Jan 19, 2026

The term "alpha peptides" appears everywhere in peptide research. Forums mention it. Vendors use it in their branding. Scientific papers reference alpha-designated compounds constantly. Yet most people searching for alpha peptides aren't entirely sure what they're looking for.
That confusion makes sense. "Alpha" in peptide terminology serves multiple purposes. It can describe a peptide's structural configuration, designate a specific compound within a family of related molecules, or simply function as part of a vendor's name. Understanding which meaning applies to your situation determines whether you'll find what you actually need.
This guide breaks down every significant meaning of alpha peptides.
You'll learn about alpha-helix structures that form the backbone of many therapeutic compounds.
You'll understand specific peptides like thymosin alpha-1 that support immune function. And you'll discover alpha-MSH derivatives that researchers study for everything from skin pigmentation to metabolic regulation. By the end, you'll know exactly which alpha peptide category matches your research goals, and which safety considerations apply to each one.
SeekPeptides has compiled research from dozens of scientific papers, clinical trials, and expert sources to create this comprehensive resource. Whether you're a researcher investigating immune modulation, someone interested in peptide science, or simply trying to understand what alpha peptides actually are, this guide provides the clarity you need.
Understanding the term "alpha" in peptide science
The Greek letter alpha appears throughout biochemistry. It typically designates something as "first" or "primary" within a classification system. In peptide science, this convention creates several distinct categories that share the alpha designation but serve completely different functions.
First, there's structural alpha. The alpha helix represents one of two primary secondary structures in proteins and peptides. When amino acids fold into a right-handed spiral pattern stabilized by hydrogen bonds, that's an alpha helix. This structural element appears in countless therapeutic peptides and determines how they interact with cellular receptors.
Second, there's naming alpha. Many peptide families contain multiple variants designated by Greek letters. Alpha-melanocyte stimulating hormone (alpha-MSH) differs from beta-MSH and gamma-MSH. Alpha defensins represent one subfamily of antimicrobial peptides, distinct from beta defensins. Thymosin alpha-1 is the primary immune-modulating thymosin, separate from thymosin beta-4.
Third, there's vendor alpha. Some peptide suppliers incorporate "alpha" into their business names. This creates search confusion when researchers look for alpha peptides as a category and instead find commercial suppliers.
Each meaning requires different understanding. Let's examine them systematically.
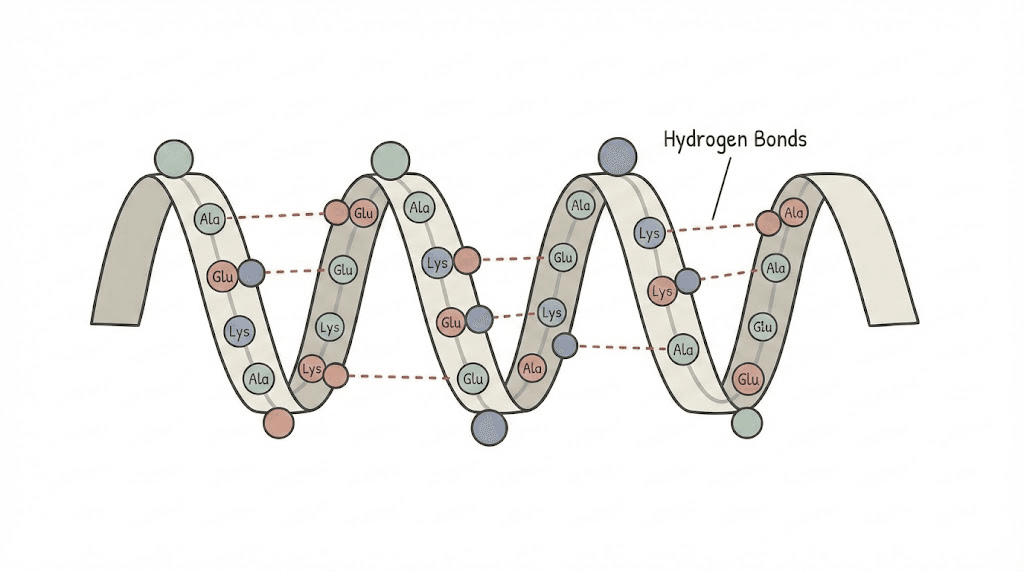
Alpha helix: the foundational peptide structure
Linus Pauling proposed the alpha helix structure in 1951, fundamentally changing how scientists understood protein organization.
This discovery earned him a Nobel Prize and established the foundation for modern peptide research.
How alpha helices form
Alpha helices arise from predictable folding patterns. Every 3.6 amino acid residues, the peptide backbone completes one full turn of the spiral. Hydrogen bonds form between the carbonyl oxygen of one residue and the amino hydrogen of a residue four positions later. This creates remarkable stability.
The structure isn't random. Certain amino acids strongly favor alpha helix formation. Alanine, glutamate, leucine, and methionine readily participate in helical structures.
Proline and glycine typically disrupt helices, creating kinks or flexibility points that can be functionally important.
Understanding this matters for peptide handling. Storage conditions, temperature changes, and reconstitution methods can affect whether therapeutic peptides maintain their intended structural conformations.
A peptide that loses its alpha helical regions may lose biological activity.
Why alpha helices matter for therapeutic peptides
Many injectable peptides rely on alpha helical regions to bind their target receptors. The helix presents amino acid side chains in specific orientations. Receptor proteins recognize these presentations and initiate downstream signaling cascades.
Consider GLP-1 receptor agonists like semaglutide. These peptides form alpha helical structures that fit into binding pockets on GLP-1 receptors. The helix orients critical residues precisely where they need to be for receptor activation. Disrupting that helix would eliminate the peptide's function.
This structural requirement influences storage protocols. Extreme temperatures denature proteins by unfolding secondary structures. Repeated freeze-thaw cycles stress peptide molecules. Proper handling preserves the alpha helical content that makes therapeutic peptides effective.
Alpha helices in cell-penetrating peptides
Some research peptides must enter cells to exert their effects. Cell-penetrating peptides often adopt alpha helical conformations upon membrane contact. This structural transition helps them cross lipid bilayers that would otherwise block entry.
The amphipathic nature of many alpha helices facilitates this. One face of the helix presents hydrophobic residues that interact favorably with membrane lipids. The opposite face displays charged or polar residues that remain soluble. This dual character enables membrane translocation.
Researchers studying nootropic peptides pay particular attention to these properties. Blood-brain barrier penetration requires specific structural features. Alpha helical content influences whether a peptide can reach its intended neural targets.
Thymosin alpha-1: the immune modulation peptide
Thymosin alpha-1 represents perhaps the most clinically significant alpha-designated peptide. Originally isolated from thymus tissue in the 1970s, this 28-amino-acid peptide plays central roles in immune system development and function.
What thymosin alpha-1 does
The thymus gland produces thymosin alpha-1 to regulate T-cell maturation. Without adequate thymosin alpha-1, T-cells fail to develop properly. This compromises the adaptive immune response.
But thymosin alpha-1 does far more than guide T-cell development. Research shows it modulates dendritic cell function, influences natural killer cell activity, and regulates inflammatory cytokine production. It doesn't simply boost immunity. It fine-tunes immune responses.
This distinction matters. Crude immune stimulation can worsen autoimmune conditions or trigger dangerous inflammatory cascades. Thymosin alpha-1's regulatory nature allows it to enhance immunity against pathogens while potentially reducing harmful inflammation. It's why researchers consider it an immunomodulator rather than a simple immune stimulant.
Clinical applications and research
Thymalfasin, the synthetic form of thymosin alpha-1, has regulatory approval in over 35 countries. Primary indications include chronic hepatitis B and hepatitis C treatment, often as an adjunct to other antiviral therapies.
Research extends into numerous other areas. Cancer immunotherapy studies examine whether thymosin alpha-1 can enhance responses to checkpoint inhibitors. Sepsis research investigates its potential to restore immune function in critically ill patients. Vaccine studies explore its use as an adjuvant to improve antibody responses, particularly in elderly populations whose immune systems respond poorly to standard vaccines.
The integrative medicine community has adopted thymosin alpha-1 for various applications. Practitioners use it to support immune function in patients with chronic infections, mold exposure, or generalized immune dysfunction.
While some applications have more robust evidence than others, thymosin alpha-1's established safety profile makes it attractive for therapeutic trials.
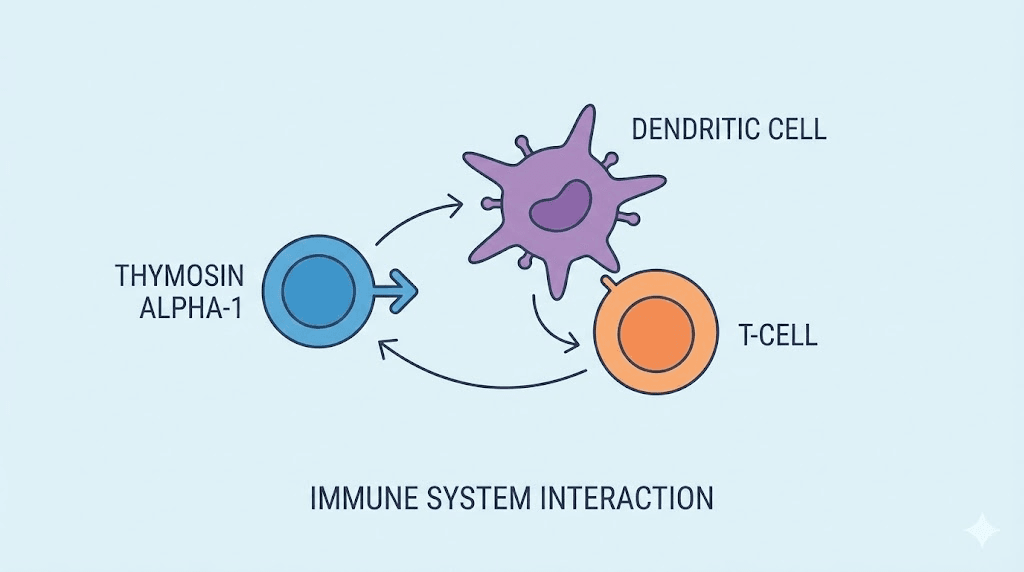
Dosing considerations for thymosin alpha-1
Standard clinical dosing typically involves 1.6mg administered subcutaneously twice weekly. This protocol derives from hepatitis treatment studies that established both efficacy and safety at this level.
Some practitioners use higher doses or more frequent administration for acute conditions. Daily dosing of 1.6mg appears in some sepsis protocols.
However, optimal dosing for various conditions remains an active research question.
Duration matters as well. Hepatitis treatment protocols often extend for months. Vaccine adjuvant use might involve only a few doses timed around immunization. Chronic immune support protocols vary widely in their recommendations.
Proper dosing requires understanding the specific research context. What works for one application may not translate directly to another.
Safety profile of thymosin alpha-1
Thymosin alpha-1 has accumulated an extensive safety record through decades of clinical use. Over 3,000 patients participated in clinical trials, and countless more have used it therapeutically since approval.
Reported adverse effects remain mild and infrequent. Injection site reactions occur occasionally. Some patients report transient fatigue or flu-like symptoms, possibly indicating immune activation.
Compared to other immune-modulating agents like interferons or interleukins, thymosin alpha-1's adverse effect profile appears remarkably benign.
This safety advantage contributes to its appeal for long-term use and its adoption in integrative protocols.
However, theoretical concerns exist for certain populations. Anyone with autoimmune conditions should approach immune modulation cautiously. Enhanced immune function could potentially worsen autoimmune attacks. Research specifically addressing autoimmune safety remains limited.
Alpha-MSH: melanocortin peptides and their analogs
Alpha-melanocyte stimulating hormone (alpha-MSH) is a 13-amino-acid peptide derived from proopiomelanocortin (POMC). It earned its name from early research showing it stimulates melanocytes to produce melanin. But subsequent research revealed functions far beyond pigmentation.
What alpha-MSH does in the body
Melanogenesis remains alpha-MSH's most visible effect. When alpha-MSH binds MC1 receptors on melanocytes, it triggers melanin production. This darkens skin and hair as a protective response to UV radiation.
But alpha-MSH also acts as a potent anti-inflammatory agent. It suppresses production of pro-inflammatory cytokines including IL-1, IL-6, TNF-alpha, and interferon-gamma. Simultaneously, it promotes anti-inflammatory IL-10. These actions make alpha-MSH a key endogenous regulator of inflammation.
The peptide also influences appetite and metabolism through hypothalamic MC4 receptors. Alpha-MSH signaling suppresses food intake and increases energy expenditure. Mutations affecting this pathway contribute to certain forms of obesity.
Sexual function represents another alpha-MSH effect. The peptide's action in the brain influences arousal pathways, leading to the development of synthetic analogs for sexual dysfunction treatment.
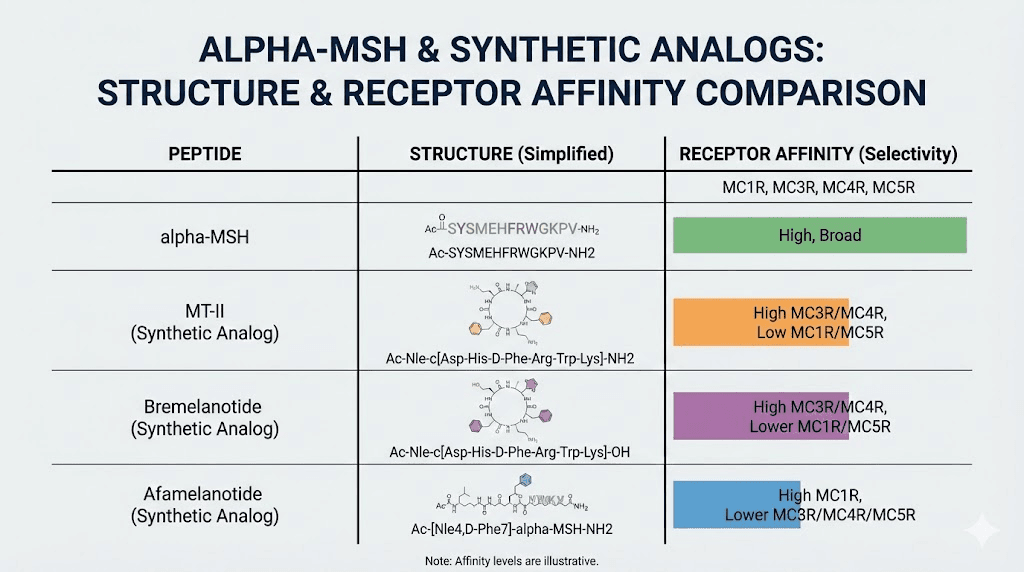
Synthetic alpha-MSH analogs
Natural alpha-MSH degrades rapidly in the body. Researchers developed synthetic analogs with improved stability and enhanced receptor selectivity. Two prominent examples have reached clinical application.
Afamelanotide (Melanotan I) is a linear analog with increased metabolic stability. It received FDA approval for erythropoietic protoporphyria, a painful photosensitivity disorder. The drug induces protective melanogenesis without requiring UV exposure, allowing patients to tolerate sunlight.
Melanotan II is a cyclic analog with broader receptor activity. It affects not only MC1 receptors but also MC3 and MC4 receptors significantly. This broader activity produces more diverse effects including appetite suppression and sexual arousal. Melanotan II never received regulatory approval due to safety concerns, but it circulates widely in research and gray-market contexts.
Bremelanotide represents a refined MC4-selective analog developed specifically for hypoactive sexual desire disorder. FDA approved it in 2019 for premenopausal women experiencing persistent lack of sexual desire causing distress.
Research and controversy surrounding melanocortin analogs
The tanning properties of alpha-MSH analogs created a market demand that outpaced clinical development. Unregulated melanotan products appeared online, used by individuals seeking enhanced tanning without sun exposure.
Health authorities expressed concern. Melanotan products sold online lack quality controls. Users inject compounds of uncertain purity at self-selected doses. Reports emerged of new or changed moles, with theoretical concerns about melanoma risk in susceptible individuals.
Research hasn't definitively linked melanotan use to melanoma development. A 2021 review suggested that any increased melanoma incidence in melanotan users likely reflects their sun-seeking behavior rather than direct drug effects. But the absence of controlled long-term safety data leaves questions unanswered.
Researchers studying melanocortin pathways for legitimate purposes must navigate this complicated landscape. The peptide family holds genuine therapeutic promise for conditions ranging from metabolic disorders to inflammatory diseases. But enthusiasm must be tempered with appropriate caution about unstudied long-term effects.
Alpha defensins: antimicrobial peptides of the immune system
Alpha defensins represent an ancient arm of innate immunity. These small cationic peptides appeared early in evolutionary history and remain crucial for host defense across species including humans.
Structure and classification of alpha defensins
Human alpha defensins are 29-35 amino acids long, containing three disulfide bonds in a characteristic pattern. This structural motif distinguishes them from beta defensins, which have a different disulfide arrangement.
Six human alpha defensins exist. Four are called human neutrophil peptides (HNP-1, HNP-2, HNP-3, HNP-4) because neutrophils store them in granules. Two are human defensins 5 and 6 (HD-5, HD-6), produced by Paneth cells in the small intestine.
The mechanism involves membrane disruption. Defensins are positively charged, while bacterial membranes are negatively charged. This electrostatic attraction brings defensins into contact with microbial surfaces.
The peptides then insert into membranes, forming pores or otherwise disrupting membrane integrity.
Antimicrobial activity
Alpha defensins demonstrate broad-spectrum activity. They kill gram-positive and gram-negative bacteria. They inactivate enveloped viruses. They combat fungi and even some parasites.
The breadth of activity stems from targeting fundamental microbial features. All bacteria require intact membranes.
By attacking this universal vulnerability, defensins remain effective against organisms that might resist conventional antibiotics targeting specific enzymatic pathways.
This has attracted interest in defensins as potential therapeutic agents. With antibiotic resistance spreading globally, peptides like defensins offer alternative approaches. Research explores both natural defensins and designed variants with enhanced potency or stability.
Defensins beyond antimicrobial function
Alpha defensins do more than kill microbes directly. They modulate immune responses in sophisticated ways. They enhance phagocytosis, helping immune cells engulf and destroy pathogens. They recruit other immune cells to infection sites. They influence cytokine production, shaping the broader inflammatory response.
Intestinal alpha defensins (HD-5 and HD-6) regulate gut microbiome composition.
By selectively killing certain bacteria while sparing others, they shape which organisms colonize the gut. HD-6 doesn't actually kill bacteria, but forms "nanonets" that trap pathogens and prevent them from invading intestinal tissue.
This gut health connection has clinical implications. Defective defensin production associates with Crohn's disease in some patients. Restoring defensin function represents a potential therapeutic strategy for inflammatory bowel conditions.
Research applications
Researchers use alpha defensins as tools and as therapeutic candidates. They serve as markers of neutrophil activation and infection. Elevated defensin levels in synovial fluid, for example, indicate prosthetic joint infection with high sensitivity and specificity.
Drug development programs explore defensin-based antibiotics.
The challenge lies in translating laboratory activity into clinical drugs. Defensins are expensive to produce, potentially toxic at high concentrations, and vulnerable to degradation.
Synthetic variants attempt to address these limitations while preserving antimicrobial potency.
The broader lesson from defensin research is that natural peptides represent an underexplored pharmacy. Evolution has refined these molecules over billions of years. Understanding them opens therapeutic possibilities that purely synthetic approaches might miss.
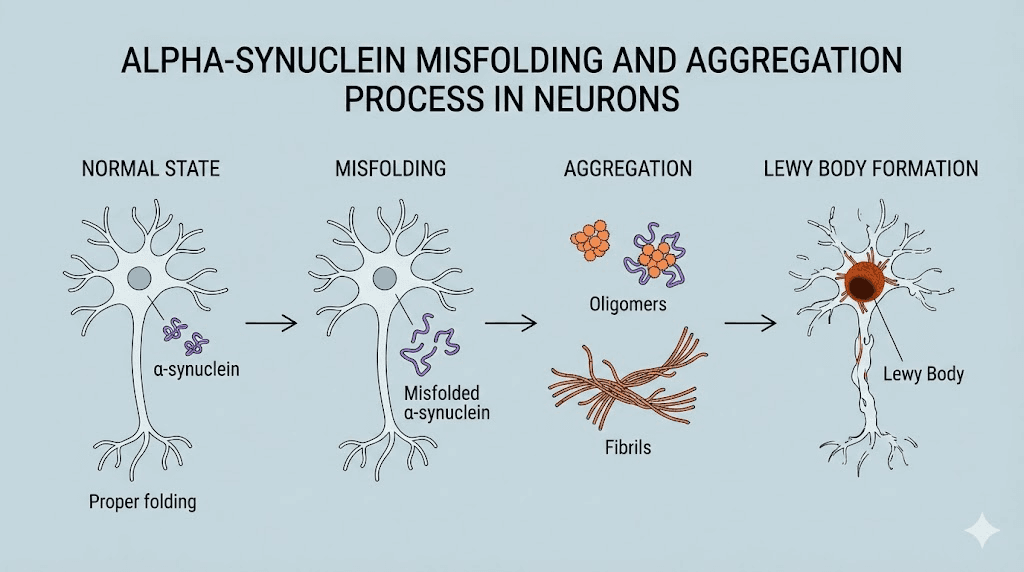
Alpha synuclein: the Parkinson's disease peptide
Alpha synuclein illustrates how a single peptide can transform from normal cellular component to disease driver. Understanding this transformation guides research into Parkinson's disease and related conditions.
Normal alpha synuclein function
Alpha synuclein is a 140-amino-acid protein abundant in brain tissue, particularly at presynaptic terminals. Under normal circumstances, it appears to regulate neurotransmitter release, synaptic plasticity, and vesicle trafficking.
The protein adopts different structures depending on its environment. In solution, it remains largely unstructured. Upon binding membranes, it adopts alpha helical conformations that facilitate its functions.
This structural flexibility becomes problematic when misfolding occurs. Misfolded alpha synuclein aggregates into toxic oligomers and eventually into insoluble fibrils.
These aggregates form the characteristic Lewy bodies found in Parkinson's disease brains.
Alpha synuclein in disease
Parkinson's disease affects over 10 million people worldwide. The hallmark symptoms, including tremor, rigidity, and movement difficulties, result from dopamine neuron death in a brain region called the substantia nigra.
Alpha synuclein aggregation correlates strongly with this neuronal death. Genetic evidence reinforces the connection. Mutations in the alpha synuclein gene cause familial Parkinson's disease. Duplications or triplications of the gene, leading to excess alpha synuclein production, also cause disease.
The aggregation appears to spread through the brain in a prion-like manner. Misfolded alpha synuclein can seed the misfolding of normal protein, propagating pathology from cell to cell. This spreading may explain Parkinson's characteristic pattern of progression.
Related "synucleinopathies" include dementia with Lewy bodies and multiple system atrophy. Each involves alpha synuclein aggregation, though with distinct cellular and regional patterns.
Peptide-based therapeutic approaches
Current Parkinson's treatments manage symptoms but don't stop disease progression. Researchers seek drugs that prevent alpha synuclein aggregation or clear existing aggregates. Peptide-based approaches show promise.
Some peptides bind to alpha synuclein and stabilize its normal conformation, preventing misfolding. Others disrupt existing aggregates. Cell-penetrating peptide conjugates can deliver these therapeutic sequences across neuronal membranes where aggregation occurs.
One innovative approach uses peptides to target alpha synuclein for degradation. The Tat-βsyn-degron peptide, for example, crosses membranes and promotes proteasomal destruction of alpha synuclein. Animal studies showed reduced alpha synuclein levels and improved outcomes.
Immunotherapy represents another peptide-related strategy. Antibodies targeting alpha synuclein can neutralize toxic species or facilitate their clearance. Several monoclonal antibodies have entered clinical trials, though results so far have been mixed.
The field remains optimistic despite challenges. Understanding alpha synuclein biology continues to deepen. New therapeutic targets emerge regularly. The eventual development of disease-modifying treatments seems achievable, even if the timeline remains uncertain.
Alpha-CGRP: the migraine peptide
Alpha-calcitonin gene-related peptide (alpha-CGRP) has transformed migraine treatment. Understanding its role led to entirely new drug classes that help patients who failed previous therapies.
What alpha-CGRP does
Alpha-CGRP is a 37-amino-acid neuropeptide produced by sensory neurons. It functions primarily as a vasodilator, relaxing blood vessels when released. The cardiovascular system depends on CGRP for normal vascular regulation.
In the trigeminal system that innervates the head and face, alpha-CGRP release triggers events associated with migraine. Blood vessels dilate. Surrounding tissue becomes inflamed. Pain signals intensify. The full cascade produces the throbbing headache, nausea, and sensory sensitivities characteristic of migraine attacks.
Researchers confirmed CGRP's central role through multiple observations. CGRP levels rise during migraine attacks. Intravenous CGRP administration triggers migraine in susceptible individuals. Blocking CGRP prevents or aborts migraine episodes.
Anti-CGRP therapies
The CGRP-migraine connection enabled development of targeted treatments. Two approaches emerged. Monoclonal antibodies neutralize CGRP itself or block its receptor. Small molecule "gepants" also block the receptor but can be taken orally.
Four monoclonal antibodies reached market approval: erenumab (which blocks the receptor), fremanezumab, galcanezumab, and eptinezumab (which target CGRP itself). These injectable preventive treatments reduce monthly migraine days significantly in clinical trials.
Gepants like rimegepant and ubrogepant work for acute treatment, aborting migraine attacks already in progress. Some also have preventive applications with regular dosing.
These therapies represent genuine breakthroughs. They work differently than triptans or traditional preventives. Patients who failed multiple previous treatments often respond. Side effects tend to be mild compared to older options.
Cardiovascular considerations
CGRP's cardiovascular importance raised initial safety concerns. Would blocking a vasodilator cause harmful cardiovascular effects? Long-term data have been reassuring, but questions remain for patients with significant cardiovascular disease.
Clinical trials excluded patients with unstable cardiovascular conditions. Post-marketing surveillance continues monitoring for unexpected cardiovascular signals. So far, the safety profile appears acceptable, but the field remains vigilant.
The broader point is that targeting naturally occurring peptides requires understanding their full physiological roles. CGRP helps regulate vascular tone. Blocking it relieves migraine but might theoretically impair adaptive vascular responses.
Such considerations inform how researchers approach peptide-targeted drug development generally.
Beta amyloid and alpha secretase: Alzheimer's disease connections
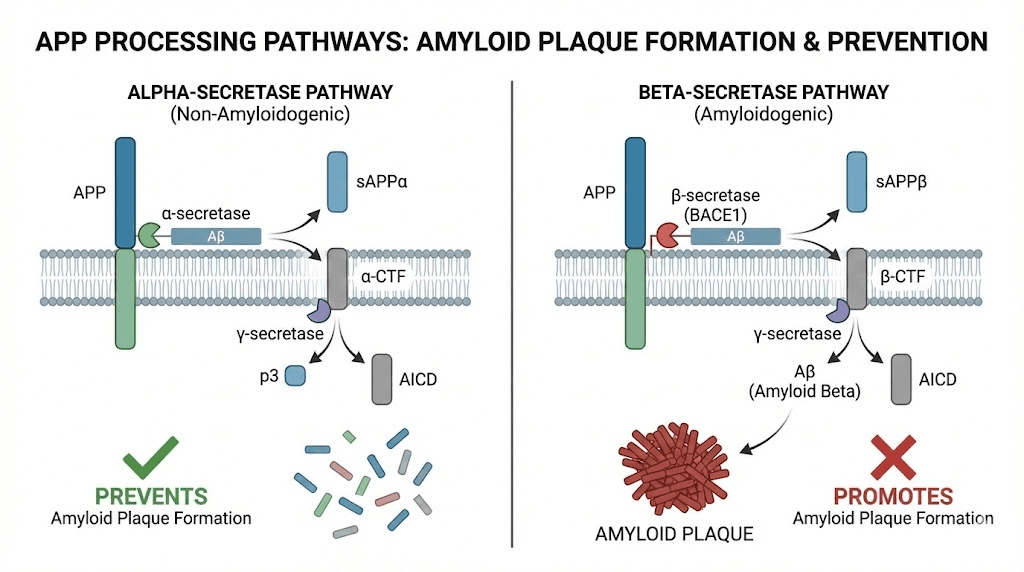
While beta amyloid causes the plaques characteristic of Alzheimer's disease, the alpha secretase enzyme that processes the amyloid precursor protein plays a protective role. Understanding this distinction matters for therapeutic development.
The amyloid hypothesis
Alzheimer's disease features two pathological hallmarks: amyloid plaques and neurofibrillary tangles. The plaques consist largely of beta amyloid peptide, a 36-43 amino acid fragment of amyloid precursor protein (APP).
APP can be processed by different enzymatic pathways. Beta and gamma secretases cleave it to produce beta amyloid. Alpha secretase cleaves within the beta amyloid sequence, preventing its formation.
The balance between these pathways influences whether toxic beta amyloid accumulates.
For decades, the amyloid hypothesis dominated Alzheimer's research. It proposed that beta amyloid accumulation causes the disease. This view guided drug development toward strategies to reduce beta amyloid production or accelerate its clearance.
Alpha secretase as therapeutic target
Enhancing alpha secretase activity represents one anti-amyloid strategy. If APP processing shifts toward the alpha pathway, less substrate remains for beta amyloid production. The non-amyloidogenic fragments produced might even have neuroprotective properties.
Several approaches attempt this. Some drugs activate alpha secretase directly. Others shift APP trafficking to compartments where alpha secretase operates more effectively. Lifestyle factors including exercise may also enhance alpha secretase activity.
The approach faces challenges. Secretase enzymes have other substrates beyond APP. Altering their activity too broadly might cause unintended consequences.
Selectivity for APP processing remains an ongoing research goal.
Recent anti-amyloid successes
After years of clinical trial failures, anti-amyloid antibodies recently achieved regulatory approval. Aducanumab was approved controversially in 2021 based on surrogate endpoints. Lecanemab followed in 2023 with stronger evidence of cognitive benefit.
These antibodies target aggregated beta amyloid, clearing plaques from the brain. Lecanemab reduced cognitive decline by 27% compared to placebo over 18 months. The effect was modest but statistically significant.
The results vindicate decades of amyloid-focused research, though debates continue about clinical meaningfulness. Critics note that plaque clearance doesn't equal cure. Supporters counter that any disease modification represents crucial progress.
For researchers interested in brain health peptides, these developments illustrate both the potential and complexity of peptide-targeted therapeutics. Simple concepts become complicated when translated to human diseases. Persistence and refinement eventually yield progress.
Alpha peptides: vendors and suppliers
"Alpha Peptides" also appears as a business name for peptide vendors. This commercial context differs entirely from the biological meanings discussed above but often appears in searches for alpha peptides.
Understanding research peptide vendors
The research peptide market includes numerous suppliers using creative naming.
Alpha Peptides, Peptides Alpha, Alpha Omega Peptide, and similar variations all serve researchers seeking laboratory compounds.
These vendors typically sell peptides designated "for research purposes only." Products include popular compounds like BPC-157, TB-500, various growth hormone releasing peptides, and melanocortin analogs.
Quality varies significantly between vendors. Reputable suppliers provide certificates of analysis showing purity testing results. Third-party testing offers additional verification. Without such documentation, researchers cannot be confident in what they're actually receiving.
Evaluating peptide vendors
Several factors distinguish quality vendors from unreliable ones. Third-party testing stands paramount. Vendors who submit products to independent laboratories for verification demonstrate commitment to quality that self-testing cannot match.
Customer reviews provide real-world feedback, though these require interpretation. A few negative reviews among many positives may indicate isolated issues rather than systemic problems. Patterns of complaints about specific issues like shipping delays, customer service, or product quality matter more than individual reports.
Transparency about sourcing, manufacturing, and testing builds confidence. Vendors willing to discuss their quality control processes typically have better processes to discuss. Evasiveness or vague claims warrant skepticism.
SeekPeptides maintains resources helping researchers navigate vendor selection. Understanding what to look for prevents disappointment and wasted resources.
Legal considerations
Research peptide legality varies by jurisdiction and intended use. In the United States, many peptides can be legally purchased for research purposes. Human use, however, often requires different regulatory pathways.
Legal status changes over time. Regulatory agencies periodically reassess peptide classifications. What's available today may not be tomorrow. Researchers should stay informed about current rules governing compounds they study.
International shipping complicates matters further. Peptides legal in one country may face import restrictions elsewhere.
Customs seizures can result in lost products and potential legal scrutiny.
Choosing the right alpha peptide for your research
With so many meanings for "alpha peptides," selecting the right one requires clarifying your actual goals. Different applications call for different compounds.
For immune research
Thymosin alpha-1 remains the primary choice for immune modulation studies. Its extensive clinical history provides benchmark data for comparison. Well-characterized mechanisms allow hypothesis testing about specific immune pathways.
Researchers might also consider alpha defensins for antimicrobial immunity work. These peptides reveal fundamental aspects of innate defense and offer potential therapeutic development opportunities.
Immune-supporting peptides extend beyond those with "alpha" designations. BPC-157 influences immune function through different mechanisms. Thymosin beta-4 addresses tissue repair with immune implications. A comprehensive research program might incorporate multiple compounds.
For metabolic research
Alpha-MSH analogs offer tools for studying appetite regulation, energy expenditure, and metabolic signaling. The melanocortin system interacts with leptin pathways, making these peptides relevant for obesity and metabolic syndrome research.
Weight loss peptides represent an active research area. Beyond melanocortins, GLP-1 agonists and dual agonists like tirzepatide demonstrate that peptide-based approaches can achieve substantial metabolic effects.
For neurological research
Alpha synuclein studies require the pathological protein itself, available from specialized suppliers. Peptide fragments of alpha synuclein serve as tools for studying aggregation, developing detection methods, and testing therapeutic interventions.
The broader field of nootropic peptides includes numerous non-alpha options. Semax, Selank, and Pinealon all target brain function through different mechanisms than alpha-designated compounds.
For pain research
Alpha-CGRP antagonists have proven effective for migraine, but the peptides themselves serve research purposes. Understanding CGRP signaling reveals basic pain mechanisms and suggests therapeutic strategies for conditions beyond migraine.
Pain management peptides include healing-focused options like BPC-157 and TB-500 that address pain through tissue repair rather than receptor blockade. Joint pain research particularly benefits from these regenerative approaches.
Administration and handling of alpha peptides
Proper handling ensures that research peptides maintain their intended activity.
Alpha peptides, like all peptides, require attention to storage, reconstitution, and administration.
Storage requirements
Most peptides ship as lyophilized (freeze-dried) powders. This form offers maximum stability. Properly stored lyophilized peptides can remain viable for years.
Correct storage means cold and dark. Refrigeration suffices for short-term storage. Freezer storage at -20°C or colder extends stability significantly. Exposure to heat, light, or moisture degrades peptides over time.
Once reconstituted, peptide solutions become less stable. Refrigerated reconstituted peptides typically remain usable for weeks to months depending on the specific compound. Freezing can extend this but risks damage from ice crystal formation.
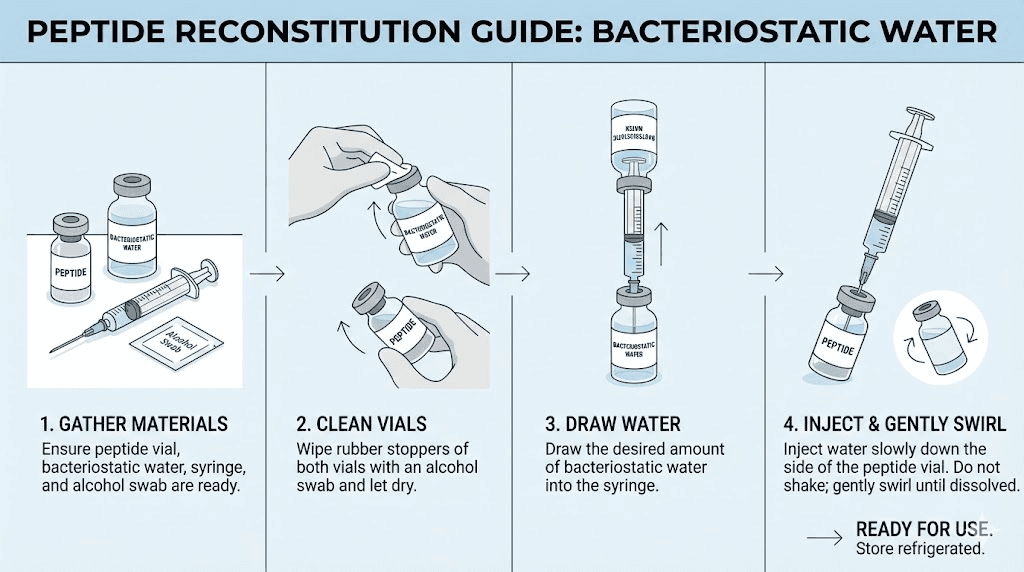
Reconstitution procedures
Proper reconstitution requires appropriate diluents. Bacteriostatic water is the standard choice for most research peptides. Its preservative content inhibits microbial growth in the reconstituted solution.
Sterile technique prevents contamination. Swab vial stoppers with alcohol. Use filtered needles or syringes. Avoid touching surfaces that will contact the solution. Contaminated peptides can produce unpredictable research results.
Calculate appropriate concentrations based on intended use. More concentrated solutions require smaller injection volumes but may have stability limitations. The SeekPeptides calculator simplifies these calculations.
Administration routes
Subcutaneous injection represents the most common administration route for research peptides. It offers good bioavailability with relatively simple technique. Proper injection practices ensure consistent dosing and minimize complications.
Some peptides work through alternative routes.
Nasal sprays deliver peptides directly to brain-adjacent tissues, potentially improving CNS uptake. Oral formulations exist for some compounds, though gastrointestinal degradation limits this approach for most peptides.
Route selection depends on the peptide's properties and research goals. Alpha-MSH analogs for tanning research typically use subcutaneous injection. Nootropic peptides might employ nasal delivery. The appropriate route influences both efficacy and safety considerations.
Safety considerations for alpha peptide research
Peptide safety varies by compound, dose, duration, and individual factors. General principles apply broadly, but specific alpha peptides carry unique considerations.
Thymosin alpha-1 safety
Thymosin alpha-1 has the most extensive safety data among alpha-designated peptides.
Thousands of patients have used it therapeutically. Adverse effects remain mild and infrequent, mostly limited to injection site reactions.
Theoretical risks exist for autoimmune conditions. Enhanced immune function might worsen autoimmune attacks. Research specifically addressing this concern remains limited. Caution is warranted for individuals with autoimmune histories.
Alpha-MSH analog safety
Approved analogs like afamelanotide and bremelanotide have documented safety profiles from clinical trials.
Side effects include nausea, facial flushing, and injection site reactions.
Unregulated melanotan products carry additional uncertainties. Purity cannot be verified without independent testing. Dosing guidelines lack clinical validation. Long-term effects remain unstudied. Users assume risks that approved pharmaceuticals have addressed through formal development programs.
Skin changes warrant attention with any melanocortin agonist. New moles, changed moles, or unusual pigmentation patterns should prompt dermatological evaluation. While direct causation of melanoma remains unproven, vigilance is appropriate.
Defensin considerations
Defensins are endogenous peptides, so moderate supplementation might seem inherently safe. However, defensins can be toxic to human cells at high concentrations.
The same membrane-disrupting properties that kill bacteria can damage host tissues.
Research applications typically use defensins at concentrations that avoid host toxicity. Therapeutic development programs must carefully establish therapeutic windows where antimicrobial activity occurs without tissue damage.
General peptide precautions
Several practices enhance safety across all peptide research. Source from reputable vendors with verified quality control. Follow established dosing guidelines where available. Monitor for unexpected reactions. Maintain sterile technique to prevent infection.
Common mistakes include using excessive doses, contaminating solutions, or failing to recognize adverse reactions. Education and careful attention prevent most problems.
Individual variation means that even well-characterized peptides can produce unexpected responses in some people. Starting with lower doses allows assessment of individual tolerance before escalating.
The future of alpha peptide research
Peptide therapeutics represent one of the fastest-growing segments of pharmaceutical development. Alpha-designated compounds participate in this trend across multiple disease areas.
Immunotherapy advances
Thymosin alpha-1 continues accumulating clinical data. Studies explore its use alongside checkpoint inhibitors for cancer, as adjuvant to vaccines in elderly populations, and for various infectious conditions.
Defensin research advances toward clinical applications. The antibiotic resistance crisis creates urgent need for alternative antimicrobials. Defensin-based drugs could eventually fill niches where conventional antibiotics fail.
Neurological breakthroughs
Alpha synuclein-targeted therapies may finally yield disease-modifying treatments for Parkinson's disease. Multiple approaches are in clinical testing. Even modest success would transform an area where current treatments only manage symptoms.
CGRP-targeted therapies continue expanding. New antibodies and gepants enter development. Combination approaches might improve outcomes beyond what single agents achieve. The migraine field now has options that didn't exist a decade ago.
Metabolic applications
Melanocortin pathway research informs obesity treatment development. MC4 receptor agonists face challenges, but the pathway remains attractive given its central role in energy balance.
Broader metabolic peptide research has already yielded transformative drugs. GLP-1 agonists dominate the obesity treatment landscape.
Their success validates peptide-based approaches and encourages further development.
Peptide delivery innovations
Delivery technologies may unlock applications currently limited by peptide instability or poor bioavailability. Oral peptide formulations using absorption enhancers or protective coatings could replace injections for some compounds.
Long-acting formulations extend dosing intervals. Monthly or quarterly injections replace daily or weekly administration for some peptides. This convenience improvement enhances patient adherence and quality of life.
Targeted delivery systems direct peptides to specific tissues. Nanoparticles, antibody conjugates, and other technologies could concentrate therapeutic peptides where they're needed while reducing systemic exposure.
Frequently asked questions
What does "alpha" mean in alpha peptides?
Alpha in peptide terminology typically indicates either a structural feature (alpha helix configuration), a designation within a peptide family (alpha-MSH vs. beta-MSH), or simply part of a vendor's business name. The specific meaning depends entirely on context. Understanding basic peptide terminology helps clarify which meaning applies.
Is thymosin alpha-1 the same as thymosin beta-4?
No. Thymosin alpha-1 and thymosin beta-4 are distinct peptides with different structures and functions. Thymosin alpha-1 (28 amino acids) modulates immune function. Thymosin beta-4 (43 amino acids) promotes tissue healing and regeneration. TB-500 is a synthetic fragment of thymosin beta-4. Despite sharing the "thymosin" name, these peptides address different research applications.
Are alpha-MSH peptides legal?
Afamelanotide and bremelanotide are FDA-approved prescription medications available through healthcare providers for specific indications. Melanotan I and II lack regulatory approval and exist in legal gray areas. Peptide legality depends on jurisdiction, the specific compound, and intended use. Research-designated products have different legal frameworks than pharmaceuticals.
How do I store alpha peptides?
Store lyophilized peptides in cold, dark conditions. Refrigeration works for short-term storage. Freezer storage (-20°C or colder) extends stability for long-term keeping. After reconstitution, store solutions refrigerated and use within the stability window for that specific peptide. Detailed storage guidelines ensure your peptides remain active.
Can I combine different alpha peptides?
Combining peptides requires understanding potential interactions. Some combinations make pharmacological sense. Others might produce unexpected effects. Peptide stacking should follow established protocols where available or proceed cautiously with monitoring. Thymosin alpha-1 combined with other immune modulators, for example, might produce additive or synergistic effects requiring dose adjustment.
What's the difference between alpha helix and beta sheet peptides?
Alpha helices and beta sheets are the two main secondary structure types in peptides and proteins. Alpha helices form right-handed spirals stabilized by hydrogen bonds along the backbone. Beta sheets form flat, extended structures with hydrogen bonds between adjacent strands. Many peptides contain both structural elements, with the ratio influencing function and stability.
Are alpha defensins available as supplements?
Human alpha defensins are not available as consumer supplements. They exist primarily as research tools for studying antimicrobial immunity. Therapeutic development programs work toward clinical applications, but approved defensin-based drugs don't yet exist. Supporting natural defensin production through gut health may represent an alternative approach, as gut-supporting peptides can influence intestinal defensin expression.
How do alpha peptides compare to other peptide types?
Alpha-designated peptides span multiple functional categories. They don't represent a single class that can be directly compared to "other" peptides. Thymosin alpha-1 compares to other immunomodulators. Alpha-MSH analogs compare to other melanocortin agonists. Alpha defensins compare to other antimicrobial peptides. Each comparison requires matching functional categories rather than Greek letter designations.
External resources
Thymosin alpha 1: A comprehensive review of the literature (PMC)
Alpha-Melanocyte Stimulating Hormone: An Emerging Anti-Inflammatory Antimicrobial Peptide (PMC)
Defensins: A Double-Edged Sword in Host Immunity (Frontiers)
For researchers serious about understanding and utilizing peptide compounds, SeekPeptides provides comprehensive educational resources, calculators for precise dosing, and guidance on best practices for peptide research.
In case I don't see you, good afternoon, good evening, and good night.