Jan 17, 2026

Your body has been fighting a quiet war. Every ache in your joints. Every flare-up of skin irritation. Every gut issue that refuses to resolve. These are not random symptoms. They are signals. And at the center of nearly every chronic health challenge sits one common enemy: inflammation.
Inflammation serves a purpose. When you cut your finger or catch a cold, the inflammatory response rushes immune cells to the scene. Redness appears. Swelling occurs. Heat radiates. These are signs that your body is working exactly as designed. The problem begins when this acute response becomes chronic, when the alarm system that should turn off after the threat passes decides to keep ringing indefinitely.
This is where KPV enters the conversation. A tripeptide derived from alpha-melanocyte stimulating hormone, KPV has emerged from research laboratories as one of the most promising anti-inflammatory compounds studied in recent years. Unlike broad-spectrum immunosuppressants that dampen your entire immune system, KPV appears to work with surgical precision. It targets the specific pathways that drive chronic inflammation while leaving your essential immune defenses intact.
The research is compelling. Studies published in Gastroenterology demonstrate KPV's ability to reduce intestinal inflammation through a unique transport mechanism. Investigations in bronchial epithelial cells show dose-dependent inhibition of key inflammatory markers. And animal models of conditions ranging from colitis to arthritis reveal anti-inflammatory effects comparable to corticosteroids, but without the troubling side effects that limit long-term steroid use.
This guide examines everything researchers and practitioners need to understand about KPV for inflammation. We will explore the molecular mechanisms that make this peptide work, the specific conditions where research shows promise, the dosing protocols that have emerged from clinical experience, and the practical considerations for anyone investigating this compound. Whether you are a researcher designing studies, a clinician exploring options for patients, or simply someone seeking to understand the science behind peptide therapy, the information that follows will provide the foundation you need.
Let us begin with the fundamentals. What exactly is KPV, and why has it captured the attention of the scientific community?
Understanding KPV: from alpha-MSH to targeted anti-inflammatory
The story of KPV begins with a larger molecule called alpha-melanocyte stimulating hormone. Alpha-MSH is a 13-amino acid peptide produced naturally in your body. It plays roles in pigmentation, appetite regulation, and importantly for our discussion, immune modulation. Scientists studying alpha-MSH noticed something interesting. The full molecule had powerful anti-inflammatory effects, but so did a much smaller fragment from its tail end.
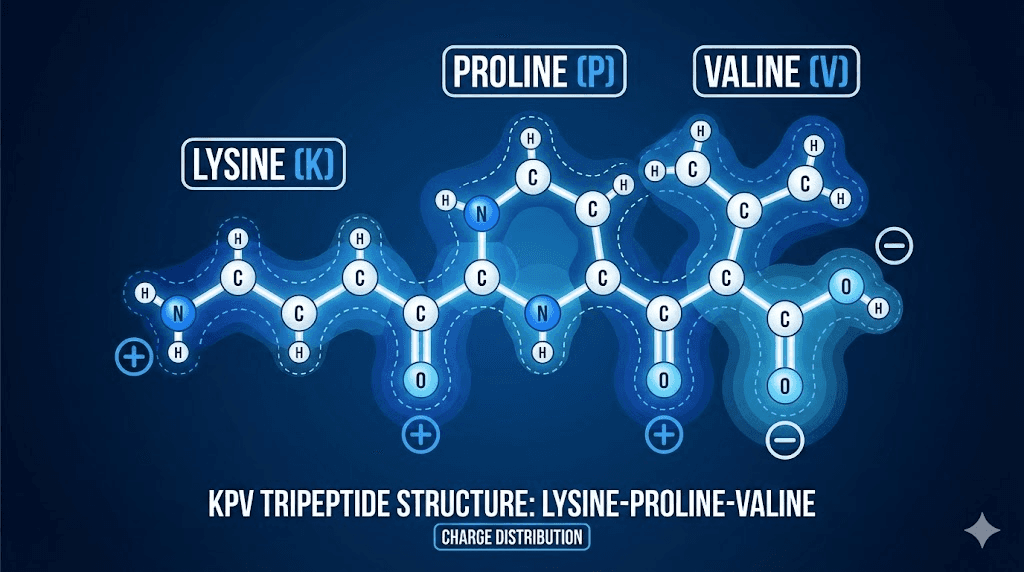
That fragment is KPV. Just three amino acids long, lysine-proline-valine, this tripeptide retains the anti-inflammatory potency of its parent molecule while shedding many of the broader hormonal effects. This makes KPV particularly attractive for research. You get targeted inflammation control without significantly affecting pigmentation or the other processes that full alpha-MSH influences. For researchers exploring what peptides are and how they work, KPV represents an elegant example of how molecular fragments can be more useful than their parent compounds.
The structure matters. Lysine provides a positive charge that influences how the peptide interacts with cell membranes and transport proteins. Proline creates a rigid kink in the molecular structure that affects binding properties. Valine adds hydrophobic character that influences tissue distribution. Together, these three amino acids create a compound small enough to cross biological barriers yet potent enough to modulate complex inflammatory cascades.
What sets KPV apart from other anti-inflammatory compounds? The answer lies in its mechanism. Most anti-inflammatory drugs work by blocking prostaglandins, inhibiting specific enzymes, or broadly suppressing immune function. KPV takes a different approach. It enters cells through specific transport proteins and directly influences the master switches that control inflammatory gene expression. This allows for more precise modulation of the inflammatory response.
The research community has taken notice. Publications in journals ranging from Gastroenterology to the British Journal of Pharmacology have explored KPV's mechanisms and therapeutic potential. This is not fringe science. These are peer-reviewed investigations from major research institutions, building a body of evidence that continues to grow. For those interested in peptide research and studies, KPV offers a fascinating case study in how basic science discoveries translate toward clinical applications.
The molecular machinery: how KPV fights inflammation
To understand why KPV works, you need to understand NF-kappa B. This transcription factor acts as a master switch for inflammation throughout your body. When activated, NF-kB moves into the cell nucleus and turns on genes that produce inflammatory cytokines, adhesion molecules, and other mediators that perpetuate the inflammatory response. In acute situations, this is helpful. In chronic inflammation, NF-kB activation becomes part of the problem.
KPV interrupts this process at multiple points. Research published in the British Journal of Pharmacology demonstrates that KPV promotes the stabilization of IkB-alpha, the protein that normally keeps NF-kB locked in the cytoplasm. When IkB-alpha remains stable, NF-kB cannot translocate to the nucleus. The inflammatory genes stay silent. The cytokine cascade never begins.
But that is not the only mechanism. The same research revealed something even more interesting. KPV accumulates in the cell nucleus, where it directly competes with NF-kB for binding to its transcriptional partners. This provides a second layer of inhibition. Even if some NF-kB makes it to the nucleus, KPV is there to block its activity. This dual mechanism, both preventing nuclear translocation and blocking nuclear activity, helps explain why KPV shows such consistent anti-inflammatory effects across different cell types and disease models.
The cytokine effects are measurable. Studies show that KPV treatment reduces production of interleukin-6, interleukin-12, interferon-gamma, and interleukin-1-beta. These are not minor players. IL-6 drives systemic inflammation and is elevated in conditions from rheumatoid arthritis to inflammatory bowel disease. IL-12 promotes Th1 immune responses that can become destructive in autoimmune conditions. Interferon-gamma amplifies inflammation and contributes to tissue damage. IL-1-beta is a key mediator of fever and inflammatory pain.
For practitioners exploring KPV peptide benefits, understanding these mechanisms provides insight into why the compound might help with such diverse inflammatory conditions. The pathways KPV targets are not disease-specific. They are fundamental to inflammation itself. This creates broad therapeutic potential while also raising important questions about appropriate use cases and patient selection.
There is another mechanism worth discussing. In intestinal epithelial cells, KPV enters through a transporter called PepT1. This is the same transporter that handles dietary peptides from protein digestion. Research from Gastroenterology showed that this PepT1-mediated uptake is actually how KPV exerts its anti-inflammatory effects in the gut. The peptide does not need to bind to receptors on the cell surface. It gets transported directly inside, where it can access the inflammatory machinery.
This finding has practical implications. It suggests that oral administration of KPV could be particularly effective for gut inflammation, since the peptide would be taken up directly by intestinal cells expressing PepT1. For systemic effects, subcutaneous or intravenous routes might be necessary to achieve adequate tissue concentrations. Understanding how peptides work at this mechanistic level helps researchers and clinicians make informed decisions about administration routes.
The melanocortin receptor question adds complexity. Early research assumed KPV worked through melanocortin receptors like MC1R, the same receptors that full alpha-MSH activates. But studies using mice with non-functional MC1R showed that KPV retained its anti-inflammatory activity. This indicates that KPV's effects, at least in some contexts, are melanocortin receptor-independent. The PepT1 transport mechanism and direct nuclear effects appear more relevant than receptor binding for inflammation control.
Research evidence: what the studies actually show
Let us examine the evidence directly. Claims about peptide benefits are easy to make. Data is harder to fabricate. What does the peer-reviewed literature actually demonstrate about KPV and inflammation?
The intestinal inflammation research is particularly robust. A study published in Gastroenterology investigated KPV in two different mouse models of colitis: DSS-induced and TNBS-induced. These models mimic different aspects of human inflammatory bowel disease. Oral administration of KPV reduced disease severity in both models. The researchers measured multiple endpoints, including inflammatory cytokine expression, tissue damage scores, and clinical symptoms. KPV showed consistent benefits across all measures.
The mechanistic work in this study went further. Using cell culture systems, the researchers demonstrated that KPV enters epithelial cells through PepT1 and that this transport is essential for its anti-inflammatory effects. They also showed that KPV reduces NF-kB activation and downstream cytokine production in a dose-dependent manner. For anyone researching peptides for gut health, this study provides foundational evidence for KPV's potential in inflammatory bowel conditions.
Airway inflammation research tells a similar story. A study in bronchial epithelial cells found that KPV inhibits NF-kB activation, reduces matrix metalloproteinase-9 activity, and decreases secretion of IL-8 and eotaxin. These are key mediators of airway inflammation and remodeling in conditions like asthma and COPD. The effects were dose-dependent, meaning higher KPV concentrations produced greater anti-inflammatory effects up to a saturation point.
Contact dermatitis models provide evidence for skin applications. Research using mouse models showed that both intravenous and topical KPV could suppress contact dermatitis reactions and induce antigen-specific tolerance. This is significant. Most anti-inflammatory treatments simply suppress symptoms. Inducing tolerance suggests a more fundamental effect on immune regulation that could have implications for autoimmune skin conditions.
The wound healing data deserves attention. A 2006 study in rabbits with corneal wounds found that topical KPV application four times daily resulted in significantly smaller wounds compared to controls after four days of treatment. Wound healing requires a delicate balance between inflammation needed for tissue repair and excessive inflammation that delays healing. KPV appears to modulate this balance favorably.
What about arthritis? Research using animal models of rheumatoid and gouty arthritis demonstrates that alpha-MSH and related peptides including KPV reduce joint inflammation with efficacy comparable to prednisolone. Critically, the peptides did not cause the weight loss commonly seen with steroid treatment. This points toward a potentially better safety profile for long-term use in chronic inflammatory joint conditions.
The comparative data is worth noting. When researchers compared KPV to corticosteroids, the anti-inflammatory effects were similar. But the side effect profiles were not. Steroids cause weight gain, bone loss, immune suppression, and metabolic disturbances with chronic use. KPV, being a naturally-occurring fragment of an endogenous hormone, appears to avoid these problems. This does not mean KPV is without limitations. It means the risk-benefit calculation may favor peptide approaches for certain applications.
Specific inflammatory conditions: where research shows promise
Inflammation manifests differently in different tissues. The same fundamental process, NF-kB activation, cytokine release, immune cell recruitment, produces distinct clinical pictures depending on location. KPV research spans multiple inflammatory conditions. Understanding the evidence for each helps clarify where this peptide might be most useful.
Inflammatory bowel disease
IBD encompasses Crohn's disease and ulcerative colitis. Both involve chronic intestinal inflammation with periods of flare and remission. Current treatments include aminosalicylates, steroids, immunomodulators, and biologics. Each has limitations. Steroids cannot be used long-term. Biologics are expensive and increase infection risk. Many patients cycle through multiple treatments without achieving sustained remission.
The KPV research in IBD is encouraging. The oral route appears effective because of PepT1-mediated uptake in intestinal cells. Animal studies show reduced inflammation, improved tissue architecture, and decreased pro-inflammatory cytokine levels. The mechanisms align with IBD pathophysiology, since NF-kB activation and cytokine overproduction are central to disease progression.
Practitioners exploring peptides for gut health applications often consider KPV for patients with IBD who have not responded adequately to conventional treatments or who cannot tolerate available options. This is an off-label use that requires appropriate clinical judgment and patient education. But the scientific rationale is sound, and anecdotal reports suggest benefit for some individuals.
Psoriasis and eczema
Skin inflammation in psoriasis involves keratinocyte hyperproliferation driven by inflammatory signaling. T cells infiltrate the skin and release cytokines that perpetuate the inflammatory cycle. Conventional treatments range from topical steroids to systemic biologics targeting specific cytokines like IL-17 or IL-23.
KPV research suggests several relevant effects. The peptide reduces inflammatory cytokine production that drives psoriatic inflammation. It may also normalize keratinocyte proliferation. Topical application has shown efficacy in dermatitis models, suggesting potential for direct skin application in psoriasis. For patients seeking alternatives to long-term steroid use, KPV offers a different mechanism without the skin thinning and other adverse effects associated with chronic topical corticosteroid exposure.
Eczema, or atopic dermatitis, involves barrier dysfunction and immune dysregulation. The research showing KPV-induced tolerance in contact dermatitis models is relevant here. If KPV can modulate aberrant immune responses in the skin rather than simply suppressing them, it might offer advantages over standard anti-inflammatory treatments. This remains an active area of investigation.
Rheumatoid arthritis
Joint inflammation in RA involves synovial proliferation, inflammatory cell infiltration, and eventual cartilage and bone destruction. TNF-alpha, IL-6, and other cytokines drive disease progression. Current biologics target these specific cytokines with good efficacy but significant cost and infection risk.
The animal data for KPV and related alpha-MSH peptides in arthritis shows anti-inflammatory effects comparable to prednisolone. The advantage is absence of steroid-related weight and metabolic effects. For researchers interested in peptides for joint pain, KPV represents a mechanistically distinct approach from current treatments. Whether it could serve as monotherapy or as an adjunct to standard disease-modifying treatments remains to be determined through clinical investigation.
The melanocortin receptor MC3R appears particularly relevant for joint inflammation. Studies suggest this receptor subtype mediates anti-inflammatory effects in arthritic tissues. While KPV may not require receptor binding for all its effects, understanding the receptor pharmacology helps predict which inflammatory conditions might respond best.
Neuroinflammation
Brain inflammation contributes to conditions from Alzheimer's disease to multiple sclerosis to depression. Microglia, the brain's resident immune cells, can become chronically activated and produce inflammatory mediators that damage neurons. The blood-brain barrier complicates treatment, since many anti-inflammatory drugs cannot reach the CNS effectively.
Alpha-MSH peptides including KPV have shown anti-inflammatory effects in brain tissue models. The small size of KPV may allow some blood-brain barrier penetration, though this requires further study. For researchers exploring nootropic peptides and brain health applications, KPV's anti-inflammatory mechanisms could be relevant to conditions where neuroinflammation plays a pathogenic role.
Dosing protocols: what research and clinical experience suggest
Dosing information for research peptides comes from multiple sources: in vitro studies that determine effective concentrations, animal pharmacokinetic studies that guide human dose translation, and clinical experience accumulated by practitioners using these compounds. For KPV, the data from these sources provides reasonable starting points for research applications.
Subcutaneous injection protocols
Subcutaneous administration is the most common route for systemic effects. The peptide is injected into fatty tissue, typically in the abdomen, and absorbs into the bloodstream over several hours. This provides sustained levels that allow KPV to reach inflammatory sites throughout the body.
Research protocols commonly use 200-500 micrograms daily. Some practitioners report using doses up to 1 milligram daily for more severe inflammatory conditions. The wide range reflects uncertainty about optimal dosing and individual variation in response. Starting at lower doses and titrating based on response is a reasonable approach. Most practitioners recommend starting around 200 mcg and increasing gradually if needed.
For those familiar with KPV peptide dosing, these ranges will be familiar. The key is matching dose to clinical goals. Lower doses may be sufficient for mild inflammation or maintenance. Higher doses might be needed during active flares or for more recalcitrant conditions.
Oral administration
The PepT1 transport mechanism makes oral KPV particularly interesting for gut inflammation. When you swallow KPV, intestinal epithelial cells take it up directly through their peptide transporters. This provides local delivery to the gut mucosa where inflammation in IBD occurs.
Oral doses are typically higher than injectable doses because of variable absorption. Research suggests 500-1000 mcg daily for oral administration. Some protocols use 1-2 mg daily. The peptide should be taken on an empty stomach to minimize degradation by digestive enzymes and maximize transporter-mediated uptake.
Compounding pharmacies can prepare oral KPV formulations. Capsules or sublingual preparations are common. The sublingual route may provide some systemic absorption in addition to the oral/gut absorption pathway. For researchers investigating injectable peptides versus oral formulations, KPV offers an opportunity to compare routes directly, since both appear effective for different applications.
Topical applications
For skin conditions, topical KPV makes mechanistic sense. The peptide reaches inflamed skin directly without requiring systemic distribution. Research in dermatitis models confirms efficacy of topical application.
Topical formulations typically contain KPV at concentrations of 0.1-0.5%. The peptide is compounded into creams or gels with appropriate bases for skin penetration. Application frequency varies from once to twice daily depending on condition severity. Some practitioners combine topical KPV with other wound-healing or anti-inflammatory peptides in customized formulations.
Reconstitution and storage
KPV typically comes as a lyophilized powder that requires reconstitution before use. The standard approach uses bacteriostatic water. For a 10 mg vial, adding 2-3 mL of bacteriostatic water creates a concentration that allows convenient dosing.
At 3 mL reconstitution volume, a 10 mg vial contains approximately 3.33 mg/mL. On a 100-unit insulin syringe, each unit equals about 33 mcg. This allows precise dosing in the 200-500 mcg range with 6-15 units per injection.
Storage matters for peptide stability. Lyophilized KPV should be stored frozen at -20°C until reconstitution. Once reconstituted, refrigerate at 2-8°C and use within 30 days. Avoid freeze-thaw cycles. Proper storage maintains potency and prevents degradation. For those new to peptides, reviewing a peptide storage guide helps avoid common mistakes that reduce effectiveness.
Cycling considerations
Should KPV be used continuously or cycled? This question lacks definitive answers. Some practitioners recommend continuous use for chronic inflammatory conditions, since the underlying disease process persists. Others suggest periodic breaks of 2-4 weeks after 8-12 weeks of use to prevent any potential adaptation or to assess baseline status.
For acute inflammatory flares, limited-duration treatment makes sense. Use KPV until the flare resolves, then discontinue or reduce to a maintenance dose. For chronic conditions, the decision depends on individual response, underlying disease trajectory, and clinical judgment. Understanding peptide cycling principles helps make informed decisions about treatment duration.
Safety profile and side effects
The safety data for KPV is reassuring, though limited by the absence of large-scale human clinical trials. Being a fragment of an endogenous hormone, KPV benefits from millions of years of evolutionary refinement. Your body already produces and recognizes this sequence as part of alpha-MSH.
What studies show
Animal studies using KPV at research doses show minimal toxicity. No significant organ damage, behavioral changes, or mortality has been reported. The peptide does not appear to accumulate with repeated dosing. It is metabolized and cleared through normal peptide degradation pathways.
Clinical experience from practitioners using KPV reports a favorable tolerability profile. Most users experience no side effects. When side effects occur, they are typically mild and transient: slight fatigue, minor headache, or temporary changes in appetite. These usually resolve without discontinuing treatment.
Comparison to alternatives
Context matters for safety assessment. Compared to what? For gut inflammation, the alternatives are 5-ASA drugs with their GI side effects, steroids with metabolic complications, immunomodulators with infection risk, or biologics with immune suppression and high cost. In this context, KPV's mild side effect profile appears favorable.
For skin conditions, long-term topical steroids cause skin atrophy, telangiectasia, and rebound inflammation. Systemic treatments for severe psoriasis carry significant risks. Topical KPV, if effective, could offer an alternative without these concerns.
For joint inflammation, NSAIDs damage GI mucosa and kidneys with chronic use. Steroids cause the constellation of Cushingoid effects. Biologics provide targeted anti-inflammatory action but suppress immunity and cost thousands of dollars monthly. KPV's different mechanism and natural origin suggest potential for a better tolerability profile in long-term use.
Contraindications and precautions
No absolute contraindications have been established for KPV, largely because formal clinical development has not occurred. Reasonable precautions based on mechanism and general peptide considerations include:
Pregnancy and breastfeeding: Insufficient safety data exists. Avoid use during pregnancy and lactation unless potential benefits clearly outweigh unknown risks.
Active infection: While KPV modulates rather than suppresses immunity, using any anti-inflammatory during active infection raises theoretical concerns. Address infections before starting KPV therapy.
Concurrent immunosuppression: Patients already on immunosuppressive medications should discuss KPV use with their prescribers. Additive effects on immune function are possible.
Autoimmune conditions: Paradoxically, while KPV may help autoimmune inflammation, its immune-modulating effects require careful consideration in complex autoimmune scenarios. Clinical judgment is essential.
For practitioners consulting resources on peptide safety, these general principles apply: start low, go slow, monitor response, and maintain open communication with patients about expected effects and any concerns that arise.
KPV in the context of peptide therapy
KPV does not exist in isolation. The peptide therapy landscape includes dozens of compounds with overlapping and complementary mechanisms. Understanding where KPV fits helps clinicians and researchers design appropriate protocols.
Comparison to BPC-157
BPC-157 is perhaps the most widely used healing peptide. It promotes angiogenesis, fibroblast migration, and tissue repair. While BPC-157 has anti-inflammatory properties, its primary mechanism differs from KPV. BPC-157 works more through growth factor modulation and tissue repair pathways. KPV works directly on inflammatory signaling cascades.
These mechanisms complement rather than duplicate each other. Some practitioners combine KPV and BPC-157 for conditions involving both active inflammation and tissue damage, like inflammatory bowel disease with mucosal ulceration. The BPC-157 guide provides more detail on this healing peptide's mechanisms and applications.
For acute injury with inflammation, BPC-157 might take priority because tissue repair is the primary goal. For chronic inflammation without significant tissue damage, KPV's targeted anti-inflammatory action might be more relevant. For conditions with both elements, combination approaches make mechanistic sense.
Comparison to thymosin beta-4
TB-500, the active fragment of thymosin beta-4, promotes healing through different pathways than either KPV or BPC-157. TB-500 affects actin cytoskeleton dynamics, cell migration, and tissue remodeling. It has anti-inflammatory effects secondary to its primary healing mechanisms.
For systemic inflammation, KPV's direct NF-kB inhibition provides more targeted anti-inflammatory action than TB-500. For localized tissue injury with associated inflammation, TB-500's healing properties combined with its secondary anti-inflammatory effects might be preferred. The TB-500 benefits article explores this peptide's unique mechanism in detail.
Again, combination protocols using multiple peptides with complementary mechanisms represent an emerging approach. The peptide stacking guide provides frameworks for thinking about multi-peptide protocols.
Comparison to conventional anti-inflammatories
How does KPV compare to drugs like ibuprofen or prednisone? The mechanisms differ fundamentally. NSAIDs block prostaglandin synthesis by inhibiting COX enzymes. This reduces one class of inflammatory mediators but does nothing to the upstream NF-kB activation or cytokine production that drives chronic inflammation.
Corticosteroids work more broadly, affecting gene transcription through glucocorticoid receptor activation. They suppress inflammation effectively but also suppress immune function, affect metabolism, and cause numerous long-term side effects.
KPV targets NF-kB specifically. This provides anti-inflammatory effects without broadly suppressing prostaglandins or activating steroid receptor pathways. The selectivity is both a strength and potential limitation. Strength because it avoids off-target effects. Potential limitation because inflammation is complex and targeting one pathway may not fully control disease in all cases.
Practical protocols for research applications
Translating research evidence into practical protocols requires integrating scientific data with clinical experience. The following represents current understanding based on available evidence and practitioner reports. These are research protocols, not medical advice.
Protocol for gut inflammation
For intestinal inflammatory conditions, oral administration exploits the PepT1 uptake mechanism. A reasonable starting protocol:
Week 1-2: 500 mcg orally once daily on empty stomach. Assess tolerance and initial response.
Week 3-4: If response inadequate and tolerance good, increase to 500 mcg twice daily.
Week 5-8: Continue effective dose. Reassess disease status.
Maintenance: If remission achieved, consider reducing to once daily dosing or every-other-day maintenance.
For those familiar with peptide dosing principles, this stepwise approach follows standard titration methodology. The goal is finding the minimum effective dose for the individual patient.
Combining with BPC-157 may provide synergistic benefits for IBD. BPC-157 at 250-500 mcg daily addresses mucosal healing while KPV addresses inflammatory signaling. This combination approach requires appropriate clinical supervision and monitoring.
Protocol for skin inflammation
Topical application provides direct delivery to inflamed skin. Systemic administration may be added for widespread or severe disease.
Topical only: Apply 0.1-0.5% KPV cream or gel to affected areas twice daily. Continue until lesions resolve. Taper to once daily for maintenance.
Combined approach: Topical application as above plus subcutaneous KPV 200-300 mcg daily for systemic anti-inflammatory effect. Consider this for extensive disease or inadequate topical response.
Duration depends on condition. Acute contact dermatitis may respond within days. Chronic psoriasis likely requires ongoing treatment with periodic reassessment.
Protocol for joint inflammation
Subcutaneous administration provides systemic distribution to reach joint tissues. Consider:
Starting dose: 200-300 mcg subcutaneously once daily.
Titration: Increase to 500 mcg daily if response inadequate after 2-4 weeks.
Duration: Continue through acute flare, then reassess. Chronic inflammatory arthritis may warrant ongoing treatment.
Combination with BPC-157 or TB-500 addresses tissue repair alongside inflammation control. The best peptides for tendon repair article discusses additional options for joint and connective tissue support.
Monitoring and assessment
Objective monitoring improves outcomes. For gut inflammation, track stool frequency, consistency, and any blood. For skin conditions, photograph lesions at baseline and intervals. For joint inflammation, assess range of motion, pain levels, and functional status.
Laboratory markers provide additional data. C-reactive protein and erythrocyte sedimentation rate reflect systemic inflammation. Fecal calprotectin specifically indicates gut inflammation. These can help assess response to treatment and guide protocol adjustments.
Clinical timelines vary. Some patients report improvement within days. Others require weeks to see significant change.
The research suggests that most responders notice improvement within 3-7 days of starting treatment. If no response occurs by 4-6 weeks at adequate doses, reassess whether KPV is appropriate for the specific condition being treated.
Sourcing quality KPV
Peptide quality varies dramatically between sources. For research applications, using properly manufactured and tested products is essential. Impure or degraded peptides may be ineffective or potentially harmful.
What to look for
Certificate of analysis: Reputable suppliers provide CoA documentation for each batch. This should show identity confirmation through mass spectrometry, purity assessment through HPLC (look for greater than 98% purity), and absence of contaminants.
Third-party testing: Independent laboratory verification adds confidence. Some suppliers include third-party test results. This is particularly valuable for newer or less established sources.
Manufacturing standards: cGMP (current Good Manufacturing Practice) manufacturing indicates quality control systems are in place. While not all research peptide suppliers have cGMP certification, those that do provide additional assurance.
Reputation and reviews: The peptide community shares information. Sources with consistent quality build positive reputations. New or problematic sources are quickly identified. Researching vendor reputation before purchasing saves problems later.
The peptide vendor guide provides additional criteria for evaluating suppliers. For any peptide including KPV, sourcing quality material is fundamental to research success.
Forms available
Lyophilized powder: The standard form. Stable when stored properly. Requires reconstitution before use. Most flexible for dosing adjustments.
Pre-mixed solutions: Some suppliers offer KPV already reconstituted. Convenient but shorter shelf life and higher cost per milligram.
Compounded preparations: Pharmacies can compound KPV into specific formulations including topical creams, oral capsules, and injectable solutions. These may include additional ingredients for stability or delivery enhancement.
Nasal sprays: Some sources offer KPV nasal spray formulations. The nasal route provides rapid absorption and potentially some CNS penetration. This format may be useful for certain applications though less studied than injection or oral routes.
Integrating KPV with lifestyle approaches
Peptides work within the context of overall health status. KPV can reduce inflammatory signaling, but if underlying drivers of inflammation persist, the peptide is fighting an uphill battle. Addressing root causes enhances peptide therapy outcomes.
Diet and inflammation
What you eat profoundly affects inflammation. Processed foods, refined sugars, and industrial seed oils promote inflammatory pathways. Whole foods, vegetables, quality proteins, and anti-inflammatory fats support resolution of inflammation.
For gut inflammation specifically, eliminating trigger foods is essential. Common culprits include gluten, dairy, and processed foods. An elimination diet followed by systematic reintroduction identifies individual triggers. Combining dietary modification with KPV addresses inflammation from both directions.
Anti-inflammatory dietary patterns like Mediterranean diet principles provide frameworks for food selection. Emphasis on olive oil, fish, vegetables, and minimal processed foods aligns with inflammation reduction goals.
Stress and inflammation
Chronic stress activates inflammatory pathways through cortisol dysregulation and autonomic nervous system imbalance. Stress reduction is not optional for inflammation control. It is mechanistically essential.
Sleep quality matters. Poor sleep elevates inflammatory markers independent of other factors. Prioritizing sleep duration and quality supports inflammatory resolution.
Mind-body practices including meditation, breathwork, and yoga reduce inflammatory markers in research studies. These are not just relaxation techniques. They influence gene expression patterns related to inflammation.
Movement and exercise
Regular moderate exercise is anti-inflammatory. It releases myokines from muscles that counter inflammatory cytokines. It improves insulin sensitivity, which reduces metabolic inflammation. It supports healthy body composition, and adipose tissue is a source of inflammatory mediators.
The key word is moderate. Excessive exercise without adequate recovery creates inflammation rather than reducing it. Balance intensity with recovery. For those with active inflammatory conditions, gentle movement may be more appropriate than intense training during flares.
Environmental factors
Toxin exposure contributes to inflammatory burden. Minimizing exposure to environmental pollutants, choosing clean personal care products, and ensuring adequate detoxification support reduces background inflammation.
These lifestyle factors do not replace peptide therapy. They work synergistically with it. A person using KPV while eating inflammatory foods, not sleeping, and under constant stress will likely see limited benefit. The same person with optimized lifestyle plus KPV creates conditions for maximum therapeutic effect.
The research frontier: where KPV science is heading
Current KPV research represents early exploration of a compound with significant potential. Several directions show promise for future investigation.
Drug delivery innovations
Novel delivery systems could enhance KPV effectiveness. Nanoparticle formulations might improve tissue targeting and extend duration of action. Sustained-release preparations could reduce dosing frequency. Transdermal patches might provide convenient non-invasive administration.
Research on iontophoretic delivery of KPV across microporated skin has already been published. This approach uses electrical current to drive peptide penetration through the skin barrier. Such technologies could make topical KPV more effective for skin conditions and potentially enable transdermal systemic delivery.
Combination therapies
Systematic investigation of KPV in combination with other treatments could expand applications. How does KPV interact with standard IBD medications? Can it reduce needed doses of immunosuppressants? Does it enhance biologic therapy effectiveness?
These questions require controlled trials to answer. But the mechanistic rationale for combinations is strong. KPV's unique mechanism of action suggests potential synergy with treatments working through different pathways.
New therapeutic applications
Inflammation plays a role in conditions beyond those currently studied. Cardiovascular disease, diabetes, neurodegenerative disorders, and even depression have inflammatory components.
KPV's fundamental mechanism could be relevant across these diverse conditions.
Ocular inflammation represents an underexplored area where topical KPV might be valuable. Periodontal disease involves chronic oral inflammation that could potentially respond to local KPV application. Each new application requires dedicated research but builds on the same mechanistic foundation.
Understanding individual variation
Not everyone responds equally to KPV. Understanding why some people respond dramatically while others see minimal benefit would improve patient selection and protocol optimization. Genetic factors in NF-kB signaling, PepT1 expression, or melanocortin receptor variants might predict response.
This personalized medicine approach requires larger datasets and sophisticated analysis. As KPV use expands and outcomes are tracked systematically, patterns may emerge that guide individualized treatment decisions.
Frequently asked questions
How quickly does KPV work for inflammation?
Most research and clinical reports suggest initial effects within 3-7 days. Some people notice improvement sooner. Full therapeutic effect typically develops over 2-4 weeks. Response time depends on condition severity, underlying causes, and individual factors. For acute inflammation, faster responses are common. Chronic inflammatory conditions may require longer treatment before substantial improvement.
Can KPV be used long-term?
Current evidence suggests KPV is safe for extended use. Being a naturally-occurring peptide fragment, it does not accumulate or cause apparent organ toxicity. Many practitioners use KPV continuously for chronic conditions. Others prefer periodic breaks. No definitive data establishes optimal long-term protocols. Clinical judgment based on individual response guides duration decisions.
Does KPV suppress the immune system?
KPV modulates rather than suppresses immunity. It reduces excessive inflammatory responses without broadly dampening immune function. This distinguishes it from corticosteroids and immunosuppressant medications. The selectivity for inflammatory pathways while preserving protective immunity is a key potential advantage. However, any immune-modulating compound should be used thoughtfully, particularly in immunocompromised individuals.
What is the best route of administration?
The best route depends on the condition being addressed. For gut inflammation, oral administration allows direct uptake by intestinal cells through PepT1 transporters. For skin conditions, topical application delivers KPV directly to inflamed tissue. For systemic inflammation affecting joints or multiple sites, subcutaneous injection provides widespread distribution. Some practitioners combine routes for comprehensive coverage.
Can KPV be combined with other peptides?
Yes, KPV can be combined with complementary peptides. BPC-157 addresses tissue healing mechanisms. TB-500 supports tissue repair through different pathways. Combining these with KPV's anti-inflammatory action creates multi-pronged approaches to complex conditions. Consult peptide stacking resources for guidance on combination protocols.
Is KPV the same as alpha-MSH?
No. KPV is the C-terminal tripeptide fragment of alpha-MSH. It retains anti-inflammatory activity but lacks many of alpha-MSH's broader effects including significant melanocyte stimulation. This makes KPV more specifically useful for inflammation without unwanted pigmentation or other hormonal effects. Think of KPV as the anti-inflammatory portion of alpha-MSH extracted for targeted use.
What is the difference between KPV morning or night dosing?
Research does not clearly favor morning versus night administration. Some practitioners prefer morning dosing to align with natural cortisol rhythms. Others prefer evening dosing for convenience or to allow nighttime tissue repair with KPV support. The KPV timing guide discusses considerations in detail. Consistency matters more than specific timing for most people.
Does KPV require a prescription?
KPV is classified as a research peptide, not an FDA-approved medication. It is available without prescription for research purposes. Clinical use falls into a gray area between research and practice. Some practitioners prescribe through compounding pharmacies. Others guide patients to research sources. Regulatory status varies by jurisdiction. Understanding local regulations is important for appropriate use.
How should KPV be stored?
Lyophilized KPV stores frozen at -20°C until reconstitution. Reconstituted peptide requires refrigeration at 2-8°C and should be used within 30 days. Avoid freeze-thaw cycles. Protect from light. Proper storage maintains potency. The peptide storage guide provides comprehensive information on maintaining peptide quality.
Are there any food or drug interactions with KPV?
No significant interactions have been documented. KPV is metabolized through standard peptide degradation pathways. It does not interact with cytochrome P450 enzymes that process most medications. Taking KPV on an empty stomach optimizes absorption, particularly for oral administration. Theoretically, other compounds using PepT1 transport might compete with KPV for uptake, but this has not been shown to be clinically significant.
External resources
These peer-reviewed sources provide additional scientific background on KPV and related research:
PepT1-Mediated Tripeptide KPV Uptake Reduces Intestinal Inflammation - Foundational research published in Gastroenterology demonstrating KPV's mechanism in gut inflammation.
Alpha-MSH Related Peptides: A New Class of Anti-Inflammatory and Immunomodulating Drugs - Comprehensive review of melanocortin peptide anti-inflammatory mechanisms.
Inhibition of Cellular and Systemic Inflammation by Melanocortin-Related Peptides - Research on KPV mechanism in bronchial epithelial cells.
Immobilized Alpha-MSH 10-13 Inhibits TNF-Alpha Stimulated NF-kB Activity - Key study on NF-kB inhibition mechanism.
Dissection of the Anti-Inflammatory Effect of Core and C-Terminal Alpha-MSH Peptides - Research comparing KPV to other alpha-MSH fragments.
Taking the next step with peptide therapy
Inflammation is complex. It involves multiple pathways, numerous cell types, and intricate regulatory networks. KPV offers a targeted approach that differs fundamentally from conventional anti-inflammatory treatments. By directly inhibiting NF-kB activation and reducing pro-inflammatory cytokine production, this tripeptide addresses inflammation at its source rather than simply masking symptoms.
The research is promising. Studies in gut inflammation, skin conditions, airway disease, and arthritis models demonstrate consistent anti-inflammatory effects. The safety profile appears favorable compared to steroids and other immunosuppressive treatments. And the natural origin of KPV, as a fragment of an endogenous hormone, suggests compatibility with normal physiology.
For those exploring peptide options for inflammatory conditions, SeekPeptides provides comprehensive resources to guide your research. Understanding mechanisms, proper protocols, and quality sourcing makes the difference between effective therapy and wasted effort. The getting started with peptides guide offers foundational knowledge for those new to peptide therapy. The peptide dosage chart provides reference information across multiple compounds. And the common peptide mistakes guide helps avoid pitfalls that undermine results.
Knowledge transforms possibility into practice. The information in this guide provides a foundation for understanding KPV's role in inflammation management. What you do with that knowledge depends on your specific situation, goals, and access to appropriate clinical guidance. For personalized protocols tailored to individual inflammatory conditions, SeekPeptides members access detailed guidance that goes beyond general information.
In case I don't see you, good afternoon, good evening, and good night.