge into effective protocol design.
Frequently asked questions
What is cerebrolysin made from?
Cerebrolysin is a mixture of low-molecular-weight neuropeptides and free amino acids derived from purified porcine (pig) brain proteins through an enzymatic hydrolysis process. The resulting peptide fragments are all below 10 kilodaltons, allowing them to cross the blood-brain barrier and exert neurotrophic effects directly in the brain.
Is cerebrolysin FDA-approved?
No. Cerebrolysin is not approved by the FDA in the United States. It is, however, approved in over 45 countries, primarily in Europe and Asia, for treating stroke, traumatic brain injury, and various forms of dementia. The regulatory status of peptides varies significantly by country and compound.
How long does it take for cerebrolysin to work?
In clinical trials, cognitive improvements were measurable by week 3-4 of treatment. For TBI recovery, neurological score improvements appeared within 7-10 days. The sustained effects, lasting months after treatment cessation, suggest that cerebrolysin triggers structural brain changes rather than providing only temporary pharmacological effects. Individual response varies based on condition, severity, and dosing protocol.
Can you take cerebrolysin orally?
No. Like most bioactive peptides, cerebrolysin is broken down by digestive enzymes before reaching the bloodstream. It must be administered via injection (intramuscular or intravenous) to maintain its biological activity. Products marketed as oral cerebrolysin alternatives are unlikely to deliver the same peptide fragments to the brain. Understanding the difference between injectable and oral peptide delivery is essential for evaluating any peptide compound.
What are the main side effects of cerebrolysin?
The most common side effects are mild and temporary: dizziness, headache, skin flushing, nausea, and fatigue. Most are related to injection speed rather than the compound itself. Slow administration significantly reduces side effect incidence. In clinical trials, adverse event rates were comparable between cerebrolysin and placebo groups, indicating a favorable safety profile.
Can cerebrolysin be combined with other peptides?
Some researchers combine cerebrolysin with Semax (for acute cognitive enhancement) and Selank (for anxiolytic effects). The mechanisms are complementary: cerebrolysin provides broad neurotrophic support, Semax upregulates BDNF, and Selank modulates emotional regulation. However, controlled clinical trials of these specific combinations are limited. Those interested in peptide stacking strategies should approach combinations cautiously.
How should cerebrolysin be stored?
Store at room temperature below 25 degrees Celsius, protected from light. Do not freeze. Cerebrolysin comes as a ready-to-use solution that does not require reconstitution. Once opened, single-use ampoules should be used immediately. Follow standard peptide storage guidelines for any multi-dose containers.
Is cerebrolysin safe for long-term use?
Clinical data supports safety for use up to three years with few adverse effects. The typical approach uses cycled treatment (4 weeks on, 8 weeks off) rather than continuous daily administration. Postmarketing surveillance across decades of clinical use in multiple countries has not revealed unexpected long-term safety concerns.
External resources
Alzheimer Drug Discovery Foundation - Cerebrolysin Brain Health Rating
Cochrane Review - Cerebrolysin for Acute Ischaemic Stroke (PubMed Central)
For researchers serious about optimizing their neuroprotective peptide protocols, SeekPeptides offers the most comprehensive resource available, with evidence-based guides, proven protocols, and a community of thousands who have navigated these exact questions.
In case I do not see you, good afternoon, good evening, and good night. May your neurons stay resilient, your synapses stay plastic, and your protocols stay evidence-based.



