Jan 5, 2026

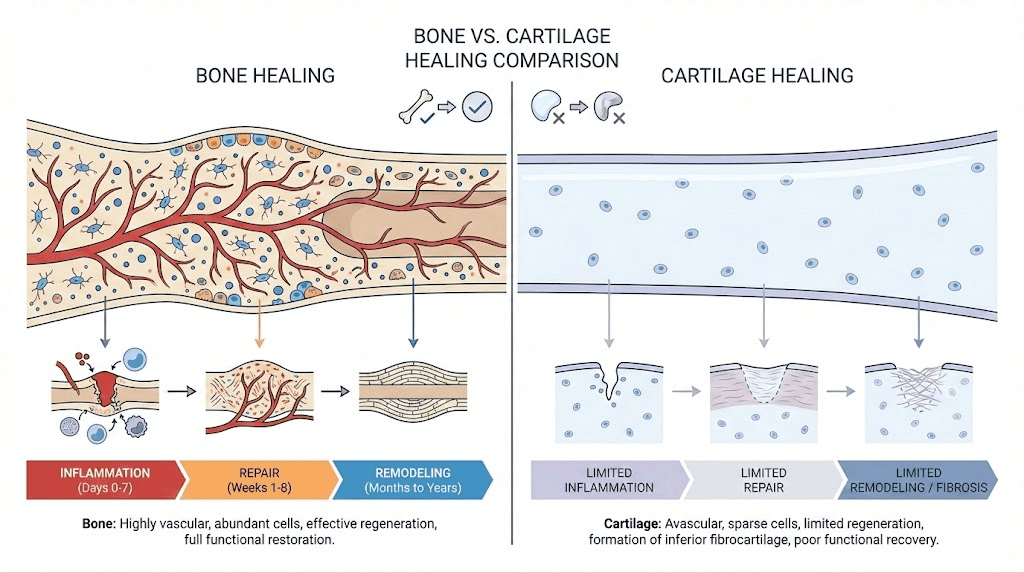
Bone, despite being highly vascularized tissue, requires precise orchestration of inflammatory phase (hematoma formation, cytokine release), repair phase (callus formation, cartilaginous bridge), and remodeling phase (ossification, mechanical adaptation) spanning weeks to months. Cartilage faces even greater obstacles given avascular nature (no blood vessels penetrating tissue), low cell density (chondrocytes sparse within extensive extracellular matrix), minimal metabolic activity (slow turnover and repair), and mechanical loading challenges (constant stress impeding healing).
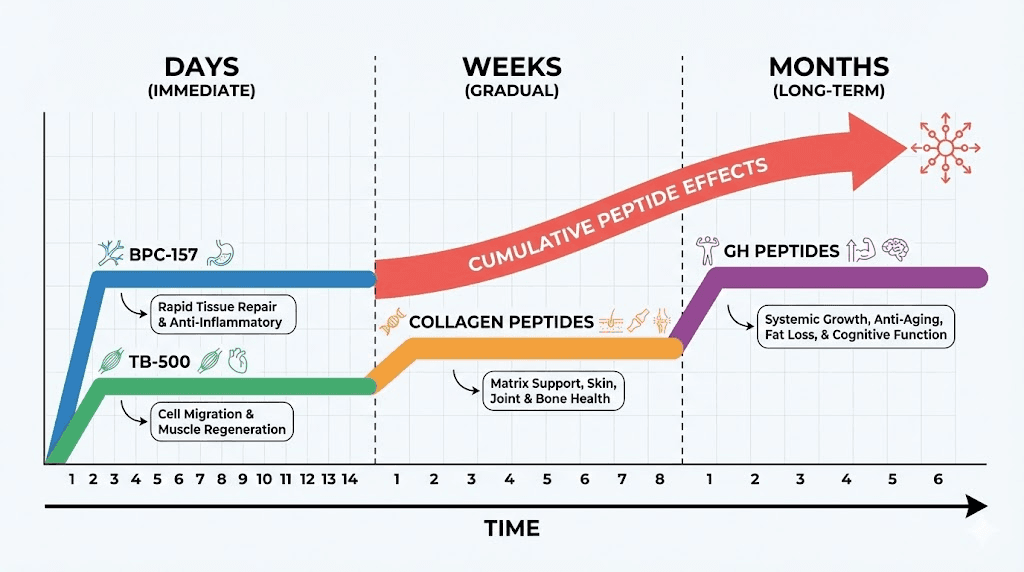
Growth hormone peptides like Ipamorelin and CJC-1295 indirectly support repair through systemic GH/IGF-1 elevation promoting overall tissue anabolism, while direct application peptides like BPC-157 target local injury sites with concentrated effects.
Acute fractures benefit from early peptide intervention (within days of injury) supporting rapid callus formation and preventing non-union complications.
Chronic conditions like osteoarthritis require sustained protocols (3-6 months minimum) addressing ongoing cartilage degradation and inflammation.
Post-surgical applications (ACL reconstruction, meniscus repair, spinal fusion) combine peptide support with rehabilitation optimizing surgical outcomes. Athletes seeking faster return to sport use aggressive protocols though must balance healing quality against timeline pressures. SeekPeptides provides comprehensive resources for musculoskeletal peptide applications tailored to individual situations.
Our complete guide covers bone and cartilage biology understanding repair challenges, specific peptides with documented musculoskeletal benefits (BPC-157, TB-500, growth factors, collagen peptides), cellular and molecular mechanisms of peptide-mediated repair, clinical and research evidence from human and animal studies, practical protocols for different injury types (fractures, cartilage damage, ligament tears, arthritis), combining peptides with conventional treatments (surgery, physical therapy, nutrition), monitoring healing progress and adjusting protocols, safety considerations for musculoskeletal applications, and realistic outcome expectations based on injury severity and tissue type. SeekPeptides serves as trusted resource for evidence-based regenerative peptide therapy guidance.
Let's examine bone and cartilage structure and healing biology to understand where peptides intervene.
Bone and cartilage biology: Understanding repair challenges
Different tissue properties create distinct healing trajectories and peptide opportunities.
Bone structure and natural healing process
Hierarchical organization: Bone consists of mineral phase (hydroxyapatite calcium phosphate crystals providing hardness), organic phase (type I collagen fibers providing flexibility), and cellular components (osteoblasts building bone, osteoclasts resorbing bone, osteocytes sensing mechanical stress). Trabecular (spongy) bone in vertebrae and long bone ends differs from cortical (dense) bone in shafts, affecting healing characteristics.
Fracture healing phases: Inflammatory phase (0-7 days) creates hematoma, recruits inflammatory cells, releases cytokines and growth factors initiating repair. Repair phase (days to weeks) forms soft callus (cartilaginous bridge) then hard callus (woven bone). Remodeling phase (months to years) converts woven bone to organized lamellar bone restoring original strength and shape. Each phase requires specific cellular activities and signaling molecules where peptides can enhance natural processes.
Vascular supply importance: Rich blood supply delivers oxygen, nutrients, inflammatory cells, growth factors, and calcium to healing sites. Bone heals better than cartilage primarily due to vascularization. However, severe fractures disrupting blood supply (displaced fractures, comminuted breaks) face healing challenges despite bone's inherent vascularity. Peptides promoting angiogenesis (BPC-157) potentially critical for revascularizing damaged bone.
Mechanical stress requirements: Wolff's Law states bone remodels in response to mechanical loading. Complete immobilization causes bone loss while appropriate loading stimulates strengthening. Healing bone requires balance between protection (preventing re-injury) and loading (stimulating proper remodeling). Peptides supporting earlier weight-bearing potentially valuable by reducing immobilization-related complications.
Cartilage structure and limited repair capacity
Avascular tissue challenges: Articular cartilage (covering joint surfaces) completely lacks blood vessels, nerves, and lymphatics. Nutrients diffuse from synovial fluid and subchondral bone creating minimal metabolic support. Injury responses limited by inability recruiting inflammatory cells and growth factors from circulation. This fundamental limitation explains poor natural cartilage healing compared to vascularized tissues.
Chondrocyte characteristics: Cartilage cells (chondrocytes) embedded in extensive extracellular matrix they produce and maintain. Low cell density (only 1-5% of cartilage volume, rest is matrix). Minimal proliferative capacity in adults. Matrix composition includes type II collagen (providing tensile strength), aggrecan proteoglycans (providing compressive resistance), and water (65-80% of tissue weight). Peptides enhancing chondrocyte activity or matrix synthesis address fundamental repair limitations.
Zonal organization: Articular cartilage has superficial zone (tangential collagen orientation, highest cell density), middle zone (random collagen orientation, largest proteoglycans), deep zone (perpendicular collagen orientation, calcified interface with bone), and tidemark (boundary between calcified and uncalcified cartilage). Injury depth determines healing potential, superficial lesions potentially healing while deep lesions rarely repair spontaneously.
Age-related changes: Young cartilage has higher cell density, better matrix quality, superior healing responses. Adult cartilage shows declining cellularity, accumulated matrix damage, reduced proteoglycan content, calcification advancement. Elderly cartilage essentially non-healing for significant injuries. Anti-aging peptides potentially slowing cartilage degeneration though cannot fully reverse age-related decline.
Inflammation's dual role in healing
Necessary acute inflammation: Initial inflammatory response clears debris, prevents infection, recruits repair cells, releases growth factors initiating healing cascade. Without adequate inflammation, healing impaired (immunosuppressed patients show delayed fracture healing). Anti-inflammatory medications (NSAIDs, corticosteroids) potentially impede bone healing if used excessively during early phases.
Problematic chronic inflammation: Sustained inflammation (lasting weeks to months) degrades healthy tissue through proteolytic enzymes, reactive oxygen species, inflammatory cytokines (IL-1β, TNF-α). In cartilage, chronic inflammation drives osteoarthritis progression destroying matrix faster than chondrocytes can rebuild. In bone, chronic inflammation may impair remodeling creating poor quality repair.
Peptide anti-inflammatory effects: BPC-157 and TB-500 modulate inflammation rather than simply suppressing it. Promote resolution of acute inflammation (shifting from pro-inflammatory to pro-repair signals) while reducing chronic pathological inflammation. This balanced approach potentially superior to conventional anti-inflammatories that broadly suppress beneficial and harmful inflammation equally.
Growth factors in musculoskeletal repair
Bone morphogenetic proteins (BMPs): Family of growth factors inducing bone and cartilage formation. BMP-2 and BMP-7 FDA-approved for spinal fusion and fracture healing (recombinant protein formulations). Extremely potent osteogenic signals triggering mesenchymal stem cell differentiation into osteoblasts. However, pharmaceutical BMP products expensive ($5,000-$10,000 per treatment) and carry side effects (ectopic bone formation, inflammation). No peptide directly mimics BMP but some enhance endogenous BMP production.
Transforming growth factor beta (TGF-β): Promotes chondrocyte proliferation and matrix synthesis. Critical for cartilage maintenance and repair. Also regulates inflammation and fibrosis. Some collagen peptides may stimulate TGF-β signaling supporting cartilage health.
Insulin-like growth factor (IGF-1): Anabolic growth factor promoting cell proliferation, protein synthesis, matrix production in bone and cartilage. IGF-1 peptides (LR3, Des) increase systemic or local IGF-1 levels potentially supporting musculoskeletal repair alongside muscle effects.
Vascular endothelial growth factor (VEGF): Critical for angiogenesis in bone healing. BPC-157 increases VEGF expression explaining improved revascularization in injured tissues. Also important for endochondral ossification (cartilage-to-bone conversion during fracture healing).
Specific peptides for bone and cartilage repair
Different peptides target distinct aspects of musculoskeletal healing.
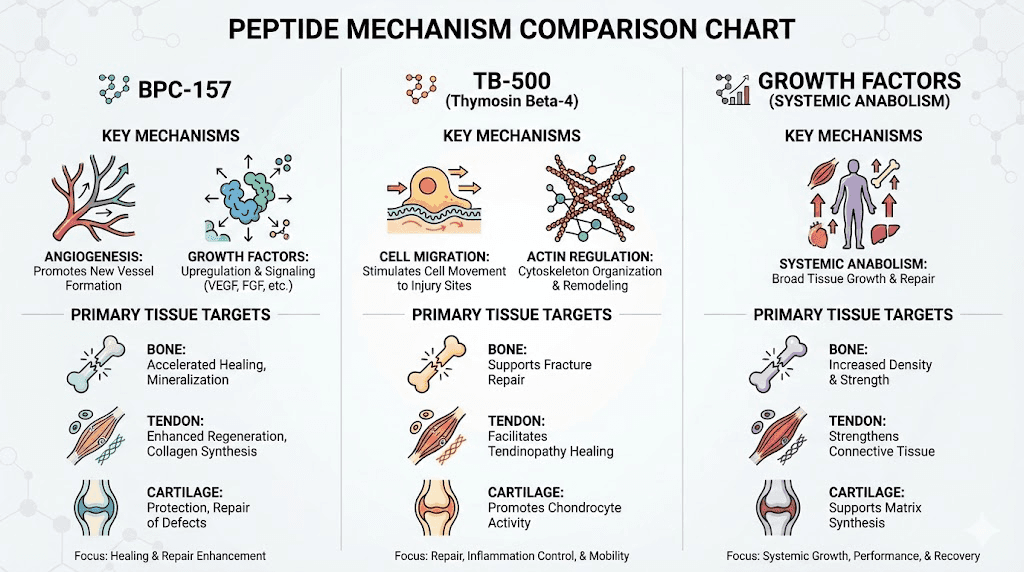
BPC-157: Multi-mechanism regenerative effects
Tendon and ligament healing: Most robust research exists for BPC-157 tendon repair. Rat studies show Achilles tendon, medial collateral ligament, and quadriceps tendon injuries healing faster with BPC-157 treatment. Mechanisms include increased growth factor expression (VEGF, FGF, EGF), enhanced fibroblast migration and proliferation, improved collagen organization, and accelerated revascularization. Tendon-to-bone healing (critical for surgical repairs like rotator cuff, ACL reconstruction) particularly benefiting from BPC-157's angiogenic effects.
Bone healing enhancement: Limited direct bone fracture research but mechanisms suggest benefits. BPC-157 promotes angiogenesis essential for bone healing blood supply. Anti-inflammatory effects reduce chronic inflammation impairing remodeling. Growth factor modulation supports osteoblast activity and callus formation. Rat studies of various tissue injuries consistently show improved healing suggesting bone fractures likely respond similarly.
Cartilage protection and repair: Studies in osteoarthritis models show BPC-157 protecting cartilage from degradation. Reduces inflammatory cytokines damaging cartilage matrix. Promotes chondrocyte survival and function. May stimulate proteoglycan synthesis though direct cartilage regeneration evidence limited. More effective preventing cartilage loss than regenerating severely damaged cartilage. BPC-157 for arthritis applications focus on slowing progression rather than reversing disease.
Joint healing: Animal studies show BPC-157 improving healing after joint injuries including meniscus damage and ligament tears. Reduces scar tissue formation promoting more organized repair. Improves biomechanical properties of healed tissue (strength, flexibility approaching normal tissue). Particularly relevant for athletes and active individuals prioritizing functional recovery over just anatomical healing.
Dosing for musculoskeletal applications: Typical protocols use 200-500mcg BPC-157 daily via subcutaneous or intramuscular injection. Some practitioners inject near injury site (local administration) versus systemic injection, though evidence for superiority of local injection limited. Duration 4-8 weeks for acute injuries, 8-12 weeks or longer for chronic conditions. SeekPeptides provides detailed BPC-157 protocols for various injury types.
TB-500 (Thymosin Beta-4): Stem cell and inflammation modulation
Mechanism of action: TB-500 primarily acts through G-actin sequestration affecting cell migration and cytoskeletal dynamics. Also modulates inflammation via NF-κB pathway inhibition. Promotes stem cell migration to injury sites (chemotactic effects). These mechanisms relevant for musculoskeletal repair where cell recruitment and organization critical.
Tendon and ligament benefits: Similar to BPC-157, TB-500 accelerates soft tissue healing. Improves collagen deposition and organization. Reduces scar tissue formation. Studies show faster return of biomechanical strength. Particularly effective for flexible tissues (tendons, ligaments, muscle) versus rigid mineralized bone. Often combined with BPC-157 for synergistic effects on connective tissue injuries.
Bone healing effects: Less researched than BPC-157 for bone specifically. Anti-inflammatory properties beneficial for healing environment. Stem cell recruitment potentially supporting mesenchymal stem cell differentiation into osteoblasts. Angiogenic effects supporting revascularization. However, direct bone healing data limited compared to soft tissue applications.
Cartilage applications: Similar to bone, limited specific cartilage research. Anti-inflammatory effects reduce cartilage-degrading cytokines. May support chondrocyte function through general cellular health effects. Not primary choice for cartilage-specific issues but reasonable adjunct supporting overall joint health when combined with more cartilage-targeted interventions.
Dosing protocols: TB-500 dosing typically 2-5mg twice weekly (loading phase) for 4-6 weeks then 2-5mg weekly (maintenance phase). Higher doses (5mg twice weekly) used for severe injuries or accelerated healing timelines. Duration similar to BPC-157, 8-12 weeks typical courses with breaks between cycles preventing receptor desensitization.
Growth hormone secretagogues: Systemic support
Indirect bone and cartilage effects: Growth hormone peptides like Ipamorelin, CJC-1295, and MK-677 don't directly heal tissues but create anabolic hormonal environment supporting repair. Elevated GH and IGF-1 promote protein synthesis, cell proliferation, tissue remodeling across all body systems including musculoskeletal. Benefits accrue over weeks to months rather than immediate effects.
Bone density and strength: GH and IGF-1 stimulate osteoblasts increasing bone formation. Clinical studies show GH treatment in GH-deficient patients improving bone density. However, effects in healthy adults with normal GH levels less dramatic. Growth hormone peptides potentially beneficial for age-related bone loss, recovery from severe injuries with systemic catabolic stress, or athletes with heavy training loads creating metabolic demands.
Cartilage metabolism: IGF-1 stimulates chondrocyte proteoglycan and collagen synthesis. May slow cartilage degradation in osteoarthritis. However, direct cartilage injection of IGF-1 more effective than systemic elevation, and systemic growth hormone peptides produce modest IGF-1 increases (not pharmacological levels). Benefits likely marginal for focal cartilage injuries though potentially valuable for systemic joint health.
Practical considerations: Growth hormone peptide protocols require months for musculoskeletal benefits versus weeks for direct-acting peptides (BPC-157, TB-500). Better suited for chronic conditions or prevention than acute injury treatment. Cost-effective for individuals also seeking body composition, recovery, or anti-aging benefits where musculoskeletal support secondary objective. Combining growth hormone peptides with direct repair peptides creates comprehensive approach.
Collagen peptides and amino acid building blocks
Hydrolyzed collagen: Breakdown of collagen protein into short peptide chains (typically 2-20 amino acids). Provides amino acids (glycine, proline, hydroxyproline) as building blocks for new collagen synthesis. Unlike intact collagen protein, short peptides absorbed intact in intestine potentially delivering specific signaling effects beyond just amino acids.
Bone and cartilage benefits: Clinical studies show collagen peptide supplementation improving joint pain, cartilage metabolism markers, bone mineral density. Type II collagen (specific to cartilage) shows benefits for osteoarthritis. Type I collagen (predominant in bone) supports bone health. Mechanisms include providing substrate for tissue synthesis, stimulating fibroblast and chondrocyte activity, and potentially specific peptide signaling.
Dosing and forms: Typical doses 10-15g daily for joint and bone support. Type II collagen (UC-II) used at lower doses (40mg daily) in some studies. Forms include bovine, marine, chicken sources with minimal difference in efficacy. Consistency important, benefits develop over 8-12 weeks continued use. Collagen peptide protocols differ from injectable peptides, oral supplementation versus injection.
Comparison to injectable peptides: Collagen peptides provide building blocks and mild stimulation. Injectable peptides (BPC-157, TB-500) create stronger signaling effects and targeted delivery. Combining both potentially synergistic, collagen providing substrate while injectable peptides provide growth signals. Cost-effective strategy using inexpensive oral collagen as foundation with targeted injectable peptides for specific injuries.
Experimental and emerging peptides
GHRP-2 and GHRP-6: Growth hormone releasing peptides with similar effects to Ipamorelin but additional hunger stimulation (ghrelin mimicry). Potential musculoskeletal benefits through GH/IGF-1 elevation. However, hunger side effects complicate use for some individuals. Less popular than Ipamorelin for musculoskeletal-specific applications.
Follistatin: Myostatin inhibitor promoting muscle growth. Minimal direct bone or cartilage effects. However, stronger muscles support joints reducing injury risk and improving rehabilitation outcomes. Experimental status with limited human data. Very expensive ($500-$2,000+ per treatment course).
Epithalon: Claimed anti-aging effects through telomerase activation. Theoretical benefits for tissue regeneration including musculoskeletal. However, minimal direct research on bone or cartilage healing. More general longevity peptide than specific repair application.
AOD-9604: Fragment of growth hormone claimed to promote fat loss while retaining GH's regenerative effects without side effects. Limited research, uncertain efficacy. Not established for musculoskeletal applications though marketed for joint health by some vendors.
Conservative approach: Stick with well-researched peptides (BPC-157, TB-500, established growth hormone peptides) rather than experimental options with minimal evidence. SeekPeptides focuses on evidence-based peptides with documented mechanisms and safety profiles.
Cellular and molecular mechanisms of repair
Understanding how peptides work at cellular level guides protocol optimization.
Osteoblast stimulation and bone formation
Osteoblast function: Bone-forming cells derived from mesenchymal stem cells. Synthesize type I collagen matrix (osteoid) then mineralize it with calcium phosphate creating hardened bone. Activity regulated by mechanical stress, hormones (GH, IGF-1, PTH), local growth factors (BMPs, TGF-β), and inflammatory signals.
Peptide effects on osteoblasts: Growth hormone peptides increase systemic IGF-1 which directly stimulates osteoblast proliferation and activity. BPC-157 potentially enhances local growth factor availability (VEGF, FGF) supporting osteoblast recruitment and function. Studies in various tissue healing models show increased cell proliferation which would include osteoblasts in bone injuries.
Mineralization process: Osteoblasts secrete alkaline phosphatase enabling calcium phosphate precipitation. Peptides don't directly affect mineralization chemistry but support cellular activity producing mineralization signals. Adequate mineral availability (calcium, phosphate, vitamin D) required regardless of peptide use, nutritional support essential foundation.
Chondrocyte matrix synthesis
Proteoglycan production: Chondrocytes synthesize aggrecan (large proteoglycan) providing cartilage's compressive resistance. Aggrecan molecules link to hyaluronic acid forming massive aggregates trapping water. Proteoglycan synthesis requires adequate amino acids (from protein intake or collagen peptides), adequate cellular energy (glucose metabolism), and growth factor signaling (IGF-1, TGF-β).
Type II collagen synthesis: Cartilage-specific collagen differs from bone's type I collagen. Requires vitamin C cofactor (ascorbic acid) for proper crosslinking. Collagen fibrils provide tensile strength complementing proteoglycans' compression resistance. Some collagen peptides may specifically stimulate type II collagen genes in chondrocytes.
Peptide enhancement: Direct chondrocyte stimulation evidence limited for most peptides. BPC-157 may support chondrocyte survival through anti-inflammatory and anti-apoptotic effects. Growth hormone pathway activation (via GH peptides) increases IGF-1 stimulating chondrocyte matrix synthesis. However, severe cartilage damage with minimal remaining chondrocytes cannot regenerate regardless of peptide support, intact cell population required.
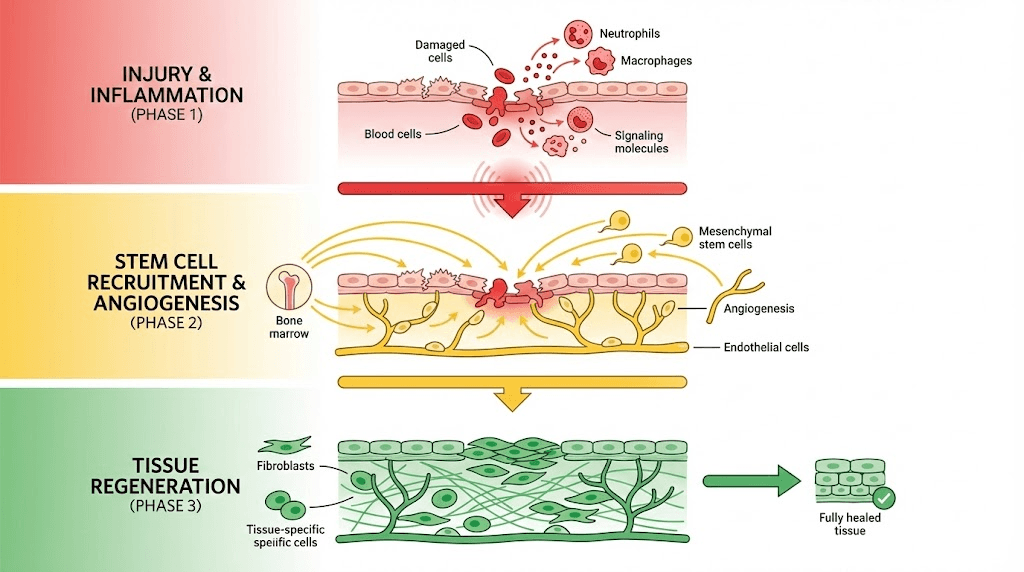
Stem cell recruitment and differentiation
Mesenchymal stem cells (MSCs): Multipotent cells capable differentiating into bone (osteoblasts), cartilage (chondrocytes), fat (adipocytes), muscle, and other mesenchymal tissues. Found in bone marrow, fat tissue, and various other locations. Recruited to injury sites by chemotactic factors where they differentiate based on local signals.
TB-500 chemotactic effects: Thymosin Beta-4 promotes stem cell migration toward injury sites. Actin dynamics regulation (TB-500's primary mechanism) critical for cell migration. Enhanced MSC recruitment potentially increasing osteoblast and chondrocyte numbers at healing sites. This mechanism particularly valuable for injuries in low-cell-density tissues like adult cartilage.
Differentiation signals: MSCs require specific signals differentiating into bone versus cartilage lineages. BMPs strongly drive osteoblast differentiation. TGF-β and specific mechanical cues promote chondrocyte differentiation. Peptides don't directly provide differentiation signals but create environment supporting natural differentiation processes. Stem cell peptides represent emerging research area.
Angiogenesis and nutrient delivery
Vascular network importance: Healing tissues require increased metabolic support (oxygen, glucose, amino acids, minerals). Angiogenesis (new blood vessel formation) delivers this support. Also provides route for immune cell and stem cell recruitment. Critical for bone healing, less relevant for avascular cartilage but important for subchondral bone supporting cartilage.
BPC-157 angiogenic mechanism: Increases VEGF expression triggering endothelial cell proliferation, migration, and tube formation. Also affects other pro-angiogenic factors (FGF, angiopoietin). Studies consistently show enhanced blood vessel formation in BPC-157 treated tissues. This mechanism explains accelerated healing across multiple tissue types including bone.
Spatial considerations: Angiogenesis must reach injury center for optimal healing. Large injuries with disrupted vasculature face greater challenges. Peptide-enhanced angiogenesis potentially critical for severe fractures, surgical bone grafts, or complex injuries where vascular ingrowth limiting factor.
Inflammation resolution and matrix preservation
Acute inflammation benefits: Initial inflammatory response (first days post-injury) clears debris, prevents infection, releases growth factors. Trying to completely suppress acute inflammation potentially counterproductive. However, excessive or prolonged inflammation damages healthy tissue.
Chronic inflammation problems: In cartilage particularly, sustained inflammation drives disease progression. IL-1β and TNF-α stimulate matrix metalloproteinases (MMPs) degrading collagen and proteoglycans faster than chondrocytes can replace. Also induce chondrocyte apoptosis reducing cell numbers. Perpetuating cycle of inflammation, matrix loss, cell death creating progressive joint destruction.
Peptide modulation: BPC-157 and TB-500 shift inflammation toward resolution phase rather than simply suppressing it. Promote M2 macrophage polarization (anti-inflammatory, tissue repair phenotype) versus M1 macrophages (pro-inflammatory, tissue damage). Reduce pro-inflammatory cytokines while maintaining beneficial aspects of immune response. This balanced approach theoretically superior to broad immunosuppression. Learn about anti-inflammatory peptides for comprehensive understanding.
Clinical and research evidence
Examining human and animal data for realistic outcome expectations.
Animal model research
Rat tendon studies: Most BPC-157 research uses rat Achilles tendon transection models. Studies show 60-80% faster healing with BPC-157 treatment versus controls. Biomechanical testing reveals improved tensile strength approaching normal tissue. Histological analysis shows better collagen organization and reduced scar tissue. These robust results across multiple independent studies suggest real biological effects not artifacts.
Bone fracture models: Limited direct studies using standardized fracture models. However, general tissue healing research suggests applicability. Some studies show improved healing in bone-tendon junctions (relevant for surgical repairs). Extrapolating from tendon data and mechanisms suggests bone healing likely benefits though direct confirmation limited.
Cartilage and osteoarthritis models: Studies in rats with induced osteoarthritis show BPC-157 reducing cartilage degradation and protecting against disease progression. Effects include reduced inflammatory markers in synovial fluid, preserved cartilage thickness on imaging, improved joint function scoring. However, established damage not reversed, benefits limited to slowing progression and preventing further deterioration.
Limitations of animal research: Rat healing faster than human, young research animals heal better than aged humans, controlled injury models don't replicate complex human injuries perfectly, dosing conversions uncertain (body weight scaling imperfect), immune system differences affect inflammation and healing. Animal data provides biological plausibility and mechanistic insights but cannot guarantee human efficacy.
Human clinical data (limited)
Case reports and series: Small numbers of patients treated with various peptides for musculoskeletal conditions. Mostly positive anecdotal reports of faster healing, reduced pain, improved function. However, no controlled conditions (placebo effects, natural healing, concurrent treatments confound results). Publications mainly in alternative medicine journals not rigorous peer-reviewed mainstream journals. Suggestive but not definitive evidence.
Lack of randomized controlled trials: No large-scale human RCTs testing peptides for bone or cartilage healing published in major medical journals. This evidence gap reflects several factors including lack of pharmaceutical company funding (peptides not patentable, unprofitable versus proprietary drugs), regulatory challenges (FDA approval process expensive), and peptide use primarily occurring outside mainstream medicine in research chemical/biohacking communities.
Indirect human data: Growth hormone studies show bone and cartilage benefits. Collagen peptide supplementation trials demonstrate joint improvements. These provide evidence for general biological principles (GH/IGF-1 axis supporting musculoskeletal health, collagen building blocks beneficial) lending credibility to targeted peptide interventions though not direct proof.
Self-experimentation reports: Thousands of individuals using peptides for injuries sharing experiences online (Reddit, forums, social media). Consistent themes emerge including faster recovery, reduced pain, improved function. However, confirmation bias, selection bias (successful users more likely reporting), and lack of objective measurements limit scientific value. Provides real-world safety data (serious side effects rare) and hypothesis-generating observations for future research.
Veterinary applications
Equine tendon injuries: Horses suffer frequent tendon and ligament injuries from racing and sports. Veterinary medicine uses various peptides and biologics for treatment. BPC-157 and similar compounds tested in horses with generally positive results. Equine studies more controlled than human case reports but less rigorous than pharmaceutical development trials.
Canine arthritis: Dogs develop osteoarthritis similar to humans. Veterinary practices use peptides, stem cells, and other regenerative therapies. Owner-reported improvements in mobility and pain though objective measurements variable. Dogs cannot report subjective pain requiring behavioral assessments.
Regulatory landscape: Veterinary medicine more permissive than human medicine for experimental treatments. Provides testing ground for therapies eventually translating to human use. However, species differences limit direct extrapolation. SeekPeptides monitors veterinary peptide research for insights applicable to human applications.
Evidence quality assessment
Strong evidence: BPC-157 accelerates tendon healing in rats (multiple independent studies, consistent results, large effect sizes). TB-500 promotes tissue repair through well-characterized actin-binding mechanism. Growth hormone/IGF-1 axis supports bone and cartilage metabolism (established endocrinology).
Moderate evidence: BPC-157 benefits cartilage in osteoarthritis models. Collagen peptides improve joint symptoms in humans. Peptides generally safe based on widespread use reports. Mechanisms biologically plausible based on known growth factor and healing pathways.
Weak evidence: Specific bone fracture healing acceleration in humans. Cartilage regeneration versus just protection. Optimal dosing protocols for different injury types. Long-term safety beyond several month treatment courses.
No evidence: Comparative effectiveness between different peptides or combinations. Head-to-head trials versus conventional treatments. Durability of healing improvements (long-term follow-up data). Cost-effectiveness analyses.
Realistic expectations account for evidence limitations. Peptides show promise warranting careful use but shouldn't replace proven treatments or create unrealistic miracle cure expectations.
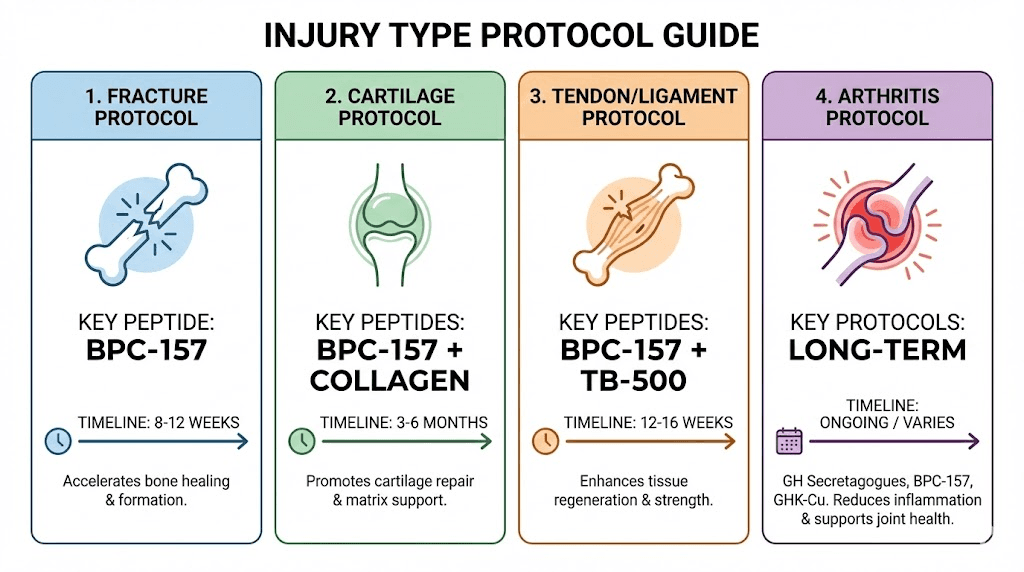
Practical protocols for different injury types
Tailoring peptide use to specific musculoskeletal conditions optimizes outcomes.
Acute fracture healing
Timing considerations: Begin peptides within first week post-fracture during inflammatory and early repair phases. Early intervention potentially most impactful for callus formation and revascularization. However, starting later (2-4 weeks post-fracture) still beneficial for remodeling phase.
Protocol example: BPC-157 300-500mcg daily subcutaneous injection for 8-12 weeks. Optional addition of TB-500 2-5mg twice weekly first 4-6 weeks (loading phase) supporting stem cell recruitment and inflammation resolution. Consider growth hormone peptides (Ipamorelin + CJC-1295) if systemic support desired though effects slower manifesting.
Injection site: Can inject near fracture site though systemic injection (abdomen, thigh) equally effective based on BPC-157's distribution. Local injection may provide psychological benefit but no convincing evidence for superior outcomes. Some practitioners prefer rotating between local and systemic sites.
Monitoring: Follow fracture healing with serial X-rays (typically 2-4 week intervals standard care). Accelerated callus formation or earlier bridging on imaging suggests peptide benefits. Clinical healing (reduced pain, increased function, weight-bearing tolerance) important markers. However, cannot definitively attribute improvements to peptides versus natural healing without controlled comparison.
Combination with conventional care: Maintain proper immobilization, weight-bearing restrictions, nutrition optimization (calcium, vitamin D, protein), and physical therapy as prescribed. Peptides augment but don't replace standard fracture management. Injury recovery protocols integrate peptides with comprehensive care.
Cartilage damage and osteoarthritis
Acute cartilage injury: Traumatic injuries (sports injuries, accidents) damaging previously healthy cartilage. Early intervention (within days to weeks) potentially preventing progression to osteoarthritis. BPC-157 300-500mcg daily for 8-12 weeks minimum. Collagen peptides 10-15g daily oral supplementation. Reduce inflammatory activities (anti-inflammatory diet, omega-3 supplementation) supporting peptide effects.
Established osteoarthritis: Chronic progressive joint disease with ongoing cartilage degradation. Peptides slow progression rather than reversing damage. Longer protocols (3-6 months) required. BPC-157 200-400mcg daily ongoing or cycled (8 weeks on, 4 weeks off). Growth hormone peptides potentially beneficial given osteoarthritis's chronic nature. Realistic expectations focusing on pain reduction and slowing progression not cartilage regeneration.
Meniscus tears: Fibrocartilage structure in knee with limited healing capacity. Peptide protocols similar to cartilage injuries. BPC-157 plus TB-500 combination potentially synergistic for fibrocartilage repair. 8-12 week protocols. However, severe tears may require surgical intervention, peptides supporting post-surgical healing not replacing surgery.
Post-surgical support: After cartilage procedures (microfracture, osteochondral grafts, chondrocyte implantation), peptides potentially optimizing outcomes. Begin immediately post-surgery continuing 8-12 weeks. Coordinate with surgeon and physical therapist ensuring peptide use doesn't conflict with rehabilitation protocols.
Tendon and ligament injuries
Acute tears: ACL, rotator cuff, Achilles, or other tendon/ligament injuries. BPC-157 and TB-500 best evidence for these conditions. Combination protocol: BPC-157 300-500mcg daily plus TB-500 2.5-5mg twice weekly for 6-8 weeks (loading), then BPC-157 maintenance 200-300mcg daily another 4-8 weeks. Total treatment 12-16 weeks.
Chronic tendinopathy: Overuse conditions like tennis elbow, patellar tendinopathy, chronic Achilles pain. Peptides address inflammation and degeneration. Longer protocols (12-16 weeks minimum) required for chronic conditions. BPC-157 200-400mcg daily potentially indefinite with periodic breaks. Combine with activity modification and eccentric exercise rehabilitation.
Post-surgical tendon repair: After surgical reattachment (rotator cuff repair, ACL reconstruction, Achilles repair). Peptides supporting tendon-to-bone healing where BPC-157 shows particular promise. Begin within days of surgery continuing through rehabilitation phase (3-6 months typically). Coordinate timing with physical therapy progression ensuring peptide-enhanced healing doesn't lead to overly aggressive activity causing re-injury.
Conservative vs surgical: Some tendon injuries managed conservatively (physical therapy, activity modification) versus surgery. Peptides potentially enabling successful conservative management avoiding surgical risks and recovery. However, complete tears generally require surgery, peptides not substitute for necessary operations.
Spinal conditions
Disc injuries: Intervertebral discs contain fibrocartilage (annulus fibrosus) and proteoglycan-rich nucleus pulposus. Disc degeneration and herniation common conditions. Limited evidence for peptide benefits but biological plausibility exists. BPC-157 potentially reducing inflammation and supporting annulus healing. However, severe herniations with nerve compression require medical intervention, peptides not substitute.
Spinal fusion procedures: Surgical joining of vertebrae treating instability, deformity, or severe degeneration. Bone healing critical for fusion success. Peptides potentially accelerating fusion and reducing non-union risk. BPC-157 300-500mcg daily for 12-16 weeks post-operatively. Some surgeons open to adjunct therapies though most conservative preferring proven protocols. Discuss with surgeon before adding peptides to post-surgical care.
Facet arthritis: Spinal joint osteoarthritis causing back pain. Similar to peripheral joint arthritis, peptides potentially slowing progression and reducing inflammation. However, spine biomechanics complex and pain often multi-factorial (muscles, discs, nerves, joints). Peptides addressing only one component.
Osteoporosis and bone density
Age-related bone loss: Peptides potentially slowing bone loss or modestly increasing density. Growth hormone peptides most relevant given GH/IGF-1 effects on bone metabolism. Long-term protocols (6-12 months) required for density changes. Combine with weight-bearing exercise, calcium, vitamin D, potentially bisphosphonates or other osteoporosis medications for comprehensive management.
Realistic expectations: Peptides unlikely producing dramatic bone density improvements matching pharmaceutical osteoporosis drugs. Modest benefits (1-3% density increases or preventing loss) more realistic. May support overall bone quality beyond just density measurements. Cost-effectiveness questionable for osteoporosis alone versus established treatments though valuable if seeking multiple peptide benefits (body composition, recovery, general health).
SeekPeptides provides comprehensive protocol guides for various musculoskeletal conditions with dosing, duration, and monitoring recommendations.
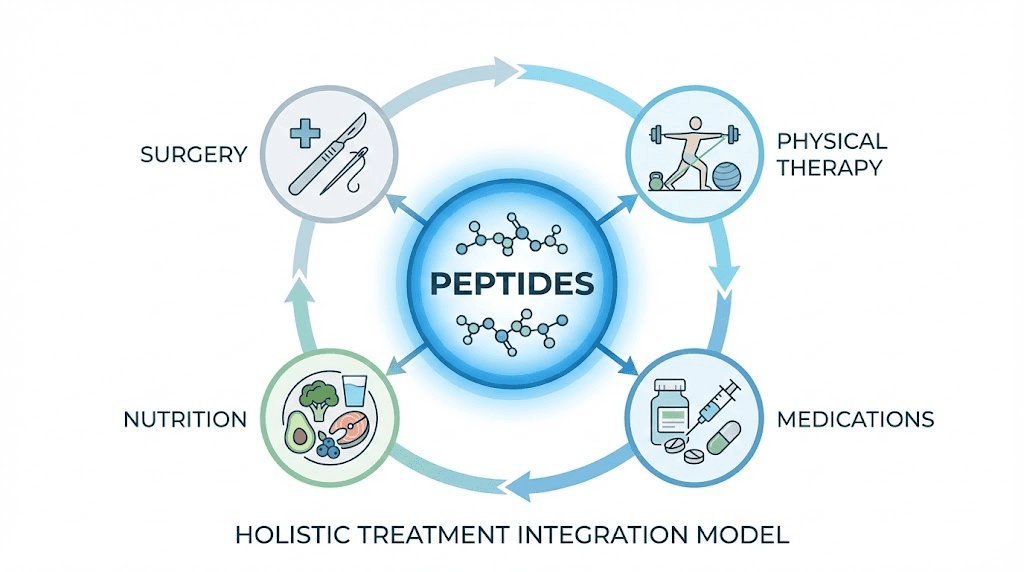
Combining peptides with conventional treatments
Integration strategies maximizing outcomes while respecting medical standards.
Post-surgical applications
Coordinating with surgeons: Most orthopedic surgeons unfamiliar with research peptides, may be skeptical or dismissive. However, increasing number acknowledge potential benefits or at minimum don't object to adjunct use. Present peptides as recovery optimization tools not alternatives to surgery. Provide research papers demonstrating mechanisms and animal data. Emphasize following all surgical protocols (immobilization, weight-bearing restrictions, physical therapy) while adding peptides as supplement.
Timing relative to surgery: Can begin peptides pre-operatively (1-2 weeks before surgery) optimizing healing environment though benefits uncertain. Post-operatively start within days once stable (after drain removal, wound sealed). Continue through active healing phase (typically 8-12 weeks) potentially extending into remodeling phase (months) for complete recovery.
Activity progression: Peptide-enhanced healing might allow earlier activity advancement. However, resist temptation progressing too quickly risking re-injury. Follow surgeon and physical therapist guidance even if feeling better than expected. Faster healing should translate to higher-quality outcome at standard timeline not necessarily faster return to activity.
Physical therapy integration
Synergistic benefits: Physical therapy provides mechanical stress stimulating tissue remodeling (Wolff's Law for bone, mechanical loading for tendon/ligament). Peptides provide biological signals enhancing cellular response to mechanical stress. Combination potentially superior to either alone. Inform physical therapist about peptide use, may affect exercise prescription or progression timing.
Loading progression: Adequate loading essential for quality tissue remodeling. Too little creates weak repair, too much causes re-injury. Peptides potentially allowing slightly more aggressive progression though individual response varies. Use pain and functional testing guiding progression regardless of peptide use.
Modalities: Physical therapy modalities (ultrasound, electrical stimulation, laser therapy) may complement peptide effects though evidence for synergy lacking. Blood flow restriction training potentially interesting combination with peptides enhancing growth factor signaling though research needed.
Nutritional support
Protein intake: Tissue repair requires amino acid building blocks. Inadequate protein intake limits healing regardless of peptide signals. Target 1.2-1.6g protein per kg body weight during active healing (higher than typical 0.8-1.0g/kg recommendations). Distribute throughout day supporting continuous protein synthesis.
Collagen supplementation: Oral collagen peptides (10-15g daily) provide specific amino acids (glycine, proline, hydroxyproline) enriched in connective tissue. May complement injectable peptides by providing substrate while injectables provide signals. Inexpensive addition ($20-$40 monthly) with modest benefits warranting inclusion.
Micronutrients: Vitamin C (cofactor for collagen synthesis), vitamin D and calcium (bone mineralization), zinc (enzyme cofactor in healing), omega-3 fatty acids (anti-inflammatory). Address any deficiencies through diet and supplementation. While not miracle cures, nutritional optimization creates foundation for peptide efficacy.
Anti-inflammatory diet: Reduce refined sugars, excessive omega-6 fats, processed foods promoting inflammation. Increase vegetables, fruits, omega-3 fatty acids (fish, flaxseed), polyphenol-rich foods supporting inflammation resolution. Combined with peptide anti-inflammatory effects creates comprehensive approach.
Pharmaceutical medications
NSAIDs caution: Non-steroidal anti-inflammatory drugs (ibuprofen, naproxen) may impair bone healing if used excessively especially early post-fracture. Use sparingly for pain control, prefer acetaminophen for non-inflammatory pain. However, short-term NSAID use (few days) unlikely causing significant problems. Peptides' anti-inflammatory effects might reduce NSAID need.
Corticosteroids concerns: Oral or injected corticosteroids (prednisone, cortisone) can impair healing and cause bone loss with prolonged use. Necessary for some conditions (rheumatoid arthritis, other autoimmune diseases) but avoid if possible during active musculoskeletal healing. Discuss with physician if taking corticosteroids and considering peptides.
Bisphosphonates: Osteoporosis medications affecting bone remodeling. No known interactions with peptides. However, complex effects on bone turnover, combining with peptides uncharted territory. Medical supervision advisable.
Blood thinners: Warfarin, novel anticoagulants, antiplatelet drugs. No direct interactions with peptides. However, fracture hematoma formation important first healing step, excessive anticoagulation potentially problematic. Medical management of anticoagulation takes priority.
Alternative therapies
Stem cell treatments: Mesenchymal stem cell injections increasingly used for joint injuries and arthritis. Combining with peptides theoretically synergistic (stem cells providing cells, peptides providing signals). However, stem cell therapies expensive ($3,000-$10,000+ per treatment) with variable quality and uncertain long-term benefits. Consider established treatments before experimental combinations.
Platelet-rich plasma (PRP): Concentrated platelets containing growth factors injected into injuries. Similar concept to peptides (delivering growth signals) but different mechanism (autologous growth factors from patient's blood). Some practitioners combine PRP and peptides though no research on synergy. Possibly redundant effects versus truly synergistic.
Prolotherapy: Injection of dextrose or other irritant solutions triggering inflammation and healing response. Theoretical conflict with peptides' anti-inflammatory effects. However, different mechanisms may not actually interfere. No research on combination.
Acupuncture, massage, other modalities: No interactions with peptides. May provide pain relief and relaxation supporting overall recovery. Integrate based on individual preferences and responses.
SeekPeptides offers guides on integrating peptides with various conventional and alternative approaches.
Monitoring healing and adjusting protocols
Objective and subjective measures guide protocol optimization.
Imaging modalities
X-rays: Standard for bone fracture monitoring. Serial X-rays every 2-4 weeks showing callus progression, fracture line bridging, eventual remodeling. Accelerated healing might show earlier callus formation or bridging though individual variation large. Cannot directly attribute faster healing to peptides without comparison but provides reassurance healing progressing appropriately.
MRI: Superior soft tissue visualization for tendons, ligaments, cartilage, menisci. Expensive ($500-$2,000 per scan) limiting serial monitoring. Baseline MRI before peptide treatment and follow-up after 3-6 months may show improved tissue integrity though subtle changes hard to interpret. More useful for surgical decision-making than routine peptide monitoring.
Ultrasound: Emerging for tendon and ligament assessment. Less expensive than MRI, no radiation unlike X-rays. Can visualize tissue structure and blood flow. Limited availability as musculoskeletal ultrasound requires operator expertise. Potentially useful for serial monitoring if accessible.
CT scans: Better bone detail than X-rays. Used for complex fractures, spinal fusion assessment, pre-surgical planning. Not routine for peptide monitoring but if obtained for other reasons provides bone healing information.
Functional assessments
Range of motion: Goniometer measurements or functional testing (how far can joint move). Improvements in flexibility suggest tissue healing progressing. Persistent restrictions indicate scarring, adhesions, or incomplete healing requiring protocol adjustment or additional interventions.
Strength testing: Manual muscle testing, handheld dynamometry, isokinetic testing. Strength improvements indicate muscle and tendon recovering. However, strength influenced by many factors (pain, atrophy, neuromuscular control) not just tissue healing.
Pain scales: Visual analog scale (0-10 rating) or numeric rating. Decreasing pain suggests inflammation resolving and tissue healing. However, subjective and influenced by expectations, medications, psychological factors. Track trends over weeks not day-to-day fluctuations.
Functional performance: Sport-specific or activity-specific tasks. Return to running, jumping, throwing, lifting as appropriate for injury type. Functional recovery ultimately most important outcome though must balance testing against re-injury risk.
Biomarkers (limited utility)
Systemic inflammation: C-reactive protein, erythrocyte sedimentation rate. General inflammation markers not specific to musculoskeletal healing. May trend downward with healing but also affected by other health factors.
Cartilage turnover markers: Urine or blood tests measuring cartilage breakdown products (CTX-II) or synthesis markers. Research tools not widely available clinically. Potentially useful for osteoarthritis monitoring though expensive and not standard care.
Bone turnover markers: Osteocalcin (bone formation), CTX (bone resorption). Used in osteoporosis management. May reflect bone healing though not specific to fracture sites. Generally not ordered for fracture healing monitoring.
IGF-1 levels: If using growth hormone peptides, serum IGF-1 monitors protocol effectiveness. Elevations confirm peptide activity though correlation with healing outcomes uncertain. Levels 300-400 ng/mL typical therapeutic targets though individual variation.
Protocol adjustments
Increasing dosing: If healing slower than expected or pain not improving after 4-6 weeks, consider increasing dose 25-50%. BPC-157 200mcg to 300-400mcg, TB-500 from 2mg to 5mg loading dose. However, very high doses unlikely providing proportionally greater benefits due to receptor saturation.
Adding complementary peptides: If using single peptide without expected results, add complementary option. BPC-157 alone becoming BPC-157 + TB-500, or adding growth hormone peptides for systemic support. However, avoid excessive polypharmacy, 2-3 peptides maximum for musculoskeletal applications.
Extending duration: Acute injuries typically improve within 8-12 weeks. Chronic conditions or slow healers may require 16-24 weeks or longer. Don't prematurely discontinue if showing gradual improvement. However, completely absent progress after 8-12 weeks suggests either ineffective protocol or injury severity beyond peptide intervention requiring medical evaluation.
Taking breaks: After successful healing, discontinue peptides rather than indefinite use. Allows assessing whether benefits sustained or healing fragile. If regression occurs during break, may need additional treatment cycle. If stability maintained, healing likely complete.
Safety considerations and realistic expectations
Responsible peptide use requires understanding limitations and risks.
Side effect profiles
BPC-157: Generally well-tolerated. Potential side effects include injection site reactions (redness, swelling, pain), mild nausea (rare), headache (uncommon), fatigue (rare). Serious side effects not reported in human use though formal safety studies lacking. Animal studies show remarkable safety even at high doses.
TB-500: Similar safety profile to BPC-157. Injection site reactions most common. Some users report lethargy or headaches. No serious adverse events documented in widespread use though systematic safety data absent.
Growth hormone peptides: Can cause water retention, joint pain, carpal tunnel symptoms, insulin resistance (with long-term high-dose use), increased hunger (especially GHRP-6, GHRP-2). Generally mild and reversible with dose reduction or discontinuation.
Collagen peptides: Excellent safety profile. Digestive upset possible with very high doses (>20g daily). Allergic reactions possible in sensitive individuals though rare.
Contraindications and warnings
Active cancer: Theoretical concern peptides promoting cell proliferation could stimulate cancer growth. No evidence this occurs but cautious approach warranted. Avoid peptides during active cancer treatment, discuss with oncologist before use in cancer survivors.
Pregnancy and breastfeeding: No safety data. Avoid all research peptides during pregnancy and lactation. Unknown effects on fetal development or infant exposure through breast milk create unacceptable risks.
Autoimmune conditions: Immune system modulation from some peptides might affect autoimmune disease activity. Uncertain whether beneficial or harmful. Medical supervision essential if using peptides with rheumatoid arthritis, lupus, or other autoimmune conditions.
Bleeding disorders: TB-500 and BPC-157 affect vascular function and potentially platelet behavior. Exercise caution with bleeding disorders or concurrent anticoagulant use. Monitor for unusual bleeding or bruising.
Allergy history: Those with peptide allergies or multiple medication allergies face higher risk of allergic reactions to new peptides. Start with very low doses testing tolerability before advancing to therapeutic doses.
What peptides cannot do
Reverse severe structural damage: Completely destroyed cartilage (bone-on-bone arthritis), displaced fractures requiring surgical fixation, complete tendon/ligament ruptures needing surgery. Peptides support healing but cannot substitute for necessary medical or surgical interventions.
Guarantee healing: Individual variation means some people respond excellently while others show minimal benefits. Genetics, age, injury severity, overall health, concurrent treatments all influence outcomes. No universal success rate data exists.
Eliminate need for rehabilitation: Even with peptides, progressive loading, range of motion work, strengthening, proprioceptive training remain essential. Biological healing must be translated to functional restoration through rehabilitation.
Provide instant results: Tissue healing requires weeks to months regardless of interventions. Expecting immediate pain relief or rapid recovery creates disappointment. Realistic timeline understanding essential for protocol adherence.
Long-term safety unknowns
Chronic use effects: Most protocols last weeks to months. Long-term continuous use (years) safety unknown. Could theoretical concerns about receptor downregulation, disrupting natural growth factor signaling, or other homeostasis disturbances exist? Prudent approach cycles peptides or limits to injury treatment periods rather than indefinite continuous use.
Interaction discoveries: Limited use creates minimal adverse interaction data. As peptide use expands, previously unknown interactions with medications, supplements, or medical conditions may emerge. Monitoring adverse event reports and maintaining communication with healthcare providers important for identifying problems.
Quality variability: Research peptide market largely unregulated. Even reputable vendors occasionally have quality issues. Bad batch risks persist requiring vigilance and testing. Peptide quality assurance remains ongoing responsibility.
Understanding individual response variability
Genetic factors: Polymorphisms in genes encoding growth factor receptors, collagen synthesis enzymes, inflammatory mediators affect healing capacity and peptide responsiveness. Some individuals genetically predisposed to robust healing responses while others heal slowly regardless of interventions. Genetic testing not standard but may explain differential peptide responses.
Age influences: Youth provides advantages including higher stem cell numbers, better vascular health, more robust inflammatory responses, faster metabolism. Elderly individuals face age-related healing impairments though peptides still beneficial, just with more modest improvements. Realistic expectations accounting for age-related limitations prevents disappointment. Peptides for aging addresses age-specific considerations.
Overall health status: Diabetes impairs healing through vascular damage, impaired immune function, advanced glycation end products. Smoking reduces blood flow and oxygenation. Obesity creates inflammatory environment and mechanical stress. Malnutrition limits building blocks for tissue synthesis. Addressing these factors optimizes peptide effectiveness. Poor health creates ceiling limiting best possible outcomes regardless of peptide quality or dosing.
Injury severity: Minor sprains and small fractures heal better than severe trauma with extensive tissue destruction. Peptides enhance natural healing processes but cannot overcome complete absence of viable tissue or massive structural deficits. Complex injuries may require surgical intervention with peptides supporting post-surgical healing rather than replacing necessary operations.
Detailed implementation: Starting your protocol
Practical guidance for beginning peptide therapy safely and effectively.
Pre-protocol preparation
Medical evaluation: Baseline assessment through healthcare provider establishing injury diagnosis, severity grading, treatment plan. Imaging studies (X-ray, MRI, ultrasound) documenting initial status for comparison. Blood work if indicated checking overall health, nutritional status (vitamin D common deficiency affecting bone health), inflammatory markers. Medical clearance especially important for those with health conditions or taking medications.
Establishing baseline measurements: Document current pain levels (0-10 scale), function (what activities possible, what limited), range of motion (degrees for joint movements), strength (manual testing or objective measurement). Photographs of affected area if visible swelling or deformity. Baseline provides comparison assessing peptide effectiveness. Progress tracking methods optimize monitoring.
Setting realistic goals: Define specific objectives (pain reduction, return to specific activities, healing verified on imaging) rather than vague "feel better." Understand realistic timeline (weeks for soft tissue, months for bone, months to years for cartilage) preventing premature disappointment. Identify success criteria (50% pain reduction, specific functional milestone) guiding protocol duration and modification decisions.
Sourcing quality peptides: Research vendors thoroughly through community testing results, vendor reviews, Certificate of Analysis verification. Consider independent testing ($75-$150) for first vendor order ensuring quality. Order adequate quantity for full protocol avoiding mid-cycle vendor changes. Budget for entire protocol cost (peptides plus supplies, testing, medical monitoring) rather than starting without financial commitment to completion. Review our peptide sourcing guide.
Supplies and preparation
Injection supplies: Insulin syringes (typically 0.5mL or 1mL with 29-31 gauge needles) for subcutaneous injection. Alcohol swabs for skin preparation. Sharps container for safe needle disposal. Bacteriostatic water for peptide reconstitution (comes with many peptide purchases or available separately). Understand proper injection technique before beginning.
Storage requirements: Freezer or refrigerator for lyophilized peptide storage maintaining potency. Dark location or aluminum foil wrap protecting from light. Refrigerator space for reconstituted peptides (separate from food for organization and safety). Cooler with ice packs if traveling with peptides. Detailed storage protocols prevent degradation.
Reconstitution process: Learn reconstitution using bacteriostatic water. Typical 2-5mL water per 5mg peptide vial though exact volume depends on desired concentration and total vial contents. Gentle swirling (not shaking) until powder dissolved. Label vial with reconstitution date and concentration for dosing accuracy. Use peptide calculator for precise measurements.
Dosing calculations: Determine daily dose based on protocol (typically 200-500mcg BPC-157, 2-5mg TB-500 twice weekly). Calculate injection volume delivering desired dose based on concentration. Mark syringes if needed for consistent volume. Recheck calculations preventing underdosing or overdosing. Detailed dosing guides prevent errors.
First injection and early protocol
Initial administration: Choose injection site (abdomen, thigh, near injury site options). Clean with alcohol swab. Pinch skin creating fold for subcutaneous injection. Insert needle at 45-90 degree angle. Inject slowly over several seconds. Remove needle, apply pressure with alcohol swab. Dispose needle in sharps container immediately. Rotate injection sites preventing buildup and irritation.
Monitoring immediate response: Watch for injection site reactions (normal mild redness or slight swelling resolves within hours). Systemic reactions (unusual fatigue, nausea, dizziness) rare but possible. Serious allergic reactions (difficulty breathing, hives, facial swelling) extremely rare, seek emergency care if occurring. First few injections most likely to reveal any sensitivity or allergy.
Establishing routine: Consistent timing (same time daily or specific days for weekly injections) maintains stable levels. Set reminders on phone preventing missed doses. Develop habit (after breakfast, before bed) for better adherence. Prepare supplies in advance reducing friction on injection days. Protocol consistency critical for optimal outcomes.
Early expectations: First 1-2 weeks unlikely showing dramatic improvements. Some users report reduced pain or inflammation within days while others notice nothing until week 3-4. Avoid discouragement from absence of immediate results. Continue protocol through initial period allowing adequate exposure time. Premature discontinuation prevents experiencing potential benefits requiring longer accumulation.
Mid-protocol assessment and adjustments
4-6 week evaluation: Assess progress against baseline measurements. Pain levels, functional capacity, subjective improvement feelings. Imaging if scheduled as part of medical care (not solely for peptide monitoring due to cost). Determine whether showing expected progress, better than expected, or disappointing results.
Positive progress response: Continue current protocol without changes. Avoid temptation increasing dose or frequency thinking "more is better." Stable effective protocol through completion then reassess need for continued treatment versus tapering. Success justifies completing full planned duration.
Minimal progress adjustments: If showing some improvement but less than hoped, consider increasing dose 25-50% (200mcg to 300mcg BPC-157, 2mg to 3-4mg TB-500). Add complementary peptide (BPC-157 alone becoming BPC-157 + TB-500). Extend planned duration (8 weeks becoming 12-16 weeks). Review adherence, technique, storage ensuring proper execution before assuming ineffectiveness.
No progress evaluation: Complete absence of any improvement after 6-8 weeks requires reassessment. Consider alternative peptides or discontinuing if specific peptide clearly ineffective. Verify peptide quality through independent testing if not previously done, possible bad batch. Medical reevaluation ensuring diagnosis accurate and no complications developed. Some injuries require interventions beyond peptides (surgery, specialized treatments).
Completion and maintenance
Protocol conclusion: After planned duration (8-16 weeks typically), discontinue daily injections. Observe 4-8 weeks off-cycle monitoring whether improvements sustained or regression occurs. Sustained benefits suggest successful healing. Regression indicates healing incomplete or condition requiring longer treatment.
Transition strategies: Gradual dose reduction (tapering) versus abrupt discontinuation both acceptable. Some prefer tapering (reducing dose 50% for 2 weeks then stopping) preventing sudden withdrawal of support. Others stop immediately without issues. Personal preference and injury severity guide approach.
Maintenance protocols: Chronic conditions (osteoarthritis) may benefit from ongoing low-dose treatment or periodic cycles. Monthly 1-week protocols, or 8 weeks on/8 weeks off cycles maintaining benefits without continuous use. Balance effectiveness against cost and convenience. Osteoarthritis particularly may require long-term management given progressive nature.
Return to activity: Gradually resume restricted activities as healing permits. Follow medical and physical therapy guidance regarding load progression and return-to-sport criteria. Peptide-enhanced healing should translate to better tissue quality and function at standard return timelines rather than necessarily faster return risking re-injury from premature loading.
Future injury prevention: Consider periodic peptide cycles for injury prevention, tissue maintenance, or recovery support even without acute injury. Some athletes use peptides proactively during intense training blocks reducing accumulated microtrauma. However, cost and time commitment substantial making this approach primarily for professional or serious amateur athletes. Most individuals reserve peptides for addressing specific injuries when they arise.
Troubleshooting common issues
Practical solutions for challenges arising during protocols.
Injection site problems
Persistent pain or swelling: Slow injection speed (take 5-10 seconds versus 1-2 seconds). Ensure peptide fully reconstituted without chunks. Warm vial to room temperature before injection (cold solutions hurt more). Rotate sites diligently avoiding repeated injections in same location. Ice area before injection numbing slightly. Most site reactions resolve spontaneously within days as technique improves and body adapts.
Bruising: Small blood vessel punctured during injection. Generally harmless though unsightly. Avoid injecting directly over visible veins. Apply pressure after injection helping clot formation. Consider using smaller gauge needles (31g versus 29g) though peptide viscosity may require larger needles. Some individuals bruise easily regardless of technique, not necessarily indicating problems.
Nodules or lumps: Injection creating small subcutaneous deposits. Usually resorb over days to weeks without treatment. Massage area gently encouraging absorption. Ensure not injecting intramuscular accidentally (too deep penetration). If persistent, inflamed, or enlarging, medical evaluation ruling out infection (very rare but possible).
Infection signs: Increasing redness, warmth, swelling, purulent drainage, fever, red streaking. Seek medical attention immediately, infections require antibiotic treatment. Prevent through strict sterile technique (clean skin, new needles, bacteriostatic water, proper storage). Infection rare with proper technique but serious complication when occurs.
Lack of therapeutic response
Verifying quality: Independent testing most definitive. However, assess other indicators like proper reconstitution behavior, appropriate vial appearance, seller reputation. Low quality or fake peptides obviously won't work. Community testing and vendor verification minimize risk though never eliminate completely.
Dosing accuracy: Recalculate ensuring mathematics correct. Verify concentration (mg peptide per mL solution). Confirm injection technique delivering full dose (not leaking). Some users accidentally underdosing through calculation errors or improper injection technique. Double-check all steps from reconstitution through administration.
Storage degradation: Review storage conditions. Frequent temperature cycling, light exposure, contamination degrade peptides. Degraded peptide retains appearance but loses potency. If suspecting degradation, obtain fresh vial from reliable source comparing response. Proper peptide storage maintains potency.
Individual non-responder: Small percentage of individuals don't respond to specific interventions regardless of quality or dosing. Genetic variability, unique disease characteristics, or unknown factors. After verifying quality, dosing, and technique, may need trying alternative peptides or accepting this particular intervention ineffective for you.
Unexpected side effects
Gastrointestinal issues: Nausea, cramping, changes in appetite rarely reported. Typically mild and transient. Take with food reducing nausea. Adjust injection timing (morning versus evening) finding better tolerated time. Reduce dose seeing if symptoms resolve while maintaining some benefit. Most GI effects resolve spontaneously after first week adaptation.
Fatigue or sleep changes: Some report increased sleepiness while others insomnia. Fatigue may indicate healing process requiring rest (growth hormone naturally increases during sleep, healing metabolically demanding). Adjust injection timing (evening dosing if causing sleepiness, morning if sleep disruption). Usually temporary effect normalizing after 1-2 weeks.
Mood alterations: Occasionally reported irritability, anxiety, or mood changes. Uncertain whether truly peptide-related versus stress of injury and treatment process. However, if significant mood symptoms emerge, consider reducing dose or discontinuing. Mental health takes priority over physical healing.
Skin reactions: Flushing, itching, or rashes. May indicate allergic sensitivity. Mild reactions (slight itching at injection site) often resolve with continued use. Widespread rashes, hives, or breathing difficulties indicate serious allergy requiring immediate discontinuation and medical attention.
Protocol interference issues
Missed doses: Single missed dose unlikely significantly impacting results. Resume regular schedule without doubling next dose. Multiple missed doses (3+ consecutive days) may slow progress though not irreversibly harmful. Restart protocol and extend duration by missed days if comfortable financially and logistically.
Illness during protocol: Acute illness (cold, flu, infection) may temporarily worsen injury symptoms through general inflammation. Continue peptides through illness unless specifically contraindicated. Fever, severe illness may necessitate temporary hold though generally safe to continue. Consult physician if major illness develops during protocol.
Surgery or procedures: Elective procedures scheduled during peptide protocol require surgical team awareness. Most likely no objection though inform anesthesiologist and surgeon. May need temporary hold perioperatively though some surgeons supportive continuing for healing support. Emergency surgery obviously takes priority over peptide considerations.
Travel or schedule disruptions: Refrigerated storage required for reconstituted peptides complicates travel. Options include bringing cooler with ice packs, planning travel during off-cycle periods, or accepting gap in protocol. Brief interruptions (3-5 days) unlikely dramatically affecting 8-16 week protocols. Resume upon return extending duration if needed.
Integration with comprehensive healing
Peptides represent one component of multifaceted recovery approach.
Sleep optimization for healing
Growth hormone release: Deep sleep triggers maximum GH secretion from pituitary. Growth hormone peptides enhance this further. Inadequate sleep blunts GH response limiting tissue repair and peptide effectiveness. Prioritize 7-9 hours quality sleep nightly during active healing.
Sleep hygiene strategies: Consistent bedtime and wake time regulating circadian rhythm. Dark, cool bedroom promoting deep sleep stages. Limit screen time before bed (blue light suppressing melatonin). Consider sleep peptides if insomnia problematic though prioritize behavioral interventions first.
Pain management for sleep: Injury pain disrupting sleep creates vicious cycle (poor sleep impairs healing which perpetuates pain). Appropriate pain medication if needed ensuring adequate rest. Positioning, supportive pillows, ice or heat before bed reducing nighttime discomfort. As peptides reduce pain and inflammation, sleep often improves creating positive feedback loop.
Stress reduction supporting recovery
Cortisol effects: Chronic stress elevates cortisol which impairs healing, increases inflammation, reduces GH and IGF-1 effectiveness. Stress management not optional luxury but essential healing component. Practices include meditation, deep breathing, gentle yoga, nature exposure, social connection.
Psychological factors: Anxiety about injury, frustration with limitations, depression from activity restrictions all common and impactful. Mental health support through counseling, support groups, or mood-supporting peptides like Selank potentially beneficial. Positive mindset correlates with better healing outcomes independent of interventions.
Movement and mechanical loading
Progressive loading principles: Adequate mechanical stress stimulates tissue remodeling creating stronger repair than unstressed healing. However, excessive loading causes re-injury. Follow physical therapy guidance progressing loading based on pain response, healing timeline, functional testing.
Blood flow benefits: Gentle movement increases circulation delivering nutrients, oxygen, peptides, and immune cells to injury sites. Complete immobilization creates stiffness and atrophy. Balance protection (avoiding re-injury) with appropriate activity (promoting healing).
Proprioceptive rehabilitation: Neuromuscular control often impaired after injury independent of structural healing. Balance exercises, controlled movements, sport-specific drills restore proprioception and coordination reducing future injury risk.
Complementary supplements
Anti-inflammatory support: Omega-3 fatty acids (fish oil, 2-3g daily EPA+DHA) provide natural anti-inflammatory effects complementing peptides. Curcumin, ginger, boswellia other anti-inflammatory botanicals. However, avoid excessive doses potentially interfering with necessary acute inflammation.
Joint-specific nutrients: Glucosamine and chondroitin for cartilage (evidence mixed but safe and inexpensive). MSM (methylsulfonylmethane) for joint health. Hyaluronic acid supporting synovial fluid. These provide building blocks similar to collagen peptides.
Bone-specific nutrients: Calcium (1000-1200mg daily from diet and supplements), vitamin D (2000-4000 IU daily achieving blood levels 40-60 ng/mL), vitamin K2 (directing calcium to bones versus soft tissues), magnesium (cofactor in bone metabolism). Standard bone health supplementation supports peptide effects.
Protein and amino acids: Adequate protein intake (1.2-1.6g per kg body weight) provides building blocks for tissue synthesis. Branching chain amino acids (leucine, isoleucine, valine) particularly important for protein synthesis signaling. Amino acid supplementation potentially enhancing peptide protocols though whole protein sources generally sufficient.
How SeekPeptides supports musculoskeletal healing
SeekPeptides provides comprehensive resources for evidence-based musculoskeletal peptide therapy.
Detailed injury-specific protocols for fractures, cartilage damage, tendon injuries, arthritis, and other conditions. Dosing, duration, monitoring, and adjustment guidelines.
Peptide comparison tools helping choose between BPC-157, TB-500, growth hormone peptides, and other options based on injury type and goals.
Community forums connecting individuals using peptides for musculoskeletal healing. Sharing experiences, troubleshooting problems, supporting recovery journeys. Collective wisdom exceeding individual knowledge.
Vendor verification database with community testing results and quality assessments. Updated regularly as new vendors emerge and existing vendors change. Reducing risk of poor quality products.
Integration guides for combining peptides with surgery, physical therapy, medications, and other treatments. Maximizing synergies while avoiding conflicts.
Progress tracking tools helping monitor outcomes objectively. Templates for recording pain, function, imaging results, adjusting protocols based on response.
Safety information covering side effects, contraindications, drug interactions, and emergency protocols. Supporting responsible use minimizing risks.
SeekPeptides remains committed to advancing musculoskeletal peptide therapy through education, community, and evidence-based guidance.
Helpful resources
Related guides worth reading
Tissue repair and healing peptides:
Future directions in musculoskeletal peptide therapy
Research continues expanding understanding and applications for bone and cartilage repair peptides.
Improved formulations: Sustained-release peptide formulations reducing injection frequency currently in development. Hydrogel or microparticle carriers releasing peptides over days to weeks from single injection. Topical preparations for superficial injuries avoiding injections entirely. While research stage currently, these innovations may improve convenience and compliance within 5-10 years.
Combination therapies: Synergistic peptide combinations optimized through systematic research rather than empirical trial. Understanding which combinations produce additive versus truly synergistic effects guides evidence-based protocols. Combining peptides with stem cells, PRP, or other biologics creating comprehensive regenerative medicine approaches. SeekPeptides tracks emerging combination therapies.
Targeted delivery systems: Nanoparticles or liposomes decorated with tissue-specific targeting ligands directing peptides specifically to bone, cartilage, or other tissues. Increases local concentration while reducing systemic exposure. Injectable scaffolds combining peptides with biodegradable matrices supporting three-dimensional tissue regeneration.
These technologies may transform peptide delivery making treatments more effective and safer.
Biomarker-guided protocols: Identifying which patients respond best to specific peptides through predictive biomarkers. Genetic testing, inflammatory profiles, or tissue imaging predicting responsiveness allowing personalized protocol selection.
Avoiding ineffective treatments and optimizing resources for individual situations.
Precision medicine approaches currently experimental but likely becoming standard within coming decades.
Regulatory pathways: As evidence accumulates, some peptides may pursue FDA approval for specific indications. BPC-157 strong candidate given robust animal data and widespread safe use. However, pharmaceutical development expensive ($100M+ for full approval process) requiring corporate investment. Most peptides likely remaining research chemicals indefinitely though select few might achieve pharmaceutical status.
Clinical trial expansion: More rigorous human trials testing peptides for orthopedic applications gradually emerging. Academic medical centers and orthopedic surgery departments increasingly interested in regenerative therapies including peptides. Next 5-10 years should produce higher-quality human data replacing current reliance on animal studies and anecdotal reports.
Clinical trial database tracks ongoing research.
External resources
In case I don't see you, good afternoon, good evening, and good night. May your bones stay strong, your cartilage stay healthy, and your healing stay progressive. Join SeekPeptides.