Feb 2, 2026
A protein in your scalp is actively blocking hair growth right now. It is called CXXC5, and until recently, nobody knew how to stop it. Researchers at Yonsei University in South Korea spent years studying why some people lose hair despite every treatment available, and what they found changed the conversation about peptide-based hair restoration entirely. The protein CXXC5 binds to another protein called Dishevelled, and when that binding happens, it shuts down the Wnt/beta-catenin signaling pathway. That pathway controls whether your hair follicles grow, rest, or die.
PTD-DBM was designed to fix this problem.
The peptide works by inserting itself between CXXC5 and Dishevelled, physically preventing the interaction that suppresses hair growth. When CXXC5 cannot bind Dishevelled, the Wnt pathway stays active. Follicles receive growth signals. Stem cells proliferate. New follicles form. The mechanism is elegant because it does not force growth through artificial stimulation or hormone manipulation. It removes a natural brake. And that distinction matters enormously for anyone who has tried minoxidil, finasteride, or other conventional approaches and found them inadequate, intolerable, or both.
This guide covers everything researchers need to know about PTD-DBM: the science behind CXXC5 inhibition, how the Wnt/beta-catenin pathway governs follicle behavior, the preclinical evidence from published studies, application protocols including the three-pronged approach with valproic acid and microneedling, realistic expectations based on current evidence, safety considerations, and how PTD-DBM compares to other hair growth peptides like GHK-Cu and Thymulin zinc.
SeekPeptides provides comprehensive protocol guidance for members navigating these complex peptide applications, but understanding the fundamentals helps anyone make informed decisions about their research approach.
Understanding the Wnt/beta-catenin pathway in hair growth
Before PTD-DBM makes sense, you need to understand what it is targeting. The Wnt/beta-catenin pathway is one of the most conserved signaling systems in human biology. It controls cell proliferation, stem cell renewal, and tissue regeneration across virtually every organ system. In the scalp, this pathway determines whether hair follicles enter the anagen (growth) phase, how long they stay there, and whether new follicles form at all.
The pathway works through a cascade of molecular events. When Wnt ligands bind to Frizzled receptors on the cell surface, they activate a protein called Dishevelled. Dishevelled then inhibits a destruction complex that would otherwise break down beta-catenin. With the destruction complex neutralized, beta-catenin accumulates in the cell, enters the nucleus, and activates genes responsible for cell growth and differentiation. In hair follicles specifically, this means stem cell activation, dermal papilla proliferation, and the transition from resting to growing phases. Researchers studying anti-aging peptide applications recognize that the same Wnt pathway governs tissue regeneration throughout the body, not just in the scalp.
Research confirms this pathway is essential. Studies demonstrate that enhancing beta-catenin signaling in dermal papilla cells produces faster and denser hair growth. Inactivation of beta-catenin, by contrast, causes dramatically reduced proliferation of hair progenitor cells and premature entry into catagen, the destructive phase where follicles shrink. Understanding how peptides interact with cellular signaling provides important context for why pathway-specific interventions like PTD-DBM represent a fundamentally different approach than conventional treatments.
CXXC5: the hair growth brake nobody knew about
Professor Kang-Yell Choi and his team at Yonsei University made a critical discovery. They found that a protein called CXXC-type zinc finger protein 5, abbreviated CXXC5, acts as a negative feedback regulator of the Wnt/beta-catenin pathway. When they examined bald scalps from individuals with androgenetic alopecia, they found significantly elevated levels of CXXC5 compared to areas with healthy hair growth.
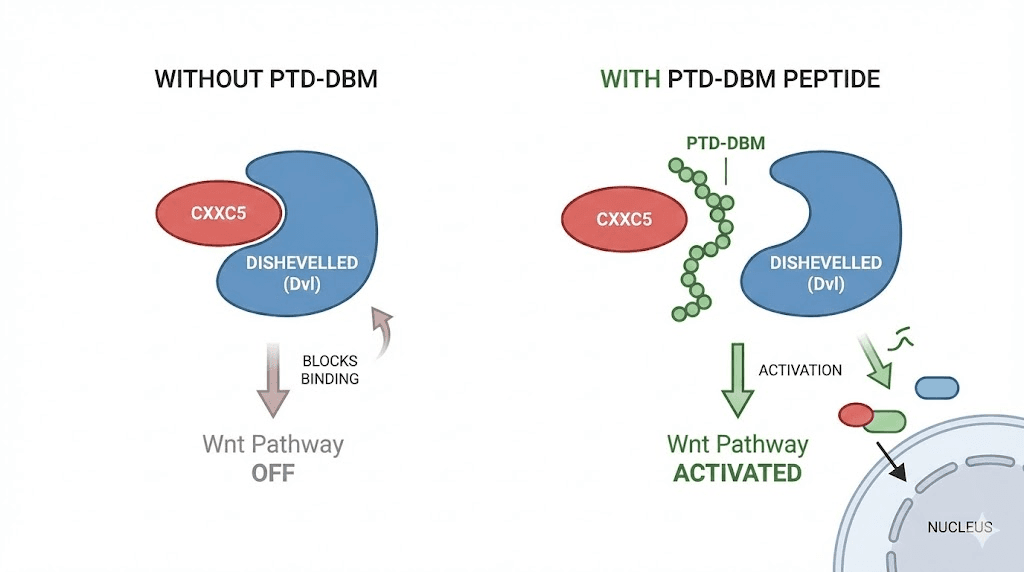
The mechanism is straightforward. CXXC5 binds directly to Dishevelled, the upstream activator of the pathway.
When CXXC5 locks onto Dishevelled, it prevents Dishevelled from doing its job. The destruction complex remains active.
Beta-catenin gets broken down before it can reach the nucleus. Hair growth signals never arrive at the genes that need them. Follicles enter telogen, miniaturize, and eventually stop producing visible hair altogether. This process is fundamentally different from the skin repair mechanisms that other peptides target, though the downstream consequences of impaired Wnt signaling affect both hair and skin quality.
This discovery explained something that had puzzled researchers for years. Treatments that activated Wnt signaling from downstream, like lithium chloride or other GSK3-beta inhibitors, showed limited effectiveness for hair growth. The reason became clear: even if you activated parts of the pathway further downstream, CXXC5 was still blocking the signal at the top. Like trying to increase water pressure in a house while someone holds the main valve shut.
The solution was obvious once the problem was identified.
Block CXXC5 from binding Dishevelled, and the entire pathway opens up.
The DHT-PGD2-CXXC5 connection
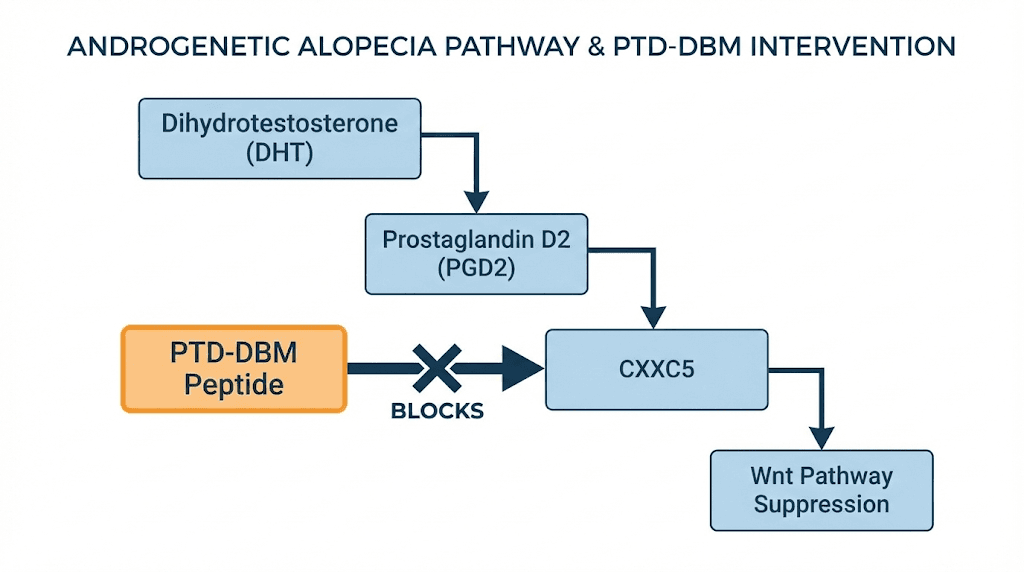
A landmark 2023 study published in the journal Cells revealed an even more significant finding. CXXC5 does not just happen to be elevated in bald scalps. It is actively induced by the same hormonal cascade that causes androgenetic alopecia.
The chain works like this. DHT (dihydrotestosterone) activates androgen receptors in scalp tissue. Those activated receptors increase expression of an enzyme called PTGDS, prostaglandin D2 synthase. PTGDS produces PGD2, prostaglandin D2, a molecule already known to be elevated in balding scalps. PGD2 then triggers BMP signaling, which induces CXXC5 expression. CXXC5 suppresses Wnt/beta-catenin signaling. Hair loss follows.
This chain, DHT to PTGDS to PGD2 to BMP to CXXC5 to Wnt suppression, represents a complete mechanistic explanation for how androgenetic alopecia works at the molecular level. And it places CXXC5 at a critical junction point. Researchers studying peptides and testosterone interactions recognize the significance of this pathway, as it connects hormonal signaling directly to follicle fate through a protein that can be specifically targeted. The peptides for men guide explores how testosterone-related pathways affect multiple aspects of male health beyond hair loss, and understanding this broader context helps explain why interventions at different points in the cascade produce different clinical effects.
The study demonstrated that when CXXC5 was knocked out in mice, PGD2 treatment no longer caused hair loss. The animals were resistant to the balding cascade. Similarly, treatment with PTD-DBM restored hair growth even in the presence of PGD2. The brake was removed, regardless of what was pushing on it.
How PTD-DBM works at the molecular level
PTD-DBM stands for Protein Transduction Domain-fused Dishevelled Binding Motif. The name describes exactly what the molecule does. It consists of two functional components: a protein transduction domain that allows it to penetrate cell membranes, and a Dishevelled binding motif that occupies the site where CXXC5 would normally attach.
Think of it as a molecular decoy.
When PTD-DBM enters the cell, it binds to Dishevelled at the same location CXXC5 would. But unlike CXXC5, PTD-DBM does not inhibit Dishevelled function. It simply sits there, occupying the binding site, preventing CXXC5 from attaching. Dishevelled remains free to do its job in the Wnt pathway. Beta-catenin accumulates. Growth signals reach the nucleus. Follicles respond.
The protein transduction domain is critical for topical application. Most peptides cannot cross cell membranes on their own, which limits their usefulness as topical treatments. Understanding peptide delivery methods reveals why this is such a significant challenge. The PTD component of PTD-DBM solves this problem by actively transporting the peptide across the membrane and into the cytoplasm where CXXC5 and Dishevelled interact. This cell-penetrating capability is what makes topical application viable, unlike many other peptide compounds that require injection for cellular delivery.
What happens downstream when CXXC5 is blocked
When PTD-DBM successfully prevents CXXC5 from binding Dishevelled, several downstream effects follow. Beta-catenin stabilizes and translocates to the nucleus. There, it activates transcription factors that drive expression of genes involved in hair follicle cycling, stem cell proliferation, and dermal papilla function.
Specific effects documented in research include proliferation of hair follicle progenitor cells, extension of the anagen (growth) phase of the hair cycle, increased hair follicle size and density, formation of new dermal papilla structures, enhanced wound-induced hair neogenesis, and prevention of follicle miniaturization. The bioregulator peptide approach of modulating existing biological pathways rather than forcing artificial responses distinguishes PTD-DBM from many conventional treatments. This modulatory mechanism aligns with how signaling peptides generally function, using precise molecular targeting rather than broad systemic effects to achieve specific outcomes.
One finding from the preclinical research deserves particular attention. PTD-DBM did not just slow hair loss or maintain existing follicles. In mouse models, topical application stimulated the formation of entirely new hair follicles, a process called neogenesis. This is different from reactivating dormant follicles. Neogenesis means creating follicles where none existed before, which has profound implications for areas of advanced hair loss where follicle counts have genuinely declined.
PTD-DBM versus downstream Wnt activators
Not all Wnt pathway activators work the same way. Understanding where each compound acts in the signaling cascade explains why some approaches succeed where others fail.
GSK3-beta inhibitors like valproic acid activate Wnt signaling by preventing the destruction complex from breaking down beta-catenin. But they work downstream of the CXXC5 blockade. If CXXC5 is still suppressing Dishevelled, the signal that tells the destruction complex to stand down never arrives properly. GSK3-beta inhibition partially compensates, but it is fighting the system rather than releasing it.
PTD-DBM works upstream. By preventing CXXC5 from blocking Dishevelled, it allows the natural signaling cascade to proceed normally. The destruction complex receives proper deactivation signals through the native pathway. This is more physiologically appropriate and likely explains why the combination of PTD-DBM (upstream release) with valproic acid (downstream activation) produces synergistic rather than merely additive effects.
The 2023 study confirmed this. Single inhibition of GSK3-beta through valproic acid or CXXC5 alone through PTD-DBM showed limited efficacy against DHT-induced hair loss. But KY19382, a small molecule that inhibits both CXXC5 function and GSK3-beta simultaneously, completely restored beta-catenin signaling even in the presence of DHT. The dual approach addressed the pathway at multiple points, producing far superior results than either intervention alone.
The preclinical evidence: what the studies actually show
Honest assessment of the evidence requires separating what has been demonstrated from what has been assumed. PTD-DBM has strong preclinical data from well-designed studies. It does not yet have large-scale human clinical trial results. Understanding this distinction is essential for setting realistic expectations.
The landmark 2017 mouse study
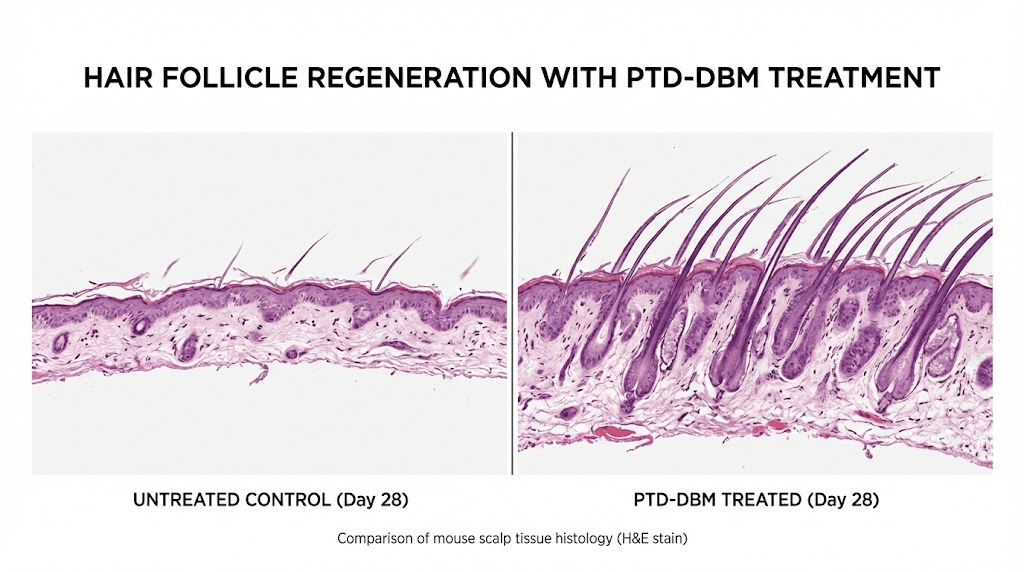
The foundational research, published in The Journal of Investigative Dermatology by Lee et al., demonstrated several key findings. When PTD-DBM was applied topically to the bare skin of laboratory mice, new hair follicle growth was observed after 28 days.
The peptide promoted both hair regrowth in depilated (shaved) areas and wound-induced hair neogenesis, the formation of entirely new follicles in healing wound sites. For researchers familiar with healing peptides, the wound-induced neogenesis finding is particularly significant because it connects the injury repair mechanism to actual follicle creation.
The study established the mechanism: PTD-DBM interferes with the binding of CXXC5 to Dishevelled, activating the Wnt/beta-catenin pathway. CXXC5 knockout mice showed accelerated hair regrowth compared to wild-type controls, validating that CXXC5 suppression drives the observed effects. The research also demonstrated that combinatory treatment with valproic acid further enhanced results, establishing the synergistic protocol that would become the standard approach.
Specific observations included new follicle formation in areas that had been completely bare, increased follicle density in treated areas versus controls, and accelerated transition from telogen to anagen. The results were sufficiently compelling that Professor Choi and his team filed patents and established a company, initially called CK Biotechnology and later renamed CK Regeon, to commercialize the approach. They raised $12 million in Series B funding in February 2021.
The 2023 DHT-PGD2-CXXC5 study
Published in Cells, this study expanded understanding of CXXC5 mechanisms and provided the most complete picture yet of how androgenetic alopecia works at the molecular level. The researchers used both in vitro cell culture (HaCaT keratinocytes and primary human dermal papilla cells) and in vivo mouse models.
Key quantitative findings included dose-dependent increases in CXXC5 expression following PGD2 treatment, corresponding decreases in beta-catenin and PCNA (a proliferation marker), restoration of Wnt signaling and cellular proliferation with PTD-DBM treatment, and complete resistance to PGD2-induced hair loss in CXXC5 knockout mice.
The study used specific concentrations in topical applications: PGD2 at 10 micrograms, PTD-DBM at 10 millimolar, DHT at 100 micromolar, and KY19382 at 2 millimolar. Treatment durations ranged from 12 to 42 days across different experimental models. The researchers employed depilation-induced hair cycling, wound-induced hair neogenesis, and vibrissa (whisker) follicle culture systems to validate findings across multiple models.
Perhaps the most clinically relevant finding was that KY19382, the dual inhibitor of CXXC5 and GSK3-beta, showed more hair follicles progressing to anagen phase compared to DHT-only controls. This dual-mechanism approach is what CK Regeon is currently developing toward clinical trials in humans.
Wound healing research
PTD-DBM shows effects beyond hair growth. The Wnt/beta-catenin pathway also governs cutaneous wound healing, and CXXC5 negatively regulates this process as well. Research demonstrates that inhibiting CXXC5 through PTD-DBM accelerates wound closure, enhances keratin 14 and collagen synthesis, and promotes wound-induced hair follicle neogenesis.
This wound healing connection is not merely academic. It has practical implications for tissue repair peptide protocols and explains why the microneedling component of PTD-DBM protocols works synergistically. Microneedling creates controlled micro-wounds that trigger wound healing cascades. PTD-DBM enhances those cascades by removing CXXC5 suppression of the Wnt pathway. The combination leverages the natural wound repair processes to generate new follicles, a process researchers call wound-induced hair neogenesis or WIHN. For those interested in injury healing through peptides, this dual application of PTD-DBM is particularly noteworthy.
The three-pronged protocol: PTD-DBM, valproic acid, and microneedling
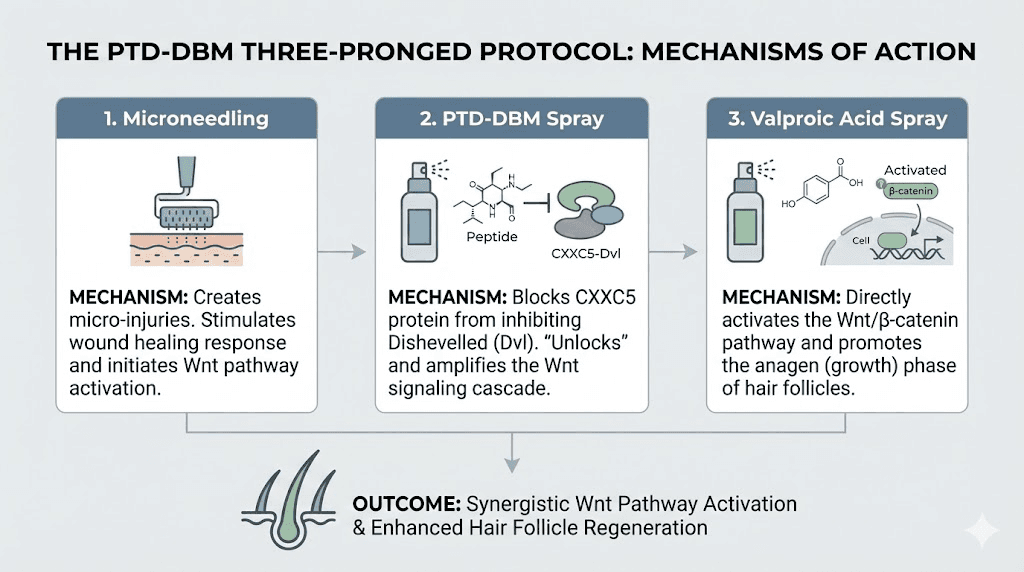
Research supports a combined approach rather than PTD-DBM alone. The three-pronged protocol addresses hair loss from three distinct but complementary angles, each targeting a different aspect of follicle regeneration.
Component one: PTD-DBM topical application
PTD-DBM is applied topically as a spray to the scalp, typically once daily. The protein transduction domain component allows the peptide to penetrate the skin barrier and enter cells where it can block the CXXC5-Dishevelled interaction. Consistent daily application maintains steady CXXC5 inhibition, keeping the Wnt pathway active.
The topical route is one of the advantages of PTD-DBM over many other research peptides. Most injectable peptides require subcutaneous or intramuscular administration because they cannot cross cell membranes effectively. The PTD component of this peptide was specifically designed to solve the delivery problem, making daily topical use practical and non-invasive. For researchers more familiar with peptide injection protocols, the topical-only application of PTD-DBM represents a significant convenience advantage.
Application is straightforward. Clean the scalp before application to remove oils and products that could impede absorption. Spray the solution directly onto affected areas. Massage gently for one to two minutes to distribute the product and enhance absorption. Allow complete absorption before applying other products or going to bed.
Component two: valproic acid topical spray
Valproic acid, typically known as an anticonvulsant and mood stabilizer, activates the Wnt/beta-catenin pathway through a different mechanism than PTD-DBM. It inhibits GSK3-beta, a component of the destruction complex that breaks down beta-catenin. By blocking GSK3-beta, valproic acid allows more beta-catenin to accumulate and reach the nucleus.
The combination makes biological sense. PTD-DBM releases the brake at the top of the pathway by blocking CXXC5. Valproic acid provides additional activation downstream by inhibiting the destruction complex. Together, they hit the pathway at two different points, producing synergistic effects that exceed what either achieves alone.
Research specifically supports this combination. Combinatory treatment of PTD-DBM with valproic acid further induced hair regrowth as well as wound-induced hair neogenesis beyond what either compound achieved independently. In the mouse studies, the combination produced faster new hair growth compared to PTD-DBM alone. The peptide stacking guide discusses how combining compounds that work through complementary mechanisms generally produces superior outcomes.
A clinical study on valproic acid alone provides additional supporting evidence. In male patients with androgenic alopecia, topical valproic acid spray applied twice daily for 24 weeks significantly increased total hair count compared to placebo.
It also increased linear hair growth rate and hair density. These results for valproic acid alone suggest the downstream Wnt activation has independent value, which combines with PTD-DBM upstream effects for enhanced outcomes.
Component three: microneedling
Microneedling serves a dual purpose in the protocol. First, it creates controlled micro-wounds that trigger wound-induced hair neogenesis. The healing process activates growth factors, stem cells, and regenerative cascades that independently support new follicle formation. Second, the micro-channels created by needling dramatically improve absorption of both PTD-DBM and valproic acid, allowing more of each compound to reach target cells.
The recommended frequency is one to two sessions per week using a dermaroller or dermapen at appropriate needle depths. For scalp applications, depths of 0.5 to 1.5 millimeters are commonly used. Shallower depths primarily enhance product absorption. Deeper depths trigger stronger wound healing responses. The microneedle peptide delivery guide covers how micro-channeling technology enhances peptide absorption across the skin barrier.
The threefold action creates a powerful combination. Microneedling wounds induce follicle generation through WIHN. Valproic acid stimulates the Wnt pathway that drives follicle development. PTD-DBM prevents CXXC5 from interfering with that development. Each component addresses a different bottleneck in the regeneration process, and removing all three bottlenecks simultaneously produces results none could achieve independently.
Timing matters. Apply PTD-DBM and valproic acid immediately after microneedling sessions. The micro-channels begin closing within minutes, and applying peptide solutions while channels remain open maximizes penetration. On non-microneedling days, standard topical application to clean, dry scalp maintains consistent CXXC5 inhibition. For researchers using other topical compounds like retinol alongside peptides, timing separation between different actives helps avoid interactions. The peptide and retinol combination guide addresses scheduling considerations for multi-product topical protocols.
How PTD-DBM compares to other hair growth treatments
Placing PTD-DBM in the context of existing treatments helps researchers understand where it fits and whether it complements or replaces their current approach.
PTD-DBM versus minoxidil
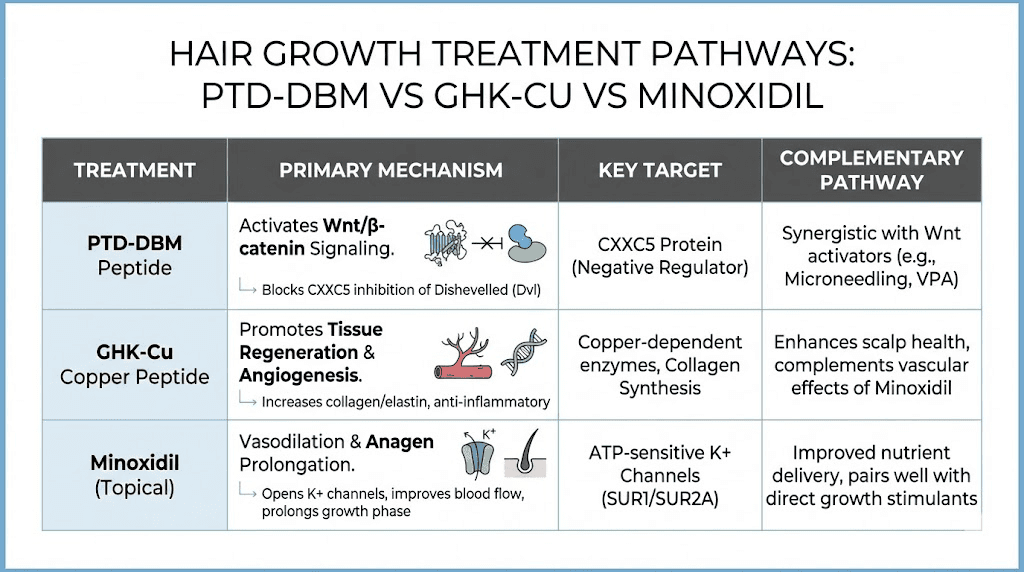
Minoxidil works primarily as a vasodilator, increasing blood flow to hair follicles. It does not address the Wnt pathway, CXXC5 suppression, or the DHT-PGD2 cascade. Its mechanism is fundamentally different from PTD-DBM, which means the two treatments target completely separate aspects of hair biology.
This difference is actually advantageous. Because they work through independent mechanisms, combining minoxidil with PTD-DBM could address both vascular supply (minoxidil) and signaling pathway restoration (PTD-DBM). They do not compete with each other or create redundancy. Researchers already using minoxidil do not need to choose between the two. The comprehensive hair loss peptide guide discusses how different treatment mechanisms can be layered for enhanced results.
However, minoxidil has decades of clinical evidence in humans. PTD-DBM does not. For evidence-weighted decision making, minoxidil remains the better-validated choice. PTD-DBM represents a more targeted mechanism but with less proof in human populations.
The peptide results documentation highlights how important evidence quality is when evaluating treatment options.
PTD-DBM versus finasteride
Finasteride blocks the enzyme 5-alpha reductase, which converts testosterone to DHT. By reducing DHT levels, it addresses the upstream hormonal trigger of androgenetic alopecia. The 2023 CXXC5 study actually demonstrated that DHT induces CXXC5 through the PGD2 pathway, meaning finasteride and PTD-DBM target the same cascade at different points.
Finasteride works at the very top: reduce DHT, and less CXXC5 gets produced in the first place. PTD-DBM works at the bottom: even if CXXC5 is present, prevent it from suppressing Wnt signaling.
The dual approach could theoretically provide superior protection, reducing the amount of CXXC5 produced (finasteride) while also blocking whatever CXXC5 remains (PTD-DBM).
Finasteride carries well-documented hormonal side effects including sexual dysfunction, which some users find intolerable. PTD-DBM is non-hormonal and applied topically, avoiding systemic hormonal effects. For individuals who cannot tolerate finasteride, PTD-DBM offers an alternative approach to addressing the same underlying pathway without hormonal manipulation. The peptides versus hormone therapy comparison provides broader context for non-hormonal intervention options.
PTD-DBM versus GHK-Cu
GHK-Cu (copper tripeptide) works through regenerative signaling, collagen stimulation, angiogenesis, and anti-inflammatory effects. It activates dormant follicles and supports overall scalp health but does not specifically target the CXXC5-Dishevelled interaction or address the Wnt pathway directly.
GHK-Cu has broader regenerative properties that extend beyond hair growth. The GHK-Cu for hair growth guide details how copper peptides support follicle function through multiple mechanisms including stem cell activation, blood vessel formation, and oxidative stress reduction. PTD-DBM is more narrowly targeted, focused specifically on removing CXXC5 suppression of Wnt signaling.
The two peptides complement each other well. GHK-Cu provides general regenerative support while PTD-DBM addresses the specific signaling blockade that causes follicle miniaturization. Some advanced protocols combine both, using GHK-Cu at standard dosages alongside the PTD-DBM/valproic acid/microneedling protocol. The copper peptides for hair page explores how these compounds interact with follicle biology through different but complementary mechanisms.
PTD-DBM versus PRP and growth factor treatments
Platelet-rich plasma (PRP) concentrates growth factors from your own blood for injection into the scalp. It provides a burst of proliferative signals that stimulate follicle activity. The approach works, but the effects are temporary and require repeated treatments.
PTD-DBM addresses a structural signaling problem rather than providing temporary growth factor supplementation. Removing CXXC5 suppression allows the body natural Wnt signaling to function normally, which is a more fundamental correction than adding external growth factors. However, the two approaches are not mutually exclusive. PRP provides concentrated growth factor stimulation while PTD-DBM ensures the Wnt pathway remains unblocked to respond to those signals.
One claimed advantage of PTD-DBM over other treatments deserves mention: some sources report that hair growth achieved with PTD-DBM does not shed when treatment stops. Unlike minoxidil, where discontinuation typically leads to loss of gains within months, PTD-DBM may produce more durable results because it promotes actual neogenesis, the creation of new follicles, rather than simply maintaining existing ones in a state of artificial stimulation. This claim requires significant caution, as long-term human data is not available to confirm durability of results. For comparison, the GHK-Cu discontinuation guide discusses how stopping other peptide treatments affects maintained results, providing a framework for thinking about long-term treatment commitment.
Combination approach comparison table
Treatment | Mechanism | Route | Human evidence | Best for |
|---|---|---|---|---|
PTD-DBM | CXXC5-Dvl blockade, Wnt activation | Topical | Limited (preclinical) | Wnt pathway restoration |
Minoxidil | Vasodilation | Topical | Extensive (FDA approved) | Blood flow improvement |
Finasteride | DHT reduction | Oral | Extensive (FDA approved) | Hormonal hair loss |
GHK-Cu | Regenerative signaling | Topical/Injectable | Moderate | General follicle health |
Valproic acid | GSK3-beta inhibition | Topical | Limited clinical | Wnt pathway enhancement |
PRP | Growth factor supplementation | Injection | Moderate | Acute follicle stimulation |
Application protocols and practical considerations
For researchers considering PTD-DBM, practical implementation details matter as much as understanding the science. The right protocol maximizes the potential benefits while the wrong approach wastes time and money.
Standard daily protocol
The basic protocol involves daily topical application of PTD-DBM spray to clean, dry scalp. Most commercial formulations come pre-mixed in spray bottles designed for direct scalp application. Application occurs once or twice daily depending on the specific product formulation and concentration.
Morning application allows the peptide to work throughout the day. Evening application provides overnight exposure when growth hormone levels peak and cellular repair processes are most active. Some users split applications between morning and evening for sustained coverage. The peptide dosing guide discusses timing considerations that may affect peptide effectiveness for various applications. Understanding peptide dosage calculations is less critical for pre-mixed topical products but becomes important if preparing custom formulations.
Consistency is more important than timing optimization. Daily application over months produces cumulative effects. Missing occasional applications is less concerning than sporadic, inconsistent use. The peptide works through sustained suppression of CXXC5 binding, which requires continuous presence. Gaps in application allow CXXC5 to resume its suppressive activity.
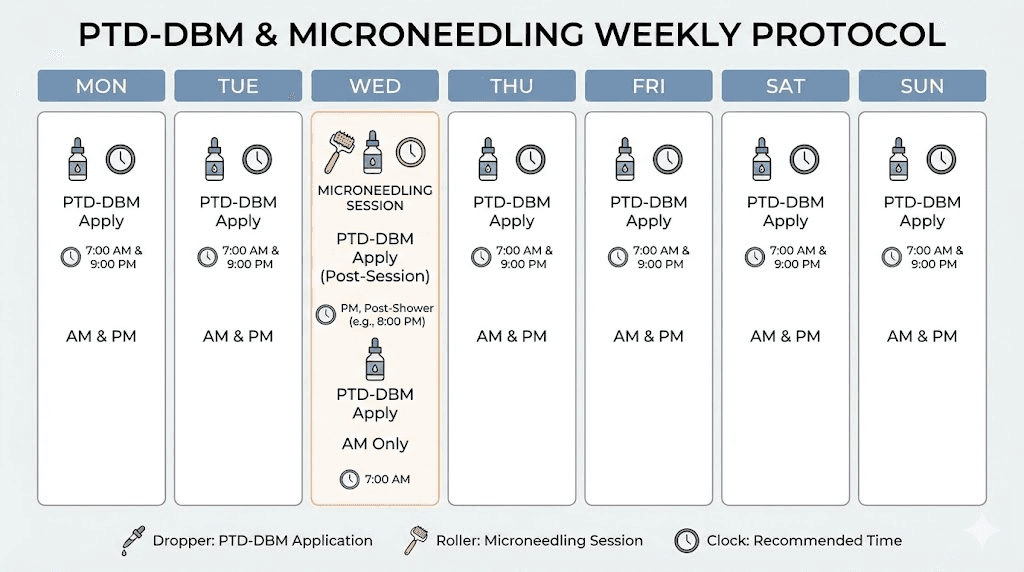
Enhanced protocol with valproic acid and microneedling
The full three-pronged approach follows this schedule:
Daily: Apply PTD-DBM topical spray to clean, dry scalp. Apply valproic acid topical spray to the same areas. Massage gently to distribute product. Allow complete absorption before other products or sleeping.
One to two times weekly: Perform microneedling session on affected areas of the scalp. Clean the scalp thoroughly before needling. Use a dermaroller or dermapen at 0.5 to 1.5 millimeter depth. Apply PTD-DBM immediately after needling while channels remain open. Follow with valproic acid application. Avoid washing the scalp for several hours after the combined session.
Protocol duration: Results from preclinical studies appeared over 28 to 42 day timeframes in mice. Human hair cycles are slower, with individual hairs taking 2 to 6 months to progress through growth phases. Minimum protocol duration should be 3 to 6 months before evaluating results. Many researchers continue for 12 months or longer for full assessment.
For those maintaining broader peptide stack protocols, PTD-DBM adds minimal complexity since it is topical rather than injectable. It does not interfere with systemic peptide protocols and can be incorporated alongside existing regimens without timing conflicts.
Product quality and sourcing considerations
The quality of PTD-DBM products varies significantly across the market. Because the peptide is not FDA-approved for hair loss treatment, products exist in a regulatory grey area. Some are sold as cosmetic hair serums. Others are available through compounding pharmacies. Research-grade peptides can be obtained from reputable peptide vendors for those who prepare their own formulations.
Key quality indicators include third-party purity testing (typically HPLC and mass spectrometry), proper storage and shipping conditions (many peptides degrade at room temperature), clear labeling of concentration and ingredients, and reputation within the research community. The peptide testing labs guide discusses how to verify product quality through independent analysis.
Some commercial products combine PTD-DBM with other active ingredients like methyl vanillate, a compound that may further support Wnt signaling. While these combinations are interesting, they make it difficult to assess PTD-DBM effects specifically. Researchers seeking to evaluate PTD-DBM as an isolated variable may prefer single-ingredient formulations.
Proper peptide storage is essential for maintaining potency. Most PTD-DBM products require refrigeration. The fridge storage duration guide and room temperature stability guide provide specific recommendations. The powder form stability guide covers lyophilized peptide longevity for those working with raw research-grade compounds. Understanding post-reconstitution storage requirements is equally important if preparing custom formulations. Peptide degradation reduces effectiveness without any visible indication that the product has lost potency, making proper storage a silent but critical factor in protocol outcomes. For those questioning whether their peptide products remain viable, the peptide expiration guide addresses shelf life concerns across different storage conditions.
Realistic expectations: separating science from hype
This is where honesty matters most. The science behind PTD-DBM is genuinely interesting. The mechanism is well-characterized. The preclinical data is compelling. But the gap between mouse studies and proven human effectiveness remains significant, and commercial marketing often obscures this gap.
What the evidence supports
The evidence firmly supports these conclusions. CXXC5 is overexpressed in bald scalps and plays a direct role in suppressing hair growth through Wnt pathway inhibition. PTD-DBM effectively blocks CXXC5 binding to Dishevelled in laboratory and animal models. Topical PTD-DBM promotes hair regrowth and neogenesis in mouse models. The combination with valproic acid produces synergistic effects in preclinical settings. The DHT-PGD2-CXXC5 axis represents a genuine mechanistic explanation for androgenetic alopecia.
These are solid scientific findings from reputable institutions published in peer-reviewed journals. The peptide research and studies overview contextualizes how preclinical evidence fits into the broader landscape of peptide development. Understanding what peptides are used for across different therapeutic areas helps appreciate the range of applications beyond hair growth where similar pathway-targeting strategies are being explored.
What the evidence does not support
Large-scale human clinical trials confirming safety and efficacy have not been published. The concentrations and formulations that work in mice may not translate directly to human scalps. Long-term safety data in humans is not available. Claims of results within weeks are not supported by published research. The durability of results after discontinuation remains unproven in humans.
This distinction between preclinical promise and clinical proof is crucial. Many peptides show remarkable effects in animal models that do not translate to equivalent human benefits. The differences between mouse and human skin, hair cycling, hormonal systems, and immune responses all affect translation. While the CXXC5 mechanism appears conserved between mice and humans (elevated CXXC5 is found in human bald scalps), the functional response to PTD-DBM treatment in humans may differ in magnitude, timeline, or both.
What community experience suggests
Anecdotal reports from online communities paint a mixed picture. Some users report positive results, particularly when combining PTD-DBM with valproic acid and microneedling. Others report no noticeable improvement after months of consistent use. The range of experiences is wider than what commercial marketing suggests.
Several factors likely contribute to variable results. Product quality differs significantly between sources. Individual variation in CXXC5 expression levels affects how much benefit CXXC5 inhibition can provide. The stage of hair loss matters, as miniaturized but living follicles respond better than areas where follicles have been lost entirely. Protocol adherence varies, and the combination approach requires consistent daily application plus regular microneedling.
Forum discussions suggest that expectations should be modest rather than transformative. The mechanism is sound, but the practical translation to visible human hair regrowth appears to be less dramatic than mouse studies might suggest.
This matches the general pattern in hair loss research, where many promising preclinical compounds show attenuated effects in humans.
The KY19382 development
Professor Choi and his company CK Regeon have shifted focus from the PTD-DBM peptide to a small molecule called KY19382. This compound inhibits both CXXC5 function and GSK3-beta simultaneously, combining the effects of PTD-DBM and valproic acid in a single molecule. It represents the next generation of the CXXC5-targeting approach.
KY19382 completed preclinical trials and was in formulation development as of the last public updates. The company was consulting with experts for clinical trial design. If KY19382 enters and succeeds in human clinical trials, it would validate the CXXC5-targeting mechanism in humans and potentially provide a pharmaceutical-grade treatment option. The timeline for clinical results remains uncertain.
This development matters because it signals that the research team behind PTD-DBM views the peptide as a proof-of-concept rather than a final product. Small molecules offer advantages over peptides for topical delivery, stability, and manufacturing scalability. The progression from PTD-DBM to KY19382 follows a typical drug development trajectory and suggests continued confidence in the underlying mechanism.
Safety considerations and potential risks
Safety data for PTD-DBM in humans is limited. Most safety information comes from preclinical studies and extrapolation from the known safety profiles of related compounds. Understanding peptide safety principles provides a framework for evaluating novel compounds like PTD-DBM.
Topical application safety
As a topical treatment, PTD-DBM avoids many systemic risks associated with oral or injectable compounds. The peptide is applied to the scalp surface and designed to penetrate locally rather than enter systemic circulation in significant amounts. This limits exposure to the treatment site and reduces potential for systemic side effects.
Reported side effects from topical application are generally mild. Skin irritation, redness, and temporary dryness at the application site are the most commonly mentioned issues. These typically resolve with continued use or dose adjustment. Allergic reactions to peptides are possible but rare. Patch testing on a small area before full application can identify sensitivity before committing to a full protocol.
The microneedling component carries its own risks independent of PTD-DBM. Infection from improper sterilization, scarring from excessive depth or pressure, and inflammation from overly frequent sessions are all possible. Proper technique, sterile equipment, and appropriate frequency minimize these risks. The common peptide mistakes guide covers errors that can compromise both safety and effectiveness.
Wnt pathway activation concerns
The Wnt/beta-catenin pathway does not only control hair growth. It is involved in cell proliferation throughout the body, and dysregulated Wnt signaling is associated with certain cancers. This raises a theoretical concern: could activating the Wnt pathway to grow hair also increase cancer risk?
Context matters here. PTD-DBM is applied topically, limiting its effects to the treatment site. Systemic Wnt pathway activation through oral or injectable drugs would raise more significant concerns than localized topical application. Additionally, PTD-DBM does not activate Wnt signaling from zero. It removes an inhibitor (CXXC5), allowing the existing pathway to function normally. The distinction between removing a brake and pressing the accelerator is important in terms of risk profile.
Nevertheless, this theoretical concern means PTD-DBM should not be used by individuals with active or recent history of Wnt-pathway-associated cancers, particularly skin cancers in the treatment area. Anyone with cancer concerns should discuss Wnt-modulating treatments with an oncologist before use.
Valproic acid considerations
Topical valproic acid has a different safety profile than oral valproic acid. Oral valproic acid carries well-documented risks including liver toxicity, teratogenicity (birth defects), and various metabolic effects. Topical application results in much lower systemic absorption, significantly reducing these risks. However, anyone who is pregnant, planning pregnancy, or has liver disease should avoid valproic acid in any form, including topical.
The interaction between topical valproic acid and other medications has not been extensively studied. Researchers using other peptide-based treatments alongside the PTD-DBM protocol should be aware that potential interactions have not been characterized. When in doubt, consulting a healthcare practitioner familiar with peptide research provides appropriate guidance.
Long-term unknowns
The most significant safety concern is simply the absence of long-term human data. PTD-DBM has not been studied in humans over years of continuous use. Potential cumulative effects, rare adverse events, and interactions that only manifest with extended use remain unknown. This is true for most research peptides and represents an inherent risk of adopting compounds before full clinical evaluation.
Researchers should weigh this uncertainty against their individual risk tolerance. Those with mild hair thinning and many available alternatives face a different risk-benefit calculation than those with advanced hair loss who have exhausted conventional options. Understanding how to approach peptide research responsibly helps frame these decisions appropriately. The comprehensive benefits and risks overview for peptides in general provides additional context for evaluating novel compounds against established safety profiles.
PTD-DBM in multi-peptide hair protocols
Advanced researchers sometimes combine multiple hair-active compounds to address follicle biology from several angles simultaneously. PTD-DBM fits into multi-peptide protocols as the Wnt pathway specialist, complementing compounds that work through other mechanisms.
PTD-DBM plus GHK-Cu stacking
This combination pairs CXXC5 inhibition (PTD-DBM) with broad regenerative signaling (GHK-Cu). PTD-DBM ensures the Wnt pathway remains unblocked while GHK-Cu provides collagen stimulation, angiogenesis, stem cell activation, and anti-inflammatory effects. The two peptides target non-overlapping mechanisms, making the combination theoretically additive or synergistic rather than redundant.
Practical implementation could involve daily topical PTD-DBM application alongside GHK-Cu injection protocols or topical copper peptide serums. The GHK-Cu dosage chart provides injection dosing reference, while the concentration guide helps with topical formulation selection. Reconstituting GHK-Cu correctly ensures consistent potency across the protocol duration.
PTD-DBM plus Thymulin zinc complex
Zinc-Thymulin targets immunological factors affecting hair follicles. Autoimmune and inflammatory contributions to hair loss operate through different pathways than CXXC5-mediated Wnt suppression. Combining PTD-DBM (Wnt pathway) with Thymulin zinc (immune modulation) addresses two independent mechanisms that both contribute to follicle health.
This combination is particularly relevant for individuals whose hair loss involves both androgenetic and inflammatory components, which is common. Scalp inflammation from various sources compounds the DHT-PGD2-CXXC5 cascade, creating multiple simultaneous threats to follicle function. A multi-target approach addresses more of these threats than any single compound. Research on anti-inflammatory peptides supports the value of addressing the inflammatory component alongside other interventions. The KPV peptide represents another anti-inflammatory option that some researchers include in scalp health protocols, and the KPV inflammation guide discusses its mechanism in detail.
PTD-DBM plus BPC-157 and TB-500
BPC-157 and TB-500 are healing peptides with broad regenerative effects. While not hair-specific, their tissue repair properties may support overall follicle health, particularly following microneedling-induced wounds. The BPC-157 and TB-500 stacking guide explains how these peptides complement each other for tissue repair.
Adding systemic healing peptides to a PTD-DBM protocol could enhance the wound-induced hair neogenesis component. BPC-157 accelerates wound healing and reduces inflammation. TB-500 promotes cellular migration to wound sites and supports tissue regeneration. Both effects could amplify the microneedling component of the PTD-DBM protocol. Understanding how BPC-157 compares to TB-500 helps determine which healing peptide best complements specific protocol goals.
However, adding injectable peptides increases protocol complexity significantly. The peptide combination guide and cycling guide help navigate multi-compound protocols. For most researchers, starting with the core PTD-DBM/valproic acid/microneedling protocol before adding additional compounds allows clearer assessment of what is working.
Comprehensive stacking protocol example
An advanced multi-peptide hair protocol might include the following components. PTD-DBM topical spray applied daily for CXXC5 inhibition. Valproic acid topical spray applied daily for Wnt pathway enhancement. Microneedling one to two times weekly for WIHN and enhanced absorption. GHK-Cu at standard injection doses two to three times weekly for regenerative support. Minoxidil applied twice daily for vasodilation. Optional finasteride for DHT reduction if tolerated.
This protocol attacks hair loss from six different angles: CXXC5 blockade, downstream Wnt activation, wound-induced neogenesis, regenerative peptide signaling, vascular supply, and hormonal reduction. The peptide stack calculator can help plan timing and dosing for multi-compound protocols. Use the peptide dosage calculator to determine exact amounts based on vial concentrations, and the reconstitution calculator to prepare injectable components correctly.
Such comprehensive protocols benefit from professional supervision. The peptide therapy clinics guide helps locate practitioners experienced with multi-peptide protocols who can monitor progress and adjust components based on response. SeekPeptides members access detailed stacking protocols and personalized guidance for complex multi-compound approaches.
Who is a good candidate for PTD-DBM
Not everyone experiencing hair loss would benefit equally from PTD-DBM. Understanding which profiles align best with the mechanism helps avoid wasted effort and expense.
Best candidates
Individuals with early to moderate androgenetic alopecia where follicles are miniaturizing but still present represent the best candidates. These follicles are being suppressed by the CXXC5-mediated Wnt pathway blockade, exactly what PTD-DBM addresses. The earlier the intervention, the more follicles remain available for rescue.
People who cannot tolerate finasteride or prefer non-hormonal approaches have particular reason to consider PTD-DBM. The peptide addresses the same downstream consequence (Wnt pathway suppression) as finasteride addresses the upstream cause (DHT production). For those who experience finasteride side effects, PTD-DBM offers an alternative route to the same therapeutic target.
Researchers already using minoxidil who want additional mechanism coverage are also good candidates. Since PTD-DBM works through an entirely different pathway, adding it to an existing minoxidil protocol provides complementary rather than redundant benefits. The hair loss peptide overview discusses how to identify which treatments address which mechanisms for your specific situation.
Poor candidates
People with complete baldness where follicles have been permanently lost are unlikely to benefit significantly. PTD-DBM can promote neogenesis (new follicle formation), but this process has limits. Areas of scarring or long-standing complete baldness where the follicular architecture has been replaced by scar tissue will not respond. The distinction between reversible and irreversible hair loss determines candidacy for any regenerative approach.
Individuals with hair loss driven primarily by non-androgenetic causes, such as autoimmune alopecia, thyroid disorders, nutritional deficiencies, or medication side effects, may see limited benefit from CXXC5 inhibition specifically. These conditions require treatment of the underlying cause rather than pathway modulation. The autoimmune peptide guide discusses approaches more suited to immune-mediated hair loss.
Conditions like eczema or scalp inflammatory conditions require different peptide strategies focused on immune modulation rather than Wnt pathway activation.
Anyone with active skin cancer on the scalp or a history of Wnt-pathway-associated malignancies should avoid PTD-DBM until cleared by an oncologist. The theoretical concern about Wnt pathway activation and cell proliferation, while not demonstrated to cause problems with topical PTD-DBM, warrants caution in these populations.
Understanding the limitations of current evidence
A responsible discussion of PTD-DBM requires clear-eyed assessment of what remains unknown. The peptide field broadly, and PTD-DBM specifically, involves compounds where marketing often outpaces evidence.
Mouse-to-human translation gaps
Mouse hair biology differs from human hair biology in several important ways. Mice have much denser hair with shorter growth cycles. Their skin structure differs. Their hormonal environment is not identical. Mouse androgenetic alopecia must be artificially induced because mice do not naturally develop the condition the way humans do.
These differences mean that impressive mouse results frequently translate to modest human outcomes. This is not unique to PTD-DBM. It is a consistent pattern across hair loss research. Researchers should expect attenuated rather than equivalent effects when extrapolating from preclinical data to human application.
Concentration and formulation uncertainty
The optimal concentration of PTD-DBM for human topical application has not been established through dose-finding clinical trials. Commercial products use various concentrations based on extrapolation from preclinical data rather than human optimization studies. This means current products may be underdosed, overdosed, or appropriately dosed, and nobody knows which.
Formulation matters enormously for topical peptides. The vehicle (what the peptide is dissolved in), penetration enhancers, pH, and stability agents all affect how much active peptide reaches target cells. Two products with identical peptide concentrations may deliver vastly different amounts to follicles depending on formulation quality. Without standardized formulations validated in human trials, comparing products or interpreting results becomes difficult.
Absence of long-term outcome data
Hair growth is slow. Meaningful assessment requires six to twelve months minimum. Long-term maintenance, the question of whether results persist, requires years of follow-up. No such data exists for PTD-DBM in humans. Whether initial improvements plateau, continue improving, or eventually reverse remains unknown.
The claim that PTD-DBM results persist after discontinuation, unlike minoxidil, is plausible based on the neogenesis mechanism but unverified in controlled human studies. New follicles formed through neogenesis should theoretically persist, but whether they remain resistant to the same CXXC5-mediated suppression that caused the original hair loss is uncertain. Long-term follow-up data would answer this question definitively.
Future directions and the path to clinical validation
The CXXC5-targeting approach is still evolving. Understanding where the research is heading provides context for current decision making.
KY19382 clinical development
CK Regeon, the company founded by Professor Choi, is developing KY19382 as a pharmaceutical product. This small molecule offers potential advantages over the PTD-DBM peptide: better stability, easier manufacturing, potentially superior skin penetration, and a path through standard pharmaceutical clinical trials. If KY19382 succeeds in Phase II and III clinical trials, it would provide the first validated pharmaceutical targeting the CXXC5-Wnt axis for hair loss.
The timeline for clinical results remains uncertain. Pharmaceutical development takes years even under the best circumstances. But the $12 million in Series B funding and ongoing research activity signal continued momentum. Successful clinical validation would not only provide a new treatment option but also retrospectively validate the CXXC5-targeting mechanism in humans, which would increase confidence in PTD-DBM as well.
Broader Wnt pathway research
PTD-DBM and KY19382 are not the only approaches targeting Wnt signaling for hair growth. Multiple research groups worldwide are investigating Wnt pathway modulation through various mechanisms. Small molecules, peptides, gene therapy approaches, and biomaterial-based delivery systems are all being explored. The peptide research landscape continues expanding as new targets and delivery methods emerge.
This broader research activity increases the likelihood that effective Wnt-based hair loss treatments will eventually reach the market, whether through the CXXC5-specific approach or alternative mechanisms. For researchers currently exploring PTD-DBM, this broader momentum provides reassurance that the underlying biological target is widely recognized as valid.
Combination therapy evolution
The future of hair restoration increasingly points toward multi-mechanism combination therapies rather than single-agent approaches. PTD-DBM represents one component of what may eventually become standardized multi-target protocols incorporating Wnt activation, DHT reduction, growth factor supplementation, anti-inflammatory agents, and regenerative peptides.
Understanding each component mechanism allows researchers to build rational combinations based on complementary biology rather than hoping different products happen to work well together. The systematic approach to peptide stacking based on mechanistic understanding will likely define the next generation of hair restoration protocols. The safe peptide selection principles that guide muscle growth protocols apply equally to hair restoration: choose compounds with well-characterized mechanisms, start conservatively, and add complexity only when justified by response.
Troubleshooting PTD-DBM protocols
When protocols do not produce expected results, systematic troubleshooting beats guesswork. Most failures trace back to a handful of identifiable issues.
No visible changes after three months
Three months of consistent use should produce at least subtle signs of response. Reduced shedding, slightly improved hair texture, or the appearance of fine vellus hairs are early indicators that the protocol is engaging follicle biology. Complete absence of any change after 12 weeks warrants investigation.
Product quality is the first variable to examine. PTD-DBM degrades with improper storage, and some commercial formulations may contain insufficient active peptide. Switching to a verified-quality source from the peptide vendor comparison eliminates this variable. Request certificates of analysis showing purity testing through HPLC or mass spectrometry. If the product cannot provide these, its quality is uncertain.
Absorption may be inadequate. Without microneedling, topical peptide delivery through intact scalp skin is limited. Adding or optimizing the microneedling component significantly increases the amount of PTD-DBM reaching target cells. Ensure needle depth is sufficient (0.5 to 1.5 millimeters) and that the peptide solution is applied immediately after needling while channels remain open. The microneedle delivery guide explains how channel duration affects absorption windows.
Protocol adherence deserves honest assessment. Skipping applications, inconsistent microneedling, or gaps in the protocol allow CXXC5 to resume its suppressive activity. The cumulative nature of the treatment means consistency over months matters more than any single application. Track your adherence objectively rather than relying on memory.
Finally, consider whether the underlying hair loss involves mechanisms beyond CXXC5-mediated Wnt suppression. Thyroid dysfunction, nutritional deficiencies, autoimmune conditions, and certain medications all cause hair loss through pathways PTD-DBM does not address. A comprehensive evaluation identifying contributing factors helps determine whether PTD-DBM alone is sufficient or whether additional interventions are needed.
Increased shedding after starting treatment
Some users report increased hair shedding in the first two to four weeks of PTD-DBM use. This phenomenon, similar to the "shedding phase" observed with minoxidil initiation, may indicate follicles transitioning from telogen (resting) to anagen (growth) phase. Old hairs must be shed before new growth emerges from reactivated follicles.
The distinction between normal transitional shedding and problematic hair loss matters. Normal transitional shedding is temporary (two to four weeks), involves hairs that were already in late telogen, and precedes visible improvement. Problematic shedding persists beyond six weeks, involves hairs from areas that were previously stable, and does not transition to improvement.
If shedding continues beyond six weeks or produces visible thinning in previously stable areas, discontinue the protocol and reassess. Not every treatment suits every individual. Persistent adverse effects indicate the approach is not working as intended for your specific biology.
Scalp irritation from microneedling or topicals
Irritation from the microneedling component usually responds to reducing frequency or needle depth. Drop from twice weekly to once weekly. Reduce depth from 1.5 to 0.5 millimeters. Allow complete healing between sessions. Using a clean, sharp device rather than dull needles that tear rather than puncture reduces unnecessary tissue trauma.
Irritation from the topical components may indicate sensitivity to inactive ingredients in the formulation rather than the peptide itself. Different manufacturers use different vehicles, preservatives, and penetration enhancers. Switching brands while maintaining the same active compounds may resolve irritation. Patch testing new products on a small area of the forearm before applying to the full scalp identifies problematic formulations before they cause widespread irritation.
Persistent irritation despite adjustments suggests the protocol may not be appropriate for your skin type. Some individuals have heightened sensitivity to topical peptide formulations regardless of vehicle or concentration. The common peptide mistakes guide covers additional troubleshooting steps for adverse reactions.
Plateau after initial improvement
Some researchers experience initial improvements that eventually plateau. This pattern may reflect receptor adaptation, where sustained CXXC5 inhibition leads to compensatory changes in the signaling network. Biological systems tend toward homeostasis, and prolonged interference with any single pathway component may trigger adaptive responses.
Cycling the protocol, taking periodic breaks of two to four weeks before resuming, may help reset receptor sensitivity. This approach parallels the cycling recommendations for many other research peptides where continuous use leads to diminishing returns. The peptide cycling guide discusses how strategic breaks maintain long-term effectiveness.
Alternatively, plateaus may indicate that PTD-DBM has produced its maximum achievable effect for your specific situation. The compound addresses one mechanism among many that govern hair growth. Once CXXC5 suppression is fully addressed, additional improvement requires targeting other mechanisms through complementary treatments. Adding GHK-Cu, optimizing minoxidil, or introducing PRP treatments at this stage addresses different bottlenecks that PTD-DBM alone cannot overcome.
Cost analysis and practical budgeting
The financial investment in a PTD-DBM protocol deserves realistic assessment. Costs vary significantly based on sourcing, additional protocol components, and duration of treatment.
PTD-DBM product costs
Commercial PTD-DBM topical products typically range from $60 to $200 per month depending on brand, concentration, and volume. Research-grade PTD-DBM peptide costs vary from $50 to $150 per vial (typically 1 to 5 milligrams), which may last several weeks to several months depending on concentration and application volume. Compounding pharmacy preparations fall in a similar range but offer potentially better quality control.
The peptide cost calculator helps estimate total protocol costs based on specific product prices, dosing frequency, and treatment duration. For a realistic assessment, calculate costs over a minimum six-month commitment, as shorter evaluation periods are insufficient to determine effectiveness.
Additional protocol component costs
Valproic acid topical preparations add approximately $30 to $80 per month through compounding pharmacies. Microneedling devices range from $20 to $150 for home dermarollers or dermapens, with replacement cartridges running $10 to $30 per month. Professional microneedling sessions cost $200 to $500 each if you prefer clinical treatment.
If combining with GHK-Cu protocols, add the cost of the peptide plus bacteriostatic water and supplies. The peptide pricing overview provides broader context for budgeting multi-peptide protocols.
Total protocol cost estimation
A basic PTD-DBM-only protocol costs approximately $60 to $200 per month. The full three-pronged protocol with valproic acid and microneedling runs approximately $100 to $350 per month. A comprehensive multi-peptide protocol adding GHK-Cu and other compounds can reach $300 to $600 per month or more.
Over a six-month minimum evaluation period, total investment ranges from $360 (basic protocol, budget sourcing) to $3,600 or more (comprehensive multi-peptide, premium products). These costs should be weighed against the incomplete evidence base. Unlike FDA-approved treatments where efficacy is established, spending on PTD-DBM carries more uncertainty about return on investment. The peptide therapy cost guide provides broader context for budgeting peptide research protocols, and the online peptide therapy options may offer more competitive pricing for some components.
One cost advantage of PTD-DBM is that it does not require ongoing clinical visits. Unlike PRP treatments that require blood draws and centrifuging at a clinic, or professional microneedling sessions, the core PTD-DBM protocol is self-administered at home after initial setup. This reduces long-term costs compared to clinic-dependent approaches, though it places more responsibility on the researcher for proper technique and consistency.
The science of hair cycling and why PTD-DBM timing matters
Understanding the hair growth cycle explains why PTD-DBM protocols require months of commitment and why results appear gradually rather than suddenly.
The four phases of hair growth
Each hair follicle independently cycles through four distinct phases. Anagen is the active growth phase, lasting two to seven years for scalp hair. During anagen, matrix cells in the follicle bulb rapidly divide, producing the hair shaft. The Wnt/beta-catenin pathway must be active for anagen to initiate and sustain.
Catagen is the transitional phase, lasting approximately two to three weeks. The follicle shrinks, the lower portion degenerates, and the dermal papilla moves upward. This phase marks the beginning of follicle regression and is triggered when growth signals diminish, precisely what happens when CXXC5 suppresses Wnt signaling.
Telogen is the resting phase, lasting approximately three months. The old hair remains in the follicle but is no longer growing. The follicle sits dormant, waiting for signals to re-enter anagen. In androgenetic alopecia, elevated CXXC5 keeps follicles trapped in extended telogen or pushes them into progressively shorter anagen phases with each cycle.
Exogen is the shedding phase where the old hair is released from the follicle, making way for a new anagen hair to emerge. This natural shedding is normal, with 50 to 100 hairs shed daily in healthy scalps.
How CXXC5 disrupts the cycle
In normal hair biology, the transition between phases is controlled by a balance of growth-promoting and growth-inhibiting signals. The Wnt/beta-catenin pathway is the primary growth promoter for anagen initiation. CXXC5 tips this balance toward inhibition.
With elevated CXXC5, several damaging patterns emerge. Anagen duration shortens with each successive cycle. Telogen extends, leaving follicles dormant for longer periods. The ratio of anagen to telogen hairs shifts toward telogen, creating visible thinning. Eventually, some follicles stop entering anagen entirely and undergo permanent miniaturization. The longevity peptide field recognizes similar degenerative cascades in other tissues, where age-related changes in signaling pathways produce progressive functional decline.
PTD-DBM interrupts this pattern by restoring proper Wnt signaling. Follicles can maintain longer anagen phases. Telogen-trapped follicles receive the activation signal to re-enter growth. But the timeline for these changes reflects the slow pace of hair cycling. A follicle that re-enters anagen today will not produce a visible hair for several months. And that hair needs additional months to reach meaningful length. This biological reality, not product inadequacy, explains why PTD-DBM protocols require extended timelines before results become apparent.
Why consistency matters more than intensity
Given the months-long timescale of hair cycling, consistent daily application matters far more than occasional intensive treatments. CXXC5 is continuously produced in response to DHT and PGD2 signaling. Any gap in PTD-DBM application allows CXXC5 to resume binding Dishevelled and suppressing Wnt signaling.
Think of it as holding a door open against a spring. The spring (CXXC5 production) is always pushing the door closed. PTD-DBM holds it open. Remove the hold even briefly, and the door closes. Follicles that were receiving growth signals during consistent application may re-enter regression if those signals are interrupted.
This continuous requirement distinguishes PTD-DBM from treatments like PRP, which provide periodic bursts of growth factors. The peptide does not create a lasting change in CXXC5 levels. It provides ongoing competitive inhibition that requires maintained presence. Understanding this helps set expectations about treatment commitment and explains why on-again, off-again protocols produce poor results.
Comparing PTD-DBM to emerging hair loss technologies
PTD-DBM exists within a rapidly evolving landscape of hair restoration research. Several emerging technologies offer complementary or alternative approaches worth understanding.
Low-level laser therapy (LLLT)
LLLT uses specific wavelengths of red and near-infrared light to stimulate cellular metabolism in hair follicles. FDA-cleared LLLT devices are available for home use. The mechanism involves mitochondrial activation and increased ATP production, which supports cellular energy for hair growth processes. Researchers interested in mitochondrial function may also find SS-31 peptide relevant, as it targets mitochondrial efficiency through a different mechanism that some advanced protocols incorporate alongside hair-specific treatments.
LLLT works through a completely different mechanism than PTD-DBM and can be used simultaneously without interference. Some researchers add laser caps or combs to their PTD-DBM protocols for additional mechanism coverage. The evidence base for LLLT in hair growth is moderate, with several clinical trials demonstrating modest improvements in hair count and density.
Exosome therapy
Exosome-based treatments deliver concentrated growth factors, cytokines, and signaling molecules derived from stem cells. These extracellular vesicles contain biological signals that promote tissue regeneration, including hair follicle activation. Exosome therapy is typically administered through scalp injections at clinical settings.
The approach differs from PTD-DBM in that it provides growth signals rather than removing growth inhibitors. The two mechanisms are complementary. PTD-DBM ensures the Wnt pathway remains unblocked while exosomes provide additional proliferative signals. However, exosome therapy requires clinical visits and is significantly more expensive than topical PTD-DBM protocols.
Gene therapy approaches
Several research groups are exploring gene therapy for permanent modification of hair growth pathways. These approaches aim to alter gene expression in follicle cells to permanently overcome the signaling defects that cause hair loss. While still largely experimental, gene therapy represents the potential for one-time treatments that produce lasting results without ongoing application.
Gene therapy is years away from practical availability and carries significant regulatory and safety hurdles. For current researchers, PTD-DBM and similar peptides represent the accessible option while more definitive solutions develop. The peptide research overview tracks how the field continues evolving toward more targeted and effective interventions.
Small molecule Wnt activators
Beyond KY19382, several small molecule compounds targeting Wnt pathway components are in development by various pharmaceutical companies. These include specific GSK3-beta inhibitors, compounds targeting other negative regulators of the pathway, and molecules that enhance beta-catenin nuclear translocation.
The advantage of small molecules over peptides is generally better oral bioavailability, improved stability, lower manufacturing costs, and easier pharmaceutical development. As these compounds progress through clinical development, they may eventually provide validated, prescription-grade alternatives to research-grade PTide treatments. For now, PTD-DBM remains one of the few commercially accessible compounds specifically targeting the CXXC5-Wnt axis.
Building a rational PTD-DBM protocol: step by step
For researchers ready to implement a PTD-DBM protocol, a systematic approach maximizes chances of success while managing risks.
Step one: baseline documentation
Before starting any treatment, document your current status. Take high-quality photographs under consistent lighting from multiple angles. Note areas of thinning, recession, or concern. Record current treatments and their duration. This baseline allows objective comparison at three, six, and twelve months.
If accessible, consider a baseline dermascopy evaluation from a dermatologist specializing in hair loss. Magnified scalp imaging reveals follicle density, miniaturization ratios, and vellus-to-terminal hair ratios that naked-eye assessment misses. These quantitative measurements provide more reliable progress tracking than visual comparison alone.
Step two: source quality products
Obtain PTD-DBM from a source that provides third-party purity verification. If using the three-pronged protocol, source valproic acid from a licensed compounding pharmacy. Purchase a quality microneedling device with appropriate needle depth for scalp use. Ensure all products are stored according to manufacturer recommendations, typically refrigerated.
The testing labs guide helps verify product quality when certificates of analysis are available. Compare vendor reputations through the vendor comparison before committing to a supplier for a months-long protocol.
Step three: start conservatively
Begin with PTD-DBM alone for the first two weeks before adding valproic acid or microneedling. This isolation helps identify any adverse reactions specific to the peptide before layering additional variables. If the scalp tolerates PTD-DBM well, add valproic acid in week three. Introduce microneedling in week four.
This staged approach lets you identify which component causes any adverse effects if they occur. Starting everything simultaneously makes troubleshooting impossible because you cannot determine which product is responsible for irritation or other issues.
Step four: maintain and document
Once the full protocol is established, maintain daily consistency. Take progress photos monthly under identical conditions. Note any changes in shedding, texture, or density. Keep a brief daily log of application to ensure adherence.
At the three-month mark, conduct your first formal assessment. Compare photographs. Evaluate subjective changes. Decide whether to continue, adjust, or discontinue based on evidence of response. At six months, conduct a more thorough evaluation. By this point, meaningful changes should be apparent if the protocol is effective for your situation.
Step five: adjust based on response
Positive response warrants continuation. Consider adding complementary treatments like GHK-Cu or minoxidil if not already using them. The peptide stack calculator helps plan expanded protocols. Some researchers also explore nasal spray peptide delivery for systemic compounds that complement topical scalp treatments, while the oral peptide delivery guide discusses another route for compounds that support hair health from within.
No response after six months suggests either product quality issues, absorption limitations, or that CXXC5 inhibition alone is insufficient for your specific hair loss pattern. Consider switching sources, adding the microneedling component if not already included, or exploring alternative approaches. The comprehensive hair loss guide discusses the full range of options for researchers who need to adjust their approach. The complete peptide reference list catalogs available compounds organized by function, which can help identify alternative candidates when a specific approach falls short.
Negative response, meaning visible worsening or persistent adverse effects, warrants discontinuation and reassessment. Not every treatment works for every individual, and continuing an ineffective or harmful protocol wastes resources and potentially delays more appropriate interventions.
For researchers serious about evidence-based peptide protocols, SeekPeptides provides comprehensive guidance including personalized protocol recommendations, community support from experienced researchers, and access to the latest evidence on emerging treatments like PTD-DBM.
Frequently asked questions
What exactly is PTD-DBM and how does it work?
PTD-DBM is a synthetic peptide consisting of a protein transduction domain (for cell membrane penetration) fused to a Dishevelled binding motif (for targeting the CXXC5-Dishevelled interaction). It blocks the protein CXXC5 from suppressing the Wnt/beta-catenin signaling pathway, which is essential for hair follicle growth, stem cell activation, and follicle neogenesis.
Is PTD-DBM FDA approved for hair loss?
No. PTD-DBM is not FDA approved for any indication. It is available through some compounding pharmacies and as research-grade peptide from various suppliers. Commercial products containing PTD-DBM are typically marketed as cosmetic scalp treatments. Understanding peptide legality helps navigate the regulatory landscape for research compounds.
How long before I might see results from PTD-DBM?
Based on preclinical data and community reports, minimum 3 to 6 months of consistent use is needed before evaluating effectiveness. Human hair cycles are significantly slower than mouse models where results appeared within 28 days. The peptide results timeline varies considerably between compounds and individuals.
Can I use PTD-DBM with minoxidil?
Yes. PTD-DBM and minoxidil work through completely different mechanisms (Wnt pathway restoration versus vasodilation). The two treatments are complementary and can be used together. Apply topical minoxidil and PTD-DBM at different times of day to avoid diluting either product.
Does PTD-DBM require injection?
No. PTD-DBM is applied topically as a spray or serum. The protein transduction domain component allows the peptide to penetrate cell membranes without injection. This is a significant advantage over many injectable peptides that require subcutaneous administration.
What is the difference between PTD-DBM and KY19382?
PTD-DBM is a peptide that blocks CXXC5 from binding Dishevelled. KY19382 is a small molecule that inhibits both CXXC5 function and GSK3-beta simultaneously. KY19382 is the next-generation development from the same research team, designed for pharmaceutical development and clinical trials. KY19382 combines the effects of PTD-DBM and valproic acid in a single compound.
Is PTD-DBM safe?
Long-term human safety data is limited. As a topical treatment, systemic exposure is minimal. Reported side effects include mild skin irritation and redness. The theoretical concern about Wnt pathway activation and cell proliferation exists but has not materialized in preclinical studies. The peptide safety guide discusses risk evaluation frameworks for research compounds.
Can women use PTD-DBM?
The mechanism targets Wnt pathway suppression, which occurs in both male and female pattern hair loss. Women may benefit from PTD-DBM, particularly since it avoids hormonal manipulation. However, women who are pregnant or planning pregnancy should avoid the protocol due to valproic acid teratogenicity concerns. The safe peptides for women guide discusses gender-specific considerations.
External resources
National Institutes of Health: CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2
MDPI Cells: CXXC5, PGD2, and Wnt/Beta-Catenin Pathway in Alopecia
Follicle Thought: PTD-DBM Update Q&A with Dr. Kang-Yell Choi
For researchers serious about understanding and implementing advanced hair restoration protocols, SeekPeptides offers the most comprehensive resource available, with evidence-based guides, personalized protocol recommendations, and a community of thousands who have navigated these exact research questions.
In case I do not see you, good afternoon, good evening, and good night. May your follicles stay active, your pathways stay unblocked, and your protocols stay consistent.



