Jan 10, 2026

The heart functions as more than a mechanical pump, serving simultaneously as an endocrine organ that produces and releases peptide hormones in response to cardiac stress and volume changes. B-type natriuretic peptide, commonly abbreviated as BNP, represents one of the most clinically significant cardiac peptides, released primarily from ventricular myocytes when the heart muscle stretches beyond normal parameters. Understanding BNP provides insights into cardiovascular health that extend far beyond simple blood pressure measurements, offering a window into cardiac wall stress, volume status, and the heart's functional capacity.
This natriuretic peptide belongs to a family that includes atrial natriuretic peptide and C-type natriuretic peptide, each playing distinct roles in cardiovascular regulation through effects on blood volume, vascular tone, and sodium excretion.
As u may know, SeekPeptides provides the best resources for understanding how peptides function throughout the body, including the critical natriuretic peptide system that maintains cardiovascular homeostasis.
Understanding BNP and the natriuretic peptide system
B-type natriuretic peptide earned its name from initial discovery in porcine brain tissue, though subsequent research revealed the heart as the primary production site in humans. The ventricles synthesize and store proBNP, the precursor molecule, which undergoes cleavage upon release to produce active BNP and the inactive NT-proBNP fragment. Both forms appear in blood tests, with different clinical applications based on their distinct half-lives and clearance mechanisms.
The natriuretic peptide system evolved as a counterregulatory mechanism against the renin-angiotensin-aldosterone system. Where RAAS promotes sodium and water retention alongside vasoconstriction, natriuretic peptides drive sodium excretion, water loss, and vasodilation. This balance maintains blood pressure and volume within physiological ranges under normal conditions.
When cardiac stress increases, whether from volume overload, pressure overload, or myocardial dysfunction, BNP release escalates proportionally. This relationship forms the foundation for BNP's clinical utility as a biomarker. Higher stress on the heart translates directly into higher circulating BNP levels, creating a measurable signal of cardiac distress.
BNP molecular structure and function
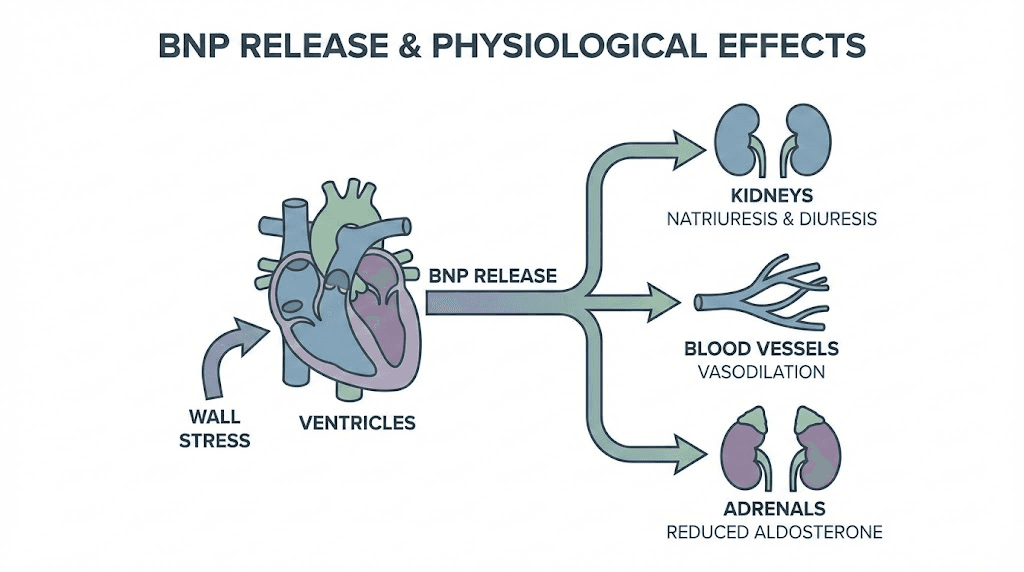
Active BNP consists of 32 amino acids arranged in a ring structure formed by a disulfide bond between cysteine residues. This configuration proves essential for receptor binding and biological activity. The ring structure allows BNP to interact with natriuretic peptide receptors on target tissues including kidneys, blood vessels, and the adrenal glands.
Three natriuretic peptide receptors mediate the effects of BNP and related peptides. NPR-A and NPR-B function as guanylyl cyclases, generating cyclic GMP as their second messenger when activated. NPR-C serves primarily as a clearance receptor, binding and internalizing natriuretic peptides to regulate their circulating levels.
The signaling cascade initiated by BNP receptor binding produces vasodilation through smooth muscle relaxation, natriuresis through kidney tubule effects, and inhibition of renin and aldosterone release. These combined actions reduce blood volume and vascular resistance, decreasing the workload on an already stressed heart.
Clinical significance of BNP levels
BNP blood tests have revolutionized the evaluation of patients presenting with dyspnea, providing objective data to differentiate cardiac from pulmonary causes of breathlessness. Emergency departments worldwide use BNP testing to rapidly assess whether heart failure contributes to a patient's symptoms, guiding treatment decisions within the critical early hours of presentation.
Normal BNP ranges
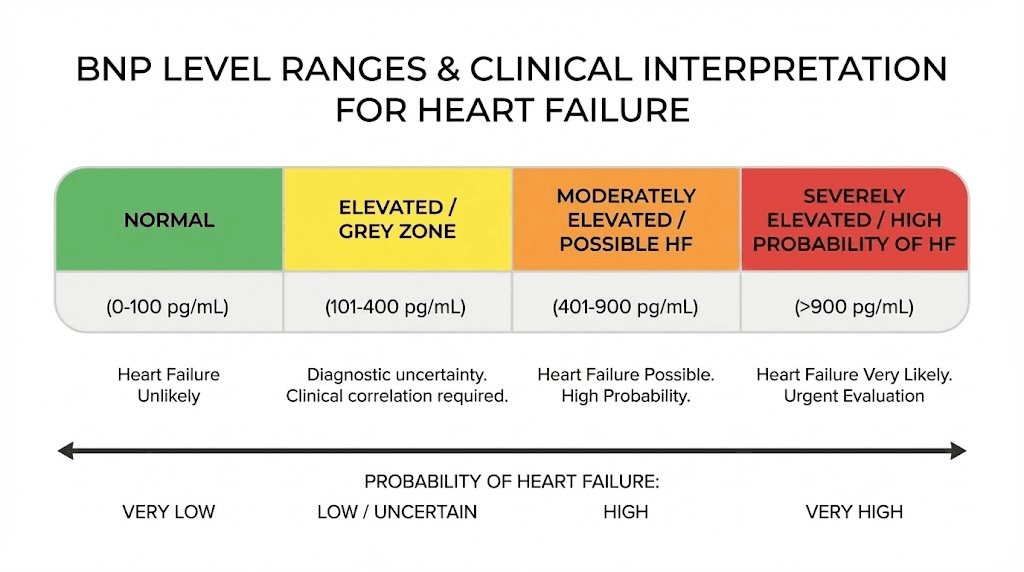
Normal BNP levels generally fall below 100 pg/mL, though reference ranges vary between assay manufacturers and laboratories. Age affects normal values, with older individuals typically showing higher baseline levels even in the absence of cardiac disease. Gender differences also exist, with women demonstrating slightly higher normal ranges than men.
NT-proBNP, the inactive fragment released alongside BNP, has different normal ranges due to its longer half-life and different clearance mechanisms. Values below 125 pg/mL in patients under 75 and below 450 pg/mL in older patients typically indicate low probability of heart failure. These thresholds help clinicians interpret results in age-appropriate context.
Body mass index influences BNP interpretation. Obese individuals often show lower BNP levels than expected for their degree of cardiac dysfunction, potentially due to increased clearance by adipose tissue receptors. This relationship requires consideration when evaluating overweight patients with suspected heart failure.
Elevated BNP and heart failure
BNP levels above 400-500 pg/mL strongly suggest heart failure as a contributing factor to patient symptoms. Values in this range prompt aggressive evaluation for cardiac causes and often initiate heart failure treatment even before confirmatory imaging. The negative predictive value of low BNP proves particularly valuable, allowing clinicians to confidently pursue non-cardiac explanations when levels remain normal.
The degree of BNP elevation correlates with heart failure severity and prognosis. Higher values indicate greater cardiac wall stress and typically predict worse outcomes. Serial BNP measurements during heart failure treatment help assess response to therapy, with decreasing levels suggesting successful volume management and reduced cardiac stress.
Not all BNP elevation indicates heart failure. Pulmonary embolism, pulmonary hypertension, sepsis, and kidney disease can all elevate BNP levels independent of primary cardiac dysfunction. Clinical context remains essential for appropriate interpretation, as isolated lab values cannot diagnose heart failure.
BNP in chronic disease monitoring
Patients with established heart failure benefit from periodic BNP monitoring to track disease stability and treatment efficacy. Rising levels may indicate disease progression or medication non-compliance before symptoms worsen, enabling proactive intervention. Outpatient BNP-guided therapy has shown promise in reducing hospitalizations and improving outcomes.
Baseline BNP levels in stable heart failure patients establish individual reference points for subsequent comparison. A patient whose typical BNP runs 200 pg/mL might warrant concern at 400 pg/mL, while another patient stable at 500 pg/mL might not alarm until reaching 800 pg/mL. Personalized thresholds improve monitoring accuracy.
Post-myocardial infarction, BNP levels help risk stratify patients for adverse outcomes. Higher levels following heart attack indicate more significant myocardial damage and predict increased risk of subsequent heart failure, arrhythmias, and death. This prognostic information guides treatment intensity and follow-up scheduling.
BNP versus NT-proBNP testing
Both BNP and NT-proBNP serve as cardiac biomarkers, but their different properties make each preferable in certain clinical situations. Understanding these distinctions helps interpret results accurately and select appropriate testing strategies.
Half-life and stability differences
BNP has a circulating half-life of approximately 20 minutes, while NT-proBNP persists for 60-120 minutes. This difference means NT-proBNP levels remain more stable throughout the day and respond more slowly to acute changes. For monitoring gradual changes, NT-proBNP's stability proves advantageous. For assessing rapid response to treatment, BNP's shorter half-life may better reflect current cardiac status.
Sample stability also differs between the tests. BNP degrades relatively quickly in collected blood samples, requiring prompt processing or specialized collection tubes. NT-proBNP remains stable longer, allowing more flexibility in sample handling. This practical consideration influences which test laboratories offer.
Kidney function considerations
NT-proBNP clearance depends significantly on kidney function, with levels rising as glomerular filtration rate declines. This relationship complicates interpretation in patients with chronic kidney disease, who may show elevated NT-proBNP levels even without cardiac dysfunction. Adjusted reference ranges for different kidney function levels help address this limitation.
BNP undergoes clearance through both receptor-mediated uptake and enzymatic degradation, making it somewhat less dependent on kidney function than NT-proBNP. However, renal impairment still affects BNP levels to some degree. Neither test perfectly distinguishes cardiac from renal causes of elevation in patients with both conditions.
Clinical application guidelines
Most clinical guidelines accept either BNP or NT-proBNP for heart failure evaluation, with appropriate adjustment of reference ranges. Consistency within individual patient monitoring proves more important than which specific test is used. Switching between tests during longitudinal follow-up complicates interpretation and should be avoided when possible.
Cost and availability influence test selection in many healthcare settings. Some institutions preferentially offer one test based on laboratory contracts or analyzer availability. Clinical utility remains comparable when appropriate cutoffs are applied, making either test acceptable for most indications.
Conditions affecting BNP levels
Multiple cardiac and non-cardiac conditions influence BNP concentrations, requiring careful consideration of the clinical context when interpreting results. Awareness of these factors prevents misattribution of elevated levels to heart failure when other causes exist.
Cardiac conditions elevating BNP
Heart failure with reduced ejection fraction produces the most dramatic BNP elevations, often exceeding 1000 pg/mL in severe cases. The stretched, volume-overloaded ventricles release large quantities of natriuretic peptides as a compensatory mechanism attempting to reduce volume and afterload.
Heart failure with preserved ejection fraction also elevates BNP, though typically to lesser degrees than systolic heart failure. The stiff, non-compliant ventricles in diastolic dysfunction experience elevated filling pressures that trigger BNP release. This finding helps identify diastolic heart failure, which might otherwise escape detection with preserved systolic function.
Atrial fibrillation independently increases BNP levels, even without ventricular dysfunction. The rapid, irregular atrial activity and loss of coordinated atrial contraction elevate cardiac pressures and trigger natriuretic peptide release. Rhythm control that restores sinus rhythm often reduces BNP levels.
Valvular heart disease, particularly mitral regurgitation and aortic stenosis, elevates BNP through their hemodynamic effects on ventricular loading. Acute valvular emergencies may produce dramatic BNP elevation that helps identify the severity of the situation.
Acute coronary syndromes increase BNP in proportion to the extent of myocardial damage. The ischemic or infarcted tissue releases natriuretic peptides, and the resulting wall motion abnormalities create additional stretch stimuli. BNP levels following myocardial infarction carry significant prognostic implications.
Non-cardiac conditions affecting BNP
Pulmonary embolism elevates BNP through right ventricular strain, providing information about hemodynamic significance that aids risk stratification. Large pulmonary emboli causing right heart dysfunction produce higher BNP levels than smaller, less hemodynamically significant emboli.
Chronic kidney disease elevates natriuretic peptide levels through reduced clearance and the cardiovascular stress that commonly accompanies renal dysfunction. Dialysis patients frequently show elevated BNP levels that complicate cardiac assessment.
Sepsis and critical illness produce BNP elevation through multiple mechanisms including myocardial depression, volume overload from fluid resuscitation, and inflammatory cytokine effects on cardiac tissue. Critically ill patients often show elevated BNP regardless of underlying cardiac status.
Hyperthyroidism increases cardiac output demands and may elevate BNP, while severe hypothyroidism can cause myxedema heart with associated natriuretic peptide elevation. Thyroid function assessment helps contextualize BNP results in patients with known or suspected thyroid disease.
Advanced age itself correlates with higher BNP levels, reflecting age-related changes in cardiac compliance and diastolic function. Age-adjusted reference ranges help prevent over-diagnosis of heart failure in elderly patients with mildly elevated levels.
The natriuretic peptide family
BNP belongs to a family of structurally related peptides that share natriuretic, diuretic, and vasorelaxant properties. Understanding the broader natriuretic peptide system provides context for BNP's specific role and the potential therapeutic applications of these peptides.
Atrial natriuretic peptide
ANP, the first discovered member of this family, is produced primarily in atrial myocytes in response to atrial stretch from volume expansion. Its 28-amino acid structure shares the characteristic ring formation with BNP. ANP release helps regulate acute volume changes through rapid natriuresis and vasodilation.
While ANP responds more quickly to volume changes than BNP, it proves less useful as a heart failure biomarker due to shorter half-life and lower circulating concentrations. BNP's ventricular origin makes it a better indicator of the ventricular dysfunction that characterizes most heart failure syndromes.
C-type natriuretic peptide
CNP differs from ANP and BNP in its primary sites of production and action. Vascular endothelium produces CNP, which acts locally on smooth muscle to promote vasodilation. This paracrine function distinguishes CNP from the endocrine actions of cardiac natriuretic peptides.
CNP also plays important roles in bone growth and development, with mutations affecting CNP signaling causing skeletal abnormalities. This function has led to therapeutic development of CNP analogs for achondroplasia treatment, demonstrating the diverse physiological roles of natriuretic peptides beyond cardiovascular regulation.
Therapeutic natriuretic peptide applications
Synthetic natriuretic peptides and related compounds have been developed for heart failure treatment. Nesiritide, a recombinant BNP, was approved for acute decompensated heart failure though its role has diminished with accumulating safety and efficacy data. The concept of supplementing the natriuretic peptide system remains therapeutically attractive.
Sacubitril, a neprilysin inhibitor, prevents the enzymatic breakdown of natriuretic peptides, effectively enhancing their activity. Combined with valsartan as sacubitril/valsartan, this approach has proven highly effective for heart failure with reduced ejection fraction, representing one of the major advances in heart failure therapy.
Research continues on natriuretic peptide system modulation for various cardiovascular conditions. Designer peptides with enhanced receptor selectivity or resistance to degradation could offer improvements over native peptide administration. The natriuretic peptide system remains an active area of cardiovascular drug development.
BNP and cardiovascular health optimization
While BNP serves primarily as a biomarker rather than a therapeutic target, understanding the factors that influence BNP levels provides insights into cardiovascular health optimization. Lifestyle and treatment interventions that reduce cardiac stress typically lower BNP levels.
Exercise and physical activity effects
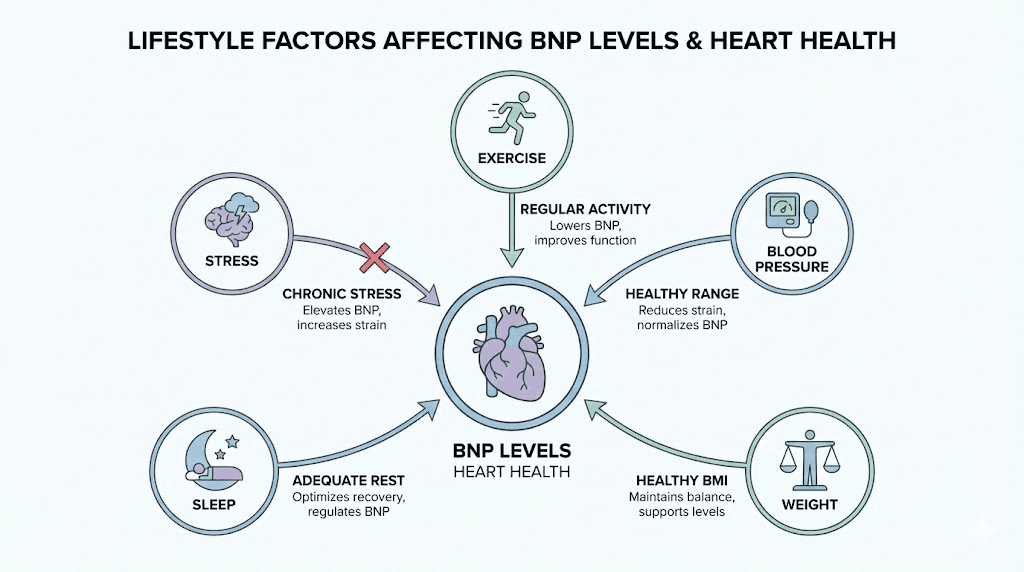
Acute exercise transiently elevates BNP, particularly with intense endurance activity. Marathon running and similar extreme exertion can produce BNP levels meeting heart failure diagnostic thresholds, though levels normalize within 24-48 hours. This physiological response reflects temporary cardiac strain rather than pathology.
Regular exercise training, conversely, may lower resting BNP levels through improved cardiac efficiency and reduced baseline wall stress. The cardioprotective effects of habitual physical activity include enhanced diastolic function and reduced filling pressures, both of which decrease natriuretic peptide release.
Athletic performance optimization through appropriate training produces cardiovascular adaptations that support healthy BNP dynamics. The athlete's heart, characterized by appropriate physiological hypertrophy and enhanced function, efficiently manages the volume and pressure demands of exercise.
Blood pressure management
Hypertension increases cardiac afterload, forcing the ventricles to generate higher pressures during systole. This increased wall stress elevates BNP levels, which decrease with effective blood pressure control. The natriuretic peptide system participates in blood pressure regulation, making this relationship bidirectional.
Antihypertensive medications, particularly those blocking the renin-angiotensin-aldosterone system, may reduce BNP levels through both direct blood pressure reduction and neurohormonal modulation. ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists all influence the balance between RAAS and natriuretic peptide systems.
Weight management and metabolic health
Obesity paradoxically associates with lower BNP levels than expected, potentially due to increased clearance through adipose tissue receptors. However, the cardiovascular stress of obesity, including volume overload, hypertension, and metabolic dysfunction, ultimately increases cardiac strain and disease risk.
Weight loss improves cardiovascular parameters that influence BNP dynamics. Reduced volume overload, improved blood pressure, and decreased metabolic stress all contribute to healthier cardiac function. Fat loss peptides like GLP-1 agonists have shown cardiovascular benefits extending beyond weight reduction.
Metabolic syndrome components including insulin resistance, dyslipidemia, and central obesity all impact cardiovascular health and indirectly influence BNP levels. Addressing metabolic dysfunction through comprehensive approaches supports cardiac health maintenance.
Sleep and recovery
Sleep apnea significantly impacts cardiac health and can elevate BNP levels through repeated nocturnal hypoxia, blood pressure surges, and increased sympathetic activation. Treatment of sleep apnea with CPAP or other modalities often reduces BNP and improves cardiovascular outcomes.
Adequate sleep quality supports cardiovascular recovery and reduces stress on the heart. Sleep optimization contributes to overall cardiac health through multiple mechanisms including blood pressure regulation and autonomic balance.
Research applications of natriuretic peptides
Beyond clinical diagnostics, natriuretic peptides serve important roles in cardiovascular research. Understanding BNP physiology informs drug development, clinical trial design, and our broader understanding of cardiac homeostasis.
Biomarker research applications
Clinical trials increasingly incorporate BNP as an outcome measure or risk stratification tool. Changes in BNP levels serve as surrogate endpoints suggesting cardiovascular benefit or harm, though relationship to clinical outcomes requires validation for each intervention studied.
BNP-guided therapy trials have explored whether titrating heart failure treatment to natriuretic peptide targets improves outcomes compared to standard care. Results have been mixed, but the concept of using biomarker guidance to intensify therapy remains promising.
Understanding cardiac physiology
Natriuretic peptide research has advanced understanding of cardiac endocrine function and the integration of cardiovascular regulation with fluid and electrolyte homeostasis. The interplay between natriuretic peptides and RAAS exemplifies complex physiological counter-regulation.
Genetic studies of natriuretic peptide system variants have identified polymorphisms associated with blood pressure, heart failure risk, and response to cardiovascular medications. This pharmacogenomic information may eventually guide personalized therapy selection.
Novel peptide development
The success of neprilysin inhibition has renewed interest in natriuretic peptide system enhancement. Novel approaches including designer peptides with improved pharmacokinetics, receptor-specific agonists, and alternative delivery methods continue under investigation.
Understanding how the body produces, releases, and clears natriuretic peptides informs peptide research more broadly. The principles governing BNP pharmacokinetics apply across many peptide hormones and therapeutic peptides.
Peptides supporting cardiovascular health
While BNP functions as a biomarker rather than a supplemental peptide, other peptides may support cardiovascular health through various mechanisms. Understanding these options provides context for comprehensive cardiovascular optimization approaches.
Tissue repair and recovery peptides
BPC-157 has demonstrated vascular protective effects in research settings, including promotion of angiogenesis and protection against various vascular insults. While cardiovascular applications remain primarily investigational, the tissue healing properties extend to vascular tissues.
TB-500 promotes tissue repair through effects on actin organization and cell migration. Research has explored cardiovascular applications including post-infarction recovery, though clinical evidence remains limited. The comparison between these healing peptides helps understand their distinct mechanisms.
Metabolic peptides with cardiovascular effects
GLP-1 receptor agonists, while developed for diabetes and weight management, have demonstrated significant cardiovascular benefits in clinical trials. Semaglutide and similar compounds reduce major adverse cardiovascular events independent of their metabolic effects.
Tirzepatide, the dual GIP/GLP-1 agonist, shows promise for cardiovascular protection alongside its potent metabolic effects. The relationship between metabolic health and cardiovascular outcomes makes these peptides relevant to cardiac wellness.
AOD-9604 and other fat loss peptides may indirectly benefit cardiovascular health through reduction of metabolically active visceral fat. Visceral adiposity contributes to inflammation, insulin resistance, and cardiovascular risk.
Growth hormone and cardiovascular function
Growth hormone signaling influences cardiovascular structure and function. GH deficiency associates with adverse cardiovascular changes including reduced cardiac mass and impaired function. Appropriate GH optimization may support cardiovascular health in deficient individuals.
Ipamorelin and other growth hormone secretagogues stimulate physiological GH release that may support cardiovascular function through effects on body composition, lipid metabolism, and cardiac tissue. The CJC-1295 and similar peptides work through related mechanisms.
The relationship between GH and cardiovascular health follows a U-shaped curve, with both deficiency and excess producing adverse effects. Physiological optimization rather than supraphysiological elevation represents the appropriate goal.
Interpreting BNP test results
Practical interpretation of BNP results requires integrating laboratory values with clinical presentation, medical history, and other diagnostic information. No single number defines health or disease, but patterns and trends provide valuable clinical guidance.
When to test BNP
Emergency department evaluation of acute dyspnea represents the most validated BNP application. Patients presenting with shortness of breath benefit from rapid differentiation between cardiac and non-cardiac causes. BNP testing in this setting is recommended by major clinical guidelines.
Outpatient screening for heart failure in asymptomatic individuals remains controversial. While elevated BNP identifies increased risk, the clinical utility of population screening has not been established. Testing is most valuable when clinical suspicion exists.
Monitoring established heart failure patients provides information about treatment efficacy and disease stability. Serial testing at consistent intervals helps identify trends that may precede symptom changes, enabling proactive management adjustments.
Understanding result variability
BNP levels fluctuate based on volume status, medications, and concurrent conditions. A single measurement provides a snapshot that must be interpreted in context. Repeated testing helps distinguish meaningful changes from normal variation.
Biological variability means that identical cardiac status may produce somewhat different BNP values on different days. Changes of less than 30-50% may not represent clinically significant differences. Larger changes warrant attention and possible intervention.
Laboratory assay differences between manufacturers mean that absolute values may not be directly comparable between different testing systems. Knowing which assay your laboratory uses helps interpret results against appropriate reference ranges.
Communicating with healthcare providers
Sharing BNP results with healthcare providers enables informed discussion about cardiovascular status and management. Trends over time prove more informative than isolated values, making longitudinal tracking valuable.
Understanding what BNP measures helps frame appropriate questions for healthcare discussions. Asking about factors that might explain elevated levels, implications for treatment, and appropriate follow-up testing demonstrates informed engagement with cardiovascular care.
BNP results do not diagnose heart failure independently. Abnormal values prompt further evaluation including echocardiography, which directly visualizes cardiac structure and function. The combination of biomarker and imaging information provides comprehensive cardiac assessment.
Future directions in natriuretic peptide research
Ongoing research continues to expand understanding of natriuretic peptide biology and clinical applications. Novel biomarkers, therapeutic approaches, and diagnostic strategies build on the foundation established by BNP research.
Novel biomarkers
Mid-regional pro-ANP and other natriuretic peptide fragments offer potential advantages for certain applications. These alternative markers may provide complementary information or prove more useful in specific clinical contexts such as renal impairment.
Combining BNP with other biomarkers including troponins, C-reactive protein, and novel markers may improve diagnostic and prognostic accuracy. Multi-marker strategies could better capture the complexity of cardiovascular disease than any single measurement.
Point-of-care testing
Rapid BNP testing at the bedside or in outpatient settings enables immediate clinical decision-making. Point-of-care devices are becoming increasingly accurate and accessible, potentially expanding BNP testing beyond traditional laboratory settings.
Home monitoring of BNP levels could enable patient-driven management of heart failure similar to home glucose monitoring in diabetes. While not yet standard practice, technological advances may eventually make this feasible.
Personalized medicine applications
Genetic variants affecting natriuretic peptide levels and response to therapies may guide individualized treatment selection. Pharmacogenomic approaches could identify patients most likely to benefit from specific cardiovascular interventions.
Individual BNP trajectories rather than population-based thresholds may better guide therapy. Machine learning approaches analyzing patterns in serial BNP measurements could improve prediction of clinical events and guide intervention timing.
Frequently asked questions
What does a high BNP level mean?
Elevated BNP indicates increased cardiac wall stress, most commonly from heart failure causing the ventricles to stretch. However, other conditions including kidney disease, pulmonary embolism, and atrial fibrillation can also raise BNP. Values above 400-500 pg/mL strongly suggest cardiac involvement in symptoms, while levels above 100 pg/mL warrant further evaluation.
What is a normal BNP level?
Normal BNP typically falls below 100 pg/mL, though age and gender influence normal ranges. Older adults and women tend to have slightly higher normal values. NT-proBNP has different reference ranges, with normal values below 125 pg/mL in younger adults and below 450 pg/mL in those over 75.
How is BNP different from NT-proBNP?
Both come from the same precursor molecule but differ in half-life, clearance mechanisms, and reference ranges. BNP has a shorter half-life of about 20 minutes, while NT-proBNP persists longer. NT-proBNP levels are more affected by kidney function. Either test can diagnose heart failure when appropriate cutoffs are used.
Can BNP levels be lowered?
Treating the underlying cause of elevated BNP, typically heart failure, reduces levels. Diuretics that reduce volume overload, medications that reduce afterload, and treatments that improve cardiac function all help lower BNP. Lifestyle modifications including weight loss, exercise, blood pressure control, and sleep optimization also support healthier BNP dynamics.
How often should BNP be tested?
Testing frequency depends on clinical context. For acute presentations, a single test often suffices for initial evaluation. Chronic heart failure patients may benefit from periodic monitoring every 3-6 months, or more frequently during treatment adjustments. Stable patients without heart failure do not need routine BNP monitoring.
How SeekPeptides supports cardiovascular wellness research
SeekPeptides provides educational resources about peptides affecting various aspects of health, including cardiovascular function. Understanding how different peptide systems work throughout the body supports informed health optimization approaches.
The peptide calculator and related tools help researchers working with various peptides, including those with potential cardiovascular applications. Accurate dosing ensures safe and effective peptide research protocols.
Educational guides covering peptide mechanisms, safety considerations, and protocol design support comprehensive understanding of peptide research. SeekPeptides serves as a resource for those seeking evidence-based peptide information.
The cardiovascular system's complexity requires integrated approaches considering multiple factors from metabolic health to tissue repair. SeekPeptides resources support understanding these interconnections.
Helpful resources
In case I don't see you, good afternoon, good evening, and good night. May your heart stay strong, your BNP stay normal, and your cardiovascular health stay optimized.
Join SeekPeptides for comprehensive peptide education and personalized wellness support.