Jan 19, 2026

What if your doctor could write you a prescription for that research peptide everyone's talking about on Reddit?
They can't. Not legally, anyway.
This is the question thousands of people ask every month.
They've heard about BPC-157 for injuries, TB-500 for recovery, or Ipamorelin for anti-aging.
They want the benefits but want to do things the right way, through their physician, with proper medical oversight.
The problem is that the regulatory landscape for peptides is far more complex than most people realize, and understanding where research peptides fit into the legal framework could save you from significant legal, financial, and health risks.
The short answer is no, doctors cannot legally prescribe research-grade peptides for human use. But that short answer obscures a massive amount of nuance about what doctors can prescribe, which peptides fall into which regulatory categories, and how you can access legitimate peptide therapy safely. This guide covers everything you need to know about peptide regulations, the difference between research and pharmaceutical-grade compounds, and your legal options for obtaining peptide therapy.
Understanding the difference between research peptides and pharmaceutical peptides
The distinction matters. It's not just labeling semantics or marketing language.
Research peptides and pharmaceutical-grade peptides exist in completely different regulatory universes. Research peptides are manufactured for laboratory use, scientific experiments, and in vitro studies. They carry labels like "research use only" or "not for human consumption," and these labels aren't suggestions. They're legal requirements that define how these compounds can be sold and used.
Pharmaceutical-grade peptides, on the other hand, are manufactured under strict FDA guidelines, tested for purity and potency, and approved for human therapeutic use. The manufacturing processes differ dramatically. The quality control standards differ. The legal status differs. And most importantly for this discussion, the prescribing rules differ entirely.
What makes a peptide "research grade"?
Research-grade peptides are synthesized for laboratory applications. They're used in cell culture studies, animal research, and academic investigations. The companies that produce them aren't held to the same manufacturing standards as pharmaceutical companies because the products aren't intended to enter human bodies.
This matters for several reasons.
Purity requirements for research peptides are lower. A research peptide might be 95% pure, which is perfectly acceptable for laboratory experiments but potentially concerning for human injection. Pharmaceutical-grade compounds typically require 98-99%+ purity with extensive documentation of exactly what comprises that remaining percentage.
Testing requirements differ too. Research peptides may undergo basic quality checks, but they don't face the rigorous stability testing, sterility verification, and batch-to-batch consistency requirements that pharmaceutical compounds must meet. When you're injecting something into your body, these differences aren't trivial, they're potentially dangerous.
The safety implications extend beyond purity. Research peptides may contain synthesis byproducts, residual solvents, or degradation products that haven't been characterized or tested for human safety. Pharmaceutical manufacturing processes specifically eliminate or control these contaminants.

What makes a peptide "pharmaceutical grade"?
Pharmaceutical-grade peptides have undergone the FDA approval process. This isn't a rubber stamp. It's years of clinical trials, extensive safety data, manufacturing facility inspections, and ongoing quality monitoring. Companies invest hundreds of millions of dollars to bring a single peptide through this process.
The FDA approval process for peptides involves multiple phases. First, preclinical studies establish basic safety and efficacy in laboratory and animal models. Then Phase 1 trials test safety in small groups of humans. Phase 2 trials evaluate efficacy and dosing. Phase 3 trials provide the large-scale data needed to demonstrate the drug works as claimed. Only after successfully completing all phases does the FDA consider approval.
Examples of FDA-approved peptide medications include semaglutide, marketed as Ozempic and Wegovy, for diabetes and weight loss. Liraglutide, sold as Victoza and Saxenda, treats similar conditions. Tirzepatide, branded as Mounjaro and Zepbound, represents the newest generation of GLP-1 receptor agonists. Insulin, perhaps the most famous peptide drug, has been saving lives for a century. Oxytocin is prescribed for labor induction. These are all peptides. They're all legal to prescribe. They're all pharmaceutical grade.
The FDA regulatory framework for peptide compounding
Understanding FDA regulations requires knowing about the 503A and 503B frameworks. These sections of the Federal Food, Drug, and Cosmetic Act govern how compounding pharmacies can prepare medications, including peptides.
Section 503A covers traditional compounding pharmacies that prepare medications in response to individual patient prescriptions. These pharmacies can compound drugs that aren't commercially available, provided they follow specific rules. Section 503B covers outsourcing facilities that can compound drugs without individual prescriptions, but with more stringent FDA oversight.
For peptides, the FDA maintains a "bulks list" of substances that pharmacies can use in compounding. This list is divided into categories that determine what's legal to compound.
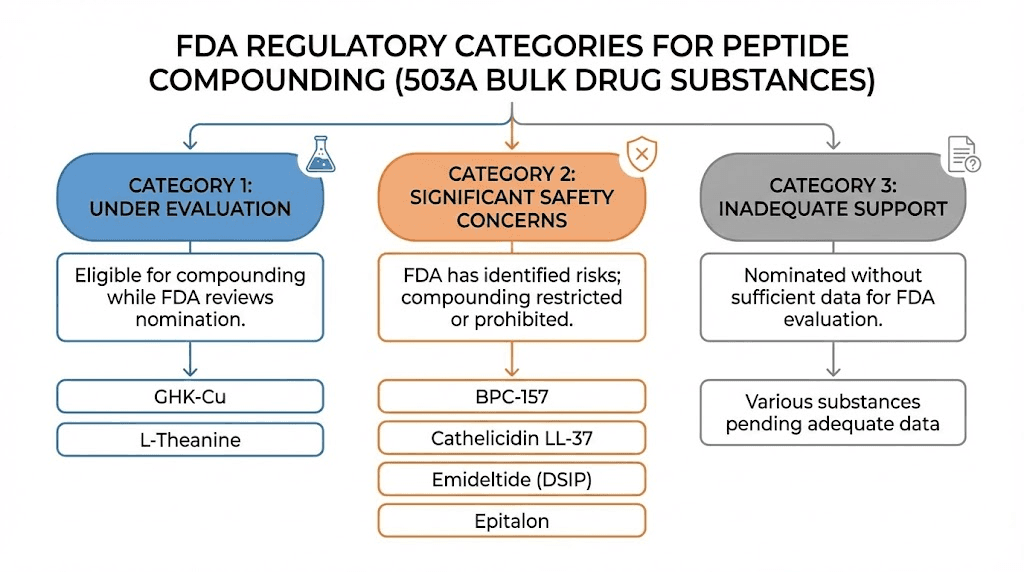
Category 1 peptides: legal for compounding
Category 1 substances have been evaluated by the FDA and deemed acceptable for use in compounding. Pharmacies can legally compound these peptides when a physician writes a prescription for an individual patient with a documented medical need.
Currently, Category 1 includes a limited number of peptides. Sermorelin is one example, used for growth hormone optimization. Gonadorelin acetate has been added to this category. GHK-Cu was placed in Category 1, though notably not for injectable administration, limiting its use to topical applications for skin health and hair growth.
NAD+ can also be compounded under this framework, though it's technically a nucleotide rather than a peptide. The Category 1 list represents the small subset of bulk substances the FDA considers safe enough for compounding use.
Category 2 peptides: banned from compounding
Category 2 is where most popular research peptides end up. These substances have been evaluated and the FDA has identified "significant safety risks" that preclude their use in compounding. Compounding pharmacies cannot legally prepare these peptides for human use, period.
The list includes many peptides you've probably heard about.
BPC-157 sits firmly in Category 2. The FDA cited immunogenicity concerns and impurity risks when making this determination. Despite the extensive anecdotal reports and animal studies suggesting benefits for injury healing, the FDA determined the safety data was insufficient for human compounding.
TB-500, also known as Thymosin Beta-4 fragment, is banned from compounding. So is AOD-9604, once touted as a fat loss peptide. Ipamorelin acetate, a growth hormone secretagogue, cannot be compounded. KPV, despite its promise for inflammation and gut health, is prohibited.
CJC-1295 with DAC is no longer available for compounding. Semax and Selank, popular for cognitive enhancement, are on the banned list. Melanotan II cannot be compounded. Epitalon, despite research suggesting longevity benefits, is prohibited.
This Category 2 designation means that even if your doctor wanted to prescribe these peptides, no legitimate compounding pharmacy can legally fill that prescription.
Category 3 peptides: insufficient data
Category 3 contains substances nominated for the bulks list but submitted without sufficient supporting information. These peptides haven't been fully evaluated, so the FDA hasn't made a determination about their safety for compounding. They exist in regulatory limbo, neither approved nor explicitly banned.
The practical effect is similar to Category 2, compounding pharmacies generally won't compound Category 3 substances because the regulatory risk is too high without clear FDA guidance.
Recent regulatory changes
The regulatory landscape shifted significantly in 2024 and 2025. The FDA made several important announcements that affected peptide availability.
In September 2024, the FDA removed several peptides from Category 2, but not because they were approved for compounding. The nominators simply withdrew their nominations. AOD-9604, CJC-1295, ipamorelin acetate, thymosin alpha-1, and Selank acetate were withdrawn from review. This doesn't mean they became legal to compound; it means their regulatory status reverted to undefined, which arguably makes them even riskier to compound.
The semaglutide and tirzepatide situation also evolved. During the drug shortages, compounding pharmacies were permitted to compound these GLP-1 medications. When the FDA declared the shortages resolved in early 2025, that permission ended. Compounded versions became unavailable by May 2025, forcing many patients to switch to brand-name versions or discontinue therapy.
The legal reality: doctors cannot prescribe research peptides
Here's the bottom line that answers our central question.
Doctors cannot legally prescribe research-grade peptides for human use. This isn't a gray area. It isn't a technicality. It's a fundamental principle of medical regulation in the United States.
Physicians can only prescribe FDA-approved medications or compounds that can be legally prepared by compounding pharmacies. Research peptides fall into neither category. They are not FDA-approved drugs. They cannot be legally compounded because they're either explicitly banned (Category 2) or not on the approved list for compounding.
The "research use only" label on these products isn't a loophole. It's a statement of their legal status. They can be sold for legitimate research purposes, laboratory experiments, scientific studies. They cannot be sold for human consumption, regardless of who's doing the prescribing.
Why the distinction matters legally
Some people argue that if a doctor prescribes something, that makes it legal. This is incorrect.
Physicians have broad prescribing authority within the scope of legal medications.
They can prescribe FDA-approved drugs off-label, meaning for conditions other than their officially approved uses. This is legal and common practice. Semaglutide prescribed for weight loss when it was only approved for diabetes was off-label prescribing, and perfectly legal because semaglutide itself was an approved drug.
Research peptides are different. They're not approved drugs being prescribed off-label. They're unapproved substances that have never gone through the FDA approval process. Prescribing them isn't off-label use; it's prescribing an unapproved drug, which violates FDA regulations regardless of the physician's intentions.
What about compounding pharmacies?
The compounding pharmacy angle confuses many people. They assume that if a compounding pharmacy will make something, it must be legal.
Legitimate compounding pharmacies operating under 503A or 503B frameworks can only compound substances that meet specific criteria. The substance must be the active ingredient in an FDA-approved drug, have a USP monograph, appear on the FDA's approved bulks list (Category 1), or have GRAS (Generally Recognized as Safe) status.
Most research peptides meet none of these criteria. BPC-157 and TB-500 are not active ingredients in approved drugs. They don't have USP monographs. They're not on the Category 1 list. They're explicitly on the Category 2 list of substances that cannot be compounded.
If a pharmacy is compounding these peptides, they're operating illegally. Several have faced enforcement actions, including criminal prosecution. The Department of Justice prosecuted Tailor Made Compounding LLC for distributing unapproved peptides including BPC-157, resulting in a $1.79 million forfeiture.

Legal risks for physicians prescribing research peptides
Doctors who prescribe or administer unapproved peptides face serious professional and legal consequences. This isn't theoretical risk; it's documented reality with case examples.
Medical malpractice exposure
The standard of care in medicine does not include injecting or prescribing unapproved experimental peptides. When a physician deviates from the standard of care and a patient is harmed, that's malpractice.
With research peptides, the malpractice risk is particularly acute. Some legal experts argue that prescribing unapproved peptides like BPC-157 is malpractice per se, meaning automatic malpractice, because it's impossible to adequately inform a patient of risks that haven't been studied in humans.
The informed consent challenge compounds the problem. Informed consent requires explaining known risks. With unapproved peptides, the risks are largely unknown. How can a doctor obtain meaningful informed consent for a substance that hasn't undergone human safety trials? This gray area makes patient consent legally and ethically murky.
Malpractice insurance issues
Here's a critical consideration that many doctors don't realize until it's too late.
Malpractice insurance policies typically exclude coverage for activities that violate federal law or fall outside the standard of care. Prescribing unapproved peptides potentially triggers both exclusions. If a patient is harmed and sues, the physician's malpractice insurance may deny the claim entirely.
This leaves the doctor personally liable for legal defense costs and any damages awarded.
Medical malpractice lawsuits can result in judgments of hundreds of thousands or millions of dollars.
Without insurance coverage, a single lawsuit could financially destroy a medical practice.
Medical board discipline
State medical boards regulate physician conduct and can take disciplinary action for unprofessional behavior. Advising patients to use unapproved drugs is generally considered unprofessional conduct that can trigger board investigation and discipline.
Potential consequences include formal reprimand, probation with practice restrictions, mandatory education requirements, fines, and license suspension or revocation. These actions become part of the physician's permanent record and are publicly searchable, damaging reputation and future employment prospects.
Recent enforcement trends show increased scrutiny. Ohio suspended four medical spa licenses in a single action for improper handling of cosmetic drugs. States are ramping up enforcement against medical and wellness practices that fail to comply with peptide regulations.
Federal criminal liability
The FDA can pursue criminal charges against physicians who distribute unapproved drugs. While individual prescribing doctors face lower prosecution risk than the companies selling these products, the risk isn't zero.
Physicians who set up practices specifically to prescribe research peptides, who buy research peptides in bulk and administer them in-office, or who work with shady compounding pharmacies could face FDA investigation and potential prosecution.
The penalties can include fines, imprisonment, and permanent exclusion from federal healthcare programs including Medicare and Medicaid.
Legal risks for patients buying research peptides
Patients face a different set of risks. The legal exposure is generally lower than for physicians, but health risks may be higher.
Personal use enforcement
The good news for individuals is that the FDA primarily focuses enforcement on sellers, not buyers. Personal possession of research peptides for self-administration rarely results in criminal prosecution.
However, "rarely" doesn't mean "never." The FDA can and does pursue enforcement when circumstances warrant. And state laws may differ from federal enforcement priorities, creating additional risk depending on your location.
The bigger concern is that purchasing unapproved drugs removes you from the legal healthcare system. You can't sue a Chinese peptide manufacturer if their product harms you. You have no legal recourse if you receive contaminated or mislabeled products. You're operating entirely outside the protective framework that pharmaceutical regulation is designed to provide.
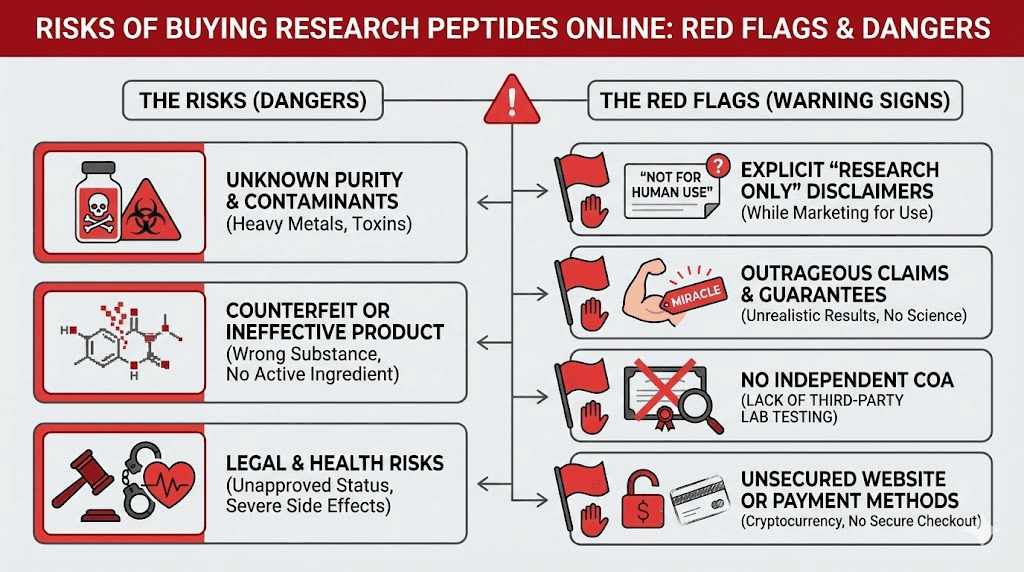
Quality and safety risks
Research-grade peptides don't come with the quality guarantees of pharmaceutical products. You're trusting unknown manufacturers to produce sterile, pure, accurately-dosed products without any regulatory oversight ensuring they do so.
A UC Davis cellular biologist put it bluntly: "Research-grade peptides are going to have junk in them. They're going to have chemicals used in the purification process and fragments of peptides that you don't want."
The rise of cheap peptides from overseas suppliers, some offering products for as little as $5 per vial, has only increased these concerns. Price points that low suggest corners are being cut somewhere in the manufacturing process.
The stability of research peptides during shipping and storage is another concern. Peptides degrade at room temperature, and international shipping rarely includes proper cold chain management. By the time that bargain peptide arrives, it may have lost significant potency or degraded into unknown compounds.
SeekPeptides provides comprehensive guidance on evaluating peptide quality, understanding storage requirements, and recognizing red flags in peptide sourcing. Members access detailed vendor evaluation frameworks and community knowledge from researchers who've navigated these challenges.
The "research use only" fiction
The peptide community has developed elaborate linguistic rituals to maintain the fiction that everyone is conducting legitimate research. Forums instruct members to say they're "researching" peptides rather than "taking" them. Vendors use "research purposes only" disclaimers as legal shields.
This isn't fooling the FDA. In warning letters, the agency has explicitly stated that despite "research use only" labels, evidence shows products are intended for human use, particularly when sold with syringes, diluents, and dosing instructions for subcutaneous injection.
Enforcement actions increasingly target this gap between stated purpose and actual use. The Department of Justice has prosecuted illegal peptide sellers, and the FDA continues issuing warning letters to companies making therapeutic claims or packaging products in ways that suggest human administration.
What peptides can doctors legally prescribe?
Despite the restrictions on research peptides, doctors have legitimate options for prescribing peptide therapy. Understanding what's available legally helps patients work with their physicians effectively.
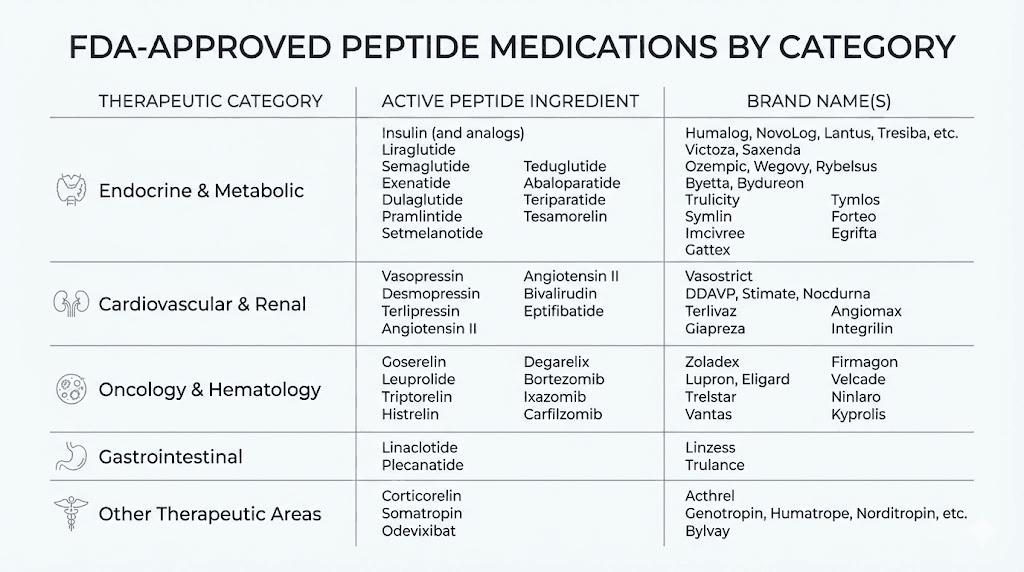
FDA-approved peptide medications
Numerous peptides have full FDA approval and can be prescribed like any other medication.
GLP-1 receptor agonists represent the most high-profile category. Semaglutide treats type 2 diabetes (Ozempic) and obesity (Wegovy). Tirzepatide offers similar benefits through a dual-action mechanism (Mounjaro, Zepbound). Liraglutide (Victoza, Saxenda) was the first in this class to achieve widespread use. These are legitimate, FDA-approved peptides that doctors prescribe millions of times each year.
Insulin is perhaps the most famous peptide medication, essential for diabetes management. Various formulations, from rapid-acting to long-acting, are available by prescription.
Oxytocin is FDA-approved for labor induction and certain postpartum applications. It's administered in hospital settings under medical supervision.
Bremelanotide (Vyleesi) is an FDA-approved peptide for hypoactive sexual desire disorder in premenopausal women. It demonstrates that even peptides affecting sexual function can achieve legitimate approval.
Teriparatide (Forteo) is an approved peptide for osteoporosis treatment, showing that bone-related peptide therapies can be legally prescribed.
Compoundable peptides
Beyond FDA-approved drugs, certain peptides can be legally compounded under the 503A framework. These require a physician's prescription for an individual patient.
Sermorelin can be compounded for growth hormone optimization. It stimulates the pituitary gland to produce more natural growth hormone, unlike synthetic HGH which replaces the body's production. Physicians prescribe it for growth hormone deficiency and sometimes off-label for anti-aging purposes.
Gonadorelin is available through compounding for fertility and hormone therapy applications. It acts on the hypothalamic-pituitary-gonadal axis to influence reproductive hormone production.
GHK-Cu can be compounded for topical use only. The FDA specifically prohibited injectable administration, but topical applications for skin rejuvenation remain legal through compounding pharmacies.
PT-141 (Bremelanotide) exists both as an FDA-approved drug and as a compoundable substance, though the regulatory status can vary. Physicians sometimes prescribe compounded versions for sexual health applications.
Off-label prescribing of approved peptides
Physicians have significant latitude to prescribe approved peptides for unapproved uses. This is called off-label prescribing, and it's completely legal when the drug itself has FDA approval.
For example, a doctor might prescribe semaglutide for weight loss even if the patient doesn't meet the strict criteria for the FDA-approved obesity indication. They might prescribe low-dose naltrexone, a peptide-like compound, for inflammation even though it's only approved for opioid addiction. These are legitimate medical decisions within the physician's professional judgment.
The key distinction: the drug must be FDA-approved for something. Off-label prescribing expands the uses of approved drugs. It doesn't make unapproved drugs legal.
How to access peptide therapy legally
For patients interested in peptide therapy, legal pathways exist. They require working within the healthcare system rather than around it.
Finding a qualified physician
The first step is finding a doctor experienced with peptide therapy. Not every physician is comfortable prescribing these medications or knowledgeable about optimal protocols.
Specialists who commonly work with peptides include endocrinologists for hormone-related therapies, obesity medicine specialists for weight loss peptides, anti-aging and longevity medicine practitioners, sports medicine physicians for performance and recovery applications, and integrative medicine doctors who take a comprehensive approach to health optimization.
When searching for a provider, look for board certification in relevant specialties, experience specifically with peptide medications, willingness to discuss treatment goals and options, and use of legitimate compounding pharmacies when appropriate.
Peptide therapy clinics have proliferated in recent years. Some are excellent, staffed by knowledgeable physicians using legal protocols. Others operate in gray areas, prescribing substances they shouldn't or sourcing from questionable suppliers. Do your research before committing to any clinic.
SeekPeptides offers resources to help members evaluate providers, treatment options, and make informed decisions about their peptide therapy journey.
Telemedicine options
Telemedicine has expanded access to peptide therapy significantly. Multiple telehealth platforms now offer consultations with physicians who can prescribe legal peptide medications.
The process typically works as follows. You complete an online health questionnaire and potentially order preliminary lab work. A physician reviews your information and conducts a video or phone consultation. If appropriate, they write a prescription sent to a pharmacy for fulfillment. The medication ships directly to your door.
Legitimate telemedicine peptide services use only FDA-approved medications or legally compoundable substances. They require proper medical oversight, including follow-up appointments and monitoring. They work with licensed pharmacies that meet all regulatory requirements.
Telemedicine is available in most states, though specific services may have geographic limitations. Ways2Well, for example, operates in 38 states. Other services have different coverage areas. Check availability in your state before assuming you can access a particular platform.
Working with compounding pharmacies
For peptides that can be legally compounded, your physician will write a prescription filled by a compounding pharmacy operating under 503A or 503B frameworks.
Legitimate compounding pharmacies should be licensed in your state and registered with the FDA. They should source pharmaceutical-grade active ingredients from registered suppliers. They should provide Certificates of Analysis documenting purity and potency. They should follow USP guidelines for sterility and quality control.
Your physician may have relationships with specific compounding pharmacies they trust. If not, ask questions about the pharmacy's credentials, sourcing, and quality assurance practices. A reputable pharmacy will be transparent about these matters.
Understanding costs
Legal peptide therapy isn't cheap. Most insurance plans don't cover peptide medications when prescribed for anti-aging, optimization, or wellness purposes. Even approved drugs like semaglutide may not be covered without specific diagnoses.
Typical costs range widely.
Brand-name GLP-1 medications can cost $1,000-$1,500 per month without insurance. Compounded peptides from legitimate pharmacies might run $150-$600 per month depending on the specific substance and dosing. Physician consultations add $200-$500 for initial visits and $100-$250 for follow-ups. Lab work for monitoring can cost $100-$500 per panel.
Annual costs for comprehensive peptide therapy might total $2,000-$10,000 or more depending on the specific protocol.
HSA and FSA accounts can often be used for peptide therapy costs when prescribed by a physician for a medical purpose. Medicare may cover certain FDA-approved peptides for qualifying diagnoses. Private insurance varies widely, so check your specific plan's formulary and coverage policies.
The cost difference between legal and gray-market peptides is significant. Research peptides from online suppliers might cost $30-$100 for what legitimate compounding charges $300-$500. But the legal, quality, and safety risks make that apparent savings illusory.
Specific peptides and their regulatory status
Understanding the regulatory status of specific peptides helps patients have informed conversations with their physicians about what's actually available.
BPC-157: not legal for prescription
BPC-157 is explicitly banned from compounding under FDA regulations. It sits in Category 2 of the 503A bulks list with cited concerns about immunogenicity and impurity risks.
Despite widespread anecdotal reports of benefits for tendon injuries, gut issues, and general healing, no human clinical trials have established safety or efficacy. The animal research, while promising, doesn't translate directly to human use, and the FDA has determined that insufficient safety data exists for compounding.
Doctors cannot legally prescribe BPC-157. Compounding pharmacies cannot legally make it. Anyone offering "prescription" BPC-157 is operating outside the law.
BPC-157 alternatives that are legal include various growth factors and healing-support compounds available through legitimate channels, though none offer the exact same mechanism of action.
TB-500: not legal for prescription
TB-500, a fragment of Thymosin Beta-4, is classified as a "Substance with Safety Concerns" and prohibited from compounding. The FDA has cited insufficient safety data for human use.
Like BPC-157, TB-500 shows promise in animal studies for injury healing and tissue repair. And like BPC-157, the lack of human clinical trials means the FDA won't approve it for compounding.
The BPC-157 and TB-500 combination popular in research circles cannot be legally prescribed or compounded in the United States.
Ipamorelin: not legal for prescription
Ipamorelin acetate was removed from the compounding pathway. It's no longer available through legitimate compounding pharmacies despite its previous popularity for growth hormone optimization.
Patients interested in growth hormone support have legal options including sermorelin, which remains compoundable, and FDA-approved synthetic HGH for qualifying diagnoses.
CJC-1295: not legal for prescription
CJC-1295 with DAC was removed from compounding availability in 2023. Like ipamorelin, it was popular for growth hormone optimization but is no longer legally available.
The ipamorelin and CJC-1295 combination that was once commonly prescribed can no longer be legally obtained.
Semaglutide: legal but restricted
Semaglutide is FDA-approved and can be prescribed by any licensed physician. It's available as brand-name Ozempic, Wegovy, and Rybelsus.
Compounded semaglutide was available during the drug shortage period but became restricted after the FDA declared the shortage resolved in February 2025. By May 2025, compounded versions were largely unavailable.
Exceptions exist for patients with documented medical needs that can't be met by standard formulations, such as specific dose requirements. However, compounding simply to save money or access the drug more easily is not permitted.
The cagrilintide and semaglutide combination (CagriSema) represents the next generation of GLP-1 therapy but is not yet FDA-approved.
Thymosin Alpha-1: regulatory status unclear
Thymosin Alpha-1 had a complex regulatory journey. It was placed in Category 2, then the nomination was withdrawn in September 2024, leaving its status undefined.
Some compounding pharmacies have interpreted this withdrawal as permission to compound. Others view it as creating additional legal risk. The safest interpretation is that without explicit Category 1 placement, compounding carries significant regulatory uncertainty.
GHK-Cu: partially legal
GHK-Cu was added to Category 1 but with a significant restriction: not for injectable administration.
This means topical formulations can be compounded for skin and hair applications, but injectable GHK-Cu cannot be legally prescribed.
For those interested in GHK-Cu for hair growth or skin rejuvenation, topical applications remain a legal option through compounding pharmacies.
Image 6 prompt: 16:9 Status chart of popular peptides showing legal vs illegal compounding status. Simple comparison table, minimalist style.
Alt: Popular peptides legal status compounding prescription chart
Recent enforcement actions and legal cases
The regulatory framework isn't just theoretical.
Enforcement actions demonstrate that the FDA and DOJ take peptide violations seriously.
Tailor Made Compounding prosecution
The Department of Justice prosecuted Tailor Made Compounding LLC for distributing unapproved peptides including BPC-157. The company pleaded guilty and forfeited $1.79 million.
This case established important precedents. Compounding pharmacies cannot hide behind the fiction of "research use." Distributing substances the FDA has banned from compounding carries criminal consequences. The financial penalties can be severe.
FDA warning letters
The FDA regularly issues warning letters to companies selling peptides with therapeutic claims. Recent letters have targeted companies making health claims about research peptides, selling peptides packaged with diluents and syringes in ways that suggest human use, and marketing peptides for specific medical conditions.
These warning letters are public records that document FDA enforcement priorities and interpretations of the law.
State enforcement actions
State authorities are increasingly active in peptide enforcement. State attorneys general have issued notices emphasizing that compounding certain peptides is no longer permitted. Medical boards have disciplined physicians prescribing unapproved substances. State pharmacy boards have taken action against compounding pharmacies violating regulations.
Ohio's suspension of four medical spa licenses for improper drug handling demonstrates that enforcement isn't limited to large compounding operations. Individual clinics and practices face scrutiny as well.
Import enforcement
The FDA expanded Import Alert 66-78 in 2025 to include additional unapproved peptides. This alert directs customs officials to detain shipments of specified peptides entering the country.
While enforcement varies, international peptide shipments increasingly face seizure risk. Buyers may lose their money with no recourse, as the products are technically contraband.
The future of peptide regulation
The regulatory landscape continues to evolve. Several factors may shape the future availability of peptide therapy.
Ongoing FDA review
The FDA continues reviewing peptides for potential inclusion on or removal from the bulks lists. The Pharmacy Compounding Advisory Committee (PCAC) meets regularly to evaluate nominated substances and make recommendations.
Some peptides currently in regulatory limbo may eventually receive Category 1 designation, making them legally compoundable. Others may be explicitly placed in Category 2, ending any ambiguity about their prohibition.
Clinical trials
Several peptides are undergoing formal clinical trials that could lead to FDA approval. If BPC-157, for example, were to complete successful human trials and receive approval, it would become a legal prescription medication regardless of its current compounding status.
These trials take years and cost hundreds of millions of dollars.
There's no guarantee any particular peptide will achieve approval.
But the pathway exists for promising compounds to eventually become legal.
Legal challenges
Industry groups have challenged FDA restrictions on peptide compounding. The Outsourcing Facilities Association filed suit regarding GLP-1 compounding restrictions, resulting in preliminary injunctions that temporarily extended compounding availability.
Future legal challenges may reshape the regulatory landscape.
Courts could rule that the FDA exceeded its authority in banning certain substances or that the Category 2 designation process was procedurally flawed.
Political factors
Healthcare policy is inherently political. Changes in FDA leadership, congressional oversight, or executive branch priorities could influence enforcement approaches and regulatory interpretations.
Some advocates push for greater access to unapproved peptides through "right to try" frameworks or reduced regulatory barriers. Others emphasize patient safety and support strict enforcement of approval requirements.
The political landscape will continue influencing how peptide regulations are interpreted and enforced.
Making informed decisions about peptide therapy
Given the complex regulatory environment, how should interested patients approach peptide therapy?
Start with legal options
Before considering gray-market alternatives, explore what's legally available. FDA-approved peptides and legally compoundable substances offer legitimate pathways to many therapeutic goals.
For weight loss, semaglutide and tirzepatide are FDA-approved options with strong efficacy data. For muscle growth and body composition, sermorelin and legally-prescribed HGH may help. For anti-aging, various approved treatments address specific concerns.
Work with a knowledgeable physician to determine what legal options might address your goals.
Understand the risks
If you're considering research peptides despite the legal issues, understand what you're accepting.
You're using products manufactured without regulatory oversight for purity, potency, or sterility. You're operating outside the medical system with no recourse if something goes wrong. You're potentially breaking federal law, even if enforcement against individuals is uncommon. You're trusting unknown suppliers with your health.
These are real risks, not scare tactics. People have been harmed by contaminated research peptides. The quality control issues are documented. The legal consequences, while rare for individuals, are possible.
Do thorough research
Whether pursuing legal or gray-market peptides, education is essential. Understand the mechanisms of action, potential side effects, proper dosing protocols, and storage requirements.
SeekPeptides provides comprehensive resources for researchers at all levels. From beginner guides to advanced protocols, from peptide databases to dosing calculators, the platform offers evidence-based information to support informed decision-making.
Consider the full picture
Peptides are one tool among many for health optimization. Before focusing exclusively on peptide therapy, consider whether you've optimized the fundamentals: sleep, nutrition, exercise, stress management.
Many people seek peptides hoping for shortcuts around basic health practices. The reality is that no peptide will overcome chronic sleep deprivation or a terrible diet. The most effective approach combines optimal lifestyle factors with targeted interventions where appropriate.
Frequently asked questions
Can my doctor write a prescription for BPC-157?
No. BPC-157 is classified as a Category 2 substance that cannot be legally compounded. Even if a doctor writes a prescription, no legitimate pharmacy can fill it. Any source offering "prescription" BPC-157 is operating illegally, and physicians who prescribe it risk malpractice liability and medical board discipline.
Is it legal to buy research peptides for personal use?
Research peptides labeled "not for human consumption" can be legally sold for legitimate research purposes. However, purchasing them with the intent to self-administer violates the intended use and may violate FDA regulations.
While enforcement against individual buyers is rare, the legal and health risks are real. For more details, see our guide on peptide legality.
What's the difference between off-label prescribing and prescribing unapproved drugs?
Off-label prescribing uses FDA-approved drugs for unapproved conditions, which is legal and common. Prescribing unapproved drugs means using substances that have never received FDA approval for any use, which violates federal regulations. Semaglutide for weight loss (when only approved for diabetes) is off-label. BPC-157 for anything is unapproved.
Why can't doctors just prescribe peptides from compounding pharmacies?
Compounding pharmacies can only prepare substances that meet specific FDA criteria: approved drug ingredients, USP monographs, Category 1 bulks list inclusion, or GRAS status. Most popular research peptides fail to meet these criteria and are often explicitly banned (Category 2). Legitimate compounding pharmacies will refuse to compound prohibited substances.
Are there any legal alternatives to banned peptides?
Yes. Sermorelin can be compounded for growth hormone support as an alternative to banned secretagogues. FDA-approved GLP-1 drugs offer effective weight loss options. Topical copper peptides are legal for skin applications.
Various other compounds may address similar goals through legal pathways.
What happens if I'm caught buying research peptides?
For individual buyers, consequences typically range from package seizure to potential customs issues. Criminal prosecution of individual buyers is rare but possible. The greater risk is health consequences from using unregulated products. There's no legal recourse if contaminated products cause harm.
Can telemedicine doctors prescribe peptides?
Telemedicine doctors can prescribe FDA-approved peptides and legally compoundable substances just like in-person physicians. They cannot legally prescribe research peptides or banned substances. Legitimate telemedicine peptide services only offer legal options and work with licensed pharmacies.
Does insurance cover peptide therapy?
Most insurance plans don't cover peptide therapy for anti-aging or optimization purposes. FDA-approved peptides may be covered for their approved indications, like semaglutide for diabetes or obesity. HSA/FSA funds can often be used for peptide therapy when prescribed by a physician. Coverage varies significantly by plan and diagnosis.
How can I find a legitimate peptide therapy provider?
Look for board-certified physicians with experience in peptide therapy, typically in endocrinology, obesity medicine, anti-aging medicine, or integrative medicine. Verify they work with legitimate compounding pharmacies. Be wary of providers who offer to prescribe substances that are banned from compounding. Check our peptide therapy guide for more information on finding qualified providers.
Will peptide regulations change in the future?
Regulations continue to evolve through FDA review processes, clinical trials, legal challenges, and political factors. Some currently banned peptides may eventually receive approval if clinical trials demonstrate safety and efficacy. Regulatory changes can also tighten restrictions. Stay informed through reliable sources like peptide regulation news.
External resources
Regulatory Status of Peptide Compounding 2025 - Frier Levitt
Deep Dive: Regulatory Status of Compounded Peptides - Holt Law
For researchers serious about optimizing their understanding of peptide therapy options, SeekPeptides provides the most comprehensive resource available.
Members access detailed regulatory guides, evidence-based protocols, and a community of experienced researchers who've navigated these complex questions.
In case I don't see you, good afternoon, good evening, and good night. Join us.