Jan 31, 2026
Deep inside every neuron in your brain, a molecular cascade determines whether that cell will grow stronger, form new connections, or slowly wither.
The protein responsible for this decision is brain-derived neurotrophic factor, commonly known as BDNF. It binds to a receptor called TrkB on the surface of neurons. Within two minutes, that receptor phosphorylates. Within thirty minutes, entire signaling pathways activate. And within hours, genes responsible for neuroplasticity, memory consolidation, and neuronal survival begin to express at dramatically different rates. This is not a slow, abstract process. It is happening right now, in your hippocampus, your cortex, and your basal forebrain, shaping how you think, remember, and adapt. When BDNF levels are high, neurons thrive. When levels drop, the consequences ripple outward through cognition, mood, and long-term brain health. Researchers have linked low BDNF to depression, anxiety, cognitive decline, and neurodegenerative conditions like Alzheimer disease.
The challenge is straightforward but not simple: full-length BDNF protein is unstable, difficult to deliver, and cannot cross the blood-brain barrier in therapeutic form. This limitation has driven the development of BDNF peptide mimetics, smaller synthetic molecules designed to activate the same TrkB pathways with greater stability and bioavailability. This guide covers everything from the cellular mechanics of BDNF signaling to the specific peptides that mimic or boost its activity, the nootropic compounds that support it naturally, and the research protocols emerging from laboratories around the world.
What is BDNF and why does it matter for brain health?
Brain-derived neurotrophic factor is a protein. More precisely, it is a neurotrophin, a class of growth factors that regulate the survival, development, and function of neurons. BDNF is encoded by the BDNF gene located on chromosome 11 in humans, and the mature protein consists of 247 amino acids with a molecular weight of approximately 27 kDa. It was first isolated in 1982 by Yves-Alain Barde and Hans Thoenen, and the decades since have revealed it to be one of the most important signaling molecules in the entire nervous system.
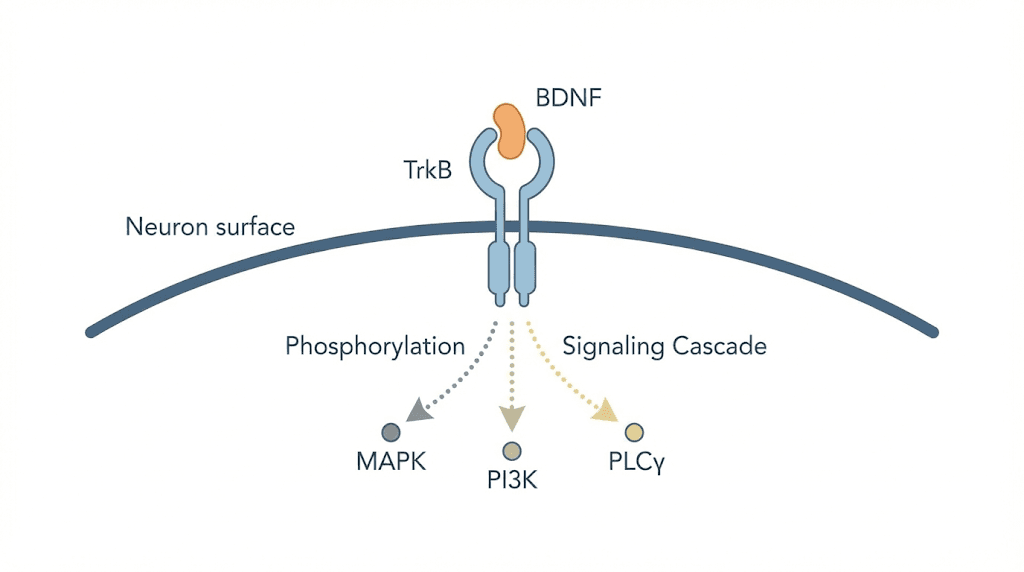
BDNF does its work primarily through a receptor called TrkB (pronounced "track B"), a member of the tropomyosin receptor kinase family. When BDNF binds to the extracellular domain of TrkB, the receptor dimerizes and autophosphorylates specific tyrosine residues in its intracellular kinase domain. This single event triggers three major downstream signaling pathways that collectively govern neuronal health: the MAPK/ERK pathway, the PI3K/Akt pathway, and the PLCgamma pathway. Each of these cascades contributes something different. ERK signaling drives neuronal differentiation and maturation. Akt signaling promotes cell survival and protects against apoptosis. PLCgamma modulates synaptic plasticity and the strengthening of neural connections.
The brain regions where BDNF exerts its strongest influence are the hippocampus, the prefrontal cortex, and the basal forebrain. These areas are central to learning, memory formation, executive function, and emotional regulation. In the hippocampus specifically, BDNF is essential for long-term potentiation (LTP), the process by which synaptic connections strengthen in response to repeated stimulation. Without adequate BDNF, LTP weakens. Memory suffers. Cognitive flexibility declines.
But BDNF is not only a brain molecule. It exists in blood platelets, skeletal muscle, the kidneys, and the retina. During exercise, skeletal muscle produces BDNF and releases lactate into the bloodstream, which crosses the blood-brain barrier and triggers additional BDNF expression in the brain. This is one reason physical activity has such profound effects on cognition and mental energy.
What makes BDNF so critical is its role as a master regulator. It does not just support individual neurons. It orchestrates the environment in which neurons communicate, adapt, and rebuild. Researchers studying peptide science increasingly focus on BDNF pathways because they represent a convergence point for nearly every cognitive function that matters: attention, learning, recall, emotional processing, and resistance to neurodegeneration.
How BDNF works at the cellular level
Understanding BDNF requires zooming in past the organ level, past the tissue level, all the way down to individual synapses. This is where the magic happens.
TrkB receptor activation
BDNF is synthesized as a precursor protein called proBDNF, which is cleaved by proteases into its mature form. The mature BDNF protein preferentially binds to TrkB, while proBDNF has a higher affinity for a different receptor called p75NTR. This distinction matters enormously. Mature BDNF binding to TrkB promotes neuronal survival and growth. ProBDNF binding to p75NTR can actually trigger apoptosis and synaptic pruning. The balance between these two forms, and the receptors they activate, determines whether a neuron strengthens or weakens.
When mature BDNF binds TrkB, the receptor dimerizes. Autophosphorylation occurs at multiple tyrosine residues within two minutes. These phosphorylated residues serve as docking sites for adapter proteins that initiate downstream signaling. The speed of this process is remarkable. TrkB activation is among the fastest receptor-mediated signaling events in the nervous system, and deactivation occurs within roughly 30 minutes in spinal cord neurons.
ERK and Akt signaling pathways
The three pathways activated by TrkB each serve distinct functions. The MAPK/ERK pathway is the primary driver of neuronal differentiation and gene expression changes. When ERK is phosphorylated, it translocates to the nucleus and activates transcription factors like CREB (cAMP response element-binding protein), which in turn drives the expression of genes responsible for synaptic plasticity and memory formation. This is not a minor effect. CREB activation through the ERK pathway is one of the most well-established molecular mechanisms underlying long-term memory consolidation.
The PI3K/Akt pathway focuses on neuronal survival. Akt phosphorylation inhibits pro-apoptotic proteins like BAD and activates anti-apoptotic mechanisms that protect neurons from oxidative stress, excitotoxicity, and metabolic damage. For anyone concerned about neuronal longevity and brain aging, the Akt pathway is the molecular mechanism that keeps neurons alive under adverse conditions.
The PLCgamma pathway modulates synaptic transmission directly. It generates second messengers that regulate calcium signaling and neurotransmitter release, fine-tuning the strength and efficiency of synaptic communication. Together, these three pathways make TrkB activation a comprehensive event that simultaneously protects neurons, promotes their growth, and optimizes their communication.
Long-term potentiation
LTP is the cellular basis of learning and memory. When a synapse is repeatedly activated, it becomes stronger. The next signal passes through more easily. BDNF is essential for this process, particularly in the hippocampus. Without BDNF, LTP is severely impaired in the CA1 region of the hippocampus, the area most critical for forming new declarative memories.
BDNF enhances LTP through multiple mechanisms. It increases the number of synaptic vesicles at the presynaptic terminal, boosts postsynaptic receptor responsiveness, and increases the overall number of synapses per neuron. It also suppresses GABAergic (inhibitory) signaling through PKC-mediated phosphorylation of GABAA receptors, which shifts the balance toward excitatory transmission and makes it easier for new memories to form.
Synaptogenesis and neurogenesis
BDNF does not just strengthen existing connections. It helps build new ones. Synaptogenesis, the formation of new synapses, depends heavily on BDNF signaling through the ERK pathway. In the adult brain, BDNF also supports neurogenesis in the hippocampal dentate gyrus, one of the few brain regions where new neurons continue to form throughout life. This process is essential for cognitive resilience during aging, and it explains why researchers interested in anti-aging strategies pay such close attention to BDNF levels.
The activity-dependent nature of BDNF expression is worth understanding. BDNF is not produced at a constant rate. Its expression increases when neurons are active, when NMDA receptors fire, and when calcium influx triggers specific kinase cascades. This means the brain produces more BDNF precisely when it is learning, adapting, or recovering from stress. It is a beautifully designed feedback loop where neural activity drives the production of the very molecule that supports more neural activity.
Signs of low BDNF levels
Low BDNF is not a diagnosis. It is a biochemical state that correlates with a wide range of cognitive and emotional symptoms. Understanding these signs can help researchers and clinicians identify when BDNF support might be beneficial.
Depression and mood disorders
Multiple meta-analyses have established a strong link between low serum BDNF and major depressive disorder. The "neurotrophin hypothesis of depression" proposes that depression results, at least in part, from reduced BDNF signaling in the hippocampus and prefrontal cortex. This is supported by the observation that most effective antidepressants, including SSRIs and SNRIs, increase BDNF expression over time. The therapeutic lag of these medications (typically 2 to 4 weeks) correlates with the time required for BDNF-driven neuroplastic changes to take effect. For those exploring peptide approaches to anxiety and mood, the BDNF connection provides a mechanistic framework for understanding how certain compounds exert their effects.
Cognitive decline and memory problems
Reduced BDNF levels correlate directly with impaired memory performance, particularly episodic and declarative memory. Studies in older adults show that lower serum BDNF corresponds to reduced hippocampal volume and slower processing speed. The decline is progressive. As BDNF drops, so does the capacity for LTP, leading to gradual but measurable deterioration in the ability to form and retrieve memories. This makes BDNF a potential biomarker for cognitive aging.
Anxiety
The relationship between BDNF and anxiety is complex but well-documented. Low BDNF in the hippocampus and amygdala is associated with increased anxiety-like behavior in animal models, and human studies show reduced serum BDNF in patients with generalized anxiety disorder. The mechanism likely involves impaired fear extinction, the process by which the brain learns that a previously threatening stimulus is no longer dangerous. BDNF is critical for this form of learning, and when it is deficient, anxiety responses persist beyond their usefulness.
Neurodegeneration risk
Low BDNF has been documented in patients with Alzheimer disease, Parkinson disease, Huntington disease, and amyotrophic lateral sclerosis. In Alzheimer disease specifically, BDNF deficits are most pronounced in the hippocampus, entorhinal cortex, and prefrontal cortex, the regions earliest and hardest hit by the disease. Reduced BDNF signaling appears to accelerate amyloid-beta accumulation, tau phosphorylation, neuroinflammation, and neuronal apoptosis. For anyone exploring neuroprotective peptide strategies, understanding the BDNF-neurodegeneration connection is foundational.
The cortisol connection
Chronic stress reduces BDNF expression through elevated cortisol. This relationship creates a destructive cycle: stress lowers BDNF, which impairs the hippocampal circuits that regulate stress responses, which leads to more cortisol production and further BDNF reduction. Breaking this cycle is one of the primary goals of stress-management peptide research and a compelling reason to explore both pharmacological and lifestyle interventions that boost BDNF.
The BDNF Val66Met polymorphism
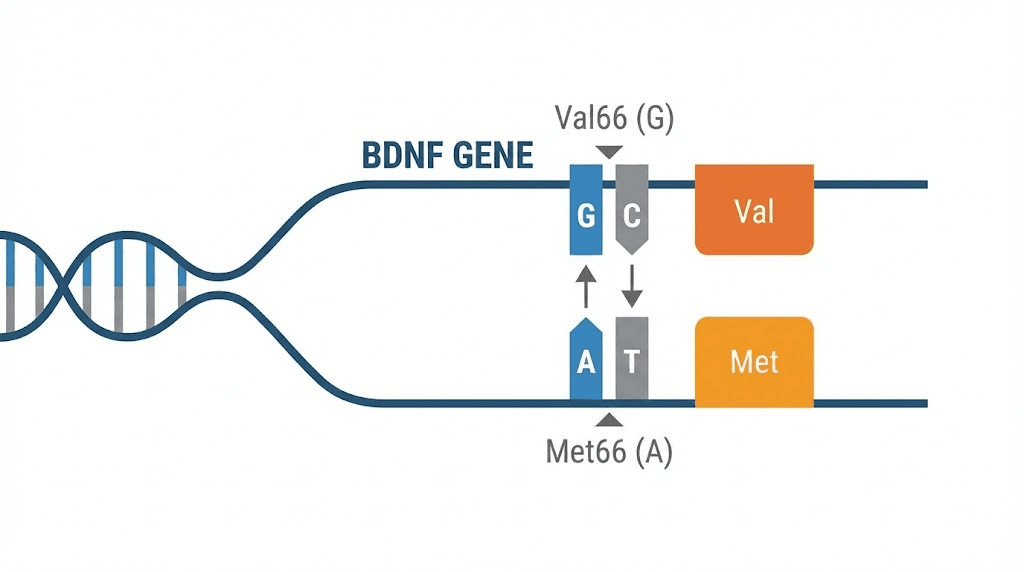
Not everyone starts with the same BDNF potential. A common genetic variant, known as Val66Met (rs6265), affects how efficiently the brain produces and secretes BDNF. Understanding this polymorphism is essential for anyone serious about optimizing cognitive function.
What it is
The Val66Met polymorphism involves a single nucleotide change in the BDNF gene that substitutes valine (Val) for methionine (Met) at position 66 of the proBDNF protein. This substitution occurs in the prodomain, the region responsible for intracellular trafficking and activity-dependent secretion of BDNF. Approximately 20 to 30 percent of Caucasian populations carry at least one Met allele, with higher frequencies in Asian populations (up to 50 percent).
How the Met allele affects BDNF secretion
The Met allele disrupts the interaction between proBDNF and sortilin, a protein that directs BDNF into regulated secretory vesicles. This means that individuals carrying the Met allele release less BDNF in response to neural activity. The constitutive (baseline) secretion remains relatively normal, but the activity-dependent secretion, the burst of BDNF that occurs during learning, exercise, and novel experiences, is significantly reduced. The implication is profound: Met carriers get less neurotrophic benefit from the very activities that should boost their brain health the most.
Impact on memory and aging
Research consistently shows that Met carriers perform worse on tests of episodic memory, particularly tasks that engage the hippocampus. One study found that the interaction between BDNF genotype and hippocampal activation during memory encoding accounted for 25 percent of the total variation in recognition memory performance. The effects become more pronounced with age. A cross-sectional study of adults aged 20 to 93 found that Met carriers showed steeper age-related declines in item memory and prospective memory compared to Val/Val homozygotes.
Sleep-dependent memory consolidation is also affected. Met carriers show stronger forgetting overnight compared to Val homozygotes, though the effect appears specific to declarative memory and does not extend to motor learning. This suggests that the polymorphism primarily impacts hippocampal consolidation processes rather than procedural memory systems.
Interaction with exercise
Here is the encouraging part. While Met carriers may produce less activity-dependent BDNF, they still respond to exercise and other BDNF-boosting interventions. Some research suggests that Met carriers may actually benefit more from regular physical activity because they have more room for improvement. Exercise remains the single most reliable natural BDNF booster regardless of genotype, and combining it with targeted peptide stacking strategies may offer compounded benefits.
Testing considerations
Genetic testing for Val66Met is available through most direct-to-consumer genomics services and can also be ordered by healthcare providers. Knowing your genotype can inform your approach to cognitive optimization. Met carriers may benefit from more aggressive BDNF-boosting strategies, including higher-intensity exercise protocols, targeted supplementation, and specific peptide dosing approaches that prioritize TrkB activation.
What are BDNF peptides?
The concept of a "BDNF peptide" refers to synthetic molecules designed to mimic the activity of full-length BDNF protein at the TrkB receptor. The need for these mimetics arises from a fundamental pharmacological challenge: BDNF itself is a terrible drug candidate.
Why full-length BDNF protein is not a practical therapeutic
BDNF is a large, 27 kDa protein that cannot cross the blood-brain barrier. It has poor metabolic stability, a short half-life in circulation, and limited bioavailability when administered systemically. Direct injection into the brain is invasive and impractical for chronic use. These limitations have driven decades of research into smaller molecules that can activate TrkB signaling with better pharmacokinetic properties. The result is a growing class of compounds known as BDNF mimetic peptides, molecules that are small enough to be practical but precise enough to trigger the same downstream pathways as the native protein.
LM22A-4
LM22A-4 is a tripeptide mimetic identified through in silico screening using a pharmacophore model based on BDNF loop domains. It binds TrkB receptors and activates Akt and ERK signaling in hippocampal neurons. In cell culture studies, LM22A-4 demonstrates approximately 89 percent of full BDNF activity at optimal concentrations. Its effective concentration ranges from the sub-nanomolar to nanomolar range, with 500 nM commonly used in vitro to ensure full TrkB activation.
When administered intranasally to adult mice at 0.22 mg/kg per day for seven days, LM22A-4 activates Trk, AKT, and ERK signaling in vivo. It has shown neuroprotective effects in cell culture models of Alzheimer disease, Huntington disease, and Parkinson disease. A study on pediatric traumatic brain injury in mice found that acute LM22A-4 treatment preserved myelin integrity and conferred significant neuroprotection. These findings make LM22A-4 one of the most promising BDNF mimetics in preclinical research, and researchers using the peptide calculator to plan protocols often consider it alongside other nootropic peptides.
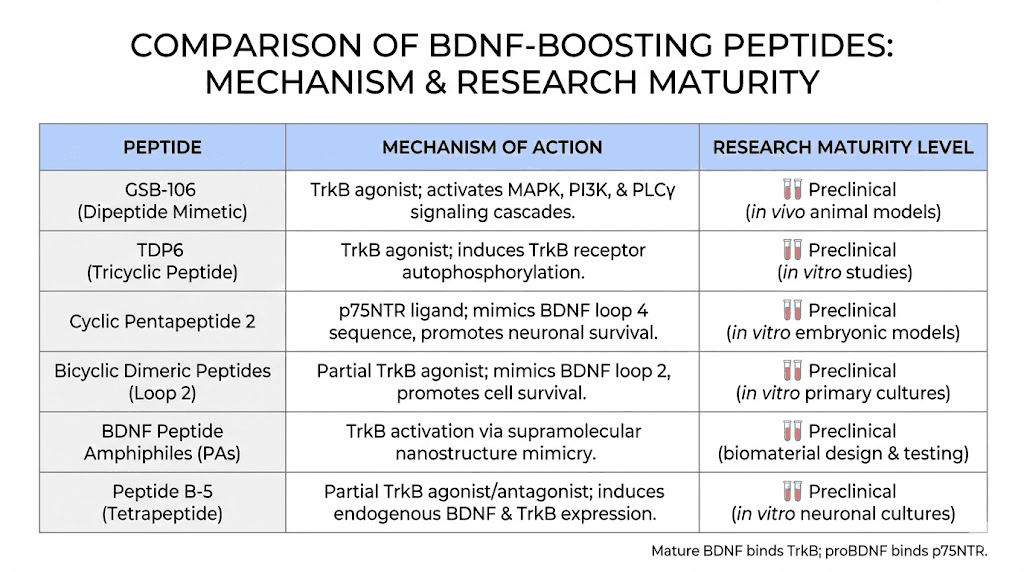
GSB-106
GSB-106, formally known as hexamethylenediamide bis(N-monosuccinyl-L-seryl-L-lysine), is a dimeric dipeptide mimetic designed to replicate the activity of BDNF loop 4. It was developed by Russian researchers as part of a systematic effort to create low-molecular-weight neurotrophin mimetics. GSB-106 activates all three major TrkB signaling pathways: MAPK/ERK, PI3K/AKT, and PLCgamma.
In the Porsolt forced swim test, GSB-106 demonstrated antidepressant activity at doses of 0.1 and 1 mg/kg administered intraperitoneally to rats. In a chronic social defeat stress model in mice, 21 days of oral administration at 0.1 mg/kg corrected depressive-like behavior, restored motor activity with a therapeutic effect of 60 percent, completely recovered anhedonia, restored synaptophysin levels (74 percent therapeutic effect), and fully restored CREB levels in the hippocampus. The effective concentration in cellular assays falls in the 10 to 100 nM window, with an EC50 of approximately 10 nM.
What makes GSB-106 particularly interesting is that it works orally. Unlike many peptides that require injection or intranasal delivery, GSB-106 has demonstrated efficacy through oral administration in animal models. This could represent a significant advantage for practical application if it advances toward clinical use.
Tetra peptides B-1 through B-5
Several tetrapeptide sequences derived from the BDNF protein loops have also been investigated. These shorter peptide fragments aim to reproduce specific structural features of the BDNF-TrkB binding interface. While less extensively studied than LM22A-4 or GSB-106, they represent an ongoing area of research into the minimum effective structure needed to activate TrkB signaling. Researchers exploring peptide formulas and structures will find this area of study particularly relevant.
Research status
All BDNF mimetic peptides remain in the preclinical stage. No human clinical trials have been completed for LM22A-4, GSB-106, or the tetra peptide variants. Safety data comes exclusively from cell culture and animal studies. TrkB signaling carries context-dependent risks, including altered seizure susceptibility, which makes human translation a careful process. Anyone involved in peptide safety research should understand that these compounds are investigational and not approved for human use.
Peptides that increase BDNF levels
While BDNF mimetics work by directly activating TrkB receptors, a separate category of peptides achieves cognitive benefits by increasing the brain natural production of BDNF. These compounds do not replace BDNF. They amplify the body own synthesis of it. Several of these peptides have more extensive research histories and, in some cases, clinical use in specific countries.
Semax
Semax is a heptapeptide derived from adrenocorticotropic hormone (ACTH). Developed at the Russian Academy of Sciences, it has been used clinically in Russia for stroke, dyscirculatory encephalopathy, and optic nerve atrophy. Its cognitive effects are well-documented and mechanistically linked to BDNF upregulation.
A single intranasal application of Semax at 50 mcg/kg body weight produces a 1.4-fold increase in BDNF protein levels in the rat hippocampus, accompanied by a 1.6-fold increase in TrkB tyrosine phosphorylation and a 3-fold increase in exon III BDNF mRNA levels. The temporal dynamics are fascinating. BDNF expression actually decreases in the hippocampus and retina 20 minutes after Semax administration, then increases significantly in the frontal cortex. By 90 minutes, BDNF levels are significantly elevated across multiple brain regions.
Clinical data from a study involving 110 ischemic stroke patients showed that treatment with Semax at 6,000 mcg daily for 10 days significantly increased plasma BDNF levels, and these elevated levels correlated with improved functional outcomes. For those interested in intranasal peptide delivery, Semax represents one of the best-studied options.
Selank
Selank is a synthetic analogue of the endogenous tuftsin molecule, extended with three additional amino acids (Pro-Gly-Pro) for improved metabolic stability. It was developed alongside Semax at the Russian Academy of Sciences and has been approved in Russia as an anxiolytic medication.
Selank rapidly elevates BDNF expression in the hippocampus following intranasal administration. In a study of ethanol-induced memory impairment, Selank at 0.3 mg/kg per day for seven days produced cognitive-stimulating effects and prevented ethanol-induced BDNF dysregulation in the hippocampus and frontal cortex. Its anxiolytic effect is comparable to low-dose benzodiazepines but without the sedation, tolerance, dependence, or cognitive impairment associated with that drug class. This makes Selank particularly attractive for researchers exploring the intersection of anxiety management and cognitive enhancement.
Noopept
Noopept (N-phenylacetyl-L-prolylglycine ethyl ester) is a dipeptide nootropic that stimulates the expression of both BDNF and nerve growth factor (NGF) in the rat hippocampus. A foundational study by Ostrovskaya and Gudasheva found that chronic 28-day administration of Noopept increased both BDNF and NGF mRNA levels in the hippocampus without tolerance development. The compound also decreased the activity of stress-induced kinases (SAPK/JNK and pERK1/2), suggesting additional neuroprotective mechanisms beyond simple neurotrophin upregulation.
Much of Noopept cognitive effect may be mediated through its metabolite cycloprolylglycine (cPG), which concentrates preferentially in the hippocampus and has been shown to reduce hippocampal cell death, increase BDNF mRNA, and modulate inflammatory cytokine expression. Noopept does not stimulate cell proliferation despite its neurotrophic effects, which addresses one safety concern associated with growth factor modulation. Those researching cognitive peptide stacking often include Noopept alongside other BDNF-boosting compounds.
Dihexa
Dihexa (PNB-0408) is an oligopeptide derived from angiotensin IV that has attracted enormous attention for its potency. In laboratory assays measuring new neuronal connections, researchers at Washington State University reported that Dihexa was seven orders of magnitude more potent than BDNF for synaptogenesis. That translates to 10 million times the synapse-forming activity at equivalent concentrations.
The mechanism is different from direct TrkB activation. Dihexa binds with high affinity (Kd of 65 pM) to hepatocyte growth factor (HGF) and potentiates its activity at the c-Met receptor. This triggers hippocampal spinogenesis and synaptogenesis similar to HGF itself. In rat models of scopolamine-induced cognitive impairment, Dihexa restored spatial learning and memory whether administered directly to the brain, orally, or by injection.
The potency comes with important caveats. The HGF/c-Met pathway is a key signaling axis in many cancers, and no long-term safety studies have been conducted. Anyone researching Dihexa should understand that it remains purely investigational and carries theoretical risks that other nootropic peptides do not share. Review the full Dihexa research profile before including it in any protocol.
PE-22-28
PE-22-28 is a synthetic peptide that activates TrkB receptors and modulates TREK-1 potassium channels. In mouse studies, it approximately doubled the population of BrdU-positive cells (a marker of new cell growth), enhanced synaptogenesis markers at both mRNA and protein levels, and rapidly increased BDNF expression in the hippocampus. Its inhibition of TREK-1 channels produces rapid antidepressant effects, distinguishing it from conventional antidepressants that require weeks to reach efficacy. PE-22-28 also appears to have seizure-protective properties rather than seizure-promoting effects, which addresses one of the theoretical concerns with TrkB agonism.
Pinealon
Pinealon is a tripeptide bioregulator (Glu-Asp-Arg) originally isolated from brain tissue extracts. It belongs to the Khavinson peptide bioregulator family and is proposed to interact directly with DNA to modulate gene expression. Research suggests Pinealon may raise the expression of BDNF and NGF in neural cell cultures while also modulating serotonin synthesis through effects on 5-tryptophan hydroxylase. Studies in prenatal rats showed significant reductions in reactive oxygen species and necrotic cells in the brain following Pinealon administration. Its unique mechanism, hypothesized to involve direct DNA interaction rather than surface receptor binding, sets it apart from other bioregulator peptides.
Comparison of BDNF-boosting peptides
The following comparison helps illustrate the relative strengths and research maturity of each compound. Researchers using the peptide stack calculator can use this information to guide protocol design.
Peptide | Primary mechanism | BDNF effect | Administration | Research maturity |
|---|---|---|---|---|
Semax | Neurotrophic modulation | 1.4-fold protein, 3-fold mRNA | Intranasal | Clinical use (Russia) |
Selank | GABAergic + neurotrophic | Rapid hippocampal elevation | Intranasal | Clinical use (Russia) |
Noopept | NGF + BDNF upregulation | Chronic hippocampal increase | Oral, sublingual | Clinical use (Russia) |
Dihexa | HGF/c-Met potentiation | 10M x more potent synaptogenesis | Oral, injection | Preclinical only |
PE-22-28 | TrkB + TREK-1 modulation | Rapid hippocampal increase | Subcutaneous | Preclinical only |
Pinealon | Gene expression modulation | NGF + BDNF upregulation | Oral, intranasal | Preclinical |
Natural strategies to increase BDNF
Peptides are powerful tools, but they work best as part of a comprehensive approach. The most effective BDNF optimization protocols combine targeted compounds with lifestyle strategies that have strong scientific support. Some of these natural approaches rival or exceed the BDNF-boosting effects of individual supplements.
Exercise: the strongest natural BDNF booster
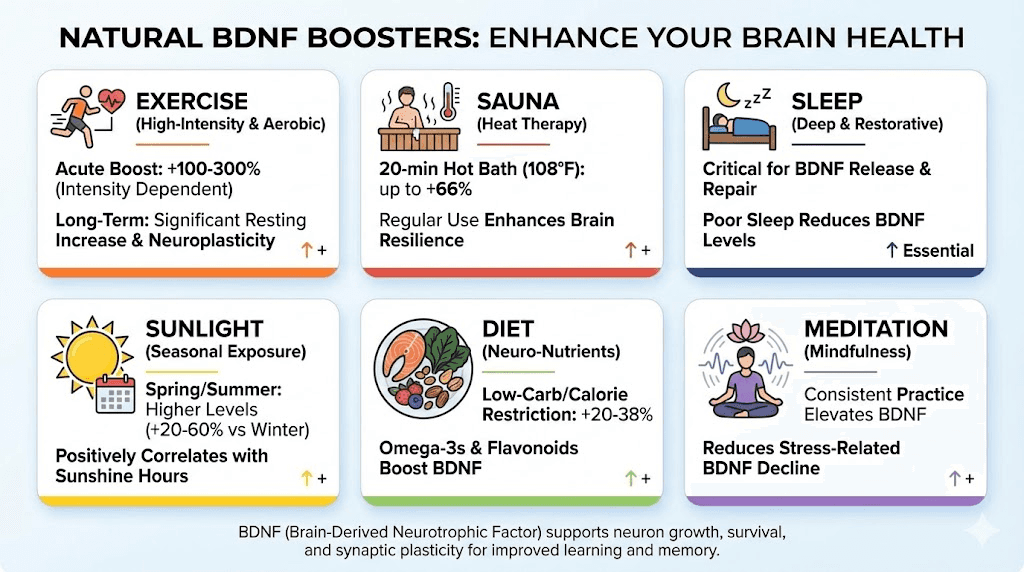
No intervention in the scientific literature matches the consistency and magnitude of exercise for increasing BDNF. The evidence is overwhelming across study types, populations, and exercise modalities.
High-intensity interval training (HIIT) produces the most dramatic acute increases. A study published in PLoS ONE found that six 40-second sprints increased plasma BDNF four to five times compared to steady low-intensity cycling. Moderate-to-vigorous cardio sessions lasting 30 to 40 minutes elevate circulating BDNF by roughly 30 percent, and these elevated levels persist for up to 24 hours.
The mechanism involves a muscle-brain communication pathway. During intense exercise, skeletal muscle produces lactate and BDNF itself. Lactate enters the bloodstream, crosses the blood-brain barrier, and triggers BDNF gene expression in hippocampal neurons. This is an elegant example of how the body connects physical effort to cognitive capacity. For researchers combining exercise with performance peptides, understanding this mechanism helps explain why exercise amplifies the cognitive benefits of compounds like Semax and Selank.
In older adults, six months of consistent dance-based aerobic training increased hippocampal volume and blood BDNF levels, demonstrating that the benefits extend well beyond young, fit populations. The message is clear: regular physical activity is the foundation upon which every other BDNF strategy builds.
Heat therapy
Sauna use and hot water immersion produce significant BDNF increases through heat-shock protein activation and core body temperature elevation. The most cited finding comes from a study of head-out hot water immersion: 20 minutes at 42 degrees Celsius (about 108 degrees Fahrenheit) increased serum BDNF by 66 percent while simultaneously reducing cortisol levels. Core body temperature rose to 39.5 degrees Celsius during this period.
Finnish sauna research, drawn from large population cohorts, has shown that sauna bathing four to seven times per week is associated with a 66 percent reduction in dementia risk. While this association is not solely attributable to BDNF, the neurotrophin pathway is a leading mechanistic candidate. Heat-shock proteins (HSPs) activated during sauna sessions stabilize cellular proteins, repair cellular damage, and influence BDNF synthesis and release. The combination of exercise followed by sauna use has been shown to increase BDNF expression beyond what either intervention achieves alone.
Sleep
Adequate sleep is essential for BDNF-dependent memory consolidation. During sleep, the hippocampus replays and consolidates memories formed during the day, a process that requires BDNF signaling. Sleep deprivation reduces BDNF expression and impairs LTP. The Val66Met polymorphism data shows that Met carriers experience stronger overnight memory decay, suggesting that sleep-dependent BDNF processes are genetically modulated. For anyone pursuing a comprehensive cognitive protocol, sleep optimization should be non-negotiable. Resources on sleep-supporting peptides like DSIP and their dosage protocols can complement sleep hygiene practices.
Sunlight exposure
Sunlight exposure influences BDNF through multiple pathways, including vitamin D synthesis, serotonin modulation, and circadian rhythm regulation. Studies show seasonal variation in serum BDNF levels, with higher concentrations during spring and summer months when sunlight exposure is greatest. Morning sunlight exposure appears particularly beneficial for setting circadian rhythms that support optimal BDNF cycling throughout the day.
Diet and specific nutrients
Omega-3 fatty acids, particularly DHA, support BDNF expression through their role in neuronal membrane composition and anti-inflammatory signaling. Flavonoids found in berries, dark chocolate, and green tea have demonstrated BDNF-boosting effects in multiple studies. Curcumin, the active compound in turmeric, increases BDNF in the hippocampus and has shown synergistic effects when combined with exercise. Caloric restriction and carbohydrate reduction also produce robust BDNF increases. When dietary modifications were combined with high-intensity interval training in one study, serum BDNF levels increased by 38 percent.
A balanced approach to nutrition that emphasizes whole foods, adequate omega-3 intake, and strategic fasting windows provides a solid dietary foundation for BDNF optimization.
Meditation and mindfulness
Regular meditation practice increases BDNF. In one study, participants who completed an eight-week mental training program showed a 26 percent increase in plasma BDNF, compared to a 13 percent decline in the control group. The mechanism likely involves stress reduction (lowering cortisol that suppresses BDNF) combined with attentional training that directly engages BDNF-dependent hippocampal and prefrontal circuits.
Intermittent fasting
Caloric restriction and intermittent fasting increase BDNF expression, likely through metabolic stress pathways that trigger adaptive neuronal responses. The BDNF increase from fasting appears to be related to the switch from glucose to ketone metabolism, as ketone bodies like beta-hydroxybutyrate directly stimulate BDNF gene expression in the hippocampus. This is one reason many cognitive researchers combine intermittent fasting with their peptide cycling strategies.
Nootropic supplements that support BDNF
Between peptide research and basic lifestyle optimization, a middle ground exists: natural supplements with demonstrated effects on BDNF expression. These compounds are widely available, generally well-tolerated, and backed by varying degrees of clinical evidence. They can complement a broader peptide research protocol or serve as standalone cognitive support.
Lion mane mushroom
Hericium erinaceus contains hericenones and erinacines, bioactive compounds that stimulate NGF synthesis and appear to support BDNF expression. A pilot study in young adults found that lion mane supplementation improved processing speed, and researchers attributed this partly to BDNF-like neurotrophic effects. In older adults with mild cognitive impairment, 16 weeks of 3 g/day supplementation produced measurable cognitive improvements, though benefits did not persist after discontinuation. This suggests that lion mane works through ongoing neurotrophic stimulation rather than permanent structural changes.
Rhodiola rosea
Rhodiola is an adaptogenic herb that improves mental performance under stress. Its effects on BDNF are believed to be mediated through cortisol reduction and modulation of stress-response pathways. By lowering the cortisol-driven suppression of BDNF, Rhodiola helps maintain neurotrophic signaling during periods of chronic stress. For researchers exploring cognitive energy and resilience, Rhodiola provides a well-studied herbal option.
Bacopa monnieri
Bacopa has been used in Ayurvedic medicine for centuries to support memory and learning. Modern research confirms that bacosides, the active compounds in Bacopa, protect hippocampal neurons against oxidative damage and amyloid-beta toxicity. In clinical trials, 150 mg of standardized Bacopa extract twice daily for six weeks significantly improved attention, working memory, and logical memory compared to placebo. The neuroprotective effects align with BDNF-supporting mechanisms, including reduced hippocampal lipid peroxidation.
L-theanine
Found naturally in green tea, L-theanine promotes relaxation without sedation and has been shown to increase BDNF expression in animal studies. Its effects on alpha brain wave production and glutamate modulation provide additional cognitive benefits that complement BDNF-driven neuroplasticity.
When combined with caffeine, L-theanine produces improvements in attention and task-switching that exceed either compound alone.
Curcumin
The primary active compound in turmeric, curcumin increases BDNF expression in the hippocampus through multiple pathways. It has anti-inflammatory and antioxidant properties that protect the neuronal environment in which BDNF operates. Bioavailability is a significant challenge with standard curcumin supplements, so formulations using piperine or lipid-based delivery systems are preferred. When paired with exercise, curcumin shows synergistic BDNF-boosting effects.
Ginkgo biloba
Ginkgo has been studied extensively for cognitive benefits in aging populations. Its effects include improved cerebral blood flow, antioxidant activity, and modulation of neurotransmitter systems. Some research suggests Ginkgo increases BDNF expression, though this effect may be secondary to its broader neuroprotective mechanisms rather than a direct neurotrophic action.
Pterostilbene
A methylated derivative of resveratrol found in blueberries, pterostilbene has demonstrated superior bioavailability compared to resveratrol and shows BDNF-supporting effects in animal models. Its ability to cross the blood-brain barrier more efficiently than resveratrol makes it a more practical choice for targeting brain BDNF pathways.
Maritime pine bark extract
Pycnogenol, extracted from maritime pine bark, contains procyanidins that improve endothelial function and cerebral blood flow. Animal studies suggest it increases BDNF expression, and human trials have shown cognitive benefits including improved attention, memory, and processing speed. The vascular improvements may enhance BDNF delivery to brain regions by improving blood flow to the hippocampus and cortex.
For researchers building comprehensive cognitive protocols, combining one or more of these supplements with targeted peptide dosages and consistent lifestyle practices creates a multi-layered approach to BDNF optimization. SeekPeptides members access detailed guides on integrating natural supplements with peptide protocols for maximum cognitive benefit.
BDNF peptide research protocols
Research protocols involving BDNF-modulating peptides vary considerably depending on the compound, the route of administration, and the research objectives. The following information summarizes what has been published in the scientific literature. All protocols described here are based on animal studies or limited clinical data and are presented for educational purposes.
LM22A-4 dosing in research
In the published literature, LM22A-4 has been administered intranasally to adult mice at 0.22 mg/kg per day for seven days. This protocol activated TrkB, AKT, and ERK signaling in vivo. Cell culture studies typically use concentrations of 500 nM to achieve maximal TrkB activation, with the EC50 in the 200 to 500 pM range. Researchers studying LM22A-4 should note that it is most commonly delivered intranasally in animal models, bypassing the blood-brain barrier through the olfactory pathway. No human dosing protocols have been established.
GSB-106 dosing in research
GSB-106 has been tested in rats at doses of 0.1 and 1 mg/kg intraperitoneally for acute antidepressant effects. For chronic administration, the mouse model used 0.1 mg/kg orally for 21 days. The effective concentration in cellular assays is 10 to 100 nM, with loss of efficacy at higher concentrations. This inverted-U dose-response pattern is important: more is not better with GSB-106. Those familiar with general peptide dosing principles will recognize this pattern from other receptor-mediated compounds.
Semax protocols for BDNF enhancement
Semax has the most clinical data of any BDNF-boosting peptide. In Russia, the standard clinical protocol uses a 0.1 percent nasal solution, with 1 to 2 drops administered two times per day for 5 to 14 days for cognitive enhancement. For stroke patients, a 1 percent solution is used at 2 to 4 drops, 3 to 4 times per day for up to 16 days. The research dose that produced the documented 1.4-fold BDNF increase was 50 mcg/kg body weight administered intranasally. Cycling is recommended, with breaks of 1 to 3 months between use periods to maintain receptor sensitivity. For specific dosing guidance, the Semax dosage guide provides detailed protocols.
Selank protocols
The effective anxiolytic and BDNF-modulating dose of Selank in animal research is 300 mcg/kg administered intranasally. Clinical protocols in Russia typically use intranasal delivery. In the ethanol memory impairment study, 0.3 mg/kg per day for seven days was sufficient to produce cognitive-stimulating effects and normalize BDNF levels. The Selank dosage guide covers both intranasal and subcutaneous protocols in detail.
Safety considerations for BDNF modulation
Any compound that activates TrkB signaling carries theoretical risks. Excessive TrkB activation could alter seizure thresholds, though PE-22-28 appears to have seizure-protective properties that somewhat mitigate this concern. The HGF/c-Met pathway activated by Dihexa is involved in cancer progression, making long-term safety a significant unknown. Proper peptide storage and reconstitution practices are essential for maintaining compound integrity and ensuring consistent dosing.
Stacking approaches
In preclinical and anecdotal settings, BDNF-boosting peptides are sometimes combined. A Semax plus Selank stack combines neurotrophic upregulation with anxiolytic effects, creating a protocol that addresses both cognitive performance and emotional regulation. Adding Noopept provides additional NGF support alongside BDNF. These combinations should be approached with caution, as the interactions between multiple TrkB-modulating compounds have not been systematically studied in clinical settings. The peptide stack calculator and the comprehensive stacking guide can help researchers plan multi-compound protocols with proper timing and dosing.
BDNF and specific health conditions
BDNF is not merely a cognitive performance molecule. Its deficiency is implicated in several serious health conditions, and understanding these connections can inform research priorities and protocol design.
Depression and mood disorders
The evidence linking low BDNF to depression is extensive. Serum BDNF levels are consistently lower in patients with major depressive disorder compared to healthy controls, and successful antidepressant treatment normalizes these levels. The hippocampal atrophy observed in chronic depression corresponds to reduced BDNF signaling and impaired neurogenesis. This is not merely correlational. Animal studies show that direct BDNF infusion into the hippocampus produces antidepressant effects, while blocking BDNF signaling prevents antidepressants from working. The success of GSB-106 in reversing depressive behaviors in animal models further supports BDNF as a causal factor. Researchers exploring mood-related peptide protocols should prioritize BDNF-boosting compounds.
Anxiety disorders
BDNF is essential for fear extinction, the process by which the brain learns that a previously threatening stimulus is safe. Individuals with the Val66Met Met allele show impaired fear extinction, and carriers of this allele respond more poorly to exposure-based therapies for anxiety disorders. Low hippocampal BDNF is associated with heightened amygdala reactivity and persistent anxiety responses. Selank and Semax both address anxiety through BDNF-dependent mechanisms, making them relevant research targets for anxiety-related investigations.
Alzheimer disease
BDNF depletion in Alzheimer disease is well-documented and appears to precede symptomatic decline. Reduced BDNF levels correlate with amyloid-beta accumulation, tau pathology, and neuronal loss in the hippocampus and cortex. Interestingly, early-stage Alzheimer patients sometimes show elevated serum BDNF, likely as a compensatory mechanism. As the disease progresses and compensatory mechanisms fail, BDNF levels drop significantly. LM22A-4 has shown neuroprotective effects in Alzheimer cell culture models, and Dihexa restored learning and memory in rats with scopolamine-induced cognitive impairment. The connection between BDNF and Alzheimer disease makes this one of the most active areas of peptide research.
Parkinson disease
BDNF deficiency in Parkinson disease is concentrated in the substantia nigra and striatum, the brain regions most affected by dopaminergic neuron loss. Reduced BDNF signaling may accelerate the degeneration of these neurons and impair the compensatory mechanisms that maintain motor function in early disease. Intranasal Noopept has shown promise in reversing motor symptoms and neurodegeneration in PINK1-knockout rats (a genetic model of Parkinson disease), with therapeutic effects associated with increased brain levels of BDNF and NGF.
Traumatic brain injury
Following traumatic brain injury, BDNF levels initially spike as part of the acute neuroprotective response, then decline as secondary injury processes unfold. Maintaining adequate BDNF signaling during the recovery window is critical for preserving neuronal survival and promoting axonal regeneration. LM22A-4 has demonstrated neuroprotection and myelin preservation in a mouse model of pediatric traumatic brain injury. For those researching tissue repair peptides, the BDNF connection to brain injury recovery adds important context.
Chronic pain
BDNF plays a complex role in pain processing. In the spinal cord, BDNF released from microglia can amplify pain signals by enhancing excitatory synaptic transmission. However, in supraspinal regions, BDNF supports the descending pain inhibitory pathways that dampen pain perception. This dual role means that BDNF modulation for pain requires careful consideration of the target tissue. Researchers working with pain-management peptides should understand this complexity before incorporating BDNF-boosting compounds into chronic pain protocols.
How to test and monitor BDNF levels
Measuring BDNF is possible, practical, and increasingly accessible. However, interpreting the results requires understanding the methodology limitations and normal reference ranges.
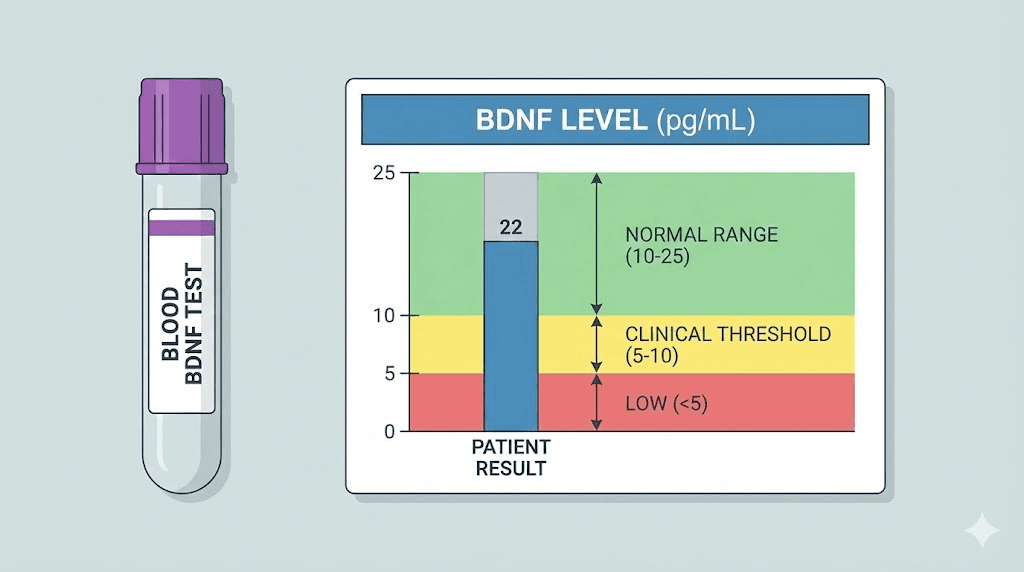
Blood serum testing
BDNF can be measured in serum, plasma, or whole blood using enzyme-linked immunosorbent assay (ELISA). Serum is the most common sample type, though it yields concentrations approximately tenfold higher than plasma because BDNF stored in platelets is released during the clotting process. This means serum and plasma values are not directly comparable, and consistency in sample type is essential for tracking changes over time.
Research has shown that blood BDNF levels correlate with brain tissue BDNF levels across species. In rats, whole-blood BDNF correlated with hippocampal BDNF (r squared = 0.44), and similar correlations were observed in pigs for plasma BDNF and hippocampal BDNF. While the correlation is not perfect, peripheral blood BDNF serves as a reasonable proxy for central nervous system BDNF status.
What the levels mean
A large validation study of 259 healthy volunteers found a mean serum BDNF value of 32.69 ng/mL with a standard deviation of 8.33. These levels remained stable over a 12-month period, suggesting that BDNF is a reliable baseline biomarker when measured consistently. No significant gender differences were observed, though a weak positive correlation with age was noted.
Lower-than-average serum BDNF is associated with cognitive impairment severity, and decreasing levels correlate with advancing Alzheimer disease. However, interpreting individual results requires caution. BDNF levels fluctuate based on time of day, recent exercise, recent meals, sleep quality, and stress levels. A single measurement provides limited information. Serial measurements over time, ideally taken under consistent conditions (same time of day, same fasting state, same exercise window), give a much more meaningful picture.
Tracking changes
For researchers monitoring the effects of peptide protocols, lifestyle interventions, or supplement regimens on BDNF, the following approach maximizes the value of blood testing. Take baseline measurements before starting any intervention, ideally two samples one to two weeks apart to establish individual variability. Then measure at regular intervals (every four to eight weeks) under identical conditions. A 20 percent change from baseline is generally considered meaningful based on power analysis of the published data, but detecting this requires sufficient sample consistency.
Pre-analytical factors significantly affect results. Blood should be drawn into appropriate tubes, left to coagulate for 30 minutes at room temperature, centrifuged at 2,000 times gravity for 10 minutes, and stored at minus 80 degrees Celsius within one hour of collection. The choice of ELISA kit also matters. Studies comparing six commercially available kits found inter-assay variations ranging from 5 to 20 percent. For consistent tracking, use the same laboratory and the same kit for all measurements. Those exploring comprehensive testing alongside their peptide testing protocols should discuss BDNF measurement with their laboratory provider to ensure proper sample handling.
BDNF stacking strategies with other peptides
Combining peptides that work through complementary mechanisms is a common approach in research settings. For BDNF optimization specifically, several stacking strategies have theoretical and preliminary experimental support.
Semax plus Selank stack
This is the most commonly discussed BDNF-focused stack. Semax provides direct neurotrophic stimulation and BDNF upregulation, while Selank adds anxiolytic effects through GABAergic modulation and additional BDNF support through serotonergic pathways. Both are administered intranasally, simplifying the protocol. The combined effect targets cognition and emotional regulation simultaneously, addressing both the "performance" and "resilience" dimensions of brain health. Researchers designing this stack can reference the comprehensive stacking guide for timing and dosing considerations.
Adding Noopept to the stack
Noopept contributes NGF upregulation alongside its BDNF effects, broadening the neurotrophic support profile. Its oral or sublingual administration makes it convenient to add to an intranasal Semax/Selank protocol without requiring additional delivery methods. The chronic nature of Noopept benefits (strengthening over 28 days without tolerance) complements the more acute effects of Semax. However, the combined effect of multiple BDNF-boosting compounds on total TrkB activation has not been systematically studied, so conservative dosing is advisable.
Dihexa considerations
Including Dihexa in a BDNF-focused stack adds a mechanistically distinct pathway (HGF/c-Met) to the mix. This creates a multi-target approach: TrkB activation through BDNF mimetics or upregulators, plus c-Met activation through Dihexa.
The theoretical advantage is that synaptogenesis and neuroplasticity are supported through independent signaling cascades, potentially producing greater effects than either pathway alone. The significant caution is that the oncogenic potential of sustained c-Met activation remains unstudied in long-term models.
Researchers interested in Dihexa should review its full research profile and understand the risk-benefit considerations before inclusion.
Combining peptides with non-peptide approaches
The most practical BDNF stacking strategy may not involve multiple peptides at all. Combining a single well-characterized BDNF-boosting peptide (such as Semax) with lifestyle interventions creates a protocol with strong scientific support and manageable complexity. For example:
Semax (intranasal, cycled) for direct BDNF upregulation
30 to 40 minutes of moderate-to-vigorous cardio, 4 to 5 times per week
Heat therapy (sauna or hot bath) 3 to 4 times per week
Lion mane mushroom supplementation (3 g/day)
Omega-3 supplementation (2 to 3 g/day with high DHA)
8 hours of quality sleep per night
Meditation or mindfulness practice (15 to 20 minutes daily)
This layered approach targets BDNF through multiple independent mechanisms, reducing dependence on any single intervention and creating redundancy that protects against the diminishing returns of receptor desensitization. SeekPeptides provides comprehensive protocol builders that help researchers integrate these approaches into coherent, evidence-based regimens.
For those looking at broader peptide protocols that include growth hormone, recovery, or inflammation management alongside cognitive optimization, the key is to ensure that the BDNF-boosting components do not interfere with other compounds. Timing separation between different peptide administrations is usually sufficient. Using the peptide reconstitution calculator ensures that each compound is prepared correctly, and the peptide cost calculator helps researchers budget multi-compound protocols effectively.
Safety considerations and side effects
BDNF modulation is not without risks. Understanding the safety landscape is essential for responsible research.
Research status of BDNF mimetics
LM22A-4 and GSB-106 remain in preclinical development. No human clinical trials have been published for either compound. Safety data is limited to cell culture and animal studies, which cannot fully predict human tolerability. The peptides that boost BDNF indirectly (Semax, Selank, Noopept) have more extensive safety profiles, including clinical use in Russia, but international regulatory approval remains lacking. Anyone involved in peptide research should review the legal status of peptides in their jurisdiction and understand the distinction between research and pharmaceutical peptides.
TrkB over-activation risks
The TrkB receptor evolved to operate within a specific dynamic range. Excessive activation could theoretically lower seizure thresholds, promote excitotoxicity, or disrupt the balance between excitatory and inhibitory neurotransmission. In practice, the BDNF mimetics studied so far have not shown seizure-promoting effects at therapeutic doses, and PE-22-28 actually demonstrates seizure-protective properties. However, the long-term consequences of sustained, supraphysiological TrkB activation remain unknown.
Cancer-related concerns with specific compounds
Dihexa activates the HGF/c-Met pathway, which is a known oncogenic signaling axis. HGF and c-Met overexpression correlate with tumorigenesis and metastasis in multiple cancer types. While no studies have directly evaluated the tumorigenic potential of chronic Dihexa use, this theoretical concern cannot be dismissed. Noopept, by contrast, has been specifically evaluated for mitogenic effects and showed no stimulation of cell proliferation in HEK293 or SH-SY5Y cell lines despite its neurotrophic activity.
Drug interactions
BDNF-boosting peptides may interact with medications that affect the same signaling pathways. SSRIs and other antidepressants increase BDNF over time, so combining them with exogenous BDNF modulators could theoretically produce additive or unpredictable effects. Benzodiazepines may interact with Selank at the GABAA receptor. Anyone taking prescription medications should consult with a healthcare provider before incorporating BDNF-modulating compounds into a research protocol. The peptide safety guide covers general interaction principles in greater detail.
Quality and sourcing
The reliability of any peptide protocol depends on compound quality. Research-grade peptides should come from verified sources with third-party testing for purity and identity. Degraded or contaminated peptides can produce inconsistent results or adverse effects unrelated to the compound mechanism. Proper storage conditions are critical: lyophilized peptides should be kept frozen and protected from moisture. Once reconstituted with bacteriostatic water, solutions should be refrigerated and used within the timeframes established for each compound. Understanding refrigerated peptide stability, powder shelf life, and room temperature limitations prevents wasted material and ensures consistent dosing. The peptide vendor guide and testing laboratory guide are essential resources for verifying compound quality.
Frequently asked questions
What is the fastest way to increase BDNF naturally?
High-intensity interval training produces the most dramatic acute BDNF increase of any natural intervention. Six 40-second sprints have been shown to increase plasma BDNF four to five times compared to low-intensity activity. Combining exercise with heat therapy (sauna or hot bath at 108 degrees Fahrenheit for 20 minutes) can further amplify the effect. For sustained elevation, regular moderate-to-vigorous cardio lasting 30 to 40 minutes, performed four to five times weekly, maintains elevated BDNF for up to 24 hours after each session. The athletic performance resources at SeekPeptides cover exercise protocols that complement BDNF optimization.
Can you take BDNF as a supplement?
Full-length BDNF protein cannot be taken as a supplement because it does not cross the blood-brain barrier and breaks down rapidly in the digestive system. However, BDNF mimetic peptides like LM22A-4, GSB-106, and PE-22-28 are designed to activate the same TrkB receptors with better bioavailability. Other peptides like Semax and Selank increase the body own BDNF production. Natural supplements like lion mane mushroom, curcumin, and omega-3 fatty acids also support BDNF expression through indirect mechanisms.
How long does it take for BDNF levels to increase with peptides?
The timeline varies by compound. Semax produces measurable BDNF increases within 90 minutes of a single intranasal dose. PE-22-28 demonstrates rapid BDNF elevation in the hippocampus within days. Noopept effects strengthen over 28 days of chronic administration. Natural interventions like exercise produce acute BDNF spikes within minutes that last up to 24 hours, while sustained lifestyle changes (regular exercise, diet optimization) produce baseline elevation over several weeks. Tracking progress with periodic blood tests helps determine individual response timelines.
Does low BDNF cause depression?
The relationship is more nuanced than simple causation. Low BDNF is consistently associated with depression, and the neurotrophin hypothesis proposes that BDNF deficiency contributes to depressive pathology. Direct BDNF infusion into the hippocampus produces antidepressant effects in animals, and nearly all effective antidepressants increase BDNF over time. However, depression is a complex condition with multiple contributing factors, and low BDNF alone is unlikely to be the sole cause. It is better understood as a significant vulnerability factor that interacts with genetics, stress exposure, and neuroinflammation to influence mood regulation.
Is BDNF testing worth it?
BDNF testing provides useful baseline data and can help track the effectiveness of interventions over time. The mean normal serum level in healthy adults is approximately 33 ng/mL. Serial measurements taken under consistent conditions (same time of day, same fasting state) are more informative than single measurements. The main limitation is assay variability between different testing kits and laboratories, so using the same testing provider consistently is essential. For researchers monitoring peptide results, BDNF levels offer one objective biomarker among several to assess cognitive protocol effectiveness.
What is the Val66Met polymorphism and should I test for it?
Val66Met is a common genetic variant in the BDNF gene that affects how much BDNF the brain releases during learning and exercise. Approximately 20 to 30 percent of Caucasian populations and up to 50 percent of Asian populations carry the Met allele. Met carriers produce less activity-dependent BDNF and may show poorer episodic memory performance and steeper age-related cognitive decline. Testing is available through consumer genomics services. Knowing your status can inform how aggressively you pursue BDNF-boosting strategies, though exercise, peptides, and lifestyle interventions benefit all genotypes.
Can BDNF peptides help with Alzheimer disease?
Preclinical evidence is encouraging but preliminary. LM22A-4 has protected neurons in Alzheimer cell culture models. Dihexa restored spatial learning and memory in rats with chemically induced cognitive impairment. Noopept increased BDNF expression in the hippocampus and decreased stress-induced kinases relevant to Alzheimer pathology. However, none of these compounds have been tested in human Alzheimer patients, and the transition from animal models to human clinical outcomes is notoriously difficult in neurodegenerative disease. The current state of peptide research suggests promise but not proof.
How many peptides can you combine for BDNF optimization?
There is no established limit, but practical considerations favor simpler stacks. Two to three BDNF-modulating peptides (such as Semax plus Selank, with or without Noopept) provide complementary mechanisms without excessive complexity. Adding lifestyle interventions (exercise, heat therapy, targeted supplementation) creates additional BDNF support through independent pathways. The guide on combining multiple peptides covers general stacking principles, and the cycling guide explains how to rotate compounds to maintain receptor sensitivity.
For researchers committed to optimizing their cognitive potential through evidence-based protocols, SeekPeptides offers the most comprehensive peptide education platform available.
Members access detailed BDNF-boosting protocols, expert-reviewed dosing guides, advanced stacking strategies, and a community of experienced researchers who have navigated these exact questions. From understanding what peptides are used for to building sophisticated cycling plans, SeekPeptides provides the resources, tools, and community that serious researchers need to make informed, confident decisions about their cognitive health journey.
In case I do not see you, good afternoon, good evening, and good night. Join us.



