Jan 10, 2026

Collagen supplement terminology creates persistent confusion across consumer markets, with manufacturers using collagen hydrolysate and collagen peptides interchangeably despite subtle processing distinctions that affect absorption kinetics, bioavailability profiles, and functional applications in tissue support.
The hydrolysis process that breaks down native collagen molecules into smaller fragments determines the ultimate molecular weight distribution, with different enzymatic treatments yielding peptide chains ranging from dipeptides containing just two amino acids to larger fragments retaining partial triple helix structures. Understanding these processing variations helps consumers navigate marketing claims, compare products effectively, and select formulations optimized for their specific goals whether targeting skin health, joint support, hair growth, or bone density maintenance.
SeekPeptides provides comprehensive resources for understanding collagen science, comparing supplement options, and developing evidence-based protocols. This guide examines the complete landscape of collagen hydrolysate versus collagen peptides including molecular differences, absorption mechanisms, sourcing considerations, quality markers, and practical selection criteria for optimizing your collagen supplementation strategy.
Understanding collagen structure and terminology
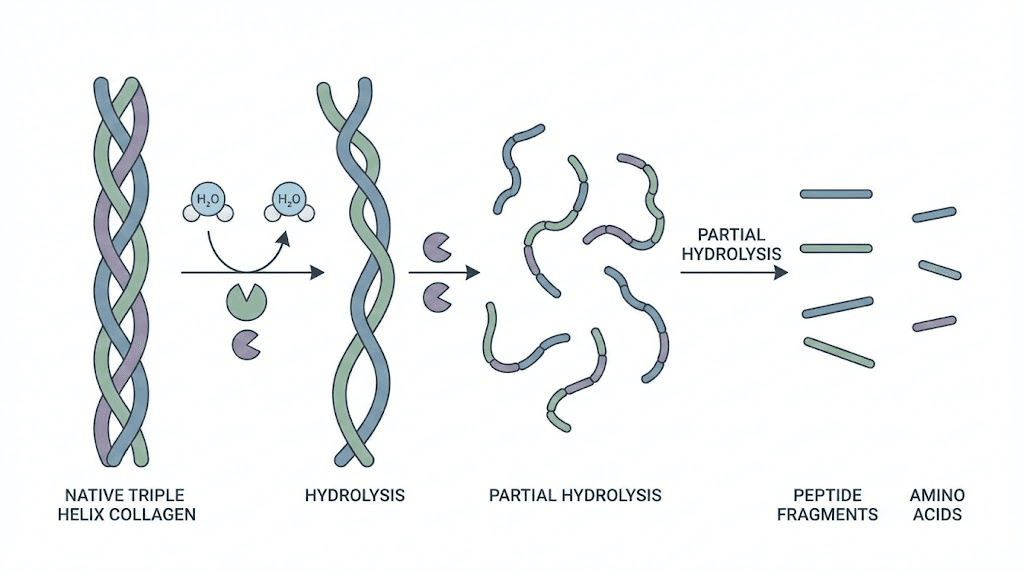
Native collagen represents the most abundant structural protein in mammalian bodies, comprising approximately 30% of total protein content and providing tensile strength to connective tissues throughout the musculoskeletal system, integumentary layers, and organ support structures. The distinctive triple helix configuration consists of three polypeptide chains wound around each other in a right-handed spiral, with glycine residues positioned at every third amino acid position to accommodate the tight central packing required for helix stability. This complex quaternary structure makes native collagen essentially indigestible in its intact form, requiring extensive breakdown before the component amino acids and peptide fragments become bioavailable for absorption and utilization.
The hydrolysis process systematically cleaves peptide bonds throughout the collagen molecule using enzymatic or chemical methods, progressively reducing molecular weight from approximately 300,000 Daltons in native collagen to fragments ranging from 2,000 to 5,000 Daltons in typical hydrolyzed products. This molecular weight reduction transforms the gelatinous, water-binding properties of partially denatured collagen into highly soluble peptide powders that dissolve readily in cold liquids without gelling or clumping.
The terminology distinction between hydrolysate and peptides reflects historical manufacturing conventions rather than fundamental chemical differences. Both terms describe collagen that has undergone enzymatic hydrolysis to break down the protein structure. European manufacturers traditionally preferred hydrolysate terminology while North American and Asian markets adopted collagen peptides as the standard descriptor. Modern usage treats these terms as synonymous in most commercial contexts.
Collagen types and their sources
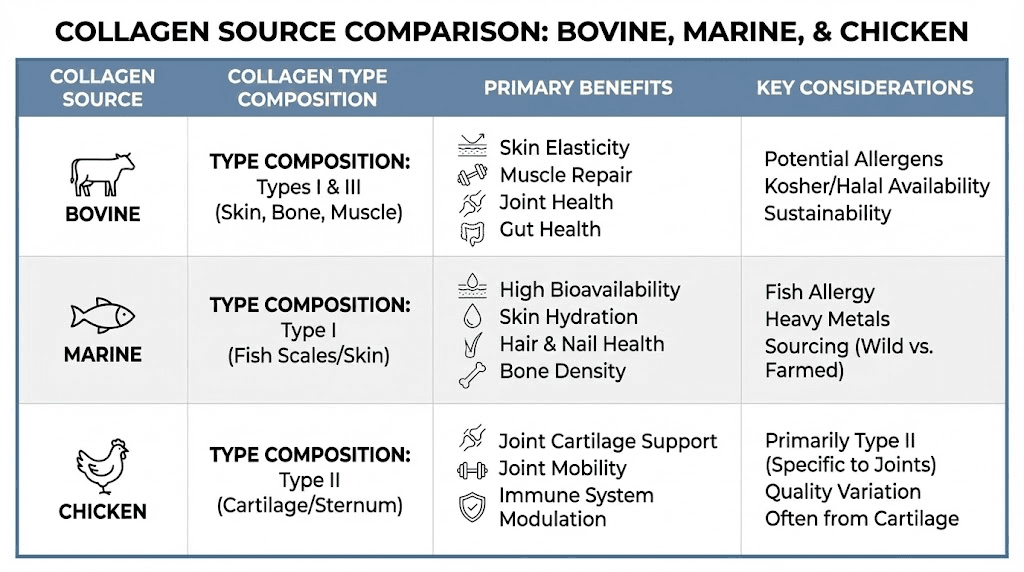
Type I collagen predominates in skin, tendons, bones, and organ structures, representing approximately 90% of total body collagen content. This type provides the primary structural support for dermal layers and contributes to skin elasticity, hydration, and wrinkle resistance. Most commercial collagen supplements derive from Type I sources including bovine hides, fish scales, and marine sources.
Type II collagen concentrates in cartilage tissue, providing compressive resistance and shock absorption capacity in joints. Chicken sternum cartilage serves as the primary commercial source for Type II supplements targeting joint health applications. The glycosaminoglycan content naturally present in cartilage-derived sources adds potential synergistic benefits for joint function.
Type III collagen often accompanies Type I in skin and blood vessel walls, contributing to tissue elasticity and structural integrity. Bovine sources typically contain both Types I and III in proportions reflecting the source tissue composition.
Understanding peptide fundamentals helps contextualize how collagen fragments function after absorption. The amino acid profile of collagen features unusually high glycine, proline, and hydroxyproline concentrations compared to other dietary proteins, with these specific amino acids playing crucial roles in collagen synthesis pathways.
Molecular weight considerations
Molecular weight directly influences absorption efficiency and bioavailability. Smaller peptides cross intestinal barriers more readily through peptide transporters that recognize di- and tripeptide sequences. Larger fragments require further enzymatic breakdown in the digestive tract before absorption becomes possible.
Commercial hydrolysates typically target molecular weight ranges between 2,000 and 5,000 Daltons, balancing absorption efficiency against manufacturing costs and functional properties. Some premium formulations achieve even lower molecular weights below 2,000 Daltons, potentially enhancing bioavailability though clinical evidence comparing specific molecular weight ranges remains limited.
The degree of hydrolysis affects not only absorption but also functional properties in various applications. Highly hydrolyzed products with very low molecular weights may sacrifice some bioactive peptide sequences that require specific chain lengths for receptor interaction. This trade-off between absorption efficiency and bioactive peptide preservation represents an ongoing optimization challenge in collagen supplement development.
The hydrolysis process explained
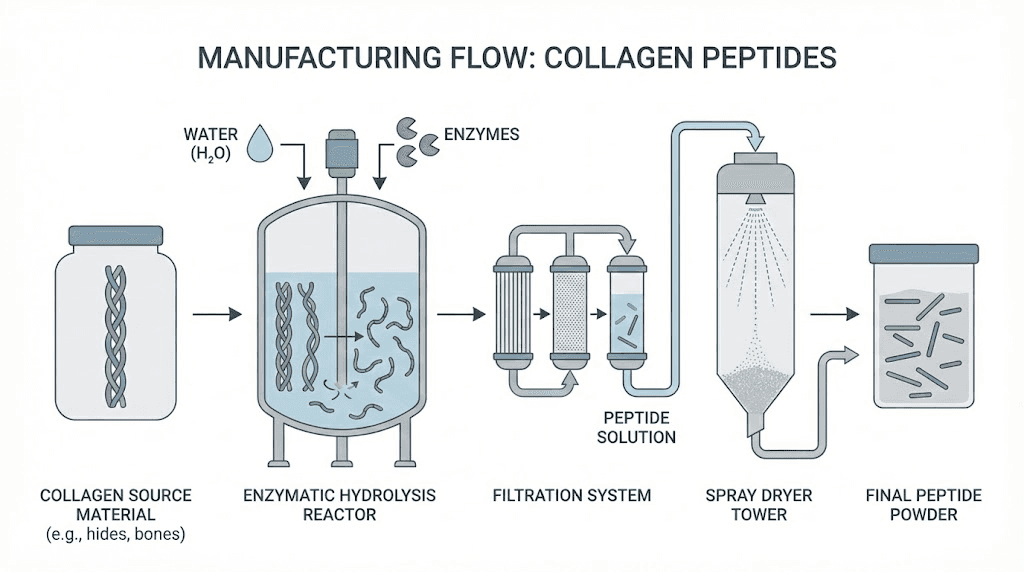
Enzymatic hydrolysis represents the industry standard for producing high-quality collagen peptides, using proteolytic enzymes to systematically cleave peptide bonds under controlled conditions that preserve amino acid integrity while achieving target molecular weight profiles. The process begins with cleaned collagen source material, typically extracted from bovine hides, porcine skins, fish scales, or fish skins through initial acid or alkaline treatments that solubilize the native protein structure.
Temperature control during hydrolysis critically affects final product quality. Excessive heat denatures the remaining helical structures prematurely while insufficient temperatures reduce enzyme activity and extend processing times. Most commercial operations maintain temperatures between 50-60°C during the enzymatic phase, optimizing enzyme kinetics while minimizing thermal degradation of sensitive amino acids.
Enzyme selection and specificity
Different proteolytic enzymes cleave peptide bonds at specific amino acid sequences, producing distinct peptide fragment populations from identical starting materials. Alcalase, neutrase, and papain represent commonly employed enzymes, each generating characteristic molecular weight distributions and peptide compositions.
Alcalase preferentially cleaves at hydrophobic amino acid residues, producing peptides with specific terminal sequences that may influence bioactivity. Papain demonstrates broader specificity, generating more diverse peptide populations. Some manufacturers employ enzyme combinations or sequential treatments to achieve particular molecular weight targets and peptide profiles.
The choice of enzyme affects not only molecular weight but also the generation of specific bioactive peptide sequences. Certain di- and tripeptides containing proline or hydroxyproline demonstrate enhanced stability against digestive enzyme degradation and may reach systemic circulation intact, potentially exerting direct signaling effects on collagen-producing cells.
Understanding how peptides work provides context for appreciating the significance of these bioactive sequences in collagen supplementation outcomes.
Quality control in manufacturing
Reputable manufacturers implement multiple quality control checkpoints throughout the hydrolysis process. Molecular weight analysis using gel permeation chromatography verifies that target specifications have been achieved. Amino acid composition testing confirms the characteristic collagen profile dominated by glycine, proline, and hydroxyproline.
Heavy metal testing ensures source materials and processing did not introduce concerning contaminant levels. Mercury, lead, cadmium, and arsenic represent primary concerns, particularly for marine-sourced collagens where bioaccumulation may concentrate these elements. Third-party testing through accredited laboratories provides independent verification of safety claims.
Microbiological testing confirms the absence of pathogenic organisms that could survive processing. While the hydrolysis conditions typically eliminate vegetative bacteria, spore-forming organisms and heat-stable toxins require specific monitoring protocols.
Absorption and bioavailability mechanisms
Collagen peptide absorption occurs primarily through the small intestine via multiple transport mechanisms that accommodate peptides of varying sizes and compositions. Di- and tripeptides enter enterocytes through the PepT1 transporter, a proton-coupled system that efficiently moves small peptides across the intestinal epithelium. Larger peptides require either paracellular transport through tight junctions or further enzymatic breakdown by brush border peptidases before absorption becomes possible.
The hydroxyproline content unique to collagen provides a useful marker for tracking collagen-derived peptide absorption and distribution. Plasma hydroxyproline concentrations rise significantly following collagen peptide ingestion, peaking within 1-2 hours and remaining elevated for 4-6 hours depending on dose and formulation. This hydroxyproline appears in both free form and as part of intact di- and tripeptides, suggesting that some collagen-specific sequences survive digestion and enter systemic circulation.
Specific bioactive sequences
Research has identified several collagen-derived peptide sequences that resist digestive enzyme degradation and may exert biological effects beyond serving as amino acid substrates for collagen synthesis. Prolyl-hydroxyproline (Pro-Hyp) and hydroxyprolyl-glycine (Hyp-Gly) represent the most abundant and extensively studied of these sequences.
These bioactive peptides appear to influence fibroblast activity directly, stimulating collagen production through receptor-mediated signaling pathways. The proposed mechanism involves peptide binding to cell surface receptors that activate intracellular cascades promoting collagen gene expression. This direct signaling effect potentially amplifies the benefits of collagen supplementation beyond simply providing amino acid building blocks.
The preservation of these bioactive sequences during hydrolysis and digestion depends on both manufacturing parameters and the digestive enzyme profile of individual consumers. Factors affecting peptide survival include molecular weight distribution of the starting product, gastric pH and residence time, and pancreatic enzyme activity levels.
Factors affecting individual response
Individual variation in collagen peptide response reflects differences in absorption efficiency, baseline collagen synthesis rates, and tissue-specific demands for collagen precursors. Age-related declines in collagen production capacity mean older adults may benefit more dramatically from supplementation than younger individuals with robust endogenous synthesis.
Digestive health significantly influences peptide absorption. Conditions affecting intestinal permeability, enzyme production, or transit time alter the efficiency of peptide uptake. Supporting gut health may enhance the benefits derived from collagen supplementation by optimizing absorption conditions.
Vitamin C status affects collagen synthesis rates, as ascorbic acid serves as an essential cofactor for prolyl and lysyl hydroxylase enzymes that modify collagen during biosynthesis. Inadequate vitamin C limits the body's ability to utilize absorbed collagen peptides for new collagen production regardless of supplementation dose.
Comparing sources: bovine, marine, and other options
Source material selection influences not only the collagen type composition but also sustainability considerations, allergenic potential, and amino acid profiles. Each major source category presents distinct advantages and limitations that should inform consumer choices based on individual priorities and restrictions.
Understanding sourcing differences helps consumers make informed decisions aligned with their health goals, ethical considerations, and any dietary restrictions they may face.
Bovine collagen characteristics
Bovine hides represent the most abundant and economical collagen source, generating significant quantities of Type I and Type III collagen from beef industry byproducts. The extensive global cattle industry ensures reliable supply chains and competitive pricing for bovine-derived supplements.
Grass-fed and pasture-raised bovine sources command premium prices based on perceived quality advantages related to animal welfare and potential nutritional differences. While grass-fed cattle may have different fatty acid profiles in their tissues, evidence that these differences meaningfully affect collagen quality remains limited. The collagen protein structure itself appears consistent across feeding regimes.
Religious dietary laws affect bovine collagen acceptability for some consumers. Halal and kosher certifications require specific processing and sourcing protocols that not all manufacturers implement. Consumers with these requirements should verify certification status before purchasing.
BSE (bovine spongiform encephalopathy) concerns historically affected bovine collagen markets, though strict sourcing regulations from countries with no recorded BSE cases and processing methods that destroy prion proteins have largely addressed these safety questions.
Marine collagen benefits
Fish-derived collagen offers Type I collagen with potentially enhanced bioavailability due to lower molecular weight distributions achievable with marine sources. Fish scales and skins from species like cod, pollock, and tilapia provide the primary commercial sources, utilizing byproducts from the fishing industry.
The amino acid composition of marine collagen closely matches human skin collagen, theoretically optimizing its utility for dermal applications. Some research suggests superior absorption of marine peptides compared to bovine sources, though study methodologies and outcome measures vary considerably across investigations.
Sustainability considerations favor marine collagen derived from certified sustainable fisheries or aquaculture operations. Wild-caught fish sources require verification that harvest methods don't contribute to overfishing or ecosystem damage. Aquaculture sources avoid wild harvest concerns but introduce questions about farming practices and environmental impact.
Fish allergies represent a significant consideration for marine collagen, though the extensive hydrolysis process typically degrades allergenic proteins. Consumers with severe fish allergies should exercise caution and potentially consult allergists before trying marine-sourced products.
Comparing marine and bovine sources parallels other supplement comparisons like bone broth versus collagen peptides, where processing methods and source materials create meaningful differences in final product characteristics.
Alternative sources and considerations
Chicken collagen from sternum cartilage provides Type II collagen targeted specifically at joint health applications. The naturally occurring glycosaminoglycans in cartilage tissue add components like chondroitin sulfate that may synergistically support joint function.
Egg shell membrane represents an emerging collagen source containing Types I, V, and X collagen along with glycosaminoglycans. Limited commercial availability restricts access to this source, though preliminary research suggests potential benefits for joint comfort.
Porcine collagen offers similar characteristics to bovine sources but faces religious dietary restrictions for Muslim and Jewish consumers. Some manufacturers prefer porcine sources for specific applications where the slightly different amino acid ratios may offer advantages.
Plant-based collagen alternatives don't actually contain collagen, as the protein occurs exclusively in animal tissues. Products marketed as vegan collagen typically provide amino acid blends, vitamin C, and botanical extracts intended to support endogenous collagen synthesis rather than supplying collagen directly.
Clinical evidence and research findings
The clinical research base for collagen supplementation has expanded significantly over recent years, with randomized controlled trials examining outcomes across skin health, joint function, bone density, and body composition domains. Interpreting this evidence requires understanding methodological variations, dose differences, and the distinction between statistically significant and clinically meaningful effects.
Most studies examine collagen peptide supplementation rather than native collagen or gelatin, reflecting the commercial dominance of hydrolyzed products. The bioavailability advantages of peptides likely contribute to their research popularity, as measurable plasma peptide levels confirm absorption and provide mechanistic insights.
Skin health outcomes
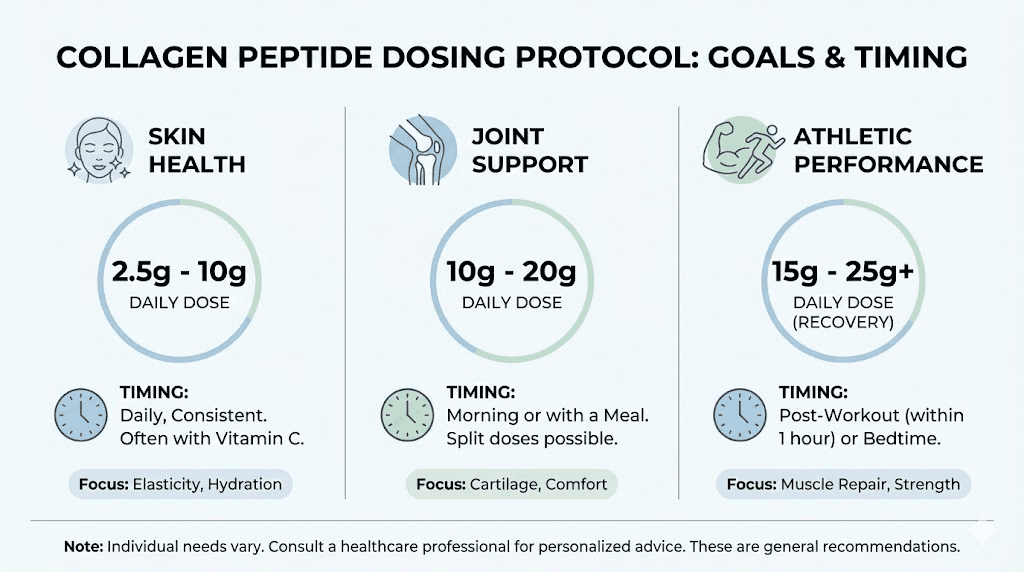
Multiple randomized controlled trials demonstrate improvements in skin hydration, elasticity, and wrinkle depth following 8-12 weeks of collagen peptide supplementation at doses typically ranging from 2.5 to 10 grams daily. The improvements generally reach statistical significance though effect sizes vary considerably across studies.
Specific peptide formulations containing characterized bioactive sequences show particularly promising results. Products standardized for prolyl-hydroxyproline content demonstrate consistent benefits across multiple trials, suggesting this sequence may represent a key active component.
Understanding peptides for wrinkles provides additional context on the various approaches available for addressing visible aging signs.
The time course of skin improvements typically shows initial hydration benefits within 4 weeks, with elasticity and wrinkle improvements requiring longer supplementation periods of 8-12 weeks. Maintenance of benefits appears to require ongoing supplementation, with improvements gradually reversing following discontinuation.
Joint health research
Clinical evidence for joint health benefits focuses on two primary populations: individuals with osteoarthritis experiencing joint pain and stiffness, and athletes experiencing activity-related joint discomfort. Different collagen types and formulations target these distinct applications.
Type II collagen from chicken cartilage demonstrates benefits for joint comfort in osteoarthritis populations, potentially through both structural support and immunomodulatory mechanisms involving oral tolerance induction. The intact Type II collagen in some formulations may interact with gut-associated immune tissue differently than fully hydrolyzed peptides.
Hydrolyzed Type I collagen supports connective tissue throughout the joint structure including tendons, ligaments, and the bone-cartilage interface. Athletes reporting activity-related knee pain show reduced symptoms following collagen peptide supplementation, potentially reflecting enhanced tendon and ligament collagen turnover.
Exploring the best peptides for tendon repair provides additional options for those focused specifically on connective tissue support.
Bone density and body composition
Preliminary evidence suggests collagen peptide supplementation may support bone mineral density, particularly in postmenopausal women at risk for osteoporosis. The organic matrix of bone consists primarily of Type I collagen, and enhanced collagen synthesis could theoretically strengthen this structural component.
Studies combining collagen peptides with resistance exercise show potential synergistic benefits for muscle mass and strength compared to exercise alone or exercise with other protein sources. The specific amino acid composition of collagen may influence signaling pathways related to muscle protein synthesis differently than complete proteins.
Body composition effects remain less consistently demonstrated than skin or joint outcomes, with some studies showing reduced fat mass and increased lean mass while others find no significant differences from placebo. Methodological factors including exercise protocols, dietary controls, and measurement techniques likely contribute to these inconsistencies.
Practical dosing considerations
Effective collagen peptide dosing varies by target outcome, individual factors, and product characteristics. The research literature provides general guidance, though optimal protocols for specific goals continue to be refined through ongoing studies.
General dosing guidelines
Most clinical trials demonstrating skin benefits use doses between 2.5 and 10 grams of hydrolyzed collagen daily. Lower doses around 2.5-5 grams appear sufficient when using products standardized for bioactive peptide content, while higher doses may be needed for products with less characterized peptide profiles.
Joint health applications typically employ similar dose ranges, with some Type II collagen formulations effective at lower doses around 40mg daily due to the proposed immune tolerance mechanism rather than simple structural support.
Athletes seeking performance and recovery support often use higher doses in the 15-20 gram range, particularly when timing supplementation around training sessions to maximize nutrient availability during the collagen synthesis response to exercise.
Using a peptide calculator helps ensure accurate dosing across different products and formulations.
Timing and administration
The optimal timing for collagen supplementation depends on intended outcomes. For general skin and joint support, consistent daily intake matters more than specific timing, with many consumers finding morning or evening routines most sustainable.
Athletes benefit from pre-workout collagen intake approximately 30-60 minutes before training, when the post-exercise collagen synthesis response can utilize circulating peptides. Vitamin C co-ingestion enhances this response by supporting the enzymatic reactions required for functional collagen production.
Collagen peptides dissolve readily in both cold and hot liquids, enabling flexible incorporation into coffee, smoothies, soups, or plain water. Heat stability allows cooking applications without degrading the peptide content, though extended high-temperature exposure should be avoided.
Combination strategies
Vitamin C represents the most important complementary nutrient for collagen supplementation, serving as an essential cofactor for collagen synthesis enzymes. Ensuring adequate vitamin C intake, either through diet or supplementation, maximizes the utility of absorbed collagen peptides.
Hyaluronic acid combines synergistically with collagen for skin hydration benefits. Some formulations include both components, while others rely on consumers to combine products. Understanding hyaluronic acid and peptide combinations helps optimize supplementation strategies.
Silicon-containing supplements like orthosilicic acid may enhance collagen cross-linking and stability in tissues. Preliminary research suggests potential synergies, though clinical evidence remains limited.
Antioxidants generally support skin health by protecting existing collagen from oxidative damage. Vitamins C and E, along with plant polyphenols, reduce collagen degradation from environmental stressors like UV radiation and pollution.
Quality markers and product selection
Navigating the collagen supplement market requires understanding the quality indicators that distinguish effective products from inferior alternatives. Price alone provides limited guidance, as premium pricing doesn't guarantee quality while some economical products deliver excellent value.
Third-party testing and certifications
Independent testing through organizations like NSF International, Informed Sport, or USP provides verification that products contain what labels claim without prohibited substances or concerning contaminants. These certifications require ongoing compliance rather than one-time testing, ensuring consistent quality across production batches.
Athletes subject to drug testing should prioritize products with Informed Sport or similar sports-specific certifications that screen for substances banned by athletic organizations. Cross-contamination from shared manufacturing facilities can introduce unintended substances that cause positive drug tests.
Heavy metal testing documentation confirms products meet safety thresholds for mercury, lead, cadmium, and arsenic. Marine-sourced collagens face particular scrutiny for mercury content due to bioaccumulation in fish tissues, though proper source selection and processing typically eliminate concerns.
Molecular weight specifications
Products specifying molecular weight distributions provide transparency about hydrolysis extent and expected bioavailability. Target ranges between 2,000 and 5,000 Daltons reflect common industry standards, while some premium products achieve lower weights for enhanced absorption.
Dalton specifications allow comparisons between products using consistent metrics. Products claiming enhanced absorption without providing molecular weight data leave consumers unable to verify these assertions.
Ultra-low molecular weight products below 1,000 Daltons may sacrifice some bioactive peptide sequences that require specific chain lengths for biological activity. The balance between absorption efficiency and bioactive peptide preservation represents an optimization challenge without clear universal solutions.
Source transparency and traceability
Reputable manufacturers provide clear information about collagen source species, geographic origin, and processing facilities. This transparency enables verification of claims about grass-fed status, sustainable sourcing, or specific processing methods.
Supply chain traceability becomes particularly important for allergen concerns and religious dietary requirements. Products blending multiple collagen sources should clearly identify all components to enable informed consumer choices.
Country of origin affects both quality expectations and regulatory oversight. Manufacturing in countries with stringent food safety regulations, such as the United States, European Union, Japan, or Australia, provides some assurance of quality standards though exceptions exist in all jurisdictions.
Potential side effects and safety considerations
Collagen peptide supplementation demonstrates an excellent safety profile across extensive clinical trial experience and widespread consumer use. The protein derives from food sources consumed throughout human history, and hydrolysis simply breaks this familiar protein into smaller, more absorbable fragments.
Common tolerability issues
Digestive symptoms including bloating, fullness, and altered bowel habits occur occasionally, particularly when starting supplementation or using higher doses. These effects typically resolve within a few days as the digestive system adjusts to increased protein intake.
Unpleasant taste or aftertaste affects some products more than others, depending on source material quality and processing methods. Marine collagen sources sometimes retain fishy flavors that manufacturing fails to completely eliminate. Flavored products or mixing with strongly flavored beverages can mask undesirable tastes.
Understanding peptide safety considerations provides broader context for evaluating any peptide supplementation approach.
Allergic considerations
Fish and shellfish allergies represent the primary allergic concern for marine collagen products. The extensive hydrolysis process typically degrades allergenic proteins, but individuals with severe allergies should exercise caution. Starting with small test doses and monitoring for reactions provides a conservative approach.
Bovine collagen may theoretically trigger reactions in individuals with rare beef allergies, though documented cases remain uncommon. Alpha-gal syndrome, a tick bite-induced allergy to mammalian meat, could potentially affect bovine collagen tolerance though research on this specific application is limited.
Egg allergies may affect tolerance of egg shell membrane-derived collagen products, though this represents a minor market segment with limited availability.
Medication interactions
Collagen peptides don't exhibit significant interactions with common medications based on current evidence. The amino acid content theoretically could affect absorption of certain drugs if taken simultaneously, though no clinically significant interactions have been documented.
Individuals taking medications affecting calcium metabolism should consult healthcare providers before combining with collagen products marketed for bone health, as the combined effects on calcium handling remain unstudied.
Those on protein-restricted diets for kidney disease should account for collagen supplementation in their total protein intake. While collagen provides lower-quality protein than complete sources, it still contributes to overall nitrogen load.
Addressing common misconceptions
Marketing claims and popular beliefs about collagen supplementation sometimes diverge from scientific evidence. Understanding these misconceptions helps consumers set realistic expectations and avoid products making unsubstantiated claims.
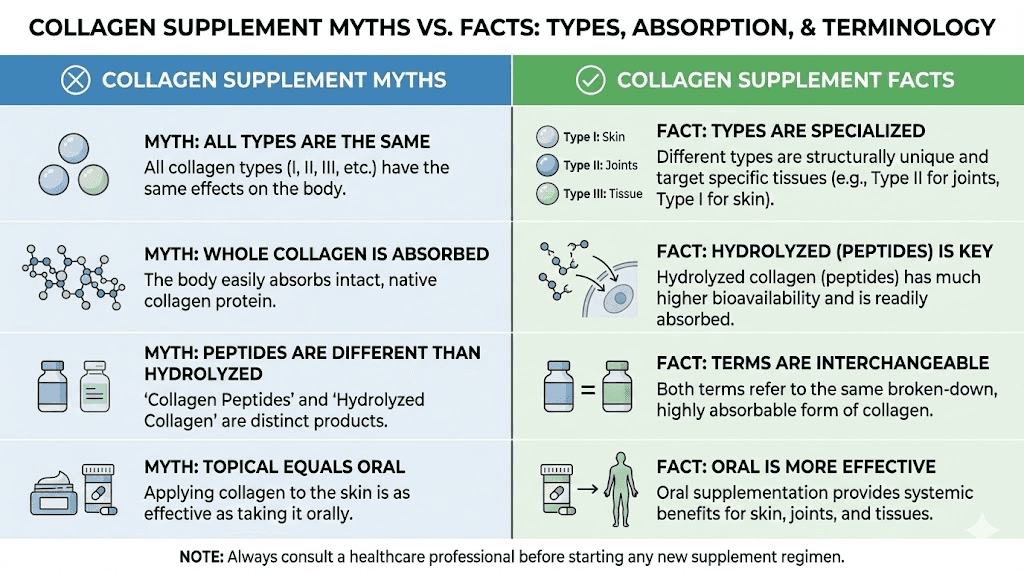
Hydrolysate versus peptides: the same thing?
The central question of this comparison, whether collagen hydrolysate and collagen peptides differ meaningfully, deserves direct address. For practical consumer purposes, these terms describe the same product category. Both refer to collagen that has undergone enzymatic hydrolysis to reduce molecular weight and enhance solubility and absorption.
Minor terminology preferences among manufacturers and regional markets created the apparent distinction. European supplement traditions favored hydrolysate terminology while American and Asian markets adopted peptides. Neither term implies superior quality or processing.
Some manufacturers use peptides specifically for lower molecular weight products and hydrolysate for less extensively processed versions, but this convention lacks industry-wide standardization. Molecular weight specifications provide more meaningful quality information than terminology choices.
Type matters less than quality
Marketing often emphasizes collagen type as the primary selection criterion, but processing quality likely matters more for most applications. A well-manufactured Type I collagen product may outperform a poorly processed Type II product even for joint applications where Type II seems theoretically superior.
The body synthesizes its own collagen types from amino acid substrates regardless of the types consumed. Supplemental collagen provides building blocks rather than pre-formed structural components that insert directly into tissues. This metabolic reality means source type influences amino acid ratios and bioactive peptide content rather than directly determining tissue deposition patterns.
Type-specific targeting makes more sense for specific bioactive formulations, such as undenatured Type II collagen for joint immune tolerance mechanisms, than for general hydrolyzed peptide products.
Absorption claims require scrutiny
Many products claim superior absorption compared to competitors without providing verification data. Absorption depends on molecular weight distribution, which varies considerably among products claiming equivalent bioavailability advantages.
Nano or ultra-hydrolyzed products commanding premium prices may not deliver proportionally better results. Once molecular weight drops below approximately 3,000 Daltons, further reduction provides diminishing absorption benefits while potentially sacrificing bioactive peptide sequences.
The most meaningful absorption claims cite specific clinical trials demonstrating increased plasma peptide levels or enhanced tissue outcomes compared to control products. Generic absorption claims without supporting data warrant skepticism.
Special population considerations
Different life stages and health conditions create varying collagen needs and supplementation considerations. Understanding these population-specific factors helps tailor recommendations appropriately.
Aging and collagen decline
Collagen synthesis naturally declines with age, beginning around age 25 and accelerating through middle age and beyond. This decline manifests as reduced skin elasticity, joint stiffness, and bone fragility as structural collagen degrades faster than replacement occurs.
Older adults may benefit more dramatically from supplementation than younger individuals, as the gap between synthesis capacity and tissue demands widens progressively. The relative improvement from supplementation appears greater in populations with more significant baseline deficits.
Exploring peptides for anti-aging provides additional strategies for addressing age-related changes in tissue quality and function.
Women over 40 experience accelerated collagen decline related to hormonal changes around menopause. Estrogen supports collagen synthesis, and declining estrogen levels during perimenopause and menopause reduce collagen production capacity. Supplementation may partially compensate for this hormonal influence on collagen status.
Athletes and active individuals
Physical activity increases collagen turnover in connective tissues, creating elevated demands for collagen precursors. Athletes experience both enhanced collagen synthesis and increased degradation, with net tissue status depending on the balance between these processes.
Training-induced micro-damage to tendons, ligaments, and cartilage requires collagen synthesis for repair and adaptation. Providing adequate collagen peptide substrate supports these repair processes and may reduce injury risk over time.
Those focused on athletic performance enhancement should consider collagen as one component of a comprehensive approach to recovery and tissue maintenance.
The timing of collagen intake around exercise sessions may enhance benefits by ensuring peptide availability during the post-exercise period when synthesis rates increase. Pre-workout supplementation approximately 30-60 minutes before training allows absorption before the exercise stimulus.
Recovery and healing contexts
Injury recovery creates dramatically increased collagen demands as tissues rebuild damaged structures. Fractures, tendon tears, ligament injuries, and surgical wounds all require substantial collagen synthesis for proper healing.
Supplementation during recovery periods may support faster and more complete healing, though clinical evidence specific to injury recovery remains less developed than for general health applications. The biological rationale strongly supports enhanced collagen availability during healing.
Those recovering from injuries should explore fast injury healing resources for comprehensive guidance on optimizing recovery.
Post-surgical recovery particularly benefits from nutritional support including adequate protein and collagen precursors. Wound healing depends on local collagen synthesis, and systemic nutrient availability affects this process significantly.
Integrating collagen with broader health strategies
Collagen supplementation works best as one component of comprehensive approaches to skin health, joint function, and overall wellness. Isolated supplementation without addressing other relevant factors limits potential benefits.
Lifestyle factors affecting collagen
Sun exposure represents the primary environmental threat to skin collagen, with UV radiation inducing matrix metalloproteinases that degrade collagen fibers. Sun protection through clothing, shade, and sunscreen preserves existing collagen while supplementation supports new synthesis.
Smoking dramatically accelerates collagen degradation through oxidative stress and impaired circulation. Smoking cessation provides one of the most impactful interventions for skin aging, exceeding any supplement benefit.
Sleep quality affects systemic repair processes including collagen synthesis. Growth hormone released during deep sleep stages supports tissue anabolism. Addressing sleep problems enhances the benefits of any regenerative supplementation approach.
Chronic stress elevates cortisol, which suppresses collagen synthesis and accelerates breakdown. Stress management through exercise, meditation, or other approaches supports collagen status indirectly by moderating this catabolic hormonal influence.
Nutritional foundations
Adequate protein intake provides the amino acid substrate for endogenous collagen synthesis. Individuals on very low protein diets may lack sufficient amino acids for optimal collagen production regardless of supplementation.
Vitamin C, zinc, and copper serve as essential cofactors for collagen synthesis enzymes. Deficiencies in these micronutrients limit the body's ability to utilize collagen peptides for new collagen production. A nutrient-rich diet or appropriate supplementation ensures these cofactors remain available.
Antioxidant-rich diets protect existing collagen from oxidative damage. Fruits, vegetables, and other whole foods provide diverse antioxidant compounds that reduce collagen degradation from environmental and metabolic stressors.
Glycine, the most abundant amino acid in collagen, may become conditionally essential under high-demand situations. Some researchers suggest glycine supplementation alongside collagen peptides when tissue demands are elevated.
Exercise and mechanical loading
Physical activity stimulates collagen synthesis in loaded tissues. The mechanical signals from exercise activate fibroblasts and other collagen-producing cells, enhancing tissue turnover and adaptation.
Resistance training benefits connective tissue throughout the musculoskeletal system. Regular strength training maintains tendon and ligament quality while supporting bone collagen matrix.
Those pursuing muscle growth should recognize that connective tissue adaptation often lags behind muscle gains, creating potential injury risk when strength exceeds structural support capacity. Collagen supplementation may help connective tissues keep pace with muscle development.
Weight-bearing exercise supports bone health through mechanical loading that stimulates bone matrix synthesis including collagen. Walking, running, and resistance training all provide beneficial bone loading.
How SeekPeptides supports collagen research
SeekPeptides provides comprehensive resources for understanding collagen science and optimizing supplementation strategies. The platform offers evidence-based guidance for navigating the complex collagen supplement landscape.
The peptide calculator helps users determine appropriate dosing based on individual factors and goals. Accurate dosing ensures effective supplementation without unnecessary expense or potential for adverse effects.
Educational resources covering peptide dosage calculation provide the knowledge foundation for informed decision-making. Understanding the principles behind dosing recommendations empowers users to adjust protocols as needed.
The peptide stacking guide explores how different peptides can be combined effectively. Synergistic combinations may enhance benefits beyond what individual components provide alone.
SeekPeptides serves as a trusted resource for evidence-based peptide therapy guidance, helping users develop personalized protocols aligned with their specific goals and circumstances.
Helpful resources
External resources
In case I don't see you, good afternoon, good evening, and good night. May your collagen stay abundant, your peptides stay effective, and your tissues stay resilient. Join SeekPeptides.