Jan 27, 2026
You have a 20mg vial of retatrutide sitting on your desk. The lyophilized powder looks deceptively simple. White. Compact. Unassuming. But between that powder and your first injection lies a series of calculations that trip up even experienced researchers. How much bacteriostatic water? What concentration do you want? How many units on your syringe equals your target dose? Get these numbers wrong and you are either wasting expensive peptide or taking doses wildly different from what clinical trials actually studied.
The frustration compounds when you search for answers.
Most resources assume you already understand peptide reconstitution basics. They throw formulas at you without context. They reference 10mg vials when you have 20mg. Or they give vague instructions like "add bacteriostatic water and inject" without specifying exactly how much water creates what concentration. When you are working with a novel triple agonist that costs hundreds of dollars per vial, guessing is not an option.
This guide eliminates the guesswork entirely.
We cover everything specific to 20mg retatrutide vials. The exact reconstitution calculations. The concentration options and why you might choose one over another. The complete titration schedule from clinical trials. How to translate milligram doses into syringe units. Storage requirements that preserve potency. And the practical details that determine whether your protocol matches what researchers actually studied in trials showing up to 24.2% body weight reduction.
SeekPeptides members access interactive calculators that handle these conversions automatically, but understanding the math yourself matters. When you know why you are drawing a specific volume, you can troubleshoot problems, adjust protocols, and make informed decisions about your research approach. Knowledge creates confidence, and confidence prevents costly mistakes.
Understanding retatrutide and the 20mg vial format
Retatrutide represents a new class of weight loss peptides called triple agonists. Unlike semaglutide which activates only GLP-1 receptors, or tirzepatide which targets GLP-1 and GIP, retatrutide simultaneously activates three hormone receptors. GLP-1 for appetite suppression and glucose control. GIP for enhanced insulin secretion and lipid metabolism. And glucagon for increased energy expenditure and fat mobilization.
This triple mechanism produces remarkable results. Phase 2 trials published in the New England Journal of Medicine showed participants losing an average of 24.2% of their body weight at the 12mg weekly dose over 48 weeks. That translates to roughly 58 pounds for someone starting at 240 pounds. The December 2025 Phase 3 TRIUMPH-4 results were even more impressive, with the 12mg group losing an average of 28.7% of body weight over 68 weeks.
The 20mg vial format exists because research protocols require flexibility.
Some researchers need higher concentrations for smaller injection volumes. Others prefer more dilute solutions for easier dose adjustments during titration. The 20mg format provides enough peptide for extended protocols while remaining practical for individual research use. It also offers better value per milligram compared to smaller vials, making it popular among serious researchers planning multi-month protocols.
Why vial size matters for your calculations
Every reconstitution calculation starts with knowing exactly how much peptide you have. With a 20mg vial, you are working with 20,000 micrograms (mcg) of retatrutide. This number becomes the numerator in your concentration formula. The bacteriostatic water volume you add becomes the denominator. Change either number and your final concentration changes proportionally.
This matters because clinical trial doses are measured in milligrams. Your syringe measures in units or milliliters. Converting between these requires knowing your concentration. A 4mg dose means something completely different depending on whether your vial contains 5,000 mcg/mL or 10,000 mcg/mL. The 20mg vial gives you flexibility to create either concentration based on your needs and injection preferences.
Understanding your starting material eliminates downstream errors. When you know you have exactly 20,000 mcg to work with, every subsequent calculation has a solid foundation. No guessing. No approximating. Just straightforward math that produces reliable results.
Reconstitution calculations for 20mg retatrutide vials
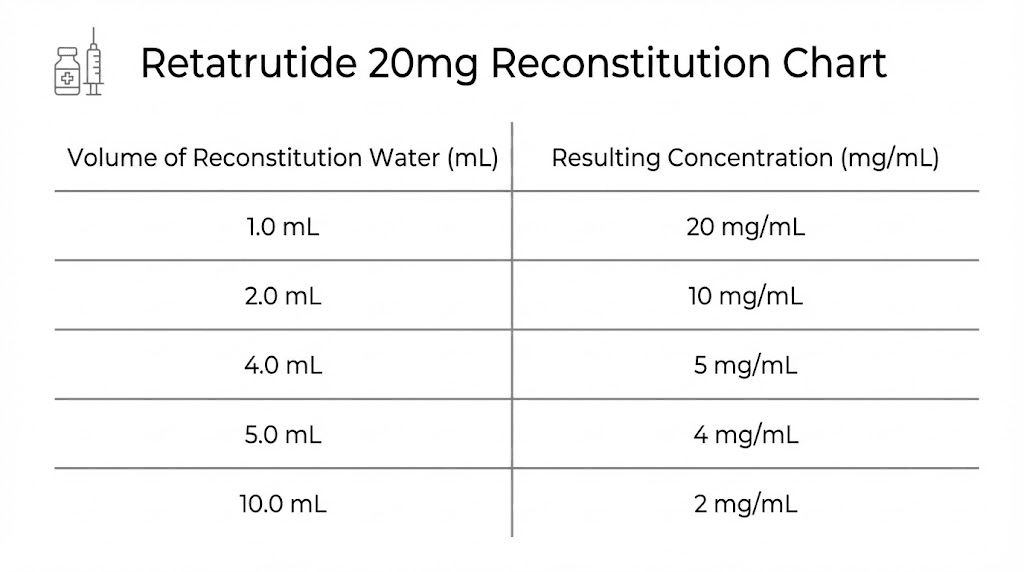
The core formula for peptide reconstitution is simple. Divide your total peptide amount in micrograms by your water volume in milliliters. The result is your concentration in mcg/mL. For a 20mg vial, you have 20,000 mcg to work with. The water volume you choose determines everything else.
Most researchers choose between two common approaches.
Option 1: Higher concentration with 2mL water
Adding 2mL of bacteriostatic water to your 20mg vial creates a concentration of 10,000 mcg/mL. This means every milliliter contains 10mg of retatrutide. On a standard U-100 insulin syringe, each unit equals 10 mcg of peptide.
Dose calculations at 10,000 mcg/mL:
1mg dose = 0.1mL = 10 units on U-100 syringe
2mg dose = 0.2mL = 20 units
4mg dose = 0.4mL = 40 units
6mg dose = 0.6mL = 60 units
8mg dose = 0.8mL = 80 units
12mg dose = 1.2mL = 120 units (requires two draws or larger syringe)
This concentration works well for lower and mid-range doses. The injection volumes remain small, which many researchers prefer for subcutaneous administration. However, doses above 8mg require either splitting across two syringes or using a larger capacity syringe. The peptide reconstitution calculator can help you verify these numbers before drawing.
Option 2: More dilute with 4mL water
Adding 4mL of bacteriostatic water creates a concentration of 5,000 mcg/mL. Each milliliter now contains 5mg of retatrutide. On a U-100 syringe, each unit equals 5 mcg of peptide.
Dose calculations at 5,000 mcg/mL:
1mg dose = 0.2mL = 20 units on U-100 syringe
2mg dose = 0.4mL = 40 units
4mg dose = 0.8mL = 80 units
6mg dose = 1.2mL = 120 units
8mg dose = 1.6mL = 160 units
12mg dose = 2.4mL = 240 units
This dilution makes higher doses more practical within a single syringe for mid-range protocols. The 4mg dose sits at a comfortable 80 units. However, the 12mg maintenance dose still requires multiple draws or a 1mL+ syringe. Some researchers find the larger injection volumes less comfortable, especially for daily protocols, though retatrutide is administered weekly which makes this less of a concern.
Step by step reconstitution process
Proper technique matters as much as correct calculations. Rushing the reconstitution process can damage the peptide or create uneven dissolution that affects dosing accuracy. Follow these steps precisely.
Step 1: Gather your materials
You need the 20mg retatrutide vial, bacteriostatic water, alcohol swabs, and a syringe for drawing water. A 3mL syringe works well for adding water. Have your injection supplies ready but set aside until the peptide fully dissolves.
Step 2: Clean both vial tops
Wipe the rubber stopper on both the peptide vial and bacteriostatic water vial with fresh alcohol swabs. Let them air dry for 10-15 seconds. This prevents introducing contaminants that could degrade the peptide or cause injection site reactions.
Step 3: Draw your chosen water volume
Pull back the syringe plunger to your target volume (2mL or 4mL depending on your concentration preference). Insert the needle into the bacteriostatic water vial and push air in to equalize pressure. Then draw the water slowly and steadily.
Step 4: Add water to the peptide vial correctly
This step is critical. Do not inject water directly onto the lyophilized powder. Instead, angle the needle so water runs down the inside wall of the vial. Add the water slowly over 30-60 seconds. The gentle approach prevents damaging the peptide structure and promotes even dissolution.
Step 5: Allow dissolution without shaking
Set the vial down and let it sit for 5-10 minutes. The powder should dissolve gradually. You can gently swirl the vial in slow circles to help mixing, but never shake it. Shaking creates bubbles and foam that can denature the peptide chains. The solution should be clear when fully dissolved. Any cloudiness or particles indicate a problem.
Step 6: Label and store properly
Write the reconstitution date, concentration, and expiration date on the vial. Store immediately in your refrigerator at 2-8 degrees Celsius. Reconstituted retatrutide remains stable for approximately 4 weeks under proper refrigeration.
Clinical trial dosing protocols for retatrutide
Understanding what doses researchers actually studied helps you make informed decisions about your own protocols. Retatrutide trials followed careful titration schedules designed to minimize side effects while achieving therapeutic levels. These schedules provide a template for safe, effective use.
The Phase 2 trial published in the New England Journal of Medicine studied doses ranging from 1mg to 12mg weekly. But participants did not start at their final dose. Everyone began at lower levels and increased gradually. This approach reduced gastrointestinal side effects and improved tolerability.
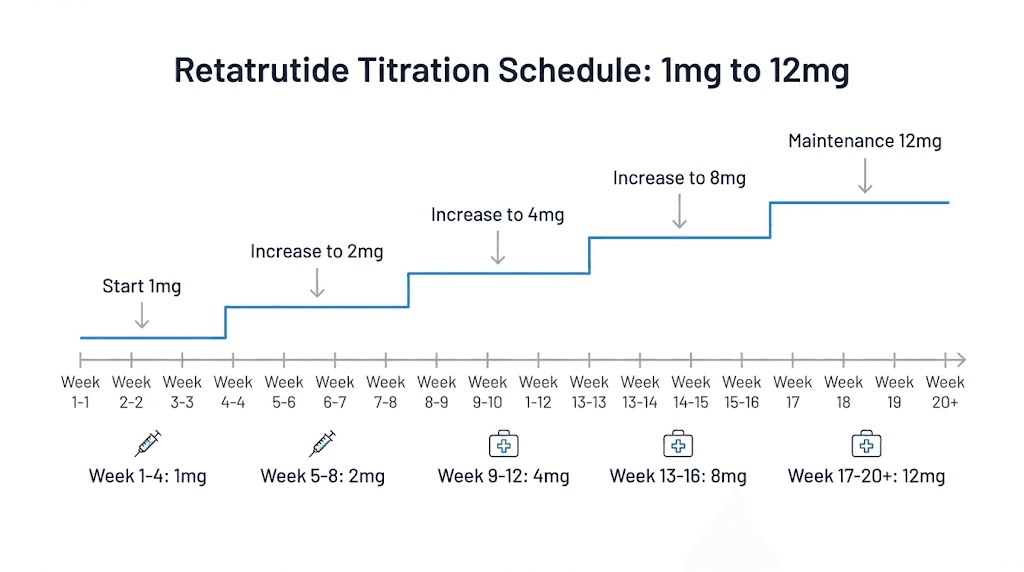
The standard titration schedule
Phase 2 protocols increased doses every four weeks following this pattern. Starting at 1mg weekly. Then 2mg. Then 4mg. Then 6mg. Then 8mg. And finally 12mg for those randomized to the highest dose group. This stepwise approach gives the body time to adjust to each dose level before increasing.
Week 1-4: 1mg weekly
Week 5-8: 2mg weekly
Week 9-12: 4mg weekly
Week 13-16: 6mg weekly
Week 17-20: 8mg weekly
Week 21+: 12mg weekly (if tolerated)
The Phase 3 TRIUMPH-4 trial used a slightly modified approach. Participants started at 2mg and increased to 4mg, then 6mg, then 9mg, and finally 12mg for the highest dose group. Both approaches share the same principle of gradual escalation.
Why titration matters for side effect management
The gastrointestinal side effects that concern most researchers occur primarily during dose escalation. Nausea affects up to 40-45% of participants at higher doses. Diarrhea occurs in roughly 33-35%. Constipation in 21-25%. These symptoms typically emerge when doses increase and diminish as the body adjusts.
Rushing through titration dramatically increases side effect severity. Clinical data shows that starting at 2mg instead of 4mg significantly reduces GI symptom frequency and intensity. The four-week intervals between increases give the digestive system time to adapt to each dose level before demanding more adjustment.
Most researchers find that gut-related side effects fade substantially after 8-12 weeks on a stable dose. The initial discomfort represents a transition period, not a permanent feature of the protocol. Patient researchers who respect the titration schedule generally report much better experiences than those who try to accelerate to higher doses.
Maintenance dosing considerations
Not everyone needs or tolerates the 12mg maximum dose. Phase 3 trials include a 4mg maintenance arm specifically because some individuals achieve excellent results at lower doses. The goal is finding the minimum effective dose for your specific response, not automatically pursuing the maximum studied amount.
Factors that influence optimal maintenance dose include starting body composition, individual metabolic response, side effect tolerance, and research goals. Someone seeking modest weight reduction might find 4-8mg weekly achieves their objectives with minimal side effects. Those pursuing maximum possible results may require the full 12mg dose.
The flexibility of the 20mg vial format supports this individualized approach. With proper reconstitution, you can accurately dose anywhere from 1mg to 12mg weekly, adjusting based on your response and tolerance.
Converting doses to syringe units
This is where many researchers make critical errors. The confusion stems from mixing measurement systems. Vials are labeled in milligrams. Concentrations are expressed in micrograms per milliliter. Syringes are marked in units or fractions of a milliliter. Without systematic conversion, mistakes happen easily.
Let me walk through the conversion process step by step.
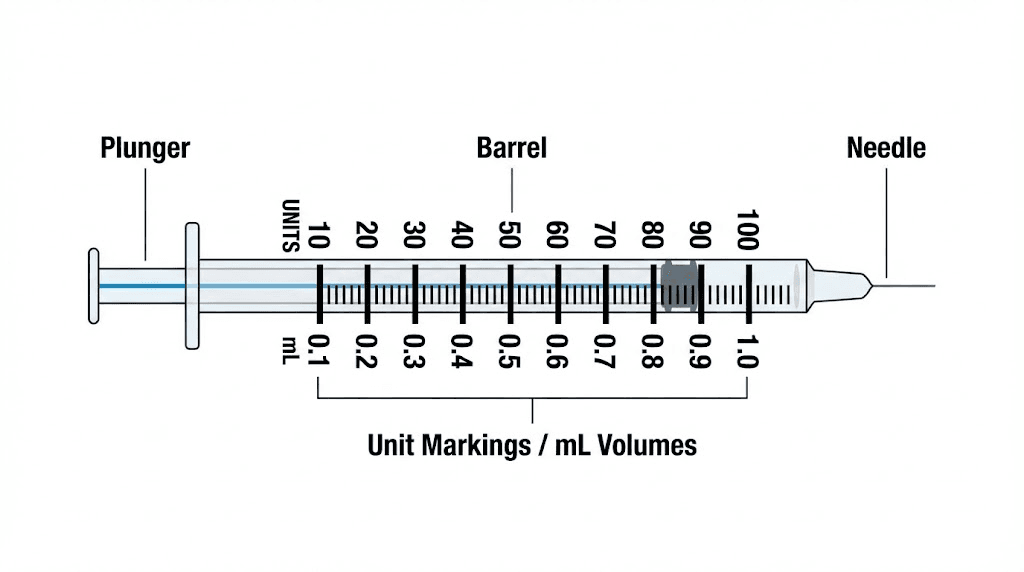
Understanding your syringe markings
Standard U-100 insulin syringes measure volume in units, where 100 units equals 1 milliliter. A syringe marked to 50 units holds 0.5mL. One marked to 100 units holds 1mL. The numbers on the syringe represent units, not milligrams or micrograms of peptide. This distinction is crucial.
When you draw to the 40-unit mark, you are drawing 0.4mL of liquid. How much retatrutide that contains depends entirely on your concentration. At 10,000 mcg/mL, 0.4mL contains 4,000 mcg (4mg). At 5,000 mcg/mL, the same 0.4mL contains only 2,000 mcg (2mg). Same syringe volume, completely different doses.
The conversion formula
To find how many syringe units you need for any dose, use this formula: (Desired dose in mcg) divided by (Concentration in mcg/mL) multiplied by 100 = units to draw.
Let me demonstrate with the 10,000 mcg/mL concentration from adding 2mL water to a 20mg vial.
For a 2mg dose:
2mg = 2,000 mcg
2,000 mcg / 10,000 mcg/mL = 0.2mL
0.2mL x 100 = 20 units
For a 6mg dose:
6mg = 6,000 mcg
6,000 mcg / 10,000 mcg/mL = 0.6mL
0.6mL x 100 = 60 units
For a 12mg dose:
12mg = 12,000 mcg
12,000 mcg / 10,000 mcg/mL = 1.2mL
1.2mL x 100 = 120 units
The same formula works at any concentration. Just substitute your actual concentration into the denominator. The peptide calculator automates these conversions, but understanding the math helps you verify your results and catch potential errors before they affect your research.
Practical tips for accurate dosing
Several practical considerations affect dosing accuracy beyond the basic math.
Air bubbles in your syringe reduce the actual peptide amount you inject. A bubble that takes up 5 units of space means you are getting 5 fewer units of solution than you think. Always tap the syringe to move bubbles to the top and push them out before injecting. This applies to all peptide injections, not just retatrutide.
Drawing from the vial consistently also matters. Insert the needle, invert the vial, and draw slowly. If you draw too fast, you may create bubbles or draw air instead of solution. Take your time. The extra 30 seconds prevents dosing errors that could affect your entire week.
Double-check your math before every injection, especially during the first few weeks when you are establishing your routine. Mistaking 40 units for 80 units doubles your dose. At higher concentrations, even small visual errors on the syringe create meaningful dose differences. SeekPeptides provides dose verification tools that help members confirm their calculations before drawing.
How long does a 20mg vial last
Understanding vial duration helps you plan your research timeline and budget. The answer depends on your target dose, titration schedule, and how long you plan to maintain at your final dose.
Duration at different dose levels
At 1mg weekly (titration start):
20mg / 1mg per week = 20 weeks from a single vial
You would never use the entire vial at this dose since titration advances every 4 weeks.
At 4mg weekly (moderate maintenance):
20mg / 4mg per week = 5 weeks per vial
A common maintenance dose for those who respond well to lower amounts.
At 8mg weekly (standard maintenance):
20mg / 8mg per week = 2.5 weeks per vial
Represents the therapeutic sweet spot identified in early trials.
At 12mg weekly (maximum studied dose):
20mg / 12mg per week = approximately 1.7 weeks per vial
The highest dose with the most dramatic results requires more frequent vial replacement.
Planning for complete protocols
A full titration from 1mg to 12mg over 20 weeks requires approximately 80mg total. That means four 20mg vials just to reach your maintenance dose. If you then maintain at 12mg weekly for 24 additional weeks (6 months), you need roughly 288mg more, or about 14-15 additional vials.
Of course, most researchers do not commit to maximum doses from the start. Many find satisfactory results at 4-8mg weekly, significantly reducing both cost and vial requirements. Planning your protocol in advance helps you budget appropriately and ensures you have sufficient supply to complete your research without interruption.
The reconstituted solution remains stable for approximately 4 weeks under proper refrigeration. At 12mg weekly doses, you will use most of a vial before stability becomes a concern. At lower doses, you may need to discard remaining solution after 4 weeks if significant amounts remain. Factor this potential waste into your planning calculations.
Storage and stability requirements
Proper storage directly impacts peptide potency and your research results. Retatrutide, like most peptides, degrades when exposed to improper conditions. Understanding these requirements helps you maintain potency throughout your protocol.
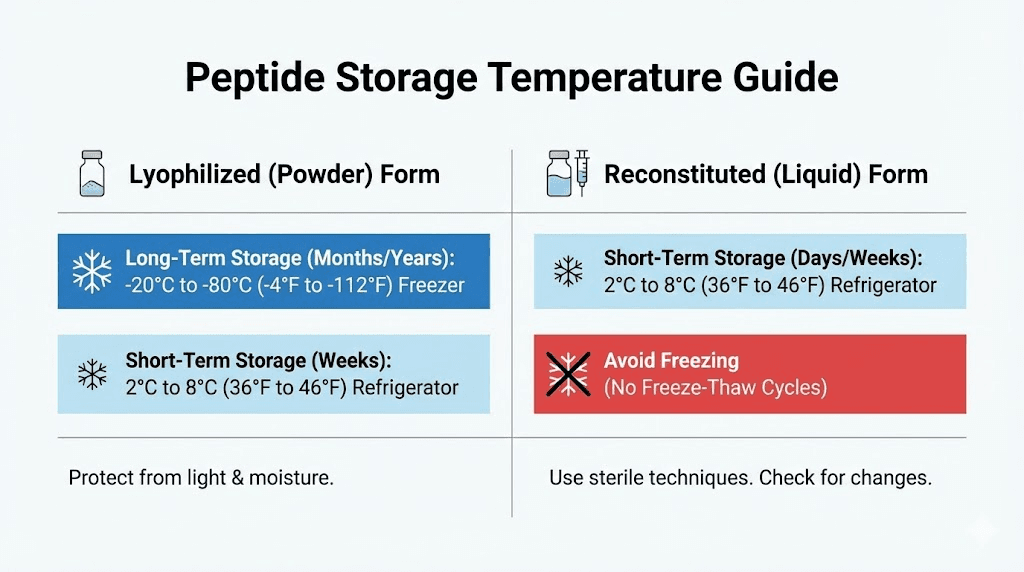
Unreconstituted powder storage
Before adding bacteriostatic water, lyophilized retatrutide is relatively stable. Store sealed vials in a refrigerator at 2-8 degrees Celsius. Protect from light by keeping vials in their original packaging or a dark container. Under these conditions, the powder remains stable for months to years depending on manufacturing date.
Room temperature storage accelerates degradation. While brief exposure during shipping rarely causes problems, prolonged storage at room temperature significantly reduces potency. If your vials arrived warm, refrigerate immediately and use them earlier in your planned protocol when potency is still highest. For more details on temperature effects, see our guide on how long peptides last at room temperature.
Freezing unreconstituted peptides is generally acceptable but unnecessary if you plan to use them within their stated shelf life. The freeze-thaw cycle can potentially damage the lyophilized cake structure, making reconstitution slightly more difficult without affecting actual peptide integrity.
Reconstituted solution storage
Once you add bacteriostatic water, storage requirements become more stringent. Refrigerate immediately at 2-8 degrees Celsius. Never freeze reconstituted peptide solutions. The ice crystal formation during freezing can physically damage peptide structures and significantly reduce potency.
Light exposure accelerates degradation in solution form. Store vials in a dark area of your refrigerator or wrap them in foil. The amber or clear glass of the vial provides some protection, but minimizing light exposure extends effective shelf life.
The bacteriostatic water itself contains benzyl alcohol as a preservative. This prevents bacterial growth but does not stop chemical degradation of the peptide. Most experts recommend using reconstituted retatrutide within 4 weeks for optimal potency. After 6 weeks, significant degradation may occur even under ideal storage conditions. For comprehensive guidance, review our reconstituted peptide storage guide.
Signs of degradation to watch for
Properly stored retatrutide solution should be completely clear and colorless. Any cloudiness, particles, discoloration, or unusual viscosity indicates potential problems. Do not use solution that appears different from when you initially reconstituted it.
Performance degradation may not be visually apparent. If you notice reduced effectiveness at doses that previously worked well, degradation is a likely culprit. This is another reason to use fresh vials and respect storage timelines rather than trying to stretch supplies beyond recommended periods.
How retatrutide compares to other weight loss peptides
Context helps you understand where retatrutide fits within the broader weight loss peptide landscape. Each option has distinct mechanisms, effectiveness profiles, and practical considerations. Understanding these differences helps you make informed decisions about your research direction.
Retatrutide versus semaglutide
Semaglutide (Wegovy, Ozempic) activates only GLP-1 receptors. This produces appetite suppression, delayed gastric emptying, and improved glucose metabolism. Clinical trials show average weight loss of approximately 13.9% at the 2.4mg weekly dose over 68 weeks.
Retatrutide adds GIP and glucagon receptor activation to the GLP-1 effects. The triple mechanism produces substantially greater weight loss. At 12mg weekly, trials show 24.2% weight loss at 48 weeks and up to 28.7% at 68 weeks in Phase 3. That represents nearly double the effectiveness of semaglutide monotherapy.
The glucagon component specifically increases energy expenditure and fat mobilization. This means retatrutide does not rely solely on appetite suppression. Even if caloric intake remains similar, the metabolic effects can drive additional fat loss compared to GLP-1 only approaches.
For a detailed breakdown, see our semaglutide versus tirzepatide comparison which provides additional context on dual versus triple agonist mechanisms.
Retatrutide versus tirzepatide
Tirzepatide (Zepbound, Mounjaro) activates both GLP-1 and GIP receptors. Clinical trials demonstrate approximately 17.8% weight loss at the 15mg dose over 68-72 weeks. This positioned tirzepatide as more effective than semaglutide when it launched.
Retatrutide adds glucagon receptor activation on top of the GLP-1 and GIP effects. The additional pathway appears to provide meaningful incremental benefit. Direct head-to-head trials are not yet available, but comparing across studies suggests retatrutide produces approximately 6-10 percentage points more weight loss than tirzepatide at equivalent treatment durations.
The glucagon effect on energy expenditure may explain some of this difference. Studies suggest retatrutide preferentially targets fat mass while preserving more lean tissue compared to calorie restriction alone. This favorable body composition effect adds value beyond the scale number.
Availability and practical considerations
An important distinction is regulatory status. Semaglutide and tirzepatide are FDA-approved and available through prescriptions. Retatrutide remains investigational, with Phase 3 trials ongoing and potential approval not expected until mid-2026 or 2027 at the earliest.
This means retatrutide is currently only available for research purposes through specialized suppliers. The 20mg vial format discussed in this guide reflects the research market, not pharmaceutical packaging that would come with eventual approval. Researchers should understand this context when sourcing materials and planning protocols.
For those exploring the research peptide landscape, our vendor guide provides information on evaluating suppliers and quality considerations.
Managing side effects during your protocol
Gastrointestinal effects represent the primary tolerability concern with retatrutide. Understanding what to expect and how to minimize discomfort helps you complete your protocol successfully. Most side effects are manageable with proper preparation and technique.
Common side effects and frequency
Phase 3 trial data from TRIUMPH-4 provides the clearest picture of what to expect at clinical doses.
At 9mg weekly:
Nausea: 38.1%
Diarrhea: 34.7%
Constipation: 21.8%
Vomiting: approximately 15-20%
At 12mg weekly:
Nausea: 43.2%
Diarrhea: 33.1%
Constipation: 25.0%
Vomiting: approximately 18-22%
These numbers look concerning, but context matters. Most symptoms are mild to moderate. They occur primarily during dose escalation. And they diminish substantially after 8-12 weeks on a stable dose. The majority of trial participants completed their protocols despite experiencing some GI symptoms.
Strategies to minimize discomfort
Follow the titration schedule
The single most effective strategy is respecting the four-week intervals between dose increases. Rushing titration dramatically increases symptom severity. Your body needs time to adapt to each dose level before taking on additional receptor activation. Patience during the first 20 weeks pays dividends throughout the rest of your protocol.
Time your injection strategically
Many researchers find that injecting in the evening before bed helps manage initial nausea. Sleeping through the peak effect period means waking up already past the worst of any symptoms. Others prefer morning injection so they can monitor their response throughout the day. Experiment to find what works for your schedule.
Adjust your eating patterns
Smaller, more frequent meals often help manage GI symptoms. The appetite suppression means you may not want large meals anyway. Avoiding fatty or heavy foods, especially in the days immediately following injection, can reduce nausea and digestive upset.
Stay hydrated
Both diarrhea and constipation can occur on retatrutide. Adequate water intake helps with both. It supports digestion, prevents dehydration from diarrhea episodes, and helps maintain regular bowel movements. Aim for at least 8-10 glasses of water daily.
Consider supportive supplements
Ginger supplements may help some researchers manage nausea. Fiber supplements can address constipation. Electrolyte drinks help if diarrhea occurs. These supportive measures do not reduce retatrutide effectiveness but can make the protocol more tolerable.
When to consider dose adjustment
Severe or persistent side effects warrant evaluation. If symptoms significantly impact daily functioning beyond the first week at a new dose, consider extending that dose level for an additional four weeks before increasing. The goal is steady progress, not racing to maximum dose while miserable.
Some researchers find their optimal dose is lower than the maximum studied amount. If 8mg weekly produces excellent results with minimal side effects, there may be no benefit to pushing to 12mg if doing so causes substantial discomfort. Individualized protocols based on personal response optimize the balance between effectiveness and tolerability.
Injection technique and administration
Proper injection technique ensures consistent peptide delivery and minimizes injection site reactions. Retatrutide is administered subcutaneously, meaning into the fat layer just beneath the skin. This is the same technique used for insulin and most other injectable peptides.
Selecting injection sites
The three most common subcutaneous injection sites are the abdomen, thighs, and upper arms.
Abdomen: Most popular site due to easy access and ample subcutaneous tissue. Inject at least 2 inches away from the navel. Avoid any areas with scars, bruises, or skin abnormalities.
Thighs: The front and outer portions of the upper thigh provide good subcutaneous access. This site works well for self-injection and is easily accessible.
Upper arms: The back of the upper arm works if you have help or good flexibility. Less convenient for self-administration but provides site rotation options.
Rotate between sites and specific locations within each site. Repeated injections in the exact same spot can cause lipohypertrophy, where fatty tissue changes texture and affects absorption. Move at least 1 inch from your previous injection location each time.
Step by step injection process
Step 1: Wash your hands thoroughly with soap and water.
Step 2: Draw your calculated dose as described earlier. Remove air bubbles by tapping the syringe and pushing them out.
Step 3: Clean the injection site with an alcohol swab. Let it air dry completely. Injecting through wet alcohol stings.
Step 4: Pinch a fold of skin at your chosen site. This lifts the subcutaneous layer away from underlying muscle.
Step 5: Insert the needle at a 45-90 degree angle depending on your body composition. Leaner individuals may need the 45 degree angle to stay in subcutaneous tissue. Those with more body fat can inject straight in at 90 degrees.
Step 6: Inject the solution slowly and steadily. There is no need to rush. Take 5-10 seconds to deliver the dose.
Step 7: Wait 5 seconds after the plunger is fully depressed before withdrawing the needle. This ensures all solution has been delivered.
Step 8: Withdraw the needle and apply gentle pressure with a clean cotton ball or gauze if there is any bleeding. Do not rub the site.
Step 9: Dispose of the used syringe in a proper sharps container. Never reuse needles.
Troubleshooting common issues
Injection site redness or swelling: Mild reactions are common and usually resolve within a day. If redness spreads, increases in size, or is accompanied by fever, seek medical evaluation.
Difficulty injecting: If the plunger is hard to push, the needle may be blocked. Withdraw and try a new needle. Never force an injection.
Leakage after injection: A small amount of solution may sometimes leak back out. This is usually negligible. Injecting more slowly and waiting before withdrawing the needle minimizes this issue.
Bruising: Occasionally unavoidable, especially if you hit a small blood vessel. Apply gentle pressure and avoid that specific spot for future injections.
Creating your complete protocol
With all the individual components understood, you can now assemble a complete retatrutide protocol tailored to your research goals. This section provides a framework you can adapt based on your specific circumstances.
Protocol planning checklist
Determine your timeline:
Most meaningful protocols run 24-48 weeks minimum. Shorter periods may show initial response but do not capture full potential. Plan for at least 20 weeks of titration plus 12-28 weeks of maintenance at your target dose.
Calculate your supply needs:
Based on your target maintenance dose and protocol duration, determine how many 20mg vials you need. Add 10-20% buffer for potential delays, degradation, or protocol adjustments.
Choose your concentration:
Decide whether the 2mL or 4mL reconstitution makes more sense for your dosing needs. Most researchers use 2mL for the higher concentration that keeps injection volumes small.
Set up proper storage:
Ensure you have reliable refrigeration. Designate a specific location for your vials. Have supplies for labeling and tracking reconstitution dates.
Establish your injection schedule:
Pick a consistent day of the week for your injections. Retatrutide is administered once weekly. Consistency helps you remember doses and allows you to recognize patterns in your response.
Sample 24-week protocol using 20mg vials
This example demonstrates how the various elements come together for a practical protocol targeting 8mg maintenance.
Weeks 1-4: 1mg weekly (4mg total, small portion of first vial)
Weeks 5-8: 2mg weekly (8mg total)
Weeks 9-12: 4mg weekly (16mg total, finish first vial and start second)
Weeks 13-16: 6mg weekly (24mg total, continue through second vial)
Weeks 17-20: 8mg weekly (32mg total)
Weeks 21-24: 8mg weekly maintenance (32mg total)
Total peptide required: 116mg over 24 weeks
Vials needed: 6 x 20mg vials with some remaining
This protocol reaches 8mg maintenance by week 17 and holds there through week 24. You could extend the maintenance phase or continue titrating to 12mg depending on your response and goals.
Tracking and documentation
Successful researchers track key metrics throughout their protocols. This data helps you make informed decisions about dose adjustments and evaluate your overall response.
Weekly measurements:
Body weight at the same time each week
Waist and hip circumference
Side effect severity (0-10 scale)
Energy levels (0-10 scale)
Appetite level (0-10 scale)
Monthly assessments:
Progress photos from consistent angles
Body fat estimates if available
Overall protocol satisfaction
Any notable changes in health markers
SeekPeptides members access comprehensive tracking templates and progress visualization tools that simplify this documentation process. The data you collect helps you optimize your protocol and provides valuable reference for future research.
Frequently asked questions
How much bacteriostatic water should I add to a 20mg retatrutide vial?
The most common approach is adding 2mL of bacteriostatic water, which creates a concentration of 10,000 mcg/mL. This makes dose calculations straightforward: each 0.1mL (10 units) contains 1mg of retatrutide. Alternatively, adding 4mL creates a 5,000 mcg/mL concentration for those who prefer larger injection volumes or easier titration between doses.
What is the standard starting dose for retatrutide?
Clinical trials typically start at 1mg or 2mg weekly. The conservative 1mg starting dose allows assessment of individual tolerance before increasing. Phase 3 protocols often begin at 2mg. Either approach is reasonable. The key is following a gradual titration schedule that increases every four weeks rather than jumping directly to higher doses.
How long does reconstituted retatrutide remain stable?
Properly refrigerated reconstituted retatrutide maintains potency for approximately 4 weeks. Store at 2-8 degrees Celsius, protected from light. After 4-6 weeks, degradation may affect effectiveness even under ideal conditions. At higher maintenance doses, you will typically use an entire vial within this window. At lower doses, plan to discard remaining solution after the stability period.
Can I use regular sterile water instead of bacteriostatic water?
Bacteriostatic water is strongly preferred because it contains benzyl alcohol as a preservative. This prevents bacterial growth during the weeks between reconstitution and complete use. Sterile water lacks this preservative, meaning the solution must be used immediately or within 24-48 hours. Given retatrutide is dosed weekly, bacteriostatic water is essentially required for safe multi-week use from a single reconstitution.
What should I do if I miss a weekly dose?
If you miss your scheduled injection day, take it as soon as you remember, then return to your regular schedule the following week. Do not double up doses to compensate. If several days have passed and your next scheduled dose is approaching, skip the missed dose and continue with your regular schedule. Consistency matters more than making up for individual missed doses.
How quickly will I see results from retatrutide?
Most researchers report noticeable appetite suppression within the first 1-2 weeks. Measurable weight changes typically appear by weeks 4-8. However, the most significant results emerge over 24-48 weeks of consistent use. Phase 2 trials showed 17.5% weight loss at 24 weeks and 24.2% at 48 weeks at the 12mg dose. Patience and consistency produce the best outcomes.
Is retatrutide approved by the FDA?
No, retatrutide remains investigational as of early 2026. It is currently in Phase 3 clinical trials. Approval is not expected until mid-2026 or 2027 at the earliest. Current access is limited to clinical trials and research peptide suppliers. This guide addresses the research context using 20mg vials available through those channels.
External resources
New England Journal of Medicine - Phase 2 Retatrutide Trial Results
ClinicalTrials.gov - TRIUMPH Phase 3 Study Information
In case I do not see you, good afternoon, good evening, and good night. May your reconstitutions stay clear, your calculations stay accurate, and your protocols stay consistent.



