Feb 5, 2026
Retatrutide and semaglutide look nearly identical on paper. Both are injectable peptides. Both target weight loss. Both emerged from the same revolution in metabolic medicine that has reshaped how researchers and clinicians think about obesity. But in practice, these two compounds produce completely different outcomes, work through fundamentally different mechanisms, and carry very different risk profiles.
That distinction matters more than most people realize.
Semaglutide arrived first. It earned FDA approval, generated billions in revenue, and became the most talked-about weight management compound in modern history. Millions of people now use it. The clinical data behind it is extensive, well-replicated, and remarkably consistent across multiple large-scale trials. For a peptide-based therapy, that track record is exceptional. But semaglutide works through a single pathway, the GLP-1 receptor, and that single-pathway approach comes with inherent limitations in how much weight it can help someone lose and what metabolic benefits it can deliver beyond appetite suppression.
Retatrutide changes the equation entirely. Instead of activating one receptor, it activates three: GLP-1, GIP, and glucagon. That triple-agonist approach is not just an incremental improvement. It represents a fundamentally different pharmacological strategy, one that early clinical data suggests could nearly double the weight loss seen with semaglutide while simultaneously delivering dramatic improvements in liver fat, metabolic markers, and body composition. The Phase 3 TRIUMPH program is ongoing, and the results so far have stunned even experienced researchers in the field.
This guide breaks down every meaningful difference between retatrutide and semaglutide. Mechanisms, clinical trial results, side effect profiles, dosing protocols, regulatory status, and practical considerations for anyone trying to understand which compound might better align with their goals. The comparison is not as simple as "newer is better." Each compound has genuine advantages the other lacks. Understanding those trade-offs requires looking at the actual data, not marketing materials or social media hype.
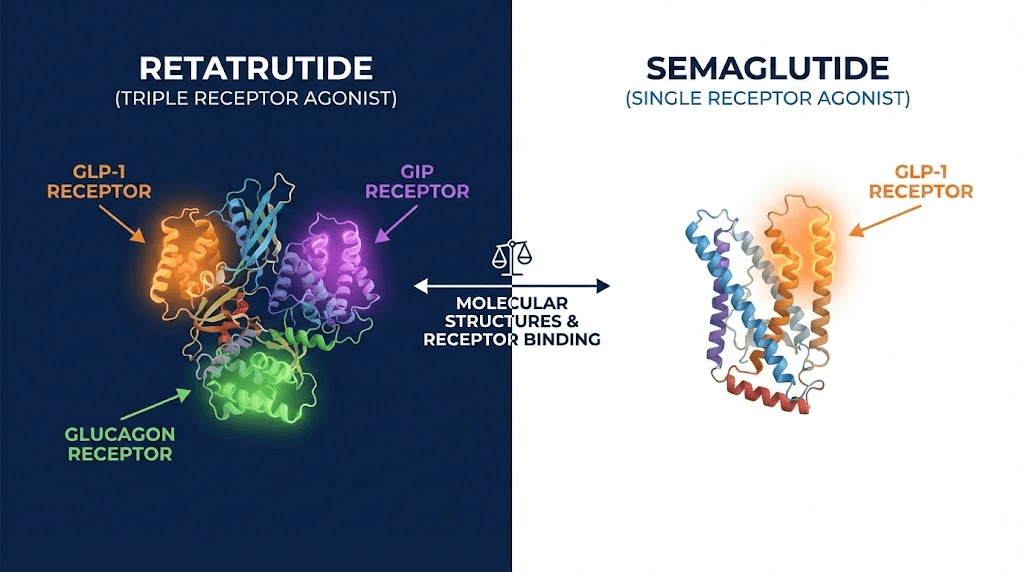
How retatrutide and semaglutide actually work
The most important difference between these two compounds is not their results. It is their mechanism of action. Understanding how each one works at the receptor level explains why their outcomes diverge so dramatically in clinical trials and why choosing between them requires more than just comparing weight loss percentages.
Semaglutide: the single-receptor approach
Semaglutide is a GLP-1 receptor agonist. That means it mimics a natural hormone called glucagon-like peptide-1, which your body produces in the gut after eating. When GLP-1 binds to its receptor, several things happen simultaneously. The stomach empties more slowly. The brain receives stronger satiety signals. Insulin secretion increases in response to glucose. Glucagon release decreases. The net effect is powerful appetite suppression combined with improved blood sugar regulation.
The compound itself is a modified version of the natural GLP-1 molecule. Researchers engineered it to resist degradation by DPP-4 enzymes, which normally break down natural GLP-1 within minutes. This modification gives semaglutide a half-life of approximately seven days, making once-weekly dosing possible. That convenience factor has been a significant driver of adoption. Anyone exploring how peptides work at the molecular level through resources like SeekPeptides will recognize this as a clever pharmacological solution to a common problem with peptide-based compounds.
But single-receptor activation has limits. GLP-1 primarily affects appetite and glucose metabolism. It does not directly increase energy expenditure. It does not directly target fat oxidation. It does not directly address the complex metabolic dysfunction that accompanies severe obesity. The weight loss it produces, while clinically significant, comes almost entirely from reduced caloric intake rather than from metabolic changes that increase how many calories the body burns at rest.
For many people considering peptides for weight loss, that single-pathway limitation is the key consideration. Semaglutide works well, but it may not work well enough for individuals with severe metabolic dysfunction or those seeking outcomes beyond simple appetite reduction.
Retatrutide: the triple-agonist revolution
Retatrutide takes an entirely different approach. Built on a 39-amino acid GIP backbone, it simultaneously activates three distinct receptor systems: GLP-1, GIP, and glucagon receptors. Each receptor contributes something unique to the overall metabolic effect, and their combined activation produces outcomes that no single-receptor agonist can match.
The GLP-1 component does what semaglutide does. Appetite suppression. Slower gastric emptying. Improved insulin sensitivity. Those benefits carry over directly.
The GIP component adds something semaglutide lacks entirely. Glucose-dependent insulinotropic polypeptide plays a complex role in metabolism. It enhances insulin secretion, yes, but it also appears to improve fat tissue function, reduce inflammation in adipose tissue, and potentially improve how the body partitions nutrients between fat storage and energy use. Tirzepatide already demonstrated the power of adding GIP agonism to GLP-1, and retatrutide builds on that foundation.
The glucagon component is what truly sets retatrutide apart from everything else in the pipeline. Glucagon is traditionally thought of as a "counter-regulatory" hormone that raises blood sugar. But glucagon receptor activation does far more than that. It directly increases energy expenditure. It stimulates hepatic fat oxidation, meaning it tells the liver to burn stored fat for fuel. It increases thermogenesis. It promotes amino acid metabolism in ways that may help preserve lean mass during weight loss. The inclusion of glucagon agonism is why retatrutide produces such dramatically different outcomes in liver fat reduction compared to GLP-1 only compounds.
Understanding this triple mechanism is essential for anyone researching the best peptides for weight loss. Retatrutide does not just make you eat less. It fundamentally alters how the body processes and stores energy at multiple points in the metabolic cascade. That is a qualitative difference, not just a quantitative one.
Why three receptors outperform one
Think of it this way. Semaglutide turns down the appetite dial. Retatrutide turns down the appetite dial while simultaneously turning up the metabolic furnace and telling the liver to empty its fat stores. The three pathways create synergistic effects that compound over time. Reduced intake plus increased expenditure plus direct fat mobilization equals dramatically greater total fat loss.
This is not theoretical speculation. The clinical trial data confirms it, as we will examine in detail in the next section. And for those exploring the broader landscape of peptides for fat loss, understanding receptor pharmacology is not just academic. It directly predicts which compounds will work best for which goals.
Weight loss results head to head
Numbers do not lie. And the numbers from clinical trials comparing these two compounds tell a story that is impossible to ignore. Let us look at the actual data from the most rigorous studies available for each compound, then put them side by side so the differences become unmistakable.
Semaglutide clinical trial results
The STEP program, a series of large-scale Phase 3 trials, established the weight loss efficacy of semaglutide at the 2.4 mg dose. These are the landmark results that led to FDA approval.
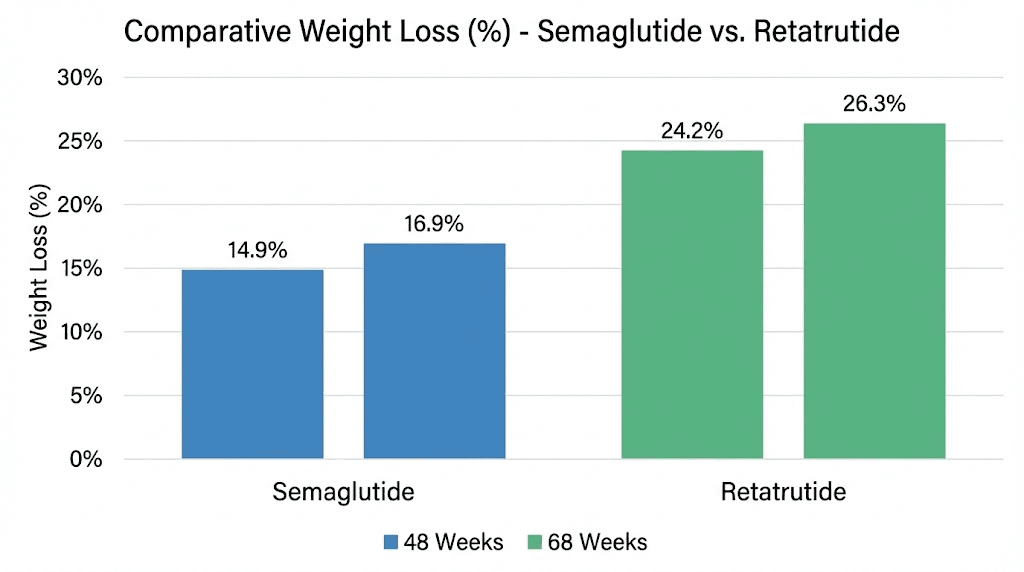
In the STEP 1 trial, participants receiving semaglutide 2.4 mg lost an average of 14.9% of their body weight over 68 weeks. That result was groundbreaking at the time. No other pharmaceutical intervention had consistently produced double-digit percentage weight loss in a large clinical population. For context, the placebo group in the same trial lost only 2.4%, making the drug-attributable weight loss approximately 12.5 percentage points.
The STEP 5 trial extended the observation period. Over 104 weeks of treatment, semaglutide produced average weight loss of 15.2%. That number is important because it shows the effect largely plateaus. Going from 68 weeks to 104 weeks added only 0.3 percentage points of additional weight loss. Most of the benefit arrives within the first year of treatment.
These results are impressive and well-documented. Anyone using a semaglutide dosage calculator to plan their protocol should understand that 14-15% body weight reduction represents the realistic ceiling for most users at the maximum approved dose. Individual results vary, of course. Some people lose 20% or more. Others lose 8-10%. But the population average centers around that 15% mark.
For someone weighing 220 pounds, 15% weight loss means losing approximately 33 pounds over about 16 months. Significant and meaningful, but perhaps not transformative for someone who needs to lose 80 or 100 pounds to reach a healthy weight.
Retatrutide clinical trial results
The retatrutide data comes from the Phase 2 trial published in the New England Journal of Medicine and the ongoing Phase 3 TRIUMPH program. The numbers are, frankly, extraordinary.
At the 12 mg dose over 48 weeks, participants lost an average of 24.2% of their body weight. That is already 60% more weight loss than semaglutide produces in a similar timeframe. But the TRIUMPH-4 Phase 3 data pushed those numbers even further. At 12 mg over 68 weeks, weight loss reached 28.7%. The 9 mg dose produced 26.4% at the same timepoint.
Read those numbers again. 28.7% average body weight loss. For that same 220-pound person, that means losing approximately 63 pounds, nearly double what semaglutide delivers. And unlike semaglutide, the weight loss trajectory with retatrutide did not appear to plateau at 48 weeks. It continued accelerating between 48 and 68 weeks, suggesting the full effect may not yet have been reached even at the trial endpoints.
For researchers following the retatrutide dosage chart and titration protocols used in these trials, the dose-response relationship is remarkably clear. Higher doses produce greater weight loss, with the 12 mg dose consistently outperforming the 8 mg and 4 mg groups. The retatrutide 20 mg dosing guide explores even higher dose ranges that some research protocols have investigated.
Direct comparison table
Metric | Semaglutide 2.4 mg | Retatrutide 12 mg |
|---|---|---|
Weight loss at 48 weeks | ~12-13% | 24.2% |
Weight loss at 68 weeks | 14.9% (STEP 1) | 28.7% (TRIUMPH-4) |
Weight loss at 104 weeks | 15.2% (STEP 5) | Data pending |
Mechanism | GLP-1 only | GLP-1 + GIP + Glucagon |
Receptor targets | 1 | 3 |
Dosing frequency | Once weekly | Once weekly |
FDA approved | Yes (Wegovy) | Not yet (Phase 3) |
Placebo-adjusted loss | ~12.5% | ~25% |
The gap is not subtle. It is not a marginal improvement. Retatrutide approximately doubles the weight loss achieved with semaglutide across comparable timeframes and trial designs. For anyone building a peptide stack for weight loss or comparing options, this magnitude of difference is unprecedented in the history of obesity pharmacotherapy.
What the percentages actually mean in real terms
Percentages can feel abstract. Let us translate them into practical outcomes for different starting weights.
A person starting at 250 pounds on semaglutide 2.4 mg can expect to lose roughly 37 pounds over 68 weeks, ending at approximately 213 pounds. That same person on retatrutide 12 mg would lose approximately 72 pounds, ending at roughly 178 pounds. The difference between those two endpoints, 213 versus 178, could be the difference between still meeting clinical criteria for obesity and reaching a normal BMI range.
At 300 pounds, semaglutide delivers roughly 45 pounds of loss (ending at 255), while retatrutide delivers roughly 86 pounds (ending at 214). Again, the practical implications are enormous. One keeps you well within the obese category. The other potentially moves you into the overweight range, a transition associated with massive reductions in mortality risk and disease burden.
These are averages, and individual responses vary significantly. Some people are exceptional responders to semaglutide and may achieve 20%+ loss. Some may respond less dramatically to retatrutide. But on a population level, the data is clear. Researchers tracking peptide dosage charts and outcomes across different compounds consistently see this pattern.
Rate of weight loss comparison
Retatrutide not only produces more total weight loss but appears to produce it faster. In the Phase 2 trial, participants on the 12 mg dose were still on a steep weight loss trajectory at 48 weeks, losing roughly 5-6 pounds per month even late in the treatment period. Semaglutide, by contrast, shows significant deceleration after 6-8 months, with monthly weight loss dropping to 1-2 pounds as patients approach the 12-15% plateau.
Speed matters for several reasons. Faster results improve adherence. Seeing consistent progress motivates people to maintain their treatment protocol. It also means reaching clinically meaningful thresholds, like a 10% or 20% reduction, sooner, which translates to earlier improvements in comorbidities like type 2 diabetes, hypertension, and sleep apnea. For anyone researching peptide calculations for weight loss, understanding these timelines helps set realistic expectations.
Side effects and safety comparison
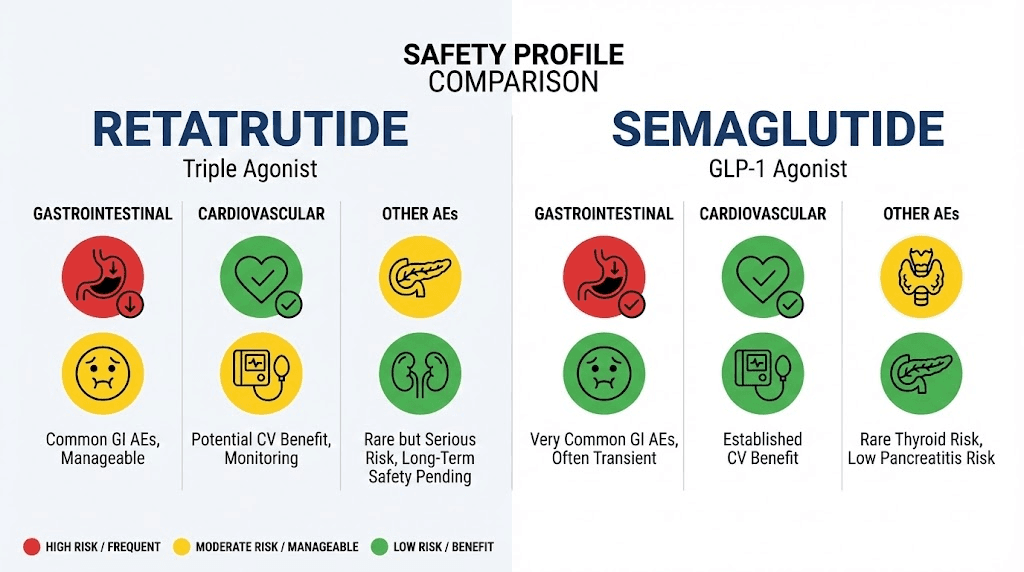
Greater efficacy often comes with greater side effects. That is a fundamental principle in pharmacology, and it applies here. Retatrutide produces more weight loss than semaglutide, and it also produces more adverse events. Understanding the specific nature and severity of those side effects is critical for making an informed decision.
Gastrointestinal side effects
Both compounds cause GI side effects. This is expected with any GLP-1 receptor agonist because slowing gastric emptying and suppressing appetite inevitably affects digestive function. But the frequency and intensity differ meaningfully between the two.
Semaglutide causes nausea in a significant percentage of users, particularly during the dose-escalation phase. Most studies report nausea rates of 40-45% at some point during treatment, though the severity is typically mild to moderate and tends to diminish over time as the body adjusts. Vomiting occurs in roughly 25% of participants. Diarrhea and constipation are also common. The slow titration schedule, starting at 0.25 mg and escalating gradually to 2.4 mg, is specifically designed to minimize these effects by giving the body time to adapt.
Retatrutide causes more GI distress, particularly at higher doses. At the 12 mg dose, nausea was reported by approximately 45% of participants. At 8 mg doses, the range was 17-60% depending on the specific trial and titration speed. These numbers are broadly comparable to semaglutide, but the intensity appears somewhat greater, likely because the glucagon component adds an additional source of gastrointestinal stimulation on top of the GLP-1 effects.
Knowing peptide safety and risks before beginning any protocol is not optional. It is essential. The GI side effects from both compounds are manageable for most people, but they can be severe enough to require dose reduction or treatment discontinuation in a meaningful minority.
Discontinuation rates tell the real story
Perhaps the most telling safety metric is not which side effects occur but how many people stop treatment because of them. Here, a significant difference emerges.
Semaglutide trials report discontinuation rates of approximately 5-7% due to adverse events. That is relatively low. It means 93-95% of people who start treatment can tolerate it well enough to continue, even if they experience some side effects along the way.
Retatrutide shows higher discontinuation rates. In the Phase 2 study, 12.2-18.2% of participants discontinued due to adverse events, depending on the dose group. That is roughly double to triple the discontinuation rate seen with semaglutide. For every 100 people who start retatrutide, somewhere between 12 and 18 will find the side effects intolerable enough to stop.
This gap has practical implications. If you are someone with a history of GI sensitivity or who has previously struggled with side effects from other medications, semaglutide may be the more tolerable option despite its lower efficacy ceiling. Tolerability is not a luxury. A compound you cannot continue taking produces zero benefit regardless of how impressive its trial data looks.
Side effect comparison table
Side effect | Semaglutide 2.4 mg | Retatrutide 12 mg |
|---|---|---|
Nausea | 40-45% | ~45% |
Vomiting | ~25% | ~25-30% |
Diarrhea | ~30% | ~25-35% |
Constipation | ~24% | ~20-25% |
Discontinuation (adverse events) | 5-7% | 12.2-18.2% |
Heart rate increase | Mild | Mild to moderate |
Injection site reactions | Mild | Mild |
Anyone reviewing common peptide mistakes beginners make will notice a consistent theme: underestimating side effects and starting at too high a dose. This applies doubly with potent compounds like retatrutide and semaglutide. Proper titration is not optional. It is the single most important factor in tolerability.
Serious adverse events
Both compounds have been associated with rare but serious adverse events in clinical trials. For semaglutide, the main concerns include pancreatitis (rare but documented), gallbladder events (including gallstones, particularly during rapid weight loss), and a theoretical concern about medullary thyroid carcinoma based on rodent studies. The SELECT cardiovascular outcomes trial actually demonstrated cardiovascular benefit rather than harm, which was reassuring.
For retatrutide, the safety database is smaller because the compound is still in Phase 3 trials. The serious adverse event profile appears broadly similar to other incretin-based therapies, but with the addition of glucagon-related effects. Glucagon receptor activation can increase heart rate slightly and may affect hepatic glucose output, which requires monitoring in diabetic populations. The long-term safety profile will not be fully established until larger and longer trials are completed.
This is an important consideration. Semaglutide has years of post-marketing safety data from millions of users. Retatrutide has data from thousands of trial participants over relatively short periods. For risk-averse individuals, that difference in safety data maturity is meaningful. Resources like the peptide research and studies compilation can help people stay current on emerging safety data.
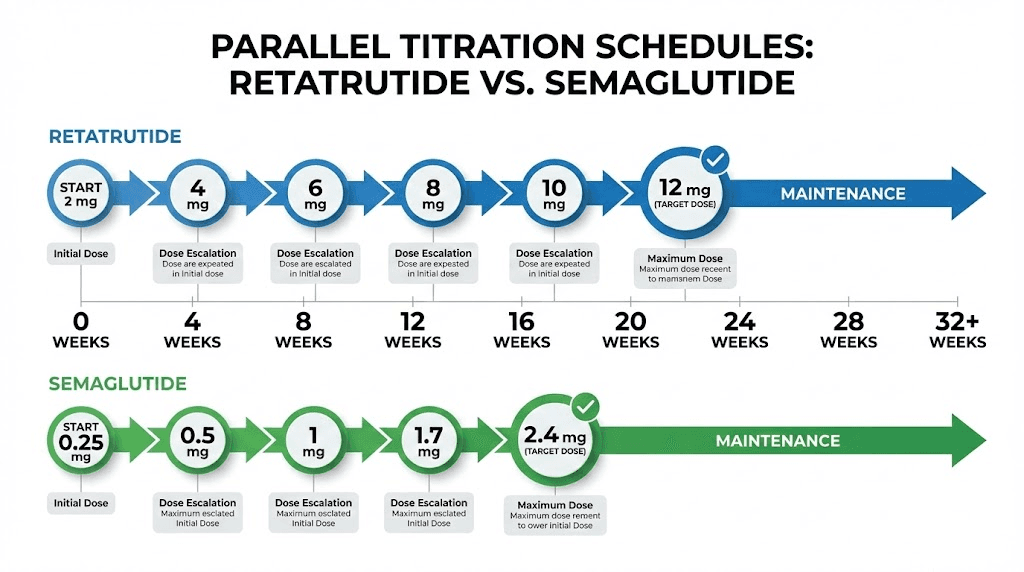
Dosing protocols and titration schedules
How you take a compound matters almost as much as which compound you take. Both retatrutide and semaglutide require careful dose titration to balance efficacy against tolerability. The protocols differ in important ways, and understanding those differences is essential for anyone planning a research protocol.
Semaglutide dosing protocol
The standard semaglutide titration for weight management follows a well-established 16-20 week escalation schedule:
Weeks 1-4: 0.25 mg once weekly
Weeks 5-8: 0.50 mg once weekly
Weeks 9-12: 1.0 mg once weekly
Weeks 13-16: 1.7 mg once weekly
Week 17 onward: 2.4 mg once weekly (maintenance dose)
This graduated approach serves two purposes. First, it allows the GI tract to adapt to the effects of GLP-1 receptor activation. Most nausea and vomiting occur during dose increases rather than at stable doses. Second, it allows clinicians to identify the minimum effective dose for each individual. Some people achieve satisfactory results at 1.0 or 1.7 mg and never need to escalate to the full 2.4 mg dose.
The semaglutide dosage calculator can help researchers plan these titration schedules and convert between dose formats. Proper dose calculation is especially important when working with research-grade materials where concentrations may differ from commercial preparations. Understanding how to calculate peptide dosages correctly prevents dangerous dosing errors.
Administration is straightforward. Semaglutide is injected subcutaneously once weekly, typically in the abdomen, thigh, or upper arm. The injection site should be rotated. The compound does not need to be administered at the same time each day, but maintaining a consistent weekly schedule improves adherence.
Retatrutide dosing protocol
Retatrutide uses a different titration approach, reflecting its greater potency and triple-receptor activity:
Weeks 1-4: 1 mg once weekly (starting dose)
Weeks 5-8: 2 mg once weekly
Weeks 9-12: 4 mg once weekly
Weeks 13-16: 8 mg once weekly
Week 17 onward: 8-12 mg once weekly (maintenance dose)
Some trial protocols use a slightly modified schedule, escalating every four weeks with dose increases of approximately double at each step. The retatrutide dosage chart provides detailed reference information for the various titration schedules used across different clinical trials.
The starting dose of 1 mg is higher in absolute terms than semaglutide starting dose, but the relative potency is comparable. Both start at a dose intended to be well below the therapeutic threshold, primarily serving as an acclimation period for the GI system. The escalation to 8-12 mg occurs over roughly the same 16-20 week period as semaglutide, though the magnitude of dose increases is proportionally larger.
For researchers working with retatrutide in a peptide research setting, proper reconstitution and storage are critical. The peptide reconstitution calculator helps ensure accurate dilution ratios, while guides on how to reconstitute peptides cover the technical process. Using the correct bacteriostatic water for peptides is equally important for maintaining compound stability and sterility.
Key dosing differences
Several practical differences stand out between the two protocols.
First, retatrutide requires larger injection volumes at maintenance doses. The 12 mg dose means injecting more solution per dose, which can cause more injection site discomfort compared to the relatively small volume needed for semaglutide 2.4 mg. Learning proper technique from a peptide injection pen guide or similar resource helps minimize this issue.
Second, the dose-response curve for retatrutide is steeper. Missing doses or inconsistent timing has a more pronounced effect on efficacy because the three-receptor system requires consistent activation to maintain its synergistic benefits. With semaglutide, the single receptor mechanism is somewhat more forgiving of timing variations.
Third, dose adjustments with retatrutide are more complex. Because three receptor systems are involved, reducing the dose to manage side effects may disproportionately affect one pathway more than others, leading to unpredictable changes in the benefit profile. With semaglutide, reducing the dose predictably reduces GLP-1 activity across the board.
Both compounds require proper storage. Resources on how to store peptides after reconstitution and how long peptides last in the fridge are relevant for anyone working with either compound in a research setting. Improper storage degrades potency and can introduce safety risks.
Switching between compounds
A question many researchers ask is whether someone can switch from semaglutide to retatrutide, or vice versa. This is not straightforward. The two compounds work through different receptor profiles, so there is no direct dose equivalence between them. Someone stable on semaglutide 2.4 mg cannot simply calculate an equivalent retatrutide dose and switch over.
The recommended approach in clinical settings is a washout period followed by a fresh titration from the starting dose of the new compound. This is conservative but safe, as it avoids the risk of excessive receptor activation that could occur if residual semaglutide activity overlaps with the initial doses of retatrutide. Planning a peptide cycle that accounts for transition periods is essential for anyone considering a switch.
Beyond weight loss: liver, cardiovascular, and metabolic effects
Weight loss is the headline number. It captures attention, drives media coverage, and dominates patient conversations. But reducing body weight is only one piece of a much larger metabolic puzzle. The most meaningful question is not just how much weight someone loses but what happens inside their body during and after that weight loss. On this front, retatrutide and semaglutide diverge in ways that may ultimately matter more than the scale number.
Liver fat reduction
This is where retatrutide data becomes truly remarkable.
Non-alcoholic fatty liver disease (NAFLD) affects an estimated 30% of the global population and is the most common cause of chronic liver disease. It progresses through stages, from simple steatosis (fat accumulation) to steatohepatitis (inflammation) to fibrosis and eventually cirrhosis. The amount of fat in the liver is the key modifiable risk factor at every stage.
Semaglutide reduces liver fat. Multiple studies have shown approximately 40-55% reductions in hepatic fat content with semaglutide treatment. That is clinically meaningful and represents a genuine therapeutic benefit. The weight loss itself accounts for some of this effect, but GLP-1 receptor activation also appears to have direct anti-inflammatory and anti-fibrotic effects in liver tissue.
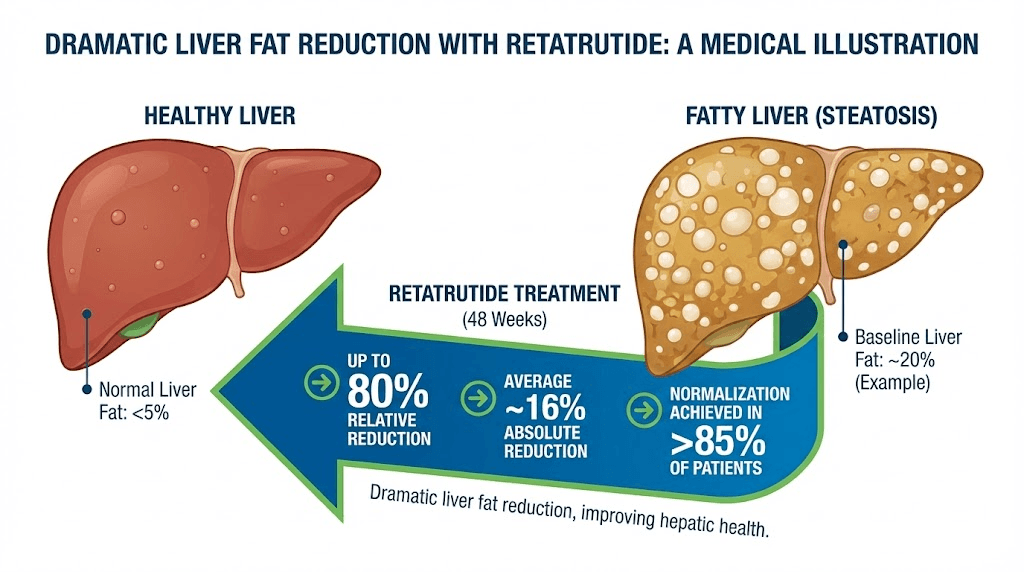
Retatrutide obliterates liver fat. In the Phase 2 trial, participants on the higher doses showed 82-86% reduction in liver fat at 48 weeks. Perhaps more impressively, 89-93% of participants achieved less than 5% liver fat content, which is the threshold for being considered free of fatty liver disease. Those numbers are not incremental improvements over semaglutide. They represent a qualitative leap in liver-directed therapy.
The glucagon receptor component explains most of this difference. Glucagon directly stimulates hepatic fat oxidation, essentially instructing the liver to burn its stored fat for fuel. This is a direct, receptor-mediated effect that occurs independently of and in addition to the weight loss driven by reduced caloric intake. It is the pharmacological equivalent of pointing a targeted spotlight at liver fat specifically and saying "burn this."
For anyone concerned about metabolic health beyond simple weight management, this liver fat data may be the single most important differentiator between the two compounds. Visceral fat loss is closely linked to liver fat reduction, and the metabolic cascade effects of clearing hepatic steatosis extend to improved insulin sensitivity, reduced systemic inflammation, and lower cardiovascular risk.
Cardiovascular outcomes
Semaglutide has a major advantage here, not because its cardiovascular effects are necessarily superior, but because they have actually been measured in a dedicated outcomes trial.
The SELECT trial enrolled over 17,000 participants with established cardiovascular disease and obesity (without diabetes) and followed them for a median of approximately 40 months. The results showed a 20% reduction in major adverse cardiovascular events (MACE), defined as cardiovascular death, non-fatal heart attack, or non-fatal stroke. That 20% reduction in MACE is a landmark finding. It established semaglutide as the first obesity medication to demonstrate cardiovascular benefit in a rigorous outcomes trial.
Retatrutide has no equivalent cardiovascular outcomes data. The compound is still in Phase 3 trials focused primarily on weight loss efficacy. A dedicated cardiovascular outcomes trial would typically come later in development, and given the earlier stage of retatrutide research, those results are likely years away.
However, there are reasons to be cautiously optimistic about retatrutide cardiovascular effects. Greater weight loss generally correlates with greater cardiovascular risk reduction. The dramatic improvements in liver fat and metabolic markers observed with retatrutide would be expected to translate into cardiovascular benefits. And the GIP receptor component has been associated with improved endothelial function in preclinical studies.
But optimism is not data. Until a proper cardiovascular outcomes trial is completed for retatrutide, semaglutide remains the only compound in this class with proven cardiovascular benefit. For individuals with existing heart disease, that distinction could be decisive. Exploring broader peptide research and clinical studies helps contextualize these findings within the larger landscape of metabolic therapy.
Blood sugar and insulin sensitivity
Both compounds improve glycemic control. Semaglutide is actually FDA-approved for type 2 diabetes (as Ozempic) at lower doses, reflecting its robust effects on blood sugar regulation. It reduces HbA1c by approximately 1.5-2.0 percentage points in diabetic populations, which is clinically excellent.
Retatrutide shows comparable or slightly superior glycemic improvements in the limited diabetic population data available. The combination of GLP-1, GIP, and glucagon receptor activity creates a complex but effective glucose-lowering effect. The GLP-1 and GIP components enhance insulin secretion and sensitivity, while the glucagon component, somewhat paradoxically, does not worsen blood sugar control because its glucose-raising effects are offset by the dominant insulin-enhancing effects of the other two pathways.
Both compounds are relevant for people exploring peptides for weight loss and muscle gain, because insulin sensitivity is a key determinant of body composition. Better insulin sensitivity means more efficient nutrient partitioning, more glucose directed to muscle rather than fat, and better preservation of lean mass during caloric deficit.
Body composition effects
One concern with any rapid weight loss intervention is the loss of lean mass alongside fat mass. Typically, roughly 25-40% of weight lost through caloric restriction comes from lean tissue rather than fat. This is metabolically counterproductive because lean mass drives basal metabolic rate, and losing it makes weight regain more likely.
Semaglutide trials have shown that approximately 30-40% of weight lost is lean mass. This is a recognized limitation that has spurred research into combination approaches with exercise and potentially anabolic agents. Understanding peptides for muscle growth is relevant here, as some researchers investigate combinations of weight loss compounds with muscle-preserving peptides.
Retatrutide data on body composition is less detailed but potentially more encouraging. The glucagon receptor component may help preserve lean mass through its effects on amino acid metabolism and protein turnover. Some researchers hypothesize that the increased energy expenditure from glucagon activation means less of the caloric deficit needs to come from dietary restriction, which may be less catabolic to muscle tissue. This remains speculative until detailed DXA body composition data from the Phase 3 trials becomes available.
For those interested in optimizing body composition during weight loss, combining either compound with resistance training is universally recommended. The peptides for athletic performance category includes several compounds that researchers study for their potential to support lean mass preservation during caloric deficit.
Availability, approval status, and practical access
Understanding the clinical data is one thing. Actually being able to access these compounds is another matter entirely. The regulatory and supply landscape for retatrutide and semaglutide could not be more different right now, and these practical realities will determine which option is available to most people for the foreseeable future.
Semaglutide: approved and available (with caveats)
Semaglutide has full FDA approval under two brand names. Ozempic is approved for type 2 diabetes at doses up to 2.0 mg weekly. Wegovy is approved for weight management at the 2.4 mg dose. Both are manufactured by Novo Nordisk and distributed through standard pharmaceutical supply chains.
However, "approved" does not mean "easily accessible." Semaglutide has experienced severe supply shortages driven by unprecedented demand. Prescriptions routinely go unfilled for weeks or months. Insurance coverage remains inconsistent, with many plans covering the diabetes indication but denying the weight loss indication. Without insurance, the monthly cost runs approximately $1,350 per month, placing it beyond reach for many people.
The supply and cost challenges have driven significant interest in alternative sources and generic equivalents. Resources comparing Ozempic alternatives help people understand what other options exist within the GLP-1 agonist class. The peptide cost calculator can help researchers estimate expenses across different sources and compounds.
Despite these challenges, semaglutide has the enormous practical advantage of being an approved, regulated pharmaceutical product. Patients can obtain it through legitimate medical channels with physician oversight, quality assurance, and ongoing monitoring. That infrastructure matters.
Retatrutide: still in clinical development
Retatrutide is not yet FDA-approved for any indication. It remains in Phase 3 clinical trials through the TRIUMPH program, which includes multiple studies examining different patient populations and endpoints. Based on the typical drug development timeline, Eli Lilly (the manufacturer) is expected to file a New Drug Application (NDA) in late 2026, with potential FDA approval in 2027 if the review process goes smoothly.
That timeline means retatrutide is at minimum 1-2 years away from being available through standard pharmaceutical channels. During this period, the only legal way to receive retatrutide in a clinical setting is through enrollment in a clinical trial.
Of course, the research peptide market exists in a legal gray area that makes retatrutide available to some researchers and individuals through non-pharmaceutical channels. The retatrutide buy guide discusses the landscape for sourcing research-grade material, while the grey market peptides guide covers the broader legal and quality considerations of obtaining peptides outside the pharmaceutical supply chain.
Anyone considering non-pharmaceutical sources must understand the risks. Without regulatory oversight, there is no guarantee of compound identity, purity, potency, or sterility. Third-party testing through peptide testing labs can verify some of these parameters, but it adds cost and complexity. Knowing whether peptides are legal in your jurisdiction is an essential first step before pursuing any non-pharmaceutical sourcing.
Cost comparison
Semaglutide currently costs approximately $1,350 per month at retail pharmacy prices for the weight management dose. With insurance coverage (where available), out-of-pocket costs vary widely from $25 to $500+ per month depending on the plan.
Retatrutide pricing is speculative since it is not yet approved, but analysts estimate it will launch at $1,100-1,400 per month, roughly competitive with semaglutide. Eli Lilly may price it at a premium given the superior efficacy data, or may price it competitively to capture market share from Novo Nordisk. The retatrutide cost and pricing guide tracks current research pricing and projected pharmaceutical pricing.
In the research peptide market, both compounds are available at significantly lower cost. However, these prices come without the quality assurance, medical oversight, and legal protections that pharmaceutical products provide. The comprehensive peptide cost guide covers pricing across different sources and quality tiers. Understanding the full peptide therapy cost landscape helps set realistic budget expectations regardless of source.
Vendor considerations for research compounds
For those pursuing research-grade peptides, vendor selection is critical. Not all peptide suppliers maintain the same quality standards, and the difference between a reputable vendor and a disreputable one can be the difference between receiving the actual compound at the labeled concentration and receiving an inert or contaminated product.
Key factors to evaluate include third-party certificate of analysis availability, HPLC purity documentation, batch-to-batch consistency, and transparent business practices. The best peptide vendors guide evaluates major suppliers across these criteria. Specific vendor reviews for retatrutide, such as the Paradigm Peptides retatrutide review, provide compound-specific sourcing insights. The ZLZ Peptide retatrutide review covers another frequently discussed source.
Making the right choice: a decision framework
There is no universally "better" compound between retatrutide and semaglutide. The right choice depends on individual circumstances, priorities, risk tolerance, and practical constraints. Let us build a decision framework that accounts for all the relevant variables.
Choose semaglutide if:
Semaglutide is likely the better option for several specific profiles.
You prioritize proven safety data. Semaglutide has years of post-marketing surveillance data from millions of users. The long-term safety profile is well-characterized, and the cardiovascular benefit is proven through the SELECT trial. If minimizing unknown risks matters most to you, semaglutide offers a much deeper safety database.
You want pharmaceutical-grade access with medical oversight. Semaglutide can be obtained through legitimate medical channels with a prescription. That means quality-controlled manufacturing, dosing standardization, and ongoing physician monitoring. For people who value the institutional support structure around approved medications, this is a significant advantage.
Your weight loss goal is moderate. If you need to lose 15-20% of your body weight, semaglutide may be entirely sufficient. Not everyone needs the maximal efficacy of a triple agonist. For many people, the 14-15% average weight loss with semaglutide, combined with its better tolerability profile, represents the right balance of benefit and risk.
You have existing cardiovascular disease. The SELECT trial data showing 20% reduction in MACE makes semaglutide the only compound in this class with proven cardiovascular benefit. Until retatrutide has equivalent data, semaglutide is the evidence-based choice for people with established heart disease.
You have a sensitive GI system. If you have a history of significant GI issues or poor tolerance to other medications, semaglutide lower discontinuation rate (5-7% vs 12-18%) suggests better overall tolerability. Starting with the gentler option and escalating only if needed is a reasonable strategy.
Resources like the getting started with peptides guide can help newcomers understand the practical considerations of beginning any peptide protocol, while online peptide therapy guides cover the telehealth pathway for obtaining semaglutide prescriptions.
Choose retatrutide if:
Retatrutide may be the better option for other specific profiles.
You need maximum weight loss. If you are severely obese and need to lose 25-30%+ of your body weight to reach a healthy range, semaglutide 14-15% average may not be enough. Retatrutide 28.7% average at the 12 mg dose is nearly double and may be the difference between meaningful results and truly life-changing outcomes.
Liver fat is a primary concern. If you have diagnosed NAFLD or NASH, the 82-86% reduction in liver fat with retatrutide is an order of magnitude beyond what any other available therapy can offer. For people whose liver health is a critical priority, this data is compelling enough to outweigh many other considerations.
You have experience with GLP-1 agonists. If you have already used semaglutide or tirzepatide and either plateaued or found the results insufficient, retatrutide triple-receptor mechanism offers a genuinely different pharmacological approach that may break through where single or dual agonists hit their ceiling.
You are comfortable with research-grade compounds. Retatrutide is not yet available through pharmaceutical channels. Using it currently requires sourcing research-grade material, which involves additional quality verification, dosing calculations, and self-administration expertise. If you are already experienced with research peptides and comfortable managing these complexities, the compound itself is accessible.
You prioritize metabolic improvement over cardiovascular proof. If your primary health goals are metabolic, including insulin sensitivity, liver health, and body composition rather than specifically cardiovascular, retatrutide broader mechanism of action may deliver more comprehensive benefits. The trade-off is that those benefits have not yet been confirmed in long-term outcomes trials.
The peptide stacks guide explores how advanced researchers combine different compounds for specific goals. For people interested in peptides for weight loss specifically for women, additional considerations around hormonal interactions apply.
The middle ground: tirzepatide and combination approaches
It is worth noting that a middle option exists. Tirzepatide (Mounjaro/Zepbound) is a dual GLP-1/GIP agonist that produces weight loss results between semaglutide and retatrutide, with approximately 20-22% average weight loss at the 15 mg dose. For people who want more than semaglutide can offer but are not ready for the more aggressive triple-agonist approach, tirzepatide occupies a useful middle ground.
The semaglutide vs tirzepatide comparison covers that specific decision in detail. Understanding the microdosing tirzepatide approach and the unit calculations for tirzepatide dosing can help people considering this intermediate option.
Some researchers also investigate combination approaches, pairing GLP-1 agonists with other peptides that target complementary pathways. The emerging research on cagrilintide combined with semaglutide (CagriSema) represents one such approach, adding amylin receptor agonism to GLP-1 for enhanced results. The CagriSema dosing guide covers the specific protocols being studied.
Other weight loss peptides work through entirely different mechanisms. AOD 9604 targets fat metabolism directly. Tesofensine works through monoamine reuptake inhibition. 5-amino-1MQ inhibits NNMT enzyme activity. FTPP adipotide targets fat tissue vasculature. Each offers a different approach, and understanding the full fat burning peptide landscape helps identify the best option for individual goals.
Questions to ask yourself before deciding
Before committing to either compound, answer these questions honestly:
How much weight do you realistically need to lose? If 15% body weight reduction achieves your health goals, semaglutide may be sufficient.
Do you have liver health concerns? If NAFLD is diagnosed or suspected, retatrutide liver fat data is exceptionally compelling.
What is your risk tolerance? Semaglutide has a deeper safety database. Retatrutide is still generating long-term safety data.
Do you need pharmaceutical-grade access? Semaglutide is available by prescription now. Retatrutide requires research-grade sourcing.
Have you tried other GLP-1 agonists? If semaglutide or tirzepatide did not produce adequate results, retatrutide different mechanism may be worth exploring.
What is your budget? Both compounds are expensive. Understand the full therapy cost implications before committing.
Do you have cardiovascular disease? Semaglutide has proven CV benefit. Retatrutide does not, yet.
Working with a knowledgeable healthcare provider, whether through in-person visits or local peptide therapy clinics or specialized clinics, is strongly recommended for anyone considering either compound. These are potent pharmaceutical agents, not supplements, and medical oversight improves both safety and outcomes.
The future of multi-receptor weight loss compounds
The competition between retatrutide and semaglutide represents something larger than two compounds vying for market share. It represents a fundamental evolution in how metabolic medicine approaches obesity. Understanding where this field is heading helps contextualize the current choice between these two agents.
The receptor count race
The progression from single to dual to triple receptor agonists follows a clear pharmacological logic. Each additional receptor target adds a new dimension of metabolic activity. Semaglutide activates one receptor. Tirzepatide activates two. Retatrutide activates three. And the clinical results have improved with each step up in receptor complexity.
This pattern will almost certainly continue. Quad-agonists and even more complex multi-receptor compounds are already in preclinical development. The GLP-3 peptide research explores some of these emerging targets. The amylin receptor agonist class represents another parallel pathway being integrated into multi-target compounds.
However, more receptors do not automatically mean better outcomes. Each additional receptor target adds complexity to the side effect profile, the dose-response relationship, and the long-term safety considerations. There is likely a point of diminishing returns where additional receptor activation produces marginal benefit at disproportionate risk. Whether retatrutide triple-agonist approach has already reached that optimal balance or whether it can be improved upon remains an open question.
Oral formulations on the horizon
Both semaglutide and retatrutide are currently administered by injection. Oral semaglutide (Rybelsus) already exists for the diabetes indication at lower doses, and higher-dose oral formulations for weight management are in late-stage development. The potential for oral retatrutide is less certain given the compound larger molecular size and more complex receptor binding requirements.
The shift from injectable to oral administration would dramatically expand access and adherence. Many people who decline injectable therapy might accept an oral alternative. The oral tirzepatide research shows that even complex peptide agonists can potentially be reformulated for oral delivery, suggesting the same may eventually be possible for retatrutide-class compounds.
For those who currently prefer non-injection routes, nasal spray peptides represent another emerging delivery mechanism, though efficacy and bioavailability challenges remain significant for large peptide molecules like these.
Combination therapies
Rather than relying on a single compound to do everything, the field is increasingly moving toward rational combinations. CagriSema (cagrilintide plus semaglutide) is the most advanced combination in development, pairing GLP-1 agonism with amylin receptor agonism. Early data suggests this combination produces weight loss competitive with retatrutide, approximately 20-25% at optimal doses.
The cagrilintide weight loss data and cagrilintide dosing information provide detailed background on this emerging combination approach. For men specifically, the cagrilintide guide for men addresses gender-specific response differences. Understanding cagrilintide dosing with tirzepatide combinations explores even more complex multi-compound protocols.
This combinatorial approach may ultimately prove more flexible than the all-in-one triple-agonist strategy, because it allows each component to be dosed independently and titrated to individual response. But it also means more injections, more complex dosing schedules, and more potential drug interactions.
What this means for researchers now
The practical takeaway is this: semaglutide is the known quantity, retatrutide is the exciting frontier, and the field will continue evolving rapidly regardless of which compound someone chooses today. Staying current with peptide research developments and maintaining a relationship with knowledgeable healthcare providers is the best strategy for navigating this rapidly changing landscape.
SeekPeptides members access regularly updated guides, protocol databases, and research summaries that track these developments as they unfold. The weight loss peptide landscape is evolving faster than any static resource can capture, which is why access to continuously updated information matters more than any single comparison.
Practical protocols and real-world considerations
Clinical trial data is generated under controlled conditions with careful patient selection, regular monitoring, and professional oversight. Real-world use introduces variables that trials do not always capture. Understanding these practical considerations helps bridge the gap between published data and actual experience.
Diet and exercise interactions
Both semaglutide and retatrutide work best when combined with dietary modification and increased physical activity. In clinical trials, all participants received lifestyle counseling alongside drug treatment. The weight loss numbers reported include the combined effect of the drug plus the lifestyle changes.
However, the nature of optimal dietary support may differ between the two compounds. With semaglutide, the primary mechanism is appetite suppression, so ensuring adequate protein intake is critical to minimize lean mass loss during the caloric deficit. A minimum of 0.7-1.0 grams of protein per pound of lean body mass is commonly recommended.
With retatrutide, the glucagon component increases energy expenditure, which means the total caloric deficit is larger even at similar intake levels. This amplified deficit increases the importance of protein adequacy and may also increase requirements for micronutrients that support metabolic processes. Proper nutrition planning is essential, and resources like the peptide dosing guide emphasize the importance of supportive nutrition alongside any peptide protocol.
Exercise selection matters too. Resistance training is critical with both compounds to preserve lean mass, but the specifics may differ. With retatrutide greater metabolic activation, some researchers suggest that higher-intensity resistance training may be better tolerated and more effective because the compound provides more metabolic support for recovery. The peptide strength protocol benefits article explores these synergies in detail.
Monitoring requirements
Both compounds require medical monitoring, but the specifics differ.
For semaglutide, standard monitoring includes regular weight checks, blood glucose and HbA1c measurements (especially in diabetic patients), periodic lipid panels, and liver function tests. The monitoring protocol is well-established because the drug has been in clinical use for years.
For retatrutide, monitoring should be more comprehensive because of the additional receptor targets. Liver function tests are particularly important given the dramatic effects on hepatic fat metabolism. Heart rate monitoring may be warranted given the glucagon component mild chronotropic effects. More frequent metabolic panels help track the complex changes across glucose, lipid, and hepatic markers that triple-agonist therapy produces.
Anyone self-administering either compound outside of formal medical supervision should establish a relationship with a physician willing to order appropriate monitoring labs. Understanding what peptide injection entails and the associated monitoring requirements is part of responsible use. The peptide vial research guide covers the practical aspects of working with research-grade materials.
Storage and handling
Pharmaceutical semaglutide (Ozempic, Wegovy) comes in pre-filled pens with clear storage instructions: refrigerate before first use, then store at room temperature for up to 56 days. The product is manufactured to pharmaceutical standards with extensive stability testing.
Research-grade retatrutide and semaglutide require more careful handling. Lyophilized (freeze-dried) peptides must be stored according to specific temperature requirements. The peptide stability in powder form and peptide stability at room temperature guides provide detailed information on optimal storage conditions. After reconstitution, proper refrigeration is essential, and understanding how long reconstituted peptides last prevents using degraded material.
Reconstitution itself requires attention to detail. Using the correct volume of bacteriostatic water and following proper mixing procedures ensures accurate dosing. The free reconstitution calculator eliminates guesswork from this process. Understanding the difference between different water types for peptide reconstitution is another fundamental skill.
Proper peptide handling also means understanding comprehensive storage protocols and whether peptides expire under different conditions. These practical details are not glamorous, but they directly impact the safety and efficacy of any research protocol. The SeekPeptides reconstitution and storage guides walk through each step with visual instructions that eliminate the guesswork.
Drug interactions and contraindications
Both compounds interact with other medications, primarily through their effects on gastric emptying and glucose metabolism.
Semaglutide slows gastric emptying, which can affect the absorption of oral medications taken at the same time. This is particularly relevant for medications with narrow therapeutic windows, such as thyroid hormones and certain antibiotics. Insulin and sulfonylureas may need dose adjustment to prevent hypoglycemia when combined with semaglutide.
Retatrutide carries the same gastric emptying interactions plus additional considerations from the glucagon component. Glucagon opposes insulin action, which creates a more complex interaction profile with diabetic medications. The GIP component may affect incretin-based medications differently than GLP-1 alone. Anyone taking multiple medications should consult with a pharmacist or physician familiar with these interactions before starting either compound.
Both compounds are contraindicated in pregnancy and should be discontinued well before planned conception due to their effects on nutrient absorption and fetal development. They are also contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2, based on preclinical findings in rodent models.
Understanding the broader context of peptide safety considerations helps frame these specific drug interaction concerns within the larger picture of responsible peptide use. Neither compound should be combined with other peptides without careful consideration of potential interactions.
Duration of treatment and what happens when you stop
This is perhaps the most important practical question, and the answer is sobering for both compounds.
Semaglutide data is clear: when treatment stops, weight regain begins. The STEP 1 extension study showed that participants who discontinued semaglutide after 68 weeks regained approximately two-thirds of the lost weight within one year. The metabolic improvements, including better insulin sensitivity and lipid profiles, also reversed upon discontinuation.
Retatrutide discontinuation data is limited because the compound is still in clinical trials and long-term follow-up after cessation has not been extensively studied. However, based on the pharmacological similarity to other incretin-based therapies, similar weight regain is expected once treatment ends. The mechanisms of action are drug-dependent rather than curative, meaning they work only while the drug is active in the body.
This has major implications for treatment planning. Both compounds should be viewed as long-term or potentially lifelong therapies rather than short-term interventions. Understanding peptide cycling approaches is relevant here, as some researchers investigate whether periodic treatment with rest intervals can maintain benefits while reducing long-term exposure. The peptide cycle planning guide covers different strategies for managing long-term use.
Frequently asked questions
Is retatrutide stronger than semaglutide?
Yes, by a significant margin. Retatrutide at the 12 mg dose produces approximately 28.7% body weight loss at 68 weeks, compared to 14.9% with semaglutide 2.4 mg at the same timepoint. This nearly double efficacy comes from retatrutide triple receptor mechanism (GLP-1, GIP, and glucagon) versus semaglutide single receptor (GLP-1 only). However, "stronger" also means stronger side effects, with discontinuation rates of 12-18% for retatrutide versus 5-7% for semaglutide. Greater efficacy comes at the cost of greater side effect burden for some users. Reviewing the best peptides for weight loss comparison helps contextualize where both compounds fit in the broader landscape.
Can I switch from semaglutide to retatrutide?
Switching is possible but not straightforward. There is no direct dose equivalence between the two compounds because they work through different receptor profiles. The recommended approach is to discontinue semaglutide, allow a washout period of at least 2-3 weeks (given semaglutide approximately one-week half-life), and then begin retatrutide titration from the standard 1 mg starting dose. Attempting to skip the washout or start retatrutide at an advanced dose risks excessive receptor activation and severe side effects. Anyone considering this transition should review the peptide cycle planning guide and ideally work with a physician experienced in these compounds.
When will retatrutide be FDA approved?
Based on the current status of the Phase 3 TRIUMPH program, Eli Lilly is expected to file a New Drug Application (NDA) in late 2026. Assuming standard FDA review timelines and no significant delays, approval could come as early as 2027. However, drug development timelines are inherently uncertain. Safety signals, manufacturing issues, or regulatory requests for additional data could push the timeline back. Until approval, retatrutide is not available through pharmaceutical channels except through clinical trial enrollment.
Which has fewer side effects?
Semaglutide has a more favorable tolerability profile overall. The individual side effect rates (nausea, vomiting, diarrhea) are similar between the two compounds, but the discontinuation rate, which reflects how many people find the side effects intolerable, is significantly lower for semaglutide (5-7%) compared to retatrutide (12-18%). Both compounds cause primarily gastrointestinal side effects that are most pronounced during dose escalation and tend to improve with continued use. Proper titration following the recommended schedule is the most important factor in minimizing side effects with either compound. Understanding common mistakes beginners make can help avoid the most frequent tolerability pitfalls.
Is retatrutide better for fatty liver disease?
The data strongly suggests yes. Retatrutide produces 82-86% reductions in liver fat at 48 weeks, with 89-93% of participants achieving less than 5% liver fat (the threshold for resolution of fatty liver disease). Semaglutide produces meaningful liver fat reductions of approximately 40-55%, but nowhere near the magnitude seen with retatrutide. The difference is primarily attributed to retatrutide glucagon receptor component, which directly stimulates hepatic fat oxidation. For individuals whose primary concern is liver health rather than just body weight, retatrutide appears to be dramatically superior. The visceral fat loss guide explores the relationship between liver fat and overall metabolic health in more detail.
Can I take retatrutide or semaglutide with other peptides?
Combining either compound with other peptides requires careful consideration. Both are potent pharmaceutical agents that significantly alter metabolic function, appetite, and gastrointestinal dynamics. Adding other peptides, particularly those that affect similar pathways, could create unpredictable interactions. Some researchers combine GLP-1 agonists with peptides that target entirely different systems, such as BPC-157 for gut health or ipamorelin for growth hormone support, but this should be done cautiously and preferably with medical oversight. The peptide stacking guide covers compatibility considerations and the peptide stack calculator helps plan multi-compound protocols.
Do these compounds show up on drug tests?
Standard employment drug tests do not screen for peptide hormones. However, specialized sports anti-doping tests (like those administered by WADA or USADA) do test for GLP-1 receptor agonists and related compounds. Both semaglutide and retatrutide would be detectable on such tests and are prohibited in competitive athletics. The peptides and drug testing guide provides comprehensive information on detection windows and testing methodologies for various peptide compounds.
What happens if I stop taking either compound?
Weight regain is the likely outcome with both compounds. Semaglutide discontinuation data from the STEP 1 extension study showed approximately two-thirds of lost weight was regained within one year of stopping treatment. Retatrutide long-term discontinuation data is still being collected, but similar weight regain is expected based on the mechanism of action. Neither compound cures obesity. They manage it. Once the drug is removed, the metabolic conditions that caused weight gain tend to reassert themselves. This is why most experts recommend viewing these as long-term therapies rather than short-term interventions. The SeekPeptides protocol guides cover strategies for managing treatment transitions, and understanding peptide transformation timelines helps set realistic expectations for both the treatment and post-treatment periods.
External resources
For those seeking the primary scientific literature and authoritative reference materials underlying this comparison, the following resources provide direct access to the most important studies and regulatory information.
New England Journal of Medicine: Retatrutide Phase 2 trial results - The landmark study establishing retatrutide efficacy and safety across multiple dose groups
New England Journal of Medicine: STEP 1 semaglutide trial results - The pivotal trial demonstrating semaglutide 2.4 mg weight loss efficacy
ClinicalTrials.gov: Retatrutide TRIUMPH program - Current status and details of all ongoing Phase 3 retatrutide trials
FDA prescribing information for Wegovy (semaglutide 2.4 mg) - Complete approved labeling including dosing, safety, and clinical data
New England Journal of Medicine: SELECT cardiovascular outcomes trial - The trial demonstrating semaglutide 20% reduction in major cardiovascular events
These primary sources should be the foundation for any serious evaluation of either compound. Secondary summaries, including this article, are no substitute for reading the actual trial data and understanding the methodology behind the headline numbers.
For those navigating the practical aspects of peptide research, SeekPeptides provides tools, calculators, and educational resources that translate complex clinical data into actionable guidance. The peptide calculator helps with dosing math. The complete peptide list catalogs every compound worth knowing about. And the before and after results database shows what real-world outcomes look like beyond clinical trial averages.
SeekPeptides members get access to detailed protocol guides, updated research summaries, and expert-curated resources that go far beyond what a single article can cover. Whether you are exploring semaglutide, retatrutide, or the broader world of peptide applications, having reliable, continuously updated information is the foundation of responsible research.
The comparison between retatrutide and semaglutide is not a simple contest with a clear winner. It is a study in trade-offs. Semaglutide offers proven safety, pharmaceutical access, cardiovascular data, and good tolerability with meaningful weight loss. Retatrutide offers dramatically greater weight loss, extraordinary liver fat reduction, and a triple-receptor mechanism that represents the cutting edge of metabolic pharmacology, but with higher side effect rates, no long-term safety data, and no current regulatory approval.
The best choice depends entirely on who is choosing. On their starting point. On their health priorities. On their risk tolerance. On their practical circumstances. There is no wrong answer between two compounds that both represent genuine advances in metabolic medicine. There is only the answer that aligns best with individual goals and constraints.
In case I do not see you, good afternoon, good evening, and good night. May your protocols stay optimized, your metabolic health stay improving, and your research stay informed by evidence rather than hype.