Jan 22, 2026
You have seen the label. Research use only. Not for human consumption. The vial looks legitimate. The price seems reasonable. And somewhere in the back of your mind, a voice asks whether this matters. Whether anyone actually follows these warnings. Whether the peptide inside is the same molecule you would get from a pharmacy, just without the markup.
That voice is asking the wrong question.
The grey market for peptides has exploded over the past decade, driven by increasing interest in therapeutic peptides, tightening regulations on compounded medications, and the simple economics of supply and demand. Millions of people now purchase peptides online, often without fully understanding what distinguishes a grey market product from a pharmaceutical-grade compound. The distinction matters more than most realize.
This guide examines everything you need to know about grey market peptides. We will explore what they actually are, how they differ from pharmaceutical and compounded alternatives, the specific risks they carry, and how to make informed decisions if you choose to use them. No judgment. No scaremongering. Just the facts you need to navigate this complex landscape.
SeekPeptides exists to provide evidence-based education about peptides, helping researchers and enthusiasts understand both the potential and the pitfalls of these compounds. Grey market sourcing represents one of the most misunderstood aspects of the peptide world, and clarity here can literally save lives.
What are grey market peptides
Grey market peptides occupy a legal and regulatory middle ground. They are not illegal to purchase or possess in most jurisdictions. They are not approved for human use. And they are not subject to the quality controls that govern pharmaceutical manufacturing.
This creates a situation where consumers assume significant risk without necessarily understanding what that risk entails.
The term grey market refers specifically to the distribution channel, not the products themselves. A peptide sold through grey market channels might be chemically identical to an FDA-approved medication. It might also be contaminated, mislabeled, underdosed, or something else entirely. The grey market designation tells you nothing about quality. It tells you everything about oversight, which is to say there is none.
The research use only loophole
When you see RUO, or Research Use Only, on a peptide vial, the vendor is making a specific legal claim. They are stating that this product is intended for laboratory research, not human consumption. This designation allows them to bypass FDA regulations that would otherwise require clinical trials, safety testing, and manufacturing oversight.
The RUO label is how vendors operate legally while selling products that everyone knows will be used by humans. It shifts all liability from the seller to the buyer. If you inject a grey market peptide and experience adverse effects, you have no legal recourse.
The label told you not to use it that way.
This loophole has enabled an entire industry to flourish. Websites sell BPC-157, TB-500, semaglutide, CJC-1295, and dozens of other peptides with detailed dosing information, user reviews, and customer support, all while maintaining that these products are exclusively for research. The cognitive dissonance is built into the business model.
How grey market differs from black market
Confusion often exists between grey market and black market peptides. The distinction is important. Black market products are illegal to sell, possess, or both. They often include controlled substances like anabolic steroids or scheduled drugs. Grey market products exist in a legal grey zone. Purchasing them is not a crime. Using them in ways not consistent with their labeling is your responsibility.
Grey market peptides can typically be shipped openly through standard mail carriers. They do not require encrypted communications or cryptocurrency payments. Vendors often accept credit cards and operate websites that would be immediately shut down if they were selling illegal substances. This apparent legitimacy contributes to the perception that these products are safe.
They may be legal. That does not make them safe.
The regulatory framework
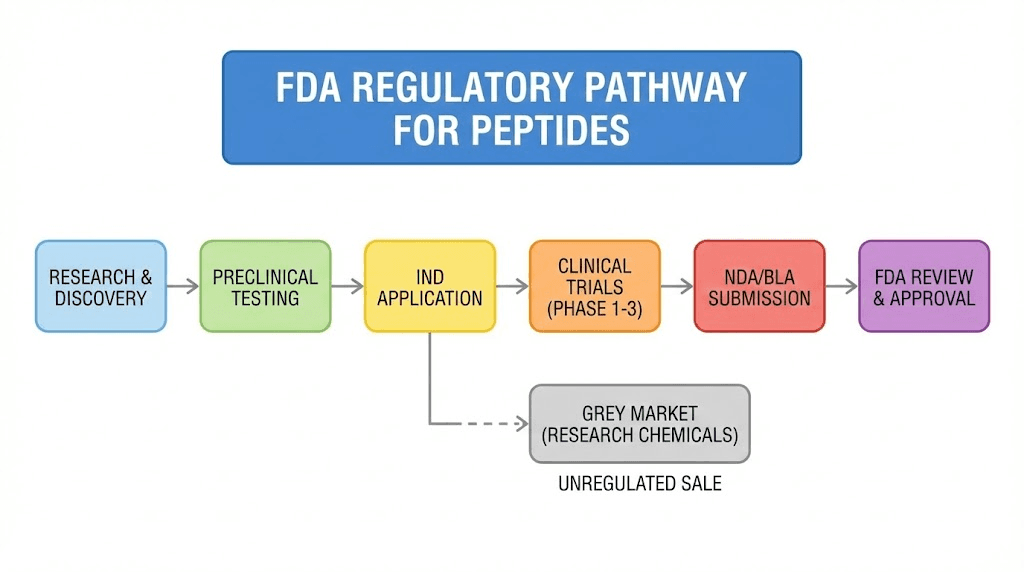
Understanding why grey market peptides exist requires understanding how the FDA regulates these compounds. According to the Federal Food, Drug, and Cosmetic Act, any substance intended to diagnose, cure, mitigate, treat, or prevent disease is classified as a drug. Drugs require FDA approval before they can be legally marketed for human use.
Most peptides have not completed this approval process. BPC-157 has never been approved for any human use. Neither has TB-500, GHK-Cu for injection, or most of the peptides popular in the biohacking community. They may have shown promise in animal studies or cell cultures. They have not demonstrated safety and efficacy in controlled human trials meeting FDA standards.
The FDA maintains something called the Bulk Drug Substances List under Section 503A of the FDCA. This list determines which compounds can be used in pharmacy compounding. Category 1 substances can be compounded for human use under certain conditions. Category 2 substances cannot. Many popular peptides, including BPC-157, KPV, Semax, and others, fall into Category 2 or have not been evaluated at all.
Why people turn to the grey market
Despite the risks, grey market peptides attract millions of users worldwide. Understanding why requires examining the factors that drive people away from conventional medical channels and toward unregulated alternatives.
Cost considerations
Pharmaceutical peptides carry substantial price tags. Brand-name semaglutide can cost over a thousand dollars per month without insurance. Even compounded versions from licensed pharmacies often run several hundred dollars. Grey market alternatives might cost a fraction of that, sometimes under fifty dollars for the same quantity of peptide.
This price differential creates powerful incentives. Someone who cannot afford traditional peptide therapy but believes it could help them faces a difficult choice. Pay prices they cannot sustain, go without, or turn to grey market sources. Many choose the third option.
The economics make sense from a supply chain perspective. Grey market vendors do not conduct clinical trials. They do not maintain FDA-compliant manufacturing facilities. They do not employ quality assurance teams or regulatory compliance officers. These cost savings get passed to consumers in the form of lower prices. What also gets passed to consumers is all the risk those safeguards were designed to prevent.
Access barriers
Some peptides simply are not available through legitimate channels at any price. BPC-157 cannot be legally prescribed in the United States because it has never received FDA approval. A physician who wants to help a patient with this compound has no legal pathway to do so. The grey market becomes the only option.
This creates an unusual situation where substances showing genuine therapeutic promise in research remain inaccessible through official medicine while being readily available online. Researchers have studied BPC-157 for wound healing, gut health, and tissue repair for decades. The compound shows remarkable effects in animal models.
Yet someone seeking these benefits must either wait indefinitely for FDA approval or venture into grey market territory.
Access issues extend beyond approval status. Geographic restrictions, insurance limitations, and physician reluctance all create barriers. Someone in a rural area might not have access to a peptide therapy clinic. Someone with inadequate insurance might not be able to afford prescriptions. Someone whose doctor dismisses peptide therapy as experimental might have nowhere else to turn.
Autonomy and self-experimentation
A significant portion of grey market users are not seeking to circumvent the medical system out of necessity. They genuinely prefer the autonomy of managing their own health decisions. The biohacking movement has embraced this philosophy, viewing the body as a system to be optimized through careful experimentation and data collection.
This perspective values personal research, community knowledge sharing, and individual choice over institutional gatekeeping. Forums and communities dedicated to peptide use share protocols, results, and warnings in ways that traditional medicine does not. Users feel empowered rather than dependent.
Whether this approach produces better outcomes is debatable. What is not debatable is that it attracts people who value control over their own bodies and are willing to accept risk in exchange for that control.
The real risks of grey market peptides
Understanding grey market risks requires moving beyond vague warnings about unregulated products. Specific hazards exist, each with distinct mechanisms and potential consequences. Some of these risks can be mitigated through careful sourcing and testing. Others cannot.
Purity and contamination
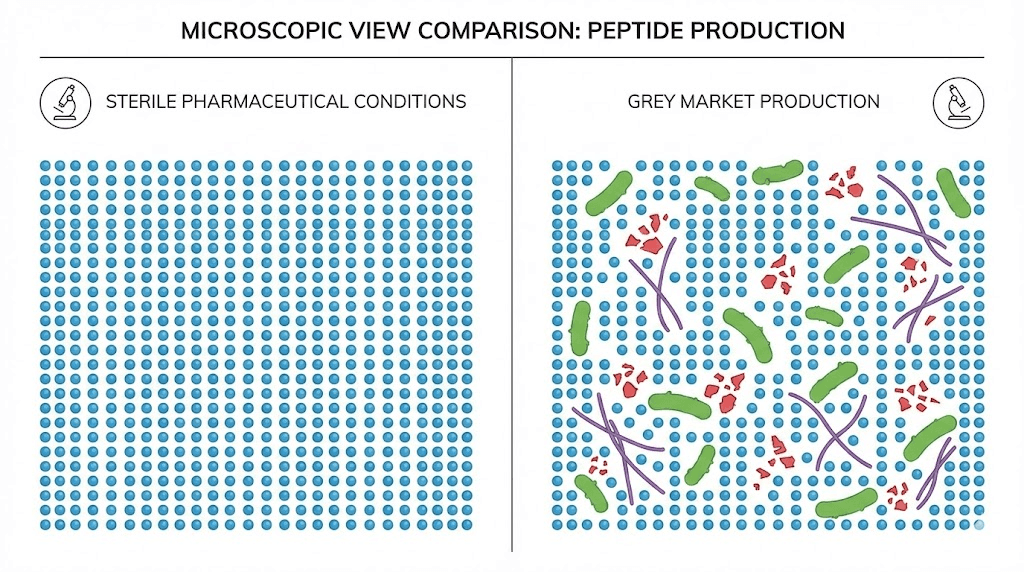
When a pharmaceutical company produces a peptide, they must demonstrate that it contains what the label claims, at the concentration specified, without harmful contaminants. Grey market products face no such requirements.
A vial labeled as containing 10mg of BPC-157 might contain 10mg. It might contain 2mg. It might contain 15mg. It might contain a different peptide entirely. Studies analyzing grey market peptides have found alarming discrepancies between claimed and actual contents. Some samples contained no detectable target peptide at all.
Chemical impurities present additional concerns. Peptide synthesis produces byproducts including truncated sequences, deletion variants, and racemic impurities. These contaminants can interfere with research outcomes at best and produce adverse biological effects at worst. Pharmaceutical-grade peptides undergo purification processes to remove these impurities. Grey market products may or may not.
The 99 percent purity claim that appears on many grey market products deserves scrutiny. This number typically comes from HPLC analysis, which measures the proportion of the target molecule relative to other peptide sequences. It says nothing about non-peptide contaminants like residual solvents, heavy metals, or bacterial endotoxins. A product can be 99 percent pure by HPLC and still contain dangerous levels of other substances.
Sterility failures
Injectable peptides must be sterile. Injecting non-sterile material into your body introduces bacteria directly into tissues, bypassing all the natural barriers that normally protect against infection. The consequences range from localized abscess to systemic sepsis.
Grey market peptides often arrive as lyophilized powder requiring reconstitution before use. The powder itself may or may not have been produced under sterile conditions. Even if it was, the reconstitution process introduces opportunities for contamination. Using non-sterile water, reusing syringes, or improper technique can turn a sterile product into a bacterial culture.
Some users employ 0.22 micron syringe filters to sterilize their reconstituted peptides. This removes bacteria and fungi but does not address endotoxins, the lipopolysaccharide fragments released by gram-negative bacteria. Endotoxins are heat stable and can survive standard sterilization processes. They trigger powerful immune responses including fever, inflammation, and in severe cases, septic shock.
Pharmaceutical manufacturing facilities test specifically for endotoxin levels using sensitive assays. Grey market products rarely undergo such testing. You cannot see endotoxins, cannot filter them out with standard equipment.
You have no way of knowing whether they are present until symptoms develop.
Dosing uncertainty
Even if a grey market peptide contains the correct compound at stated purity, dosing remains problematic. Calculating accurate peptide dosages requires knowing exactly how much active compound is in each vial. If the labeled quantity is inaccurate, every dose you calculate will be wrong.
Consider the implications. You purchase what is labeled as 5mg of BPC-157. You reconstitute it in 2ml of bacteriostatic water, planning to inject 250mcg per dose, which should give you 20 doses. But the vial actually contains 3mg. Every injection delivers 150mcg instead of 250mcg, sixty percent of your intended dose. Your results suffer and you have no idea why.
The reverse scenario presents equal problems. That same vial might contain 8mg instead of 5mg. Now every injection delivers 400mcg, significantly more than intended. With peptides where dosing matters, like tirzepatide or retatrutide, overdosing can produce serious side effects.
SeekPeptides provides dosage calculators and reconstitution tools that help users determine accurate doses based on labeled contents. But these tools assume the label is truthful. When dealing with grey market products, that assumption may not hold.
Legal and professional risks
While purchasing grey market peptides is generally legal, using them can create legal exposure in certain contexts. Athletes face sanctions for using substances prohibited by WADA or other governing bodies. Many peptides including growth hormone releasing peptides appear on banned substance lists regardless of their regulatory status.
Healthcare professionals risk their licenses by using or recommending grey market products. Recent enforcement actions have targeted medical spas and wellness clinics that administer peptides obtained from non-pharmacy sources. Practitioners have faced license suspension and civil penalties.
Employers conducting drug testing may question the presence of certain compounds. While most peptides do not appear on standard drug panels, questions could arise in occupations requiring medical clearance or security investigations. The RUO label offers no protection in these situations.
Understanding peptide quality testing
If you choose to use grey market peptides, understanding how quality is assessed, and the limitations of that assessment, becomes essential. Testing can reduce certain risks while doing nothing about others.
HPLC analysis explained
High Performance Liquid Chromatography, or HPLC, serves as the primary tool for assessing peptide purity. The technique separates compounds based on their chemical properties, producing a chromatogram that shows peaks corresponding to different molecular species.
The main peak represents your target peptide. Smaller peaks represent impurities like truncated sequences or synthesis byproducts.
Purity is calculated by comparing the area under the main peak to total peak area. A 98 percent purity means 98 percent of detected material corresponds to the intended molecule.
What HPLC does not tell you is equally important. It cannot identify what molecule produces each peak, only that different molecules are present. It does not detect substances that do not absorb at the wavelength being monitored. It says nothing about sterility, endotoxin levels, or non-peptide contaminants.
When evaluating a Certificate of Analysis, look for HPLC purity above 95 percent for research applications and above 98 percent for anything approaching pharmaceutical quality. But remember that this number represents only one dimension of quality.
Mass spectrometry confirmation
Mass spectrometry, often abbreviated MS or written as LCMS when combined with liquid chromatography, provides identity confirmation that HPLC cannot. By measuring the mass-to-charge ratio of molecules, mass spec confirms that the compound in your vial matches the expected molecular weight of the target peptide.
This addresses one of the most significant grey market risks: receiving something other than what you ordered. A mass spec report showing the correct molecular weight confirms identity in a way that purity testing alone cannot. If you ordered BPC-157 and the mass spec shows a 1419 Da molecular weight, you have the right compound.
Reputable grey market vendors provide both HPLC and mass spec data. The combination tells you that the product contains the claimed molecule at the stated purity.
What it still does not tell you is whether the product is sterile, free from endotoxins, or accurately dosed.
Third-party testing services
The grey market has developed its own quality assurance ecosystem. Testing services like Janoshik and others offer independent verification of peptide products. Users can submit samples from their purchases and receive objective data about identity and purity.
Community testing platforms aggregate results across vendors and products, creating crowdsourced quality data. These platforms identify consistently high-quality sources and flag vendors selling substandard products. They represent the grey market's attempt at self-regulation in the absence of official oversight.
Using these services costs money and adds friction to the purchasing process. Many users skip testing and simply trust vendor claims or community reputation. This works until it does not. The testing infrastructure exists for those willing to use it.
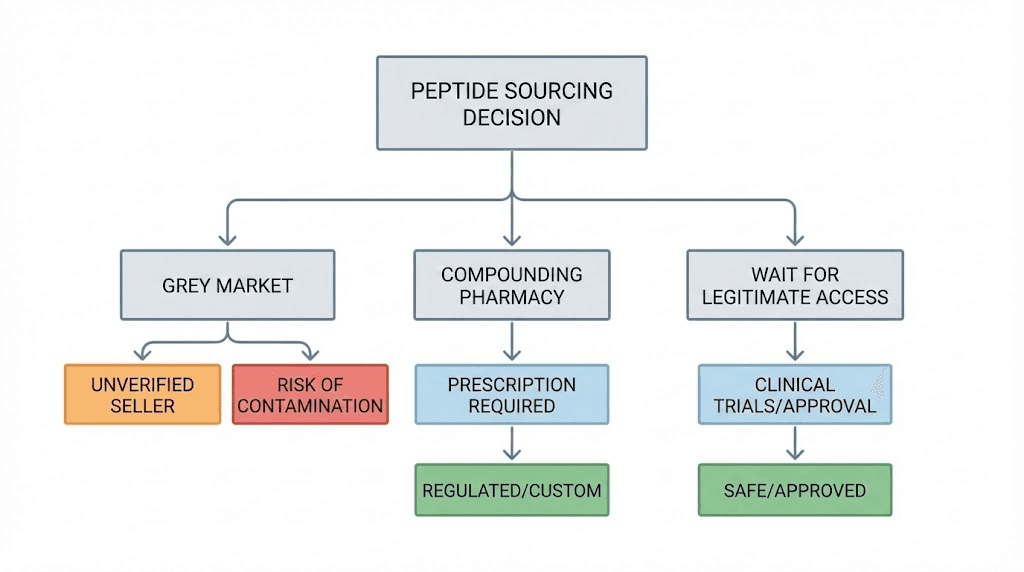
Grey market versus compounding pharmacies
Understanding the alternative to grey market sourcing clarifies what you gain and lose with each approach. Compounding pharmacies represent the regulated middle ground between FDA-approved pharmaceuticals and grey market research chemicals.
What compounding pharmacies offer
Licensed compounding pharmacies operate under FDA and state board of pharmacy oversight. They must follow USP Chapter 797 standards for sterile compounding, maintain proper facilities, and submit to regular inspections. When problems occur, accountability exists.
A 503A pharmacy compounds patient-specific medications based on individual prescriptions. A 503B outsourcing facility can produce larger batches for office use by healthcare providers. Both operate under regulatory frameworks designed to ensure quality and safety.
Compounded peptides from licensed pharmacies come with certificates of analysis documenting potency and sterility testing.
They are produced in environments designed to prevent contamination and are subject to recall if problems are discovered. None of this is true for grey market products.
Limitations of compounding
Compounding pharmacies face significant limitations on which peptides they can produce. The FDA Bulk Drug Substances List restricts compounding to approved substances. Many popular peptides fall outside this list. A compounding pharmacy cannot legally produce BPC-157, no matter how much demand exists.
Compounded medications also require prescriptions. You need a physician willing to prescribe the peptide, which in turn requires a medical justification. Not everyone has access to such physicians. Not every physician is willing to prescribe peptide therapies.
Cost remains a factor. Compounded peptides typically cost more than grey market alternatives, though less than brand-name pharmaceuticals. The regulatory overhead adds expense that grey market vendors avoid.
Making the choice
If a compounded version of the peptide you want exists and you can obtain a prescription, using a licensed pharmacy reduces risk substantially. You gain quality assurance, sterility guarantees, and accurate dosing. You lose the cost savings and access advantages of grey market sourcing.
If the peptide you want cannot be legally compounded, the choice becomes more complicated. You can accept that you cannot access it safely. You can pursue grey market sources while taking whatever risk mitigation steps are available. Or you can wait and hope that regulatory landscapes change.
There is no universally correct answer. Different people with different risk tolerances and different needs will make different choices. What matters is that the choice is informed.
How to reduce grey market risks
If you decide to use grey market peptides despite the risks, certain practices can reduce but not eliminate those risks. Think of this as harm reduction rather than risk elimination. The safest approach remains not using unregulated injectables. These guidelines assume you have already made a different choice.
Vendor selection
All grey market vendors are not equal.
Some maintain reasonable quality standards despite lacking regulatory oversight. Others sell whatever they can source at the lowest possible cost. Distinguishing between them requires research.
Look for vendors who provide third-party testing results, not just internal certificates of analysis. Examine the testing documentation for consistency and detail. Vendors who post individual batch results demonstrate more commitment to quality than those who recycle the same generic COA for every product.
Community forums dedicated to peptide use maintain vendor reputation threads. Users share testing results, experiences, and warnings. These sources provide real-world quality data that no amount of vendor marketing can match. Spend time in these communities before making purchases.
Be suspicious of prices that seem too good to be true. Peptide synthesis has real costs. A vendor dramatically undercutting market prices is either losing money, using inferior materials, or misrepresenting their products. Sometimes all three.
Testing your purchases
Submit samples to independent testing services before using any grey market peptide. Yes, this costs money. Yes, it delays use. The alternative is injecting substances of unknown composition into your body.
At minimum, request identity confirmation through mass spectrometry. This ensures you received the compound you ordered. Purity testing via HPLC provides additional confidence about contamination levels.
Endotoxin testing is available but rarely performed by individual users due to cost and complexity. If you use grey market peptides regularly, understanding this limitation is important. You cannot test for endotoxins practically, which means you cannot verify their absence.
Proper handling and reconstitution
Proper reconstitution technique can prevent contamination during preparation. Use only bacteriostatic water or sterile water for injection, never tap water or non-sterile saline. Work in a clean environment. Do not touch syringe tips or vial stoppers with bare hands.
Store reconstituted peptides properly. Most require refrigeration and lose potency over time. Using a peptide that has degraded due to improper storage introduces another source of dosing uncertainty.
Never reuse syringes or needles. Never share vials between users. These basic sterile practices prevent contamination regardless of product quality.
Starting conservatively
When trying a new batch or new vendor, start with lower doses than you might otherwise use. This limits exposure if something is wrong with the product. Observe carefully for any unexpected reactions before proceeding to full doses.
Keep records of what you use, when, and from which source.
If problems develop, this information helps identify the cause. It also allows you to track which vendors and batches work well.
Specific peptide categories and their grey market status
Different peptide categories present different risk profiles and availability considerations. Understanding where specific compounds fit helps inform sourcing decisions.
Healing and recovery peptides
Peptides like BPC-157 and TB-500 remain unavailable through legitimate channels despite years of research interest. Both show remarkable healing effects in animal studies. Neither has completed human trials meeting FDA standards.
Grey market versions of these compounds vary widely in quality. The relatively simple structure of BPC-157 makes synthesis straightforward, but quality control during production still matters. TB-500, being derived from thymosin beta-4, presents more complex manufacturing challenges.
Users of healing peptides often stack these compounds for synergistic effects. When sourcing multiple peptides, quality concerns multiply. Each additional grey market product adds its own risk profile.
Weight management peptides
The explosion of interest in GLP-1 agonists like semaglutide and tirzepatide has created enormous grey market demand. These peptides exist as FDA-approved medications, but supply shortages and high costs drive many toward unregulated alternatives.
Grey market semaglutide carries particular risks because dosing is critical. Unlike some peptides where imprecise dosing causes minimal problems, GLP-1 agonists produce dose-dependent gastrointestinal side effects.
Taking more than intended can cause severe nausea, vomiting, and in rare cases, pancreatitis.
Compounded semaglutide remains available through licensed pharmacies during shortage periods. For most users, this represents a better risk-benefit trade-off than grey market sources, even at higher cost.
Growth hormone secretagogues
Peptides like Ipamorelin, CJC-1295, and similar growth hormone releasing peptides have long grey market histories. These compounds stimulate natural growth hormone production and have attracted users interested in anti-aging, muscle building, and recovery enhancement.
The category is well-established in grey market channels with relatively consistent quality from reputable sources. That said, no grey market source eliminates the fundamental risks of unregulated injectable products.
SeekPeptides provides detailed information on stacking these peptides and understanding their mechanisms of action. Education helps users make informed decisions regardless of sourcing choices.
Cognitive and nootropic peptides
Semax, Selank, and related nootropic peptides occupy an interesting niche. Some are approved medications in Russia and other countries, making them grey market in the US despite established pharmaceutical use elsewhere.
These peptides are often administered intranasally rather than by injection, which eliminates some sterility concerns while introducing others. Nasal spray formulations still require proper preparation and storage.
The cognitive peptide space attracts users interested in memory enhancement, focus improvement, and anxiety reduction. The grey market serves this demand in the absence of approved alternatives.
The future of peptide regulation
Regulatory landscapes evolve. Understanding where peptide policy might be heading helps contextualize current grey market dynamics and informs long-term planning.
FDA enforcement trends
Recent years have seen increased FDA scrutiny of peptide compounding. The agency has placed several popular peptides on its Category 2 list, effectively banning their use in compounding. This removes legitimate access to compounds that were previously available through licensed pharmacies.
Warning letters to companies marketing peptides for human use have increased. The FDA has signaled that it views the current grey market situation as problematic and is taking steps to address it. Whether these efforts reduce grey market activity or simply push it further underground remains to be seen.
State-level actions
States have begun their own enforcement efforts against grey market peptide use, particularly in medical settings. Clinics and spas administering peptides from non-pharmacy sources face license actions and civil penalties. This creates risk for practitioners while doing little to affect individual consumers.
Some states have considered legislation that would more clearly define peptide regulatory status. These efforts have produced mixed results.
The patchwork of state and federal regulations creates confusion that the grey market exploits.
Research and approval pathways
Clinical trials for peptides like BPC-157 continue in various countries. Positive trial results could eventually lead to FDA approval and legitimate access. This process takes years or decades and offers no guarantees.
Some advocates push for expedited approval pathways for peptides with strong safety profiles and established research bases. Whether such pathways emerge depends on political will and regulatory philosophy that can shift with administrations.
For now, many promising peptides remain in regulatory limbo, too interesting to ignore but lacking the clinical data required for approval. The grey market fills this gap, for better and worse.
Making informed decisions
Grey market peptides present a genuine dilemma. They offer access to compounds that may provide real benefits. They also carry risks that legitimate pharmaceuticals do not.
No one can make this choice for you. What we can do is ensure you understand what you are choosing.
Questions to ask yourself
Before purchasing grey market peptides, consider honestly whether the potential benefit justifies the risk. Is there a legitimate alternative available? Have you exhausted conventional options? Are you comfortable with the level of uncertainty involved?
Consider your personal risk factors. Immunocompromised individuals face greater danger from potential contamination. Those with existing health conditions may react differently to impure products. Athletes risk career-ending sanctions. Healthcare workers risk professional licenses.
Think about your ability to mitigate risks. Can you afford third-party testing? Do you have the knowledge to practice proper sterile technique? Will you actually start conservatively and monitor carefully, or are you likely to skip these steps?
The role of education
Whatever choice you make, education improves outcomes. Understanding peptide safety, proper dosing protocols, and realistic timeline expectations helps whether you use pharmaceutical-grade products or grey market alternatives.
SeekPeptides exists to provide this education. Our guides for beginners, detailed dosage charts, and common mistake warnings help users of all experience levels make better decisions. Knowledge does not eliminate risk. It does help you manage it.
When to walk away
Sometimes the right choice is to forgo a peptide rather than source it from grey market channels. If testing is unavailable or unaffordable, if vendor reputation is unclear, if the specific compound has known quality control issues, walking away may be wiser than proceeding.
Patience has value. Regulatory situations change. Research progresses. Compounding options expand. A peptide unavailable today through legitimate channels may become accessible tomorrow. Waiting costs you time. It may save you from worse outcomes.
Frequently asked questions
Are grey market peptides legal to buy?
In most jurisdictions, purchasing peptides labeled for research use only is legal. The compounds themselves are not controlled substances. However, using them in ways inconsistent with their labeling, meaning human consumption, is your responsibility. The legal grey zone protects vendors more than consumers.
You assume all risk once the package arrives.
How can I tell if a grey market peptide is safe?
You cannot definitively determine safety without comprehensive testing that most individuals cannot perform or afford. Identity and purity testing through third-party labs provides some assurance. Sterility and endotoxin levels remain difficult to verify. The honest answer is that grey market peptides carry inherent uncertainty that cannot be fully resolved.
What is the difference between grey market and research peptides?
These terms are often used interchangeably. Research peptides refers to compounds sold for laboratory use, which typically means they are grey market products not approved for human consumption. Some vendors prefer the term research peptides because it sounds more legitimate. The regulatory status and risks are identical.
Why are some peptides only available on the grey market?
FDA approval requires extensive clinical trials demonstrating safety and efficacy, costing hundreds of millions of dollars. Most peptides have not gone through this process because companies have not invested in trials. Some peptides are also specifically placed on FDA lists prohibiting their use in compounding. This leaves grey market sources as the only access point for these compounds.
Can my doctor prescribe grey market peptides?
Physicians can only prescribe FDA-approved medications or compounds available through licensed compounding pharmacies. Grey market peptides are neither. A physician prescribing grey market products would face serious legal and professional consequences.
If your doctor suggests obtaining peptides from grey market sources, this raises significant concerns about their judgment.
How do grey market peptide prices compare to pharmaceutical versions?
Grey market peptides typically cost a fraction of pharmaceutical equivalents, often 50 to 90 percent less. This price difference reflects the absence of regulatory compliance costs, quality assurance overhead, and liability insurance. The savings come with corresponding increases in risk that consumers must evaluate for themselves.
What should I do if I have a bad reaction to a grey market peptide?
Seek medical attention immediately for serious reactions. Be honest with healthcare providers about what you used, including the source. Retain the product and any documentation for potential testing. Report the issue to community platforms to warn others. Accept that legal recourse against vendors is essentially nonexistent.
Are there any grey market peptides that are safer than others?
Risk profiles vary by compound, synthesis complexity, and stability. Simple peptides with established synthesis protocols tend to have more consistent quality than complex molecules. Peptides requiring cold chain shipping present more opportunities for degradation. However, all grey market products share the fundamental risks of unregulated manufacturing and distribution.
External resources
In case I do not see you, good afternoon, good evening, and good night. May your sourcing stay informed, your vials stay sterile, and your decisions stay clear-eyed.



