Feb 3, 2026
At the cellular level, epidermal growth factor triggers a cascade that most people never think about. A single 53-amino-acid protein binds to a receptor on the cell surface. That receptor activates. Kinase domains fire. And within seconds, a chain reaction ripples through the PI3K/AKT pathway, the ERK/MAPK pathway, and multiple transcription factors that control whether a cell divides, migrates, or repairs itself. This is not some obscure laboratory curiosity.
This is one of the most fundamental signaling mechanisms in human biology, and it earned Stanley Cohen the Nobel Prize in Physiology or Medicine in 1986. EGF is the reason wounds close. It is the reason skin regenerates after a burn. It is a critical factor in hair follicle cycling, gut mucosal integrity, and the production of collagen that keeps skin looking firm and resilient. Yet despite decades of research and clinical application, most people interested in peptides for anti-aging and tissue repair have never explored what EGF can actually do. This guide covers everything. The molecular mechanisms behind EGF signaling. The clinical evidence for wound healing, skin rejuvenation, hair growth, and gastrointestinal protection. The delivery methods that actually work versus those that waste your investment. And the safety profile that separates legitimate concern from unfounded fear.
Whether you are evaluating peptides for skin health or researching growth factors for injury healing, understanding EGF is essential. SeekPeptides has assembled the most thorough breakdown available, drawing from peer-reviewed studies, clinical trial data, and the latest formulation science. Let us get into the details.
What is EGF peptide
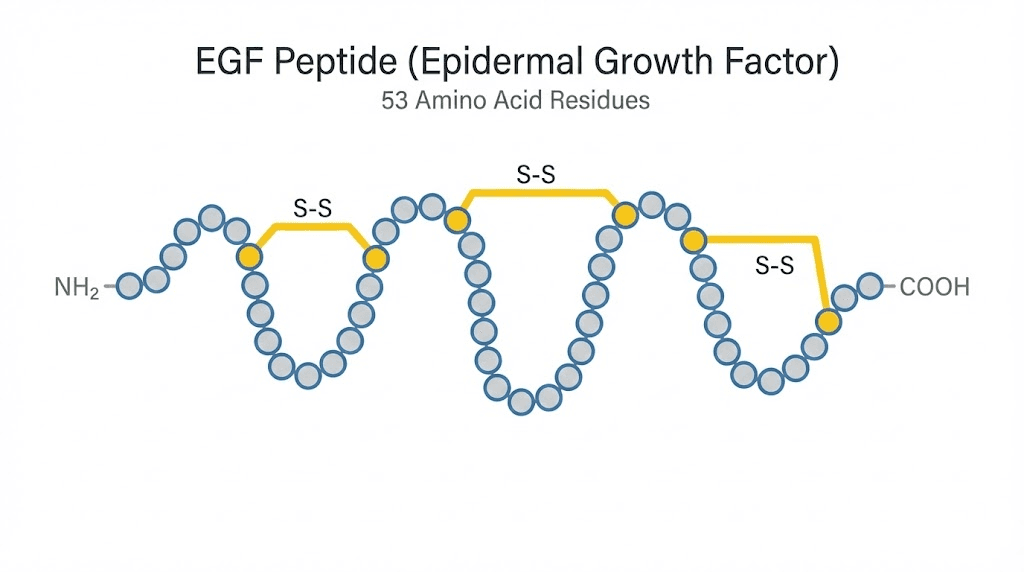
Epidermal growth factor is a small polypeptide consisting of 53 amino acids with a molecular weight of approximately 6.2 kilodaltons. It belongs to the EGF family of growth factors, a group that also includes transforming growth factor alpha (TGF-alpha), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, betacellulin, epiregulin, and epigen.
Each of these ligands binds to the EGF receptor (also known as EGFR, ErbB1, or HER1), though with varying affinities and biological outcomes.
The discovery story matters. Stanley Cohen first isolated EGF from mouse submaxillary glands in 1962 while studying nerve growth factor. He noticed that newborn mice injected with gland extracts opened their eyes earlier and erupted teeth faster than controls. The responsible agent turned out to be a small, heat-stable protein that stimulated epidermal cell proliferation. Cohen spent the next two decades characterizing EGF and its receptor, work that culminated in the 1986 Nobel Prize alongside Rita Levi-Montalcini.
Structurally, EGF contains three intramolecular disulfide bonds that create a compact, stable tertiary structure. These bonds are critical. They protect the peptide from rapid degradation and allow it to maintain biological activity across a range of conditions. The human form, designated hEGF, is produced naturally by the salivary glands, kidneys, duodenal glands (Brunner glands), and the mammary glands during lactation. It circulates in blood, saliva, urine, and breast milk.
Today, recombinant human EGF (rhEGF) is produced through genetic engineering using bacterial expression systems like Escherichia coli, yeast systems like Saccharomyces cerevisiae, or even genetically modified barley plants. This recombinant form is identical in amino acid sequence to the naturally occurring peptide and serves as the basis for both clinical wound healing products and cosmetic skincare formulations.
How EGF works at the cellular level
Understanding how EGF produces its effects requires a closer look at receptor binding and the downstream signaling cascades it activates. This is where the real power of this peptide becomes clear.
EGFR binding and activation
EGF exerts its biological effects by binding to the epidermal growth factor receptor (EGFR), a transmembrane glycoprotein with intrinsic tyrosine kinase activity. EGFR belongs to the ErbB family of receptors, which includes four members: ErbB1 (EGFR), ErbB2 (HER2), ErbB3, and ErbB4. When EGF binds to the extracellular domain of EGFR, the receptor undergoes a conformational change that promotes dimerization, the pairing of two receptor molecules. This dimerization can be homodimerization (two EGFR molecules) or heterodimerization (EGFR paired with another ErbB family member like HER2).
Dimerization activates the intracellular tyrosine kinase domain. The receptor phosphorylates itself at specific tyrosine residues on its cytoplasmic tail. These phosphorylated residues serve as docking sites for adaptor proteins and signaling molecules, launching multiple parallel signaling pathways simultaneously.
The PI3K/AKT pathway
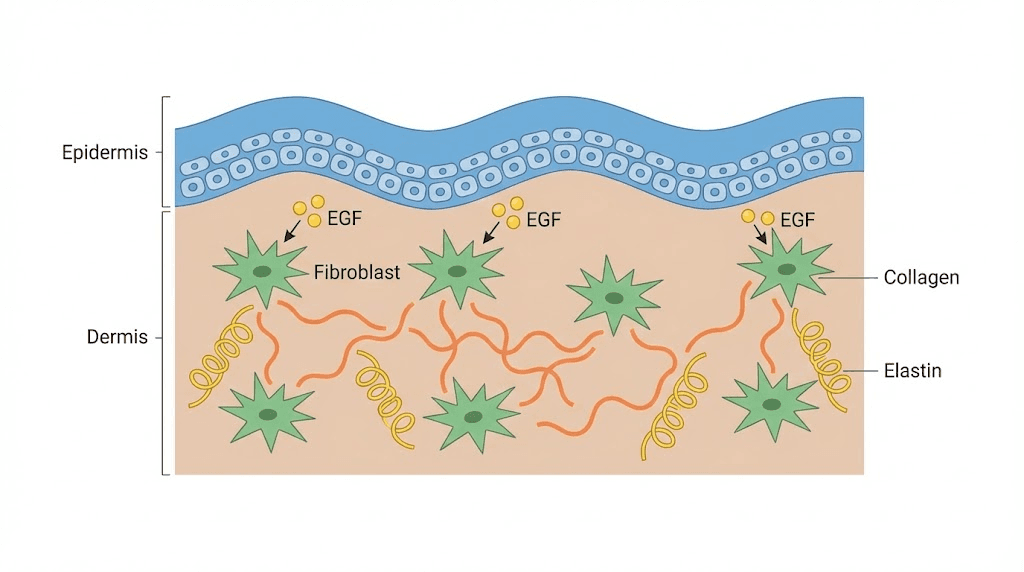
One of the primary cascades activated by EGF-EGFR binding is the PI3K/AKT pathway. This pathway promotes cell survival by inhibiting pro-apoptotic proteins and activating survival signals. For anti-aging applications, this matters enormously. The PI3K/AKT pathway regulates cell metabolism, protein synthesis, and the cellular stress response. It is also critical for collagen production in fibroblasts, the cells responsible for maintaining the structural integrity of skin and connective tissue.
The ERK/MAPK pathway
The second major cascade is the Ras/Raf/MEK/ERK signaling pathway, commonly referred to as the MAPK pathway. This is the primary driver of cell proliferation. When EGF activates this pathway, it triggers a phosphorylation cascade that ultimately reaches the cell nucleus, where it activates transcription factors controlling genes involved in cell division, migration, and differentiation. For wound healing, the MAPK pathway is the main engine. It drives keratinocyte migration across wound beds, stimulates fibroblast proliferation, and promotes the formation of new blood vessels through angiogenesis-related signaling.
The PLC-gamma/PKC pathway
A third important pathway involves phospholipase C-gamma (PLC-gamma) and protein kinase C (PKC). This cascade regulates intracellular calcium levels, which are essential for cell adhesion, tight junction formation, and the assembly of the cytoskeleton. In the context of gut health, this pathway is particularly relevant because it governs the integrity of epithelial barriers. EGF-mediated PKC activation helps maintain the tight junctions between intestinal epithelial cells, preventing unwanted permeability.
The convergence of these three pathways, PI3K/AKT for survival, ERK/MAPK for proliferation, and PLC-gamma/PKC for barrier integrity, explains why EGF has such broad biological effects. It is not doing one thing. It is coordinating an entire cellular response program that spans protection, repair, growth, and differentiation.
Wnt/beta-catenin signaling in hair follicles
In hair follicle biology, EGF also interacts with the Wnt/beta-catenin signaling pathway, a critical regulator of the hair growth cycle. This pathway determines whether follicles enter the anagen (growth) phase or remain in telogen (rest). EGF promotes proliferation of outer root sheath cells through Notch pathway cross-talk, and the interplay between EGF concentration and Wnt signaling determines whether hair follicles activate or remain dormant. This dual role makes EGF a fascinating molecule for hair growth research.
EGF peptide benefits for skin
Skin is where EGF research has generated the most consumer interest, and for good reason. The peptide addresses multiple mechanisms of skin aging simultaneously. But the details matter far more than the marketing claims suggest.
Collagen and elastin stimulation
As skin ages, fibroblast activity declines. Collagen production drops by approximately 1% per year after the mid-twenties, and elastin synthesis follows a similar trajectory. EGF directly addresses this decline by stimulating fibroblast proliferation and upregulating the genes responsible for collagen and elastin production. Research published in peer-reviewed dermatology journals has demonstrated that EGF-treated fibroblasts show increased expression of type I and type III collagen, the primary structural proteins in skin.
This is not the same as applying collagen topically. Topical collagen sits on the surface and hydrates. EGF signals your own cells to manufacture new collagen from the inside. The difference is fundamental. If you are exploring natural peptides for skin rejuvenation, EGF represents one of the most direct approaches to rebuilding the dermal matrix.
Wrinkle reduction and skin firmness
Clinical studies evaluating rhEGF for facial rejuvenation have shown measurable improvements in wrinkle depth, skin thickness, and overall texture. A systematic review published in PMC examining EGF in aesthetics and regenerative medicine found consistent evidence of wrinkle reduction across multiple study designs. Importantly, the review noted that intradermal delivery of EGF produced greater and longer-lasting improvements compared to topical application alone. This finding has significant implications for how EGF should be used, which we will explore in the delivery methods section.
For those comparing peptides for wrinkles, EGF offers a different mechanism than neurotoxin-mimicking peptides like Snap-8 or muscle-relaxing peptides like Syn-Ake. Those peptides work by reducing muscle contraction. EGF works by rebuilding the skin itself.
Hyaluronic acid production
EGF also stimulates keratinocytes to produce hyaluronic acid, the molecule responsible for skin hydration and plumpness. Each molecule of hyaluronic acid can hold up to 1,000 times its weight in water, making it one of the most effective natural humectants in the body. By boosting endogenous production rather than applying it topically, EGF helps restore hydration from within. This pairs naturally with topical hyaluronic acid peptide formulations for a comprehensive hydration strategy.
Skin barrier strengthening
A weakened skin barrier leads to transepidermal water loss (TEWL), increased sensitivity, and vulnerability to environmental stressors. Clinical research has shown that EGF-containing creams significantly reduce TEWL while increasing skin moisture and elasticity within as little as one week of application. For anyone dealing with barrier dysfunction, whether from aggressive skincare actives, environmental damage, or conditions like eczema, EGF offers a repair-focused approach that strengthens the barrier at a cellular level.
Hyperpigmentation and skin tone
Some research suggests that EGF can improve hyperpigmentation by accelerating the turnover of melanin-laden keratinocytes. As new cells are produced more rapidly, older cells carrying excess pigment are shed faster. This is particularly relevant after procedures like laser treatments and chemical peels, where post-inflammatory hyperpigmentation is a concern. Combined with vitamin C and peptide protocols, EGF can support a more even skin tone through accelerated cell renewal.
Acne and inflammatory skin conditions
A clinical study demonstrated that rhEGF cream applied for six weeks improved both inflammatory and non-inflammatory acne lesions. The mechanism involves EGF suppression of pro-inflammatory cytokines including IL-1 alpha, IL-8, and TNF-alpha induced by Cutibacterium acnes in keratinocytes. For those exploring peptides for acne, EGF provides an anti-inflammatory pathway that works differently from conventional acne treatments. It does not dry, peel, or irritate. It heals.
Research in atopic dermatitis models has shown that EGF treatment significantly reduces TEWL, epidermal thickness, and allergen-specific immunoglobulin E levels. EGF suppressed allergen-induced expression of IL-17A and neutrophil accumulation in atopic skin, suggesting a protective role against Th17-mediated inflammation.
This opens possibilities for EGF in managing inflammatory skin conditions beyond traditional approaches.
EGF for wound healing and tissue repair
Wound healing is where EGF research has the deepest roots and the strongest clinical evidence. This is not speculative. This is decades of controlled trials.
The landmark clinical evidence
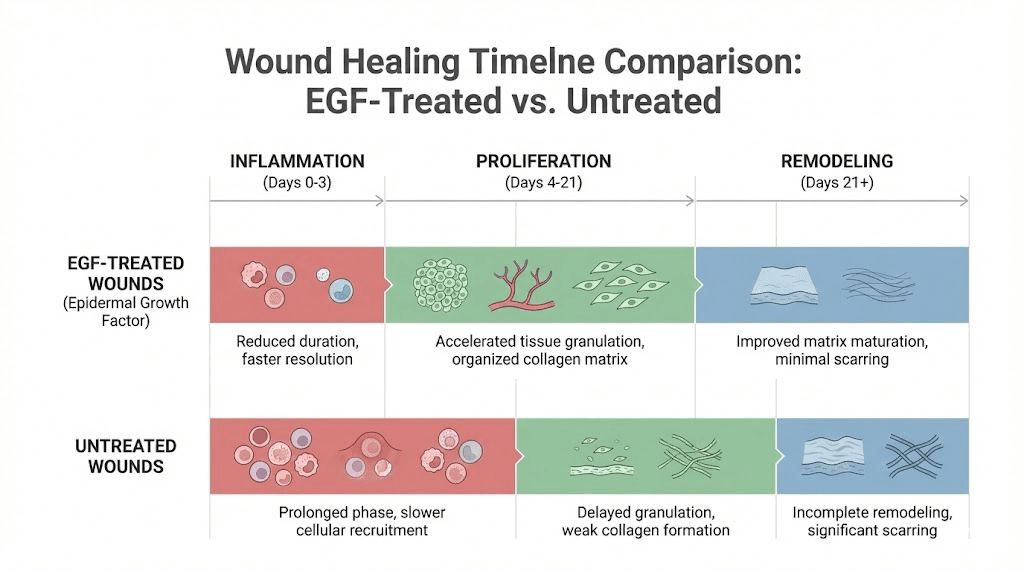
The foundational clinical work appeared in the New England Journal of Medicine in 1989. Researchers demonstrated that topical application of EGF accelerated epidermal regeneration in all 12 patients with partial-thickness skin wounds. The treated wounds showed faster re-epithelialization compared to controls, establishing for the first time in a rigorous clinical setting that EGF could meaningfully accelerate human wound healing.
Since then, the evidence has only grown stronger. A systematic review and meta-analysis published in Burns and Trauma analyzed 229 papers including 281 studies and found that growth factor administration, including EGF, significantly shortened healing time for superficial burn injuries, deep burn injuries, and surgical wounds. The analysis also found increased healing rates, decreased scar scores, and fewer adverse reactions in the growth factor treatment groups compared to controls.
Mechanisms of wound repair
EGF accelerates wound healing through four interconnected processes. First, it promotes re-epithelialization by stimulating keratinocyte migration across the wound bed. Keratinocytes at the wound edge respond to EGF by flattening, extending lamellipodia, and migrating as a coordinated sheet across the exposed tissue. Second, EGF stimulates angiogenesis, the formation of new blood vessels that supply oxygen and nutrients to the healing tissue. Third, it activates myofibroblasts, the contractile cells responsible for wound contraction. And fourth, it promotes extracellular matrix deposition, providing the structural scaffold for new tissue.
These processes overlap and reinforce each other. The result is a coordinated repair response that is faster and more organized than what occurs without EGF supplementation. For anyone researching peptides for injury recovery, EGF targets the fundamental biology of tissue repair rather than simply managing symptoms.
Diabetic foot ulcer evidence
Perhaps the most compelling clinical application of EGF is in diabetic foot ulcers, chronic wounds that resist conventional treatment and frequently lead to amputation. A pivotal study involving 29 diabetic patients with high-grade, poor-prognosis ulcers (Wagner scale III and IV) used intralesional EGF injections three times weekly. Of the 17 patients who completed 5 to 8 weeks of treatment, all achieved complete re-epithelialization with no recurrence during a 12-month follow-up period.
A meta-analysis by Rahim et al. in 2023 compared EGF to placebo across eight randomized controlled trials including 620 patients. After four weeks of treatment, the healing rate was 34% in the EGF group versus 17% in the placebo group. The conclusion was unambiguous: EGF significantly promotes wound healing in diabetic foot ulcers.
The recurrence data is equally impressive. With intralesional EGF treatment, ulcer recurrence did not exceed 5% at 12-month follow-up. Compare that to the 40% recurrence rate typically observed after conventional healing of diabetic ulcers. This is not a marginal improvement. It is a fundamental shift in outcomes.
Burns and surgical wounds
Meta-analyses have confirmed that EGF shortens healing time by 3 to 5 days for burns and surgical wounds. While this may sound modest, in clinical practice those days translate to reduced infection risk, lower healthcare costs, and significantly less patient suffering. For researchers interested in tendon repair and broader injury healing protocols, EGF represents one component of a comprehensive growth factor strategy that can be combined with peptides like BPC-157 and TB-500 for synergistic effects.
Post-procedure recovery
EGF has gained particular traction in post-procedure skincare. After microneedling, laser resurfacing, and chemical peels, the skin barrier is compromised and the healing response is activated. Applying EGF during this window takes advantage of the temporarily increased permeability (allowing better penetration) while accelerating the repair process. Many dermatologists now incorporate EGF-containing products into their post-procedure protocols specifically because it supports barrier repair without the irritation associated with other active ingredients.
EGF peptide and hair growth
Hair biology is complex. And EGF role within it is more nuanced than most sources acknowledge.
The biological switch concept
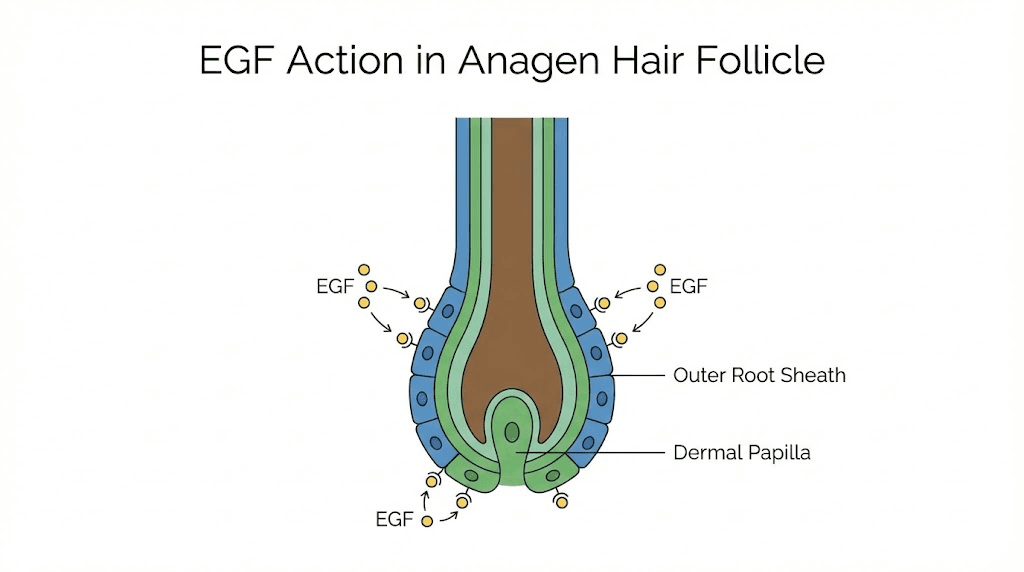
EGF acts as a concentration-dependent biological switch for hair follicle behavior. At certain concentrations, it promotes proliferation of outer root sheath cells and supports the anagen (growth) phase. At other concentrations, it can actually suppress follicle activity. This dual nature explains why simply applying more EGF does not necessarily produce better hair growth results. The dose-response relationship is not linear. It is biphasic.
Research has demonstrated that EGF promotes dermal papilla cell proliferation via the Notch signaling pathway. Dermal papilla cells are the master regulators of hair follicle cycling. They determine whether a follicle enters growth, regression, or rest.
By stimulating these cells at appropriate concentrations, EGF can tip the balance toward active growth. For those researching peptides for hair loss, understanding this concentration sensitivity is critical.
EGF and androgenetic alopecia
Androgenetic alopecia, the most common form of hair loss, involves androgen-mediated suppression of hair follicle activity. A study published in Frontiers in Pharmacology investigated EGF in combination with Jagged1 (a Notch pathway ligand) in a mouse model of androgen-induced hair loss. The results were telling.
Neither EGF nor Jagged1 alone significantly promoted hair growth in androgen-suppressed follicles. But when combined, the two molecules produced significant hair regeneration (p less than 0.05). The combination therapy was associated with increased cell proliferation in hair follicles and altered expression of genes important for follicular cell differentiation. Critically, the combination also suppressed testosterone-induced inflammatory gene expression, suggesting it can overcome the androgen-mediated inflammatory response that contributes to follicle miniaturization.
This finding has important implications.
EGF alone may not be sufficient for androgenetic alopecia. But as part of a combination strategy involving Notch pathway activation, it becomes a powerful component. Researchers exploring GHK-Cu for hair and PTD-DBM for hair growth may find that EGF complements these other growth factor approaches through its distinct mechanism of action.
The alopecia areata paradox
Here is where the story gets complicated. A case-control study found that serum EGF levels were significantly higher in alopecia areata patients compared to healthy controls. Moreover, EGF levels correlated with disease severity: patients with severe alopecia areata had higher EGF than those with moderate disease, who in turn had higher levels than those with mild disease.
This does not mean EGF causes alopecia areata. It likely reflects a compensatory response, the body producing more growth factor in an attempt to counteract the autoimmune attack on hair follicles. But it does underscore that more EGF is not always better.
Context matters. Concentration matters. And the underlying condition matters. This is why a one-size-fits-all approach to growth factor therapy falls short, and why personalized protocols through platforms like SeekPeptides offer a more rational framework for hair growth optimization.
Combining EGF with other hair growth peptides
The most promising approach to EGF-based hair growth involves combining it with complementary growth factors and peptides. Copper peptides like GHK-Cu for hair growth address the anti-inflammatory and vascular remodeling aspects of follicle health. Peptide shampoos can deliver growth factors topically during daily cleansing. And nanoliposome delivery systems loaded with multiple peptides have shown enhanced follicular penetration in preclinical studies.
The future of peptide-based hair growth is combination therapy, not single-agent approaches.
EGF and gut health
Most people do not realize that EGF is a natural component of saliva and breast milk. It is not an exotic laboratory compound. Your body produces it every time you eat.
Natural EGF in the digestive system
Salivary glands produce EGF continuously, and the act of chewing delivers this growth factor directly to the gastrointestinal tract. EGF is also secreted into the gut lumen by Brunner glands in the duodenum. Breast milk contains significant concentrations of EGF, which is why breastfed infants have lower rates of necrotizing enterocolitis and other gut-related complications compared to formula-fed infants.
The salivary EGF system is more important than most people appreciate. Research using sialoadenectomized rats (rats with salivary glands removed) showed that deprivation of salivary EGF caused a 31 to 36 percent reduction in mucus coat thickness and a 38 to 43 percent reduction in adherent mucin content.
The mucus coat is the first line of defense protecting the gut epithelium from acid, enzymes, and pathogens. Without adequate salivary EGF, this protective barrier deteriorates rapidly.
Intestinal barrier protection
EGF regulates intestinal permeability through multiple mechanisms. It activates EGFR-PLC-gamma-PKC and EGFR-ERK/MAPK signaling pathways to increase tight junction protein expression between epithelial cells. It stimulates goblet cell differentiation, increasing mucin (Muc2) production. And it induces DUOX2 expression, which generates reactive oxygen species that help maintain microbial homeostasis at the epithelial surface.
For anyone dealing with increased intestinal permeability, sometimes called leaky gut, EGF targets the exact cellular mechanisms that maintain barrier integrity. This is a fundamentally different approach from simply coating the gut lining with mucilaginous herbs or probiotics. EGF tells the cells themselves to tighten their junctions and produce more protective mucus. The category of peptides for gut health includes several options, but EGF mechanism of action is unique in its directness.
Oral EGF and mucosal healing
Oral administration of EGF has demonstrated remarkable effects in animal studies. Rats fed EGF at 10 times the estimated daily intake from human milk showed increases in specific activity of various brush-border hydrolases, the enzymes responsible for nutrient absorption. EGF also lengthened intestinal microvilli within minutes of administration, acutely increasing the absorptive surface area of the gut.
In models of intestinal injury, oral EGF accelerated mucosal repair and restored enzyme activity in damaged brush border cells. It also inhibited pathogenic bacterial adhesion to epithelial cells, reducing adhesion by up to 94.7% in some studies. This anti-adhesion effect means EGF does not just repair damage. It actively prevents pathogens from gaining a foothold in the first place.
Clinical applications in gut disease
In experimental models of necrotizing enterocolitis (NEC), oral EGF at physiological doses significantly reduced both the incidence and severity of the condition. EGF prevented tight junction disruption in neonatal intestinal tissue, preserving barrier function during the critical early period when NEC typically develops.
Systemic EGF administration also reduced mucosal damage and inflammation in colitis models, providing evidence for its role in inflammatory bowel disease management. For researchers studying inflammation peptides and their application to gastrointestinal conditions, EGF represents one of the most well-characterized options available. Its natural presence in saliva and breast milk provides a strong biological rationale for oral supplementation strategies.
EGF and ulcer healing
EGF inhibits acid secretion in the stomach while simultaneously exerting a trophic effect on gastroduodenal mucosa. It protects gastric mucosa against injury, mediates mucosal adaptation to chronic stress, and accelerates gastric and duodenal ulcer healing by stimulating cell migration and proliferation at the ulcer margins. The protective effect is partly mediated by increases in prostaglandin E2 and mucus production, but EGF also has a direct cytoprotective effect independent of these mediators.
Intraduodenal administration of EGF can partly prevent formation of cysteamine-induced duodenal ulcers and accelerate healing of chronic duodenal ulcers. This evidence, combined with the natural salivary delivery system, suggests that adequate EGF exposure throughout the digestive tract is essential for maintaining mucosal health. Thorough chewing of food may literally nourish the gut by delivering salivary EGF to the intestinal lining.
EGF versus other peptides and actives
Choosing between growth factors and active ingredients requires understanding what each one actually does at the cellular level. Marketing claims blur the differences. Science clarifies them.
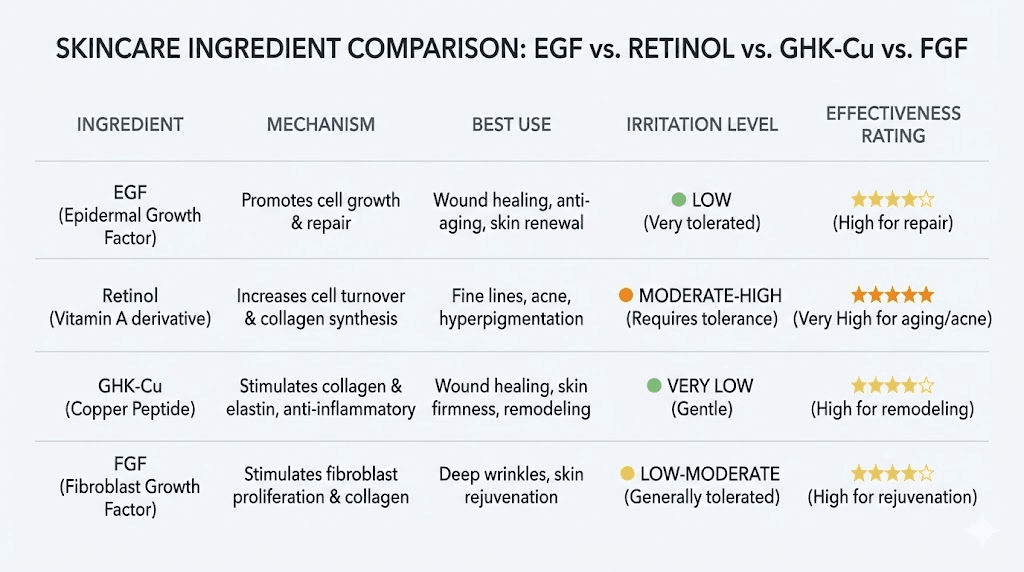
EGF versus retinol
Retinol (vitamin A) and EGF both stimulate cell turnover, but through completely different mechanisms. Retinol binds to nuclear retinoic acid receptors and modulates gene transcription across hundreds of genes involved in differentiation, proliferation, and sebum production. It is highly effective for texture improvement, acne, and photoaging. But it comes with a cost: irritation, dryness, peeling, and sun sensitivity, especially during the adjustment period.
EGF operates through receptor tyrosine kinase signaling and is fundamentally non-irritating. It does not thin the stratum corneum. It does not increase photosensitivity. And it does not cause the retinization response that makes many people abandon retinol prematurely. For sensitive skin, compromised barriers, or post-procedure recovery, EGF is the gentler yet still effective option.
Where retinol wins: texture refinement, severe acne, and established photoaging. Where EGF wins: wound healing, barrier repair, sensitive skin, and stimulating new collagen without irritation. Can you use both? Yes. Many protocols alternate them or use them in separate routines. Our guide on using peptides and retinol together covers the details of combining these actives safely.
EGF versus copper peptides (GHK-Cu)
GHK-Cu and EGF are often compared because both promote collagen synthesis and wound healing. But their mechanisms differ substantially. GHK-Cu is a tripeptide-copper complex that modulates gene expression across over 4,000 genes. It has strong anti-inflammatory properties, stimulates decorin production (which organizes collagen fibers), and promotes angiogenesis through distinct pathways from EGF.
EGF is more directly mitogenic. It triggers cell division more aggressively than GHK-Cu. For acute wound healing where rapid cell proliferation is the priority, EGF has an edge. For chronic anti-aging protocols where organized collagen remodeling and anti-inflammatory effects matter more, GHK-Cu may be preferable. Both are gentle. Both boost collagen. But they arrive at similar outcomes through different biological routes. Combining them is a strategy many advanced researchers employ, and our copper peptide skincare routine guide covers how to integrate multiple growth factors effectively.
EGF versus FGF (fibroblast growth factor)
FGF and EGF are both growth factors, but they target different cell populations. EGF primarily acts on ectodermal cells, which include keratinocytes, epidermal cells, and epithelial cells lining the gut and respiratory tract. FGF primarily targets mesodermal and endodermal cells, including fibroblasts, chondrocytes, and endothelial cells.
In practical terms, EGF is more effective for surface-level repair, re-epithelialization, barrier function, and epidermal regeneration. FGF is more effective for deeper tissue repair, angiogenesis in the dermis, and fibroblast-mediated collagen synthesis. The ideal wound healing or anti-aging approach uses both, which is why multi-growth-factor formulations have gained popularity. Understanding this distinction helps you choose the right peptide formula for your specific goals.
EGF versus biomimetic peptides
Biomimetic peptides like palmitoyl pentapeptide-4 (Matrixyl) and argireline mimic fragments of larger proteins to trigger specific cellular responses. They are synthetic, smaller, more stable, and generally less expensive than full-length growth factors like EGF. However, they also tend to be less potent.
A full-length EGF molecule activates its receptor with maximum affinity and triggers the complete downstream signaling cascade. A biomimetic peptide that mimics one aspect of collagen signaling produces a narrower, weaker response. The trade-off is cost and stability versus potency and breadth of effect. For comprehensive skin peptide protocols, combining biomimetic peptides with growth factors like EGF captures the advantages of both approaches.
EGF versus ceramide-based approaches
Ceramides are lipids, not peptides. They physically fill the spaces between corneocytes in the stratum corneum, restoring barrier function through structural repair. EGF restores barrier function through biological signaling, telling cells to produce their own ceramides, tight junction proteins, and mucins. The comparison between ceramides and peptides is not an either-or choice. Ceramides provide immediate structural support while EGF builds long-term biological resilience. Using both addresses barrier dysfunction from two complementary angles.
Delivery methods and protocols
Having the right peptide is only half the equation. Delivering it effectively to the target tissue is the other half, and with EGF, delivery is the make-or-break factor.
The delivery challenge
EGF faces two fundamental delivery obstacles. First, it has a relatively short half-life in biological environments. Proteases in the skin and bloodstream rapidly degrade the peptide, limiting its duration of action. Second, at 6.2 kilodaltons, EGF is significantly larger than most topical skincare ingredients. For context, retinol has a molecular weight approximately 22 times smaller. This size makes passive diffusion through the intact stratum corneum extremely limited.
These challenges mean that simply putting EGF in a cream and applying it to intact skin may not deliver the concentrations needed for meaningful biological effects. The formulation and delivery method matter enormously. Understanding this is essential before investing in any EGF product or protocol.
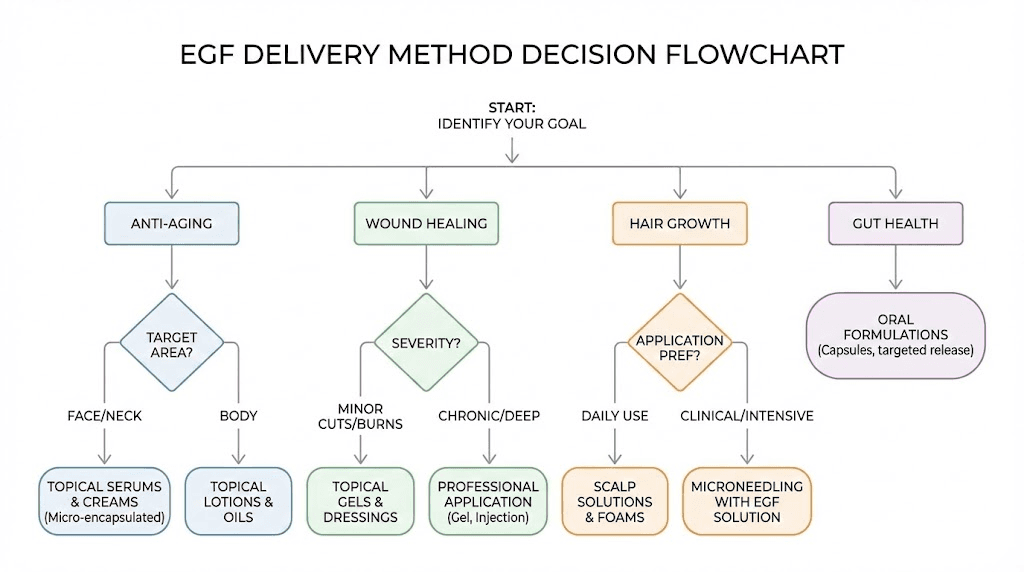
Topical application
Topical EGF products are the most accessible and widely available option. Commercial serums typically contain EGF at concentrations between 10 and 100 parts per million (ppm). Clinical trials have used concentrations up to 4,000 nanograms per gram. The evidence shows that topical EGF does produce measurable effects on skin hydration, barrier function, and mild wrinkle improvement, but the magnitude of effect is generally less than what is achieved through intradermal delivery.
The primary route of topical EGF entry into the skin is through hair follicles and sweat glands, not through the stratum corneum itself. This means topical application is more effective on hair-bearing skin and less effective on glabrous (hairless) areas. For those using topical EGF products, application after gentle exfoliation or on slightly compromised skin (such as after a mild peel) can improve penetration. Our glow peptides guide discusses how to layer growth factors with other actives for maximum effect.
Microneedling-enhanced delivery
Microneedling dramatically improves EGF delivery by creating thousands of micro-channels through the stratum corneum. Needles of 0.5 to 2.0 mm in length create temporary channels that allow large molecules like EGF to bypass the barrier entirely and reach the viable epidermis and upper dermis where EGFR receptors are concentrated.
The combination of microneedling and EGF produces synergistic effects. Microneedling itself triggers a wound healing response that upregulates EGFR expression, making the tissue more responsive to exogenous EGF applied immediately afterward. Several clinical studies have demonstrated superior anti-aging outcomes with microneedling plus EGF compared to either treatment alone. Those interested in microneedle peptide patches will find that EGF is one of the growth factors best suited to this delivery approach.
Mesotherapy and intradermal injection
For maximum efficacy, intradermal delivery of EGF via mesotherapy (superficial microinjections into the dermis) produces the strongest and longest-lasting results. The systematic review on EGF in aesthetics specifically noted that intradermal delivery showed greater reduction of wrinkles, folds, and longer response duration compared to topical application.
Mesotherapy bypasses all delivery challenges entirely by placing EGF directly where it needs to act. However, it requires professional administration, involves multiple sessions, and carries the standard risks of injection-based procedures (bruising, infection, discomfort).
For those already using peptide injections in their protocols, adding EGF mesotherapy for facial rejuvenation is a logical extension. A proper peptide injection pen makes the process more controlled and consistent.
Advanced delivery systems
Research is rapidly advancing alternative delivery technologies that could overcome EGF limitations without requiring injections.
Hyaluronate-EGF conjugates chemically bond EGF to hyaluronic acid, creating a larger complex that is more stable and has extended release kinetics. The hyaluronic acid component also enhances skin hydration, creating a dual-benefit formulation.
Liposomal delivery encapsulates EGF within lipid vesicles that fuse with cell membranes, delivering the peptide directly into cells. Liposomes protect EGF from protease degradation and significantly improve cellular uptake compared to free EGF in solution.
Freeze-dried EGF dressings are used in clinical wound care. The lyophilized format preserves EGF stability during storage and releases it upon contact with wound exudate, providing sustained delivery to the wound bed. If you are interested in proper peptide preservation techniques, our lyophilized versus liquid peptides guide explains the science behind these formats.
Hydrogel systems embed EGF within a gel matrix that controls release rate and maintains moisture at the application site. These systems are particularly effective for wound healing applications where sustained EGF exposure over hours or days is needed.
Protocol considerations
There is no universally standardized EGF protocol. The optimal approach depends on the application.
For topical skincare, most formulations suggest once or twice daily application to clean skin. EGF is typically applied after cleansing and before heavier moisturizers or occlusives. Some practitioners recommend cycling, using EGF for 8 to 12 weeks followed by a rest period, though the evidence for cycling versus continuous use is not definitive.
For wound healing, clinical protocols typically involve application or injection 2 to 3 times per week at concentrations far higher than what is found in cosmetic products. The clinical trial using intralesional EGF for diabetic foot ulcers administered injections three times weekly for 5 to 8 weeks.
For hair growth, the research on EGF plus Jagged1 used topical application to the scalp in combination with other bioactive compounds. The concentration-dependent biphasic response of hair follicles to EGF means that more is not necessarily better. Using a peptide calculator to determine appropriate concentrations based on formulation volume and target tissue area can help avoid the paradoxical inhibitory effects of excessive EGF exposure.
Safety profile and considerations
The safety question around EGF inevitably centers on one concern: cancer. The logic seems straightforward. EGF makes cells grow. Cancer is uncontrolled cell growth. So does EGF cause cancer? The answer, supported by extensive research, is more nuanced and more reassuring than the question implies.
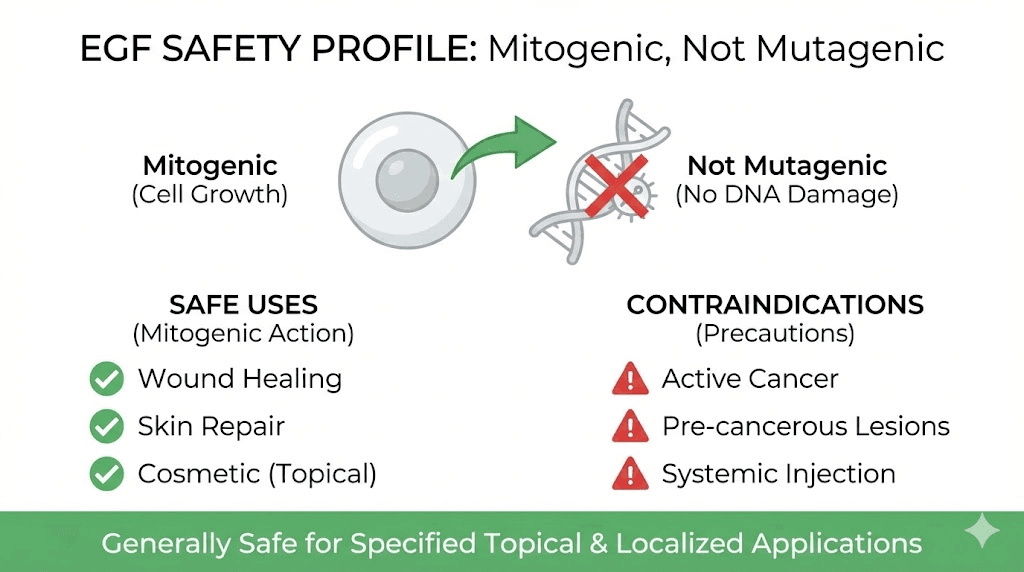
Mitogenic but not mutagenic
This distinction is the foundation of EGF safety. Mitogenic means it stimulates cell division. Mutagenic means it causes DNA mutations. EGF is the former but not the latter. An extensive battery of preclinical studies has documented that EGF does not induce genotoxicity, mutagenicity, or cytotoxicity. It does not damage DNA. It does not cause the genetic errors that transform healthy cells into cancerous ones.
Think of it this way. EGF tells cells to divide. But it does not change what those cells are. A normal cell exposed to EGF divides into two normal cells. The growth signal is temporary, regulated by receptor downregulation and intracellular feedback mechanisms that prevent sustained overstimulation. This is fundamentally different from a mutagen like UV radiation, which damages DNA and creates cells that can no longer control their own growth.
Tumor promotion versus tumor initiation
Classic experiments in oncology established that EGF can promote growth of tumors that have already been initiated by chemical or viral carcinogens. This is an important finding. But promotion is not initiation. A promoter accelerates the growth of cells that are already abnormal. An initiator creates the abnormality in the first place. EGF is a promoter, not an initiator.
Moreover, the body has multiple endogenous mechanisms that protect against non-programmed mitogenic events. EGF bioavailability is tightly controlled by local peptidases. Receptor downregulation occurs rapidly after ligand binding, reducing sensitivity to prolonged stimulation. And PKC-mediated counter-regulation of tyrosine kinase activity provides an additional brake on excessive signaling. These built-in safety mechanisms mean that exogenous EGF application in healthy tissue does not result in uncontrolled proliferation.
Topical EGF and skin cancer
No cases of skin cancer attributable to topical EGF use have been reported in the medical literature. Given that EGF-containing skincare products have been available for decades and have been used by millions of people, the absence of any documented causal link between topical EGF and cutaneous malignancy is highly reassuring. The scientific consensus among dermatologists and skin biologists is that topical EGF application on healthy, non-cancerous skin does not pose a meaningful cancer risk.
When EGF is contraindicated
There are situations where EGF should be avoided. In many human epithelial tumors, the EGFR is amplified or overexpressed with deregulated signaling. In this context, any therapy with EGF or any other growth factor is absolutely contraindicated. This includes active cancers, pre-cancerous lesions (such as severe actinic keratoses), and situations where the skin contains genetically damaged cells from extensive UV exposure or other carcinogen exposure.
A careful assessment should be performed based on personal and family cancer history before initiating EGF therapy. Individuals with a history of EGFR-overexpressing cancers, multiple dysplastic nevi, or active skin malignancies should avoid EGF products entirely. Those with psoriasis, a condition related to abnormal epidermal proliferation, should also exercise caution with mitogenic growth factors.
For anyone navigating the safety considerations of peptide research, understanding individual risk factors is essential. The peptide safety and risks guide covers the broader framework for evaluating peptide safety profiles, and SeekPeptides provides member-exclusive safety resources that address individual circumstances.
Cytoprotective effects
Interestingly, some research shows EGF acting in a protective capacity against cancer. EGF has been shown to protect against rectal cancer induction in azoxymethane-exposed rats and has demonstrated cytoprotective effects in mucositis associated with cancer chemotherapy. In animals with intestinal mucosal atrophy that received repeated co-administrations of EGF and KGF, the intestinal tissue was rapidly repaired without evidence of abnormal growth, malignant, or pre-malignant transformation.
These findings suggest that EGF role in tissue homeostasis is more complex than simple growth stimulation. In the right context, it may actually support the orderly repair processes that prevent rather than promote malignant change.
How to choose an EGF product
The market for EGF products ranges from rigorously tested clinical-grade formulations to marketing-driven products with questionable concentrations and stability. Knowing what to look for separates effective choices from wasted money.
Concentration matters
Clinical trials have typically used EGF at concentrations around 4,000 nanograms per gram for wound healing applications. Commercial skincare products range from 10 to 100 parts per million. The problem is that many products do not disclose their EGF concentration, making it impossible to evaluate whether they contain enough peptide to produce biological effects.
When evaluating products, look for those that specify EGF concentration or at least list EGF (often labeled as sh-Oligopeptide-1 in INCI nomenclature) high on the ingredient list. Products where EGF appears near the bottom of a long ingredient list likely contain trivial, non-functional concentrations. Using a peptide cost calculator can help you evaluate the value proposition of different products based on their actual peptide content versus price.
Stability and formulation
EGF stability is a significant concern. The peptide degrades with exposure to heat, light, oxygen, and extreme pH. Products in clear containers, stored at room temperature, or with long shelf lives may have reduced EGF activity by the time they reach you. Look for products with airless pump packaging, amber or opaque containers, and cold-chain shipping when possible.
The formulation base also affects efficacy. EGF in a simple water-based serum has limited skin penetration. Formulations that incorporate penetration enhancers, liposomal technology, or hyaluronate conjugation will generally deliver more active EGF to target tissues. This is the same principle that applies to peptide storage in general: environmental conditions and formulation design directly impact potency.
Source and production method
Recombinant human EGF (rhEGF) produced in bacterial or yeast expression systems is the gold standard. Plant-derived EGF from genetically modified barley (used by some Icelandic brands) offers an alternative with potentially different impurity profiles. The key question is purity and biological activity, which should ideally be verified through third-party testing. The principles outlined in our peptide testing labs guide apply to EGF products just as they do to injectable peptides.
Product format considerations
EGF is available in multiple formats. Serums provide the highest concentration in the lightest vehicle. Creams offer occlusivity that may enhance peptide absorption. Sheet masks deliver a concentrated burst of EGF over an extended contact time. Freeze-dried or lyophilized formats offer maximum stability until activated. And microneedle patches combine delivery enhancement with the convenience of a patch format.
For anti-aging protocols, a serum applied twice daily to clean skin is the most common approach. For wound healing, clinical-grade freeze-dried dressings or hydrogels are preferred. For hair growth, a targeted scalp serum or mesotherapy solution is most appropriate. The delivery format should match your specific goal. Evaluating the full range of peptide formulas available helps you find the right match.
Combining EGF with other ingredients
EGF works well with most other skincare ingredients. It pairs naturally with hyaluronic acid (enhanced hydration plus barrier repair), niacinamide (complementary barrier support and anti-inflammation), vitamin C (antioxidant protection plus collagen synthesis support), and other growth factors (multi-pathway stimulation).
EGF should be used with caution alongside strong acids (AHAs, BHAs at high concentrations) and retinoids in the same routine, not because of chemical incompatibility but because these actives compromise the skin barrier, which can alter EGF penetration and effect. Using them in separate routines (EGF in the morning, retinol at night) is a common strategy. Our guide on peptides and retinol protocols covers the details of timing and layering for maximum benefit.
Setting realistic expectations
EGF is not a miracle ingredient. Topical EGF applied to intact skin will not produce the same dramatic results as intradermal EGF injection. Anti-aging improvements from topical use typically become visible after 4 to 8 weeks of consistent application. Wound healing acceleration requires clinical-grade concentrations and appropriate delivery methods. Hair growth effects depend on concentration, delivery, and whether EGF is combined with complementary agents.
The most common mistake is expecting overnight transformation from a cosmetic serum. EGF works at the cellular level, and cellular processes take time. Collagen synthesis takes weeks to months. Barrier remodeling requires multiple cell cycles. Hair follicle cycling occurs over months. Patience and consistency are as important as product selection. For those who want to track their progress systematically, peptide before and after documentation can help set benchmarks and measure real outcomes over time.
Frequently asked questions
Is EGF peptide safe for everyday use?
For most people, topical EGF is safe for daily application. It is non-irritating, does not increase sun sensitivity, and does not cause the adjustment period associated with retinoids. The primary contraindications are active cancers, pre-cancerous lesions, and EGFR-overexpressing conditions. If you have no history of skin cancer and no active malignancies, daily topical EGF use on healthy skin is considered safe based on decades of clinical use and the absence of documented adverse events. For a broader understanding of peptide safety, consult the detailed safety guide.
Can EGF cause cancer?
EGF is mitogenic (stimulates cell division) but not mutagenic (does not cause DNA mutations). It does not initiate cancer in healthy tissue. However, it can promote the growth of cells that are already cancerous or pre-cancerous. This is why it is contraindicated in people with active malignancies or known EGFR-overexpressing tumors. In healthy individuals with no cancer history, the scientific evidence does not support a causal link between EGF use and cancer development.
What concentration of EGF is effective?
Clinical wound healing trials have used concentrations around 4,000 nanograms per gram. Commercial skincare products typically contain 10 to 100 parts per million. For topical anti-aging, higher concentrations generally produce better results, but the delivery method matters as much as concentration. Microneedling or intradermal delivery at moderate concentrations can outperform topical application at much higher concentrations. Use the peptide calculator to assess concentration-to-volume relationships for your specific formulation.
Can I combine EGF with retinol?
Yes, but ideally in separate routines rather than the same application. EGF works best on intact or minimally compromised skin where it can bind to EGFR receptors and initiate signaling. Retinol temporarily compromises the barrier and alters cell turnover patterns. Applying EGF in the morning and retinol at night is a common approach that captures the benefits of both without interaction concerns. The complete guide on using peptides and retinol together covers specific protocols for combining these actives.
How long before I see results from EGF?
Skin hydration and barrier improvements can be measurable within one week. Visible wrinkle reduction and firmness improvements typically require 4 to 8 weeks of consistent use. Wound healing acceleration depends on wound type and severity but is generally measurable within days to weeks. Hair growth effects, where applicable, require 3 to 6 months due to the length of the hair growth cycle. Patience is essential. The most reliable way to track progress is through consistent documentation, as outlined in the peptides before and after guide.
Does EGF help with hair loss?
EGF has a complex, dose-dependent relationship with hair follicle biology. At optimal concentrations, it promotes outer root sheath cell proliferation and supports the growth phase of the hair cycle. Research on androgenetic alopecia shows that EGF combined with Jagged1 produced significant hair regeneration in androgen-suppressed mouse models, though EGF alone was less effective. For hair loss, EGF is best used as part of a combination approach alongside other growth factors. Our peptides for hair growth guide covers the full range of peptide-based hair loss strategies.
Can EGF help with gut health?
Yes. EGF is a natural component of saliva and breast milk that plays a critical role in maintaining intestinal barrier integrity. It strengthens tight junctions between epithelial cells, stimulates mucin production by goblet cells, accelerates mucosal repair after injury, and inhibits pathogenic bacterial adhesion. Oral EGF has shown promising results in animal studies for ulcer healing, necrotizing enterocolitis prevention, and restoration of brush border enzyme activity. The peptides for gut health category includes EGF among the most well-characterized options for gastrointestinal support.
How does EGF compare to other anti-aging peptides?
EGF is a full-length growth factor that activates a complete receptor-mediated signaling cascade. This makes it more potent than smaller biomimetic peptides (like Matrixyl or argireline) that mimic only fragments of signaling molecules. However, it is also less stable, more expensive, and more challenging to deliver effectively. Compared to copper peptides, EGF is more directly mitogenic while GHK-Cu offers broader gene modulation and stronger anti-inflammatory effects. The best anti-aging protocols often combine multiple peptide types for synergistic effects, and our peptide stacking guide covers how to design effective multi-peptide regimens.
External resources
Epidermal Growth Factor in Aesthetics and Regenerative Medicine: Systematic Review (PMC)
The Use of Epidermal Growth Factor in Dermatological Practice (PMC)
Epidermal Growth Factor and Intestinal Barrier Function (PMC)
For researchers serious about understanding growth factor biology and building effective peptide protocols, SeekPeptides offers the most comprehensive resource available. Members access in-depth growth factor protocols, evidence-based skincare and healing guides, personalized dosing strategies, and a community of experienced researchers who have worked with EGF and dozens of other peptides across every application covered in this guide.
In case I do not see you, good afternoon, good evening, and good night. May your growth factors stay active, your skin barriers stay strong, and your healing stay swift.



