Jan 30, 2026
Somewhere in the Amazon rainforest, a waxy monkey frog sits motionless on a branch. It looks unremarkable. Green skin, sticky toes, big eyes. But this frog, Phyllomedusa sauvagei, harbors something extraordinary in its skin secretions. A seven-amino-acid chain that changed our understanding of natural peptides forever.
That chain is dermorphin.
When Italian pharmacologist Vittorio Erspamer and his research team isolated dermorphin in 1981, what they found defied expectations. This tiny peptide turned out to be one of the most potent naturally occurring pain-relieving substances ever discovered, roughly 30 to 40 times more powerful than morphine by weight and up to 2,170 times more potent when delivered directly to the brain. It binds to mu-opioid receptors with an affinity that makes most synthetic compounds look clumsy by comparison. And it achieves all of this through a structural trick that scientists had never seen in a vertebrate before: a D-amino acid embedded in position two of its sequence.
Understanding dermorphin matters for anyone serious about peptide research. Not because you will find it on a shelf next to BPC-157 or TB-500, but because this peptide reveals how nature engineers molecular precision that synthetic chemistry still struggles to replicate. The story of dermorphin involves frog biology, peptide safety considerations, receptor pharmacology, a forgotten clinical trial, a horse racing scandal, and ongoing questions about whether one of the most promising pain compounds of the twentieth century was abandoned too soon.
This guide covers everything researchers need to know. The science. The history. The controversy. And the future potential that keeps dermorphin relevant decades after its discovery.
What is dermorphin?
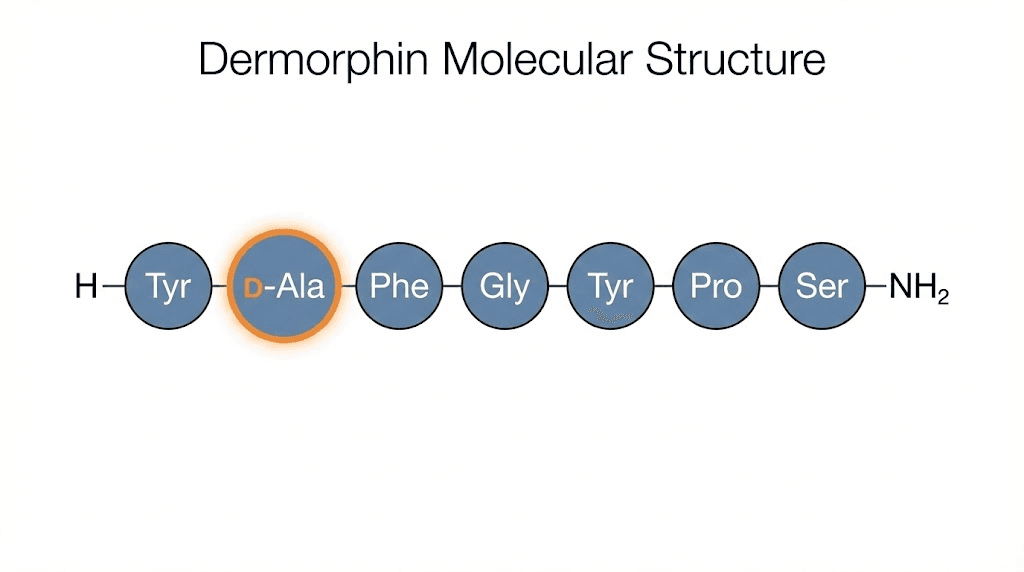
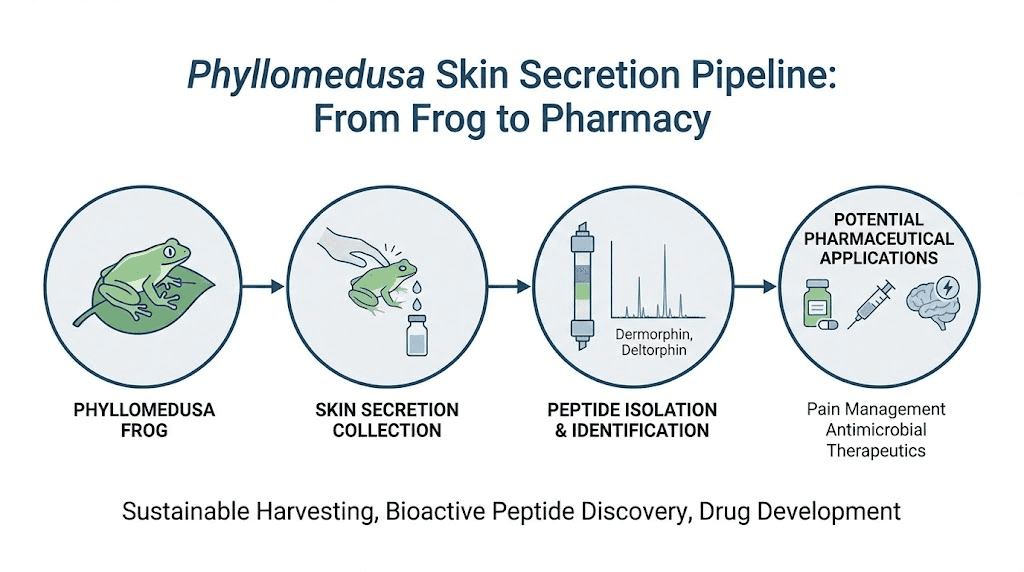
Dermorphin is a naturally occurring heptapeptide, meaning it consists of exactly seven amino acids arranged in the sequence H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2. It belongs to a class of bioactive peptides found exclusively in the skin secretions of South American tree frogs from the subfamily Phyllomedusinae. The name itself comes from "derm" (skin) and "morphin" (a nod to its morphine-like activity), reflecting both its source and its function.
What makes dermorphin unusual is not just its potency. It is the D-alanine residue sitting at position two in the chain.
To understand why that matters, consider this: nearly all peptides used in research contain L-amino acids exclusively. That is the standard configuration in vertebrate biology. D-amino acids, their mirror images, show up in bacteria and some invertebrates. Finding a D-amino acid in a vertebrate peptide was, at the time of discovery, like finding a left-handed glove in a factory that only makes right-handed ones. It simply was not supposed to happen.
The presence of D-alanine gives dermorphin several critical advantages. It resists enzymatic degradation far better than peptides built entirely from L-amino acids. Enzymes that would normally chew through a peptide chain do not recognize the D-configuration, so dermorphin survives longer in biological systems. This structural quirk also contributes to its extraordinary pain-relieving potency and its high selectivity for a specific type of opioid receptor.
The frog produces dermorphin as part of a defensive secretion cocktail. Phyllomedusa sauvagei and related species secrete dozens of bioactive peptides from granular glands in their skin. These include antimicrobial compounds, tachykinins, bradykinins, and multiple opioid peptides. Dermorphin is just one weapon in an impressive chemical arsenal that evolution spent millions of years perfecting.
The amino acid sequence in detail
Breaking down dermorphin residue by residue reveals why each position matters for biological activity.
Position 1, Tyrosine (Tyr): This aromatic amino acid is critical for opioid receptor binding. The hydroxyl group on tyrosine interacts directly with the mu-opioid receptor binding pocket. Remove it, and activity drops to near zero.
Position 2, D-Alanine (D-Ala): The star of the show. This D-amino acid is essential for high-affinity receptor binding and enzymatic resistance. Replace it with L-alanine, and dermorphin loses most of its potency. The D-configuration forces the peptide backbone into a conformation that fits the mu-receptor perfectly.
Position 3, Phenylalanine (Phe): Another aromatic residue that contributes to receptor recognition. Together with tyrosine at position 1, it creates the pharmacophore, the minimum structural feature needed for opioid activity.
Position 4, Glycine (Gly): The simplest amino acid provides flexibility to the peptide backbone. This flexibility allows the C-terminal portion to adopt different conformations depending on the environment.
Positions 5 through 7 (Tyr-Pro-Ser-NH2): These residues form the "address" portion of the molecule. While the N-terminal tetrapeptide (positions 1 through 4) provides the core opioid activity, positions 5 through 7 fine-tune receptor selectivity, pushing dermorphin toward mu-opioid receptors specifically rather than delta or kappa subtypes.
Research has shown that the minimum active fragment of dermorphin is the N-terminal tetrapeptide H-Tyr-D-Ala-Phe-Gly. This four-residue sequence retains significant opioid activity, though the full heptapeptide shows superior potency and selectivity. Scientists have used this knowledge to create dozens of synthetic analogs, each tweaking different positions to explore how structure determines function in peptide formulas.
How the frog makes a D-amino acid
One of the most fascinating aspects of dermorphin biology is how the frog produces a D-amino acid from standard genetic instructions. The mRNA that codes for prodermorphin, the precursor protein, contains the standard codon GCG for L-alanine at position 2. There is no genetic code for D-alanine. None exists in any known organism.
Instead, the frog relies on a post-translational modification. After the ribosome assembles the precursor protein with L-alanine in place, a specialized enzyme converts that L-alanine into its D-isomer. This enzyme, an isomerase, has never been fully characterized, and its mechanism remains one of the unsolved puzzles in peptide biochemistry. Scientists published the genetic evidence for this conversion in Science in 1989, confirming that the D-amino acid is genuinely derived from its L-counterpart after translation.
This discovery has implications far beyond frog biology. It suggests that other organisms might use similar post-translational modifications to create D-amino acid-containing peptides that have gone undetected. Traditional peptide isolation methods often destroy or overlook D-amino acids, meaning there could be an entire hidden pharmacology waiting to be uncovered.
Discovery and history of dermorphin
The story of dermorphin begins with one of the most prolific pharmacologists of the twentieth century.
Vittorio Erspamer spent decades cataloging bioactive compounds from animal sources. His laboratory in Rome had already identified dozens of important molecules, including serotonin in rabbit intestinal tissue. When his team turned their attention to South American tree frogs in the late 1970s, they found what Erspamer would later describe as a "treasure store" of bioactive peptides.
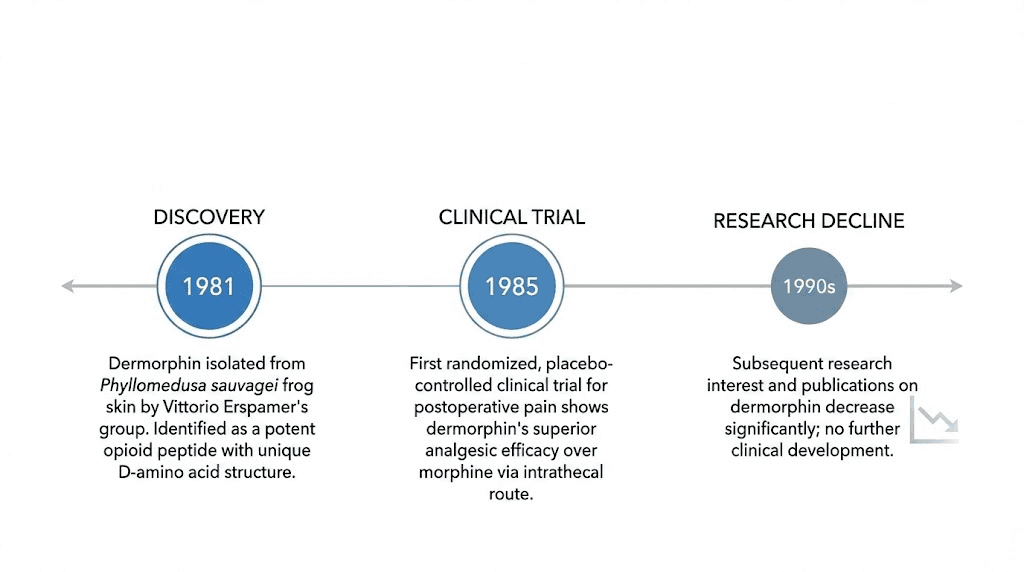
In 1981, Montecucchi, Erspamer, and colleagues published the isolation and characterization of dermorphin from the skin of Phyllomedusa sauvagei. The paper described a new heptapeptide with striking opioid properties, including pain-relieving activity that exceeded morphine by orders of magnitude in certain assays. The compound was named for its dermal origin and its morphine-like activity.
The scientific community reacted with skepticism. A D-amino acid in a vertebrate peptide? Unprecedented. An opioid more potent than anything found in mammals? Extraordinary claims require extraordinary evidence. Erspamer himself noted that his team's initial findings "were met with a great deal of skepticism among his peer group."
But the data held up. Independent laboratories confirmed the structure, the D-amino acid, and the pharmacological potency. By 1985, dermorphin research had reached its peak publication year, with groups around the world exploring its research applications.
The forgotten clinical trial
Perhaps the most remarkable chapter in dermorphin history is a clinical trial that the medical world essentially ignored.
In 1985, Italian clinicians from hospitals in Aquila, Teramo, and Rome conducted a randomized, placebo-controlled study of intrathecal dermorphin for postoperative pain. The trial compared three groups: patients receiving 20 micrograms of dermorphin intrathecally, patients receiving 500 micrograms of morphine intrathecally, and a control group receiving 30 milligrams of pentazocine intramuscularly with a sham spinal puncture.
The results were striking.
Dermorphin provided pain relief lasting an average of 43.41 hours (plus or minus 1.64 hours). Morphine lasted 34.45 hours (plus or minus 2.35 hours). The control group needed additional pain medication after just 10.79 hours (plus or minus 2.23 hours). At one twenty-fifth the dose of morphine, dermorphin outperformed it by nearly nine hours.
Only 22 percent of dermorphin patients needed additional pain medication, compared to 58 percent in the morphine group and 88 percent in the control group. Hospital stays were shorter too: 5.6 days for dermorphin patients versus 6.3 for morphine and 8.7 for control.
No respiratory depression occurred in the dermorphin group. Side effects, including urinary retention, nausea, and vomiting, were comparable across all three groups.
The authors concluded that a single intrathecal administration of dermorphin adequately relieved pain in four out of five patients for the entire five-day postoperative period.
And then, nothing happened.
A 2018 review in the Journal of Pain Research by Keppel Hesselink and Schatman searched for citations of this landmark trial. They found only 15 papers referencing it, and not a single one was written from a clinical perspective. No follow-up trials were conducted. No pharmaceutical company pursued development. The most potent natural opioid peptide ever tested in humans was effectively forgotten.
Why dermorphin was abandoned
Several factors contributed to this abandonment. The results were published in a pharmacological journal specializing in peptide research, not in a mainstream medical journal where clinicians would see them. The intrathecal route of administration, while effective, was not commercially attractive in the 1980s when the pharmaceutical industry preferred oral and subcutaneous formulations. There may also have been economic considerations. As Keppel Hesselink wrote, "perhaps a sense that the profitability of such an endeavor would not be as great as that associated with mass-producing accepted, more traditional mu agonist agents."
Additionally, dermorphin cannot cross the blood-brain barrier when administered peripherally, which was confirmed in 1982. This limitation ruled out oral or subcutaneous delivery for central pain relief, making it less commercially viable compared to existing injectable peptide options that the pharmaceutical industry was pursuing at the time.
The absence of patent protection for a naturally occurring compound likely played a role as well. Without a patent, no company had financial incentive to fund the expensive clinical trials needed for regulatory approval.
Mechanism of action: how dermorphin works
Understanding how dermorphin produces its effects requires a look at the opioid receptor system, one of the most important signaling networks in mammalian biology.
The human body contains three main types of opioid receptors: mu (MOR), delta (DOR), and kappa (KOR). Each type mediates different effects. Mu receptors are primarily responsible for pain relief, euphoria, and respiratory depression. Delta receptors contribute to analgesia and mood regulation. Kappa receptors mediate pain relief but also produce dysphoria and sedation.
Dermorphin is a highly selective mu-opioid receptor agonist. Its affinity for mu receptors is approximately 100-fold higher than its affinity for delta or kappa receptors. This selectivity is not just a laboratory curiosity. It has real implications for the benefits and risks of peptide compounds in research settings.
Receptor binding and activation
When dermorphin encounters a mu-opioid receptor on a neuron's surface, the N-terminal portion of the peptide, specifically the Tyr-D-Ala-Phe sequence, inserts into the receptor binding pocket. The D-alanine at position 2 is crucial here. Its mirror-image configuration forces the peptide backbone into a specific three-dimensional shape that fits the mu-receptor binding site like a key designed for one particular lock.
Once bound, dermorphin activates the receptor's intracellular signaling cascade. The receptor is coupled to a G-protein (specifically Gi/Go proteins), and activation triggers several downstream events. Adenylyl cyclase activity decreases, reducing cyclic AMP levels. Potassium channels open, hyperpolarizing the neuron. Calcium channels close, reducing neurotransmitter release. The net result is a dampening of pain signal transmission along neural pathways.
The selectivity for mu receptors over other opioid subtypes appears to relate to the C-terminal portion of dermorphin. While positions 1 through 4 provide the core opioid activity, positions 5 through 7 (Tyr-Pro-Ser) act as an address code that directs the peptide preferentially toward mu receptors. The charged amino acid residues within the transmembrane domains of the mu-opioid receptor protein create an electrostatic environment that favors dermorphin binding over peptides with different charge distributions.
Potency compared to other compounds
The raw numbers tell a compelling story about dermorphin's potency. In standardized pharmacological assays, dermorphin outperforms several well-known compounds by wide margins.
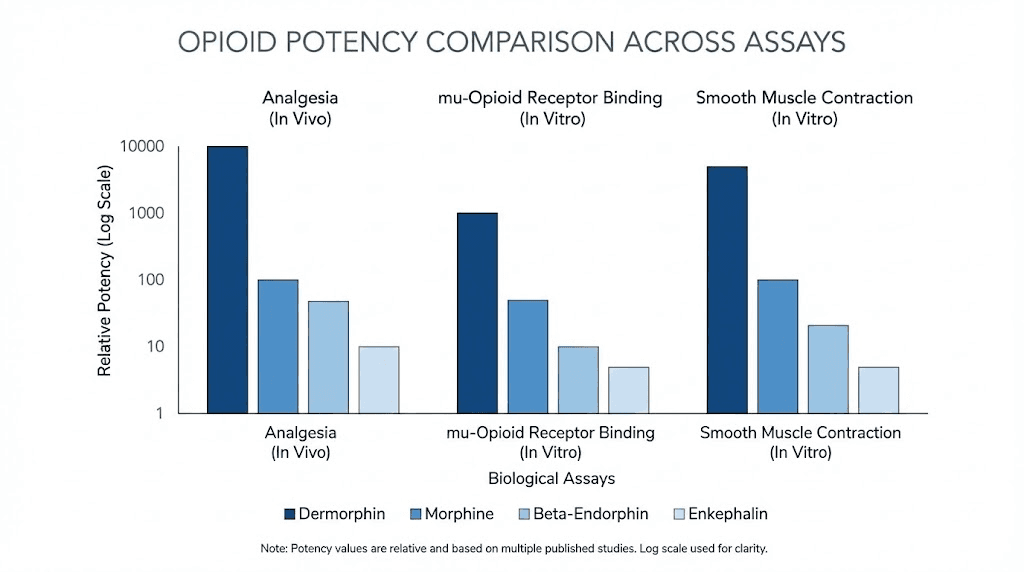
On the guinea pig ileum preparation, a standard test for opioid potency, dermorphin is 57 times more potent than met-enkephalin, 294 times more potent than leu-enkephalin, 18 times more potent than beta-endorphin, and 39 times more potent than morphine.
When administered intracerebroventricularly (directly into the brain) in rats, the gap widens dramatically. On the tail-flick test, dermorphin is 752 times more potent than morphine. On the hot-plate test, it is 2,170 times more potent. Spinal administration shows dermorphin is 3,000 to 5,000 times more active than spinal morphine.
When given intravenously in mice, the potency ratio drops to about 11:1 compared to morphine, reflecting the peptide's limited ability to cross the blood-brain barrier from the peripheral circulation.
For context, until the discovery of mammalian endomorphins by Zadina and colleagues in the 1990s, dermorphin and its relatives represented the most potent and selective mu-opioid receptor agonists identified in any living organism. That is a significant distinction in the broader world of peptide research and studies.
Biphasic effects on the body
One of the more interesting pharmacological features of dermorphin is its dose-dependent biphasic activity.
At low doses (10 to 100 picomoles per kilogram administered intracerebroventricularly in rats), dermorphin increases locomotor activity, respiratory rate, tidal volume, and respiratory minute volume. These stimulatory effects appear to be mediated through mu-1 opioid receptor subtypes.
At higher doses, dermorphin produces the more expected opioid effects: catalepsy, sedation, and respiratory depression. These effects are mediated through mu-2 receptor subtypes.
This biphasic response pattern, stimulation at low doses and depression at high doses, is not unique to dermorphin, but it is more pronounced with this peptide than with many other opioid compounds. The distinction between mu-1 and mu-2 mediated effects has been an area of active research, with implications for developing compounds that could provide pain relief (a mu-2 effect) without respiratory depression (also a mu-2 effect at higher receptor occupancy).
Dermorphin peptide benefits: what the research shows
The potential benefits of dermorphin have been explored across multiple research domains. While this peptide is not approved for medical use in humans and remains a research compound, the scientific literature documents several noteworthy properties that continue to generate interest among researchers studying peptide dosing and pharmacology.
Exceptional analgesic potency
The most extensively documented benefit of dermorphin is its pain-relieving capability. In animal models, dermorphin consistently demonstrates superior analgesic potency compared to morphine across multiple pain assessment methods including the tail-flick test, hot-plate test, and acetic acid writhing test.
The 1985 clinical trial confirmed this translates to humans. A single 20-microgram intrathecal dose provided pain relief lasting over 43 hours in postoperative patients. For comparison, 500 micrograms of intrathecal morphine, a dose 25 times larger, lasted about 34 hours. This is relevant for researchers studying peptides for injury recovery and healing, as understanding pain management pathways informs broader recovery research.
The analgesic effect of dermorphin is fully reversed by naloxone, confirming that it works through the opioid receptor system rather than through some novel or unknown mechanism.
Reduced tolerance development
One of the most significant findings about dermorphin comes from chronic exposure studies. When rats and mice were exposed to continuous infusions of either dermorphin or morphine for four days, the results diverged dramatically.
After four days of continuous morphine infusion, only 10 percent of rats still showed analgesic responses. Tolerance had developed rapidly, as it does in most morphine exposure scenarios. But after four days of continuous dermorphin infusion, 65 percent of rats still maintained analgesic responses. Dermorphin produced less tolerance than morphine by a wide margin.
This finding has profound implications. Tolerance to opioid medications is one of the primary challenges in pain management, forcing dose escalation that increases side effect risk. A compound that provides superior analgesia with slower tolerance development addresses one of the central problems in the field.
Lower physical dependence potential
Withdrawal studies tell a similar story. When naloxone was administered to precipitate withdrawal symptoms in animals chronically exposed to dermorphin versus morphine, the dermorphin group showed significantly less severe withdrawal.
Morphine-treated rats exhibited more than 20 shakes per 15-minute observation period during precipitated withdrawal. Dermorphin-treated rats never exceeded 10 shakes, with a mean of about 6 per 15-minute period. While neither group was withdrawal-free, the severity difference was substantial and consistent across studies.
The reduced dependence profile likely relates to dermorphin's receptor selectivity. Morphine activates mu, delta, and kappa receptors to varying degrees, creating a complex pattern of receptor adaptation. Dermorphin's highly selective mu-receptor activation may produce a cleaner pharmacological signal that the body adapts to more slowly.
Favorable side effect profile
Clinical data, though limited, suggest that dermorphin's side effect profile compares favorably to morphine. In the 1985 trial, the rate of urinary retention was 26 percent for dermorphin versus 30 percent for morphine. Nausea and vomiting occurred in 22 percent of dermorphin patients versus 18 percent for morphine and 14 percent for control. Critically, no respiratory depression was observed in the dermorphin group.
Animal studies support this clinical observation. The analog [Lys7]dermorphin showed that the ratio of analgesic to respiratory-depressant doses was 17 times more favorable than the same ratio for morphine. This wider therapeutic window, the gap between the dose that relieves pain and the dose that suppresses breathing, is exactly what researchers hope to find in any new analgesic compound.
For those exploring the broader landscape of peptide safety and risks, dermorphin's profile offers useful context about how structural modifications can influence a compound's therapeutic index.
Gastrointestinal effects
Research has documented several effects of dermorphin on the gastrointestinal system. The peptide inhibits gastric acid secretion, delays gastric emptying, and slows intestinal and colonic propulsion. These effects are consistent with mu-opioid receptor activation in the gut and mirror the constipating effects of other opioid compounds.
While gastrointestinal slowing is typically considered a side effect, there are research contexts where controlled modification of gut motility is desirable. The interaction between opioid peptides and gut health pathways remains an active area of investigation.
Endocrine effects
Dermorphin also stimulates thyrotropin (TSH) secretion in normal subjects, suggesting it interacts with hypothalamic-pituitary pathways that regulate thyroid function. This endocrine effect was documented in early human studies and adds to the complex pharmacological profile of this peptide.
The interaction between opioid peptides and endocrine function is well established. Endogenous opioids like beta-endorphin play regulatory roles in hormone secretion, and dermorphin's effects on TSH secretion fit within this broader biological framework. Researchers interested in how peptides influence hormonal pathways, including those exploring peptides and testosterone, find the opioid-endocrine connection relevant to understanding systemic peptide effects.
The dermorphin peptide family
Dermorphin does not exist in isolation. It is the founding member of a family that has grown to include seven naturally occurring peptides and nearly 30 synthetic analogs. Understanding this family provides context for dermorphin's place in the broader landscape of bioregulator peptides and opioid research.
Natural family members
The dermorphin peptide family includes the parent compound and six closely related natural variants. All share the characteristic N-terminal Tyr-D-amino acid sequence and all are isolated from Phyllomedusinae frog skin.
Dermorphin: H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2. The prototype. Highly selective for mu-opioid receptors.
[Hyp6]-dermorphin: Contains hydroxyproline at position 6 instead of proline. Similar potency to dermorphin with slightly different pharmacokinetic properties.
The remaining five family members represent variations on the core structure, each with slightly different receptor binding profiles and potency characteristics. Together, these seven peptides provided the template for decades of structure-activity relationship studies.
Deltorphins: the delta-selective cousins
While dermorphin targets mu-opioid receptors, the same frogs produce peptides that selectively target delta-opioid receptors. These are the deltorphins, discovered by Kreil and colleagues in 1989 and by Erspamer shortly thereafter.
Deltorphins share the unusual D-amino acid at position 2, but their sequences diverge from position 3 onward, creating a completely different receptor selectivity profile. Deltorphin I and Deltorphin II remain among the most selective delta-opioid receptor agonists known to science.
The fact that a single frog species produces both the most potent mu-selective and the most selective delta-selective opioid peptides known is remarkable. It suggests that these frogs evolved a sophisticated chemical defense system that targets multiple opioid receptor subtypes in potential predators, and it has given researchers powerful pharmacological tools for studying opioid receptor systems.
Synthetic analogs of dermorphin
The structural simplicity of dermorphin, just seven amino acids, makes it an ideal template for analog design. Scientists have created nearly 30 synthetic variants, each modifying one or more residues to explore how changes affect potency, selectivity, and pharmacokinetic properties.
DALDA (H-Tyr-D-Arg-Phe-Lys-NH2): A tetrapeptide analog that shows particular promise. Unlike morphine, systemic DALDA does not impair motor coordination in rotarod tests, even at the highest doses tested. It also does not reduce locomotor activity in open-field tests, suggesting a more favorable side effect profile for certain research applications.
DMTP (H-Tyr-D-Arg-Phe-Gly-NH2): Another tetrapeptide derivative that has progressed through Phase I and Phase II clinical studies. Results demonstrated high analgesic efficacy with a more favorable safety profile compared to morphine. DMTP is described as a highly selective agonist of the mu-1 opioid receptor subtype.
[Lys7]-dermorphin: A full-length analog with lysine replacing serine at position 7. Studies of its respiratory and cardiovascular effects in awake rats showed that the ratio of analgesic to respiratory-depressant doses was 17 times more favorable than the same ratio for morphine.
These analogs demonstrate a principle central to peptide formula research: small structural changes can dramatically alter a compound's pharmacological profile. Each substitution teaches researchers something new about the relationship between molecular structure and biological function.
Dermorphin in the context of the opioid system
To appreciate dermorphin's significance, it helps to understand where it fits among the body's own opioid peptides and the broader landscape of pain-modulating compounds.
The body's natural opioid peptides
Mammals produce their own opioid peptides, collectively known as endogenous opioids. The three main families are:
Endorphins: Derived from pro-opiomelanocortin (POMC), beta-endorphin is the most studied member. It has broad affinity across mu and delta receptors and plays roles in pain modulation, stress response, and reward pathways.
Enkephalins: Met-enkephalin and leu-enkephalin are pentapeptides with moderate affinity for both mu and delta receptors. They function primarily as local neurotransmitters in pain pathways.
Dynorphins: Derived from prodynorphin, these peptides preferentially activate kappa-opioid receptors and are involved in pain modulation, stress responses, and dysphoric states.
Endomorphins: Discovered in 1997 by Zadina and colleagues, endomorphin-1 and endomorphin-2 are the only known mammalian peptides with high selectivity for mu-opioid receptors. Before their discovery, dermorphin held the distinction as the most potent and selective mu-receptor agonist from any living source.
Dermorphin surpasses all of these endogenous peptides in potency at the mu receptor. It is 57 times more potent than met-enkephalin, 18 times more potent than beta-endorphin, and significantly more selective for mu receptors than any endogenous mammalian opioid except the endomorphins.
Why does a frog make opioid peptides?
The evolutionary question is fascinating. Why would a frog evolve to produce peptides that interact with mammalian opioid receptors?
The answer likely involves predator defense. When a predator, such as a snake or bird, bites or attempts to eat a Phyllomedusa frog, the skin secretion cocktail is released. The opioid peptides would cause disorientation, sedation, or altered behavior in the predator. Combined with the other bioactive peptides in the secretion (which cause pain, inflammation, and cardiovascular effects), the cocktail creates a powerfully unpleasant experience that the predator learns to associate with the frog's appearance.
The presence of both mu-selective (dermorphin) and delta-selective (deltorphin) opioid peptides in the same secretion suggests a strategy of hitting multiple receptor targets simultaneously for maximum defensive effect. This is a form of chemical warfare refined over millions of years of evolution.
Interestingly, indigenous Amazonian peoples recognized the bioactive potential of frog skin secretions long before Western science did. The Matses, Katukina, Kaxinawa, and other tribes have used the secretion of Phyllomedusa bicolor (a close relative) in a ritual called Kambo or Sapo for centuries, applying the dried secretion to small skin burns to induce intense physiological effects including pain modulation and heightened alertness.
The horse racing controversy
Dermorphin entered public consciousness in 2011, and not for reasons scientists would have preferred.
Intelligence obtained from several North American race tracks suggested that trainers were using dermorphin to enhance horse performance during races. The substance had gone undetected until that point because existing drug tests did not screen for it. Dermorphin was not on anyone's list of expected doping agents because it is a naturally occurring frog peptide, not a pharmaceutical product.
When Industrial Laboratories in Colorado and Louisiana State University's equine drug-testing laboratory developed detection methods, approximately 30 positive tests emerged from tracks in the American Southwest. The National Horsemen's Benevolent and Protective Association (NHBPA) responded forcefully. CEO Phil Hanrahan declared: "Dermorphin is doping. Those who use dermorphin should be severely punished."
Why dermorphin appealed to horse dopers
From a pharmacological standpoint, dermorphin had three properties that made it attractive for illicit use in horse racing.
First, it directly stimulates locomotor activity at low doses, potentially making horses run harder. Second, it suppresses pain perception, allowing horses to push through discomfort or injuries during a race. Third, it suppresses the perception of exhaustion at the end of a race, potentially extending peak performance duration.
The fact that dermorphin produces less tolerance and dependence than morphine also made it practical for repeated use without the declining efficacy that would come with other opioids.
Regulatory and legal consequences
The Racing Commissioners International (RCI) classified dermorphin as a Class I drug, the most serious category for banned substances in equine competition. This classification carries the harshest penalties available under racing regulations.
Penalties were significant. Trainers faced suspensions of six months or more. In 2018, a Louisiana veterinarian was sentenced to 15 months in jail for selling dermorphin for use in horses.
The dermorphin scandal also accelerated broader reform efforts in horse racing drug testing. Veterinary researchers at Penn developed novel detection methods that could identify dermorphin metabolites even when they were not included on the original testing panel. The Horseracing Integrity and Safety Act (HISA), which established federal oversight of drug testing in horse racing, cited cases like dermorphin as evidence of the need for stronger enforcement.
For researchers following peptide regulation news, the dermorphin horse racing episode illustrates how potent bioactive peptides can create regulatory challenges that extend far beyond the laboratory.
Dermorphin safety and side effects
Any discussion of dermorphin benefits must be balanced against a thorough understanding of safety considerations. As a potent mu-opioid receptor agonist, dermorphin carries risks inherent to all opioid compounds, though research suggests some of these risks may be modulated compared to conventional opioids.
Known side effects from research
Clinical and preclinical data identify several side effects associated with dermorphin:
Nausea and vomiting: In preliminary open-label studies, 50 percent of patients receiving intrathecal dermorphin reported nausea or vomiting. In the controlled trial, this dropped to 22 percent, comparable to morphine at 18 percent. Pretreatment with domperidone, an anti-emetic, reduced the incidence to below 20 percent.
Urinary retention: Reported in 26 percent of dermorphin patients versus 30 percent for morphine. This is a common mu-opioid receptor mediated effect and was not significantly different between active treatment groups.
Gastrointestinal slowing: Dermorphin inhibits gastric acid secretion, delays gastric emptying, and slows colonic propulsion. These effects mirror standard opioid-induced constipation.
Catalepsy at high doses: In animal studies, higher doses of dermorphin produce catalepsy, a state of muscular rigidity and decreased voluntary movement. This effect is dose-dependent and reversible with naloxone.
Respiratory depression at high doses: While the 1985 clinical trial reported no respiratory depression, animal studies clearly demonstrate dose-dependent respiratory depression at higher doses. The biphasic nature of dermorphin's effects means low doses can actually stimulate respiration while high doses suppress it.
Tolerance and dependence: While less severe than with morphine, dermorphin does produce both tolerance and physical dependence with chronic exposure. These effects are reversible upon discontinuation but represent real risks for any compound in this pharmacological class.
Safety considerations for researchers
Researchers working with dermorphin or studying its effects should understand several important safety parameters. The compound is not approved for human use by any regulatory agency. It has never been approved for medical use in humans or animals, and it holds no pharmaceutical registration anywhere in the world.
The potency of dermorphin means that biologically active amounts are extremely small. Microgram quantities delivered intrathecally produce profound effects. This potency demands precise handling protocols and dosing equipment, considerations that apply broadly to anyone involved in calculating peptide dosages.
Cross-tolerance exists between dermorphin and morphine. Animals tolerant to morphine show reduced responses to dermorphin and vice versa, confirming that both compounds act through overlapping receptor mechanisms. Researchers studying either compound need to account for prior opioid exposure in their experimental designs.
As with any peptide safety evaluation, the route of administration dramatically affects both the potency and the side effect profile. Intrathecal dermorphin is thousands of times more potent than intravenous dermorphin due to the blood-brain barrier limitation of peripheral administration.
Dermorphin versus other pain-related peptides
Placing dermorphin in context with other peptides studied for pain-related applications helps clarify its unique position in peptide research.
Dermorphin versus BPC-157
BPC-157 is a 15-amino-acid peptide derived from human gastric juice that has gained significant attention for its tissue-healing properties. While both dermorphin and BPC-157 are peptides studied in pain-related contexts, they work through completely different mechanisms.
BPC-157 promotes tissue repair through angiogenesis, collagen production, and growth factor modulation. It does not interact with opioid receptors. Dermorphin provides pain relief by activating mu-opioid receptors without directly promoting tissue healing.
Researchers studying injury recovery might encounter both compounds in the literature but should understand that they address fundamentally different aspects of the pain and healing equation. BPC-157 targets the underlying tissue damage. Dermorphin modulates the perception of pain arising from that damage.
Dermorphin versus DSIP
DSIP (Delta Sleep-Inducing Peptide) is a nonapeptide studied for its effects on sleep architecture and pain modulation. While both dermorphin and DSIP influence pain pathways, DSIP works through mechanisms distinct from the opioid system, including modulation of GABAergic transmission and stress hormone regulation.
DSIP may help manage chronic pain indirectly through improved sleep quality and stress reduction, whereas dermorphin provides direct, rapid analgesia through opioid receptor activation. The compounds occupy different niches in pain research.
Dermorphin versus KPV
KPV peptide is a tripeptide fragment of alpha-melanocyte-stimulating hormone studied for its anti-inflammatory properties. KPV reduces pain indirectly by modulating inflammation through NF-kB pathway inhibition. Dermorphin does not significantly affect inflammatory pathways but directly suppresses pain signal transmission.
These represent fundamentally different approaches to pain management: anti-inflammatory (KPV) versus analgesic (dermorphin). Understanding this distinction is important for researchers designing studies that might involve inflammation-modulating peptides.
Dermorphin versus SS-31
SS-31 (Elamipretide) is a mitochondria-targeted peptide being studied for various conditions including pain associated with mitochondrial dysfunction. While dermorphin works through opioid receptors in the nervous system, SS-31 targets mitochondrial cardiolipin to improve cellular energy production and reduce oxidative stress.
The comparison illustrates how different peptides approach pain through entirely different biological pathways. Dermorphin targets the symptom (pain perception) while compounds like SS-31 target potential underlying cellular dysfunction.
The Kambo connection
Any complete discussion of dermorphin must address its relationship to Kambo, the traditional Amazonian frog secretion ceremony that has gained global attention in recent years.
What is Kambo?
Kambo (also called Sapo) is a ritual practice using the dried skin secretion of Phyllomedusa bicolor, a close relative of the dermorphin-producing P. sauvagei. The secretion is applied to small burn marks on the skin, allowing the bioactive peptides to enter the lymphatic system directly.
Indigenous Amazonian tribes including the Matses, Katukina, Kaxinawa, and Mayoruna have practiced Kambo ceremonies for centuries. Traditional uses include preparation for hunting (to improve stamina, alertness, and pain tolerance), purification rituals, and treatment of various ailments including malaria, snake bites, and lethargy.
Dermorphin's role in Kambo
The secretion of Phyllomedusa bicolor contains dermorphin and deltorphins among dozens of other bioactive peptides. The opioid peptides in the secretion contribute to the pain-modulating and consciousness-altering effects that Kambo practitioners report.
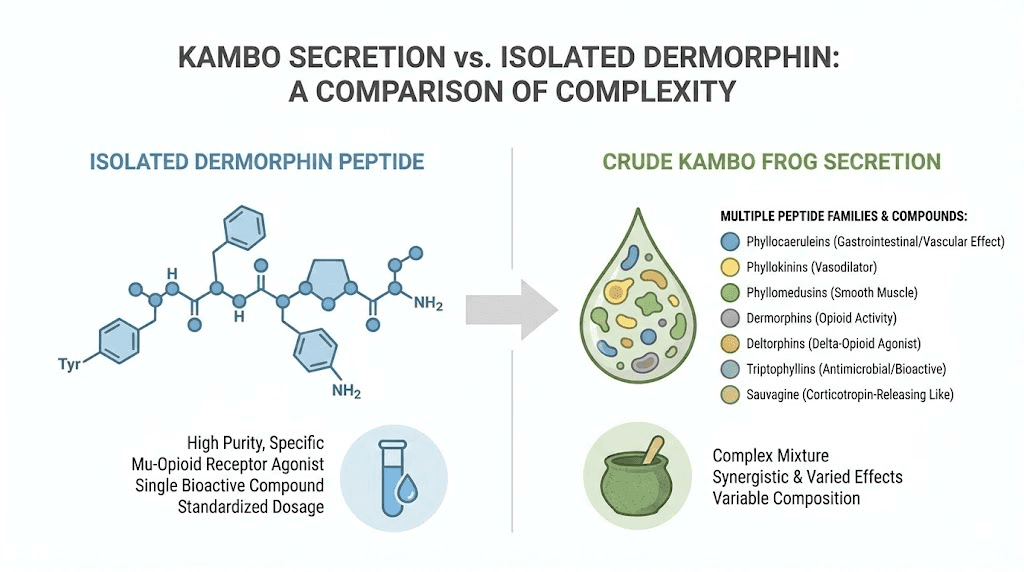
However, it is important to understand that Kambo is not the same as dermorphin administration. The frog secretion contains at least seven families of bioactive compounds including caeruleins (which cause intense smooth muscle contraction), tachykinins (which trigger nausea and vasodilation), bradykinins (which cause intense pain and inflammation), sauvagine (a potent vasodilator), antimicrobial dermaseptins, and the opioid peptides (dermorphin and deltorphins).
The intense physiological response that Kambo produces, including rapid onset of facial swelling, sweating, nausea, vomiting, and bowel movements followed by a period of altered perception, results from the combined action of all these peptide families, not from dermorphin alone.
Safety concerns with Kambo
Kambo use has been associated with serious adverse events including sudden cardiac death, esophageal rupture, syndrome of inappropriate antidiuretic hormone secretion (SIADH), acute renal failure, dermatomyositis, severe psychosis, toxic hepatitis, and deaths. A systematic review documented multiple fatalities associated with Kambo ceremonies.
Brazil banned the sale and marketing of Kambo in 2016. Australia listed it as a Schedule 10 poison in 2021 following two deaths during Kambo rituals. These regulatory actions reflect the genuine dangers of uncontrolled exposure to a complex mixture of potent bioactive peptides.
The Kambo context reinforces a fundamental principle in peptide safety: the difference between a purified, characterized compound administered in precise doses and a crude mixture applied without dosing control is the difference between research and recklessness. Researchers studying dermorphin or any other peptide must maintain rigorous protocols for purity, dosing, and administration, standards that are impossible to achieve in a ceremonial context.
The case for rediscovering dermorphin
In 2018, Jan Keppel Hesselink and Michael Schatman published a provocative paper titled "Rediscovery of old drugs: the forgotten case of dermorphin for postoperative pain and palliation." Their argument is worth examining in detail because it raises questions relevant to anyone involved in peptide research.
The argument for re-exploration
Keppel Hesselink and Schatman identified several factors that make dermorphin worth revisiting.
First, intrathecal drug delivery technology has improved enormously since 1985. Modern intrathecal pumps are far more precise and reliable than those available when dermorphin was first tested clinically. The route of administration that was considered impractical four decades ago is now standard practice for chronic pain management.
Second, the opioid crisis has created urgent demand for alternatives to conventional opioids. A compound that provides superior pain relief with less tolerance development, less physical dependence, and potentially less respiratory depression directly addresses the most pressing problems in modern pain medicine.
Third, dermorphin's selectivity for mu receptors over kappa and delta receptors may translate to fewer "off-target" effects. Morphine activates all three receptor types to varying degrees, and many of its side effects relate to kappa and delta receptor activation. A cleaner pharmacological profile could mean fewer complications.
Fourth, dermorphin's use in palliative care for cancer patients receiving intrathecal therapy deserves specific exploration. Cancer pain management often requires high-dose intrathecal opioids, and the limitations of morphine in this context (pH changes in cerebrospinal fluid, granuloma formation at catheter tips) create clinical problems that a different compound might avoid.
Barriers to rediscovery
Despite the compelling arguments, several barriers stand in the way of dermorphin's return to clinical investigation.
The absence of patent protection means no pharmaceutical company has financial incentive to fund the required clinical trials. Phase III trials typically cost hundreds of millions of dollars, and without the prospect of exclusive marketing rights, the return on investment is unclear.
Regulatory requirements have also increased substantially since 1985. Modern clinical trials require extensive preclinical toxicology packages, manufacturing under Good Manufacturing Practice (GMP) conditions, and multi-center randomized controlled trials that would demand resources well beyond what any academic group could assemble.
There is also the challenge of peptide stability and delivery. Like many peptide storage concerns, dermorphin must be handled carefully to maintain biological activity. Developing a stable pharmaceutical formulation for intrathecal delivery would require significant formulation science work.
Public perception presents another hurdle. Dermorphin is associated with both frog secretion ceremonies (Kambo) and horse racing doping. Neither association helps the case for legitimate clinical development, even though the pharmaceutical compound would have nothing to do with either context.
Current research directions
Despite the barriers, dermorphin-related research continues along several avenues that may eventually produce clinical applications.
Novel analog development
The most active area of dermorphin research involves creating new analogs with improved pharmacological properties. Recent work published in the International Journal of Molecular Sciences describes novel dermorphin analogs with neurotropic activity that are active after systemic and even noninvasive administration, addressing the blood-brain barrier limitation of the parent compound.
Other researchers are exploring peripherally restricted dermorphin analogs that cannot enter the brain at all, targeting pain relief through peripheral opioid receptors without central nervous system effects. The DALDA analog demonstrated this concept: it provides analgesia for neuropathic pain through peripheral mu-opioid receptors while showing no motor impairment or sedation at effective doses.
This approach, targeting peripheral rather than central opioid receptors, could produce pain relief without the sedation, euphoria, and addiction potential associated with centrally acting opioids. For researchers following developments in peptide research, peripherally restricted opioid peptides represent one of the most promising frontiers.
Quality control and analytical methods
Researchers have also published work on comprehensive quality control approaches for dermorphin derivatives. A study in the journal Scientia Pharmaceutica describes methods for quality control of a dermorphin derivative representative of synthetic opioid peptides with non-narcotic type analgesia. These analytical advances are necessary groundwork for any eventual clinical development.
Receptor pharmacology tools
Dermorphin continues to serve as an important pharmacological tool for studying mu-opioid receptor biology. Researchers at the University of Kentucky developed dermorphin-based affinity labels with subnanomolar affinity for mu-opioid receptors, creating tools that help map receptor structure and function at the molecular level.
These tools contribute to our understanding of how opioid receptors work, knowledge that informs the development of better pain medications across the entire field, not just dermorphin-related compounds.
Dermorphin and the broader peptide landscape
For researchers and enthusiasts following the world of peptides, dermorphin offers several important lessons.
Nature as a source of pharmacological innovation
Dermorphin demonstrates that natural sources can produce compounds with properties that synthetic chemistry has difficulty matching. The D-amino acid incorporation, the receptor selectivity, the potency, these features emerged through millions of years of evolutionary optimization. The pharmaceutical industry has increasingly recognized the value of natural product-derived compounds, and amphibian skin peptides remain one of the richest sources of novel bioactive molecules.
From the skin of Phyllomedusa frogs alone, researchers have cataloged more than 200 peptide sequences. These include antimicrobial compounds that could address antibiotic resistance, wound-healing peptides, and vasodilators, all in addition to the opioid peptides. The biodiversity of amphibian skin secretions remains largely unexplored, and conservation of these species is essential for future pharmacological discovery.
The importance of D-amino acids in peptide design
Dermorphin's D-alanine residue taught the peptide chemistry field a fundamental lesson: mirror-image amino acids can dramatically improve a peptide's pharmacological properties by increasing enzymatic stability and altering receptor binding geometry.
This principle is now widely applied in the design of therapeutic peptides. Many modern peptide drugs incorporate D-amino acids or other non-natural modifications specifically to improve metabolic stability, an approach that can be traced directly back to observations about dermorphin's unusual durability in biological systems. Researchers working with any peptide formula can draw on these insights.
The gap between discovery and development
Perhaps dermorphin's most sobering lesson is how a compound with genuinely superior clinical performance can fall through the cracks of the pharmaceutical development system. A single poorly placed publication, an unfashionable route of administration, lack of patent protection, these non-scientific factors can determine whether a promising compound reaches patients or remains a laboratory curiosity.
This pattern repeats across the peptide research landscape. Many peptides with demonstrated biological activity face development challenges related to delivery, stability, manufacturing costs, and commercial viability rather than lack of efficacy. The dermorphin story serves as a cautionary tale about how the structure of pharmaceutical development can fail to capture the value of naturally derived compounds.
Practical considerations for researchers
Researchers approaching dermorphin or its analogs in laboratory settings should understand several practical aspects that affect experimental outcomes.
Handling and storage
Dermorphin, like most bioactive peptides, requires careful handling to maintain potency. The peptide should be stored lyophilized (freeze-dried) at minus 20 degrees Celsius or below for long-term storage. Once reconstituted, solutions should be used promptly or aliquoted and frozen to prevent degradation. Repeated freeze-thaw cycles degrade peptide quality.
Standard peptide storage protocols apply. Use sterile technique during reconstitution. Reconstitute with appropriate solvents, typically bacteriostatic water or sterile saline depending on the application. Store reconstituted solutions in glass vials rather than plastic to minimize adsorption losses.
The stability of peptides in powder form varies by compound, and dermorphin's D-amino acid content may confer additional stability compared to all-L-amino-acid peptides of similar size, though researchers should still adhere to strict storage protocols. Understanding how long reconstituted peptides last in the fridge is essential for any laboratory working with these compounds.
Reconstitution and preparation
Researchers unfamiliar with peptide preparation can reference guides on how to reconstitute peptides for general best practices. The peptide reconstitution calculator can help determine appropriate dilution volumes to achieve target concentrations.
Dermorphin's high potency means that biologically active concentrations are in the picomolar to nanomolar range for central administration and micromolar range for peripheral assays. Accurate preparation requires calibrated micropipettes and precise weighing of the lyophilized peptide. Serial dilutions from a concentrated stock solution are typically more reliable than attempting to weigh out the tiny amounts needed for individual experiments.
Assay considerations
Standard opioid receptor binding assays, GPI (guinea pig ileum), MVD (mouse vas deferens), and radioligand displacement assays all work reliably with dermorphin. For in vivo studies, the tail-flick, hot-plate, and acetic acid writhing tests are established methods for assessing dermorphin's analgesic activity.
Researchers should be aware of cross-tolerance effects. Animals previously exposed to morphine or other opioids will show reduced responses to dermorphin, and vice versa. Washout periods are essential for clean experimental results.
The naloxone reversibility of dermorphin effects provides a useful control in any experimental protocol. Administration of naloxone should fully reverse dermorphin's analgesic, locomotor, and respiratory effects, confirming that observed effects are opioid receptor-mediated.
Understanding dermorphin's regulatory status
Dermorphin occupies an unusual regulatory position that researchers need to understand.
The peptide is not classified as a controlled substance in the same way that morphine or fentanyl are. It has no pharmaceutical registration, no approved medical use, and no DEA scheduling. However, its potent opioid activity means that any attempt at clinical use would face intense regulatory scrutiny.
In the equine industry, dermorphin is classified as a Class I banned substance by the Association of Racing Commissioners International, carrying the most severe penalties for violations. This classification reflects the compound's potent pharmacological activity and its potential for misuse in competitive settings.
For research purposes, dermorphin can typically be obtained from peptide synthesis companies as a research chemical. It is available as a custom synthesis or catalog item from various suppliers, though researchers should ensure they are working with properly tested peptide materials from reputable sources. Verification of purity through HPLC and mass spectrometry is standard practice, and peptide quality verification should never be skipped.
Researchers interested in the broader regulatory landscape for research peptides can explore our comprehensive guide on whether peptides are legal and the evolving peptide regulation landscape.
The Phyllomedusa pharmacopeia: beyond dermorphin
Dermorphin is just one member of an extraordinary collection of bioactive compounds found in Phyllomedusa frog skin. Understanding the broader pharmacopeia provides context for how these frogs evolved such a remarkable chemical defense system and why they continue to interest researchers across multiple disciplines.
Seven peptide families from one frog
The skin of Phyllomedusinae frogs contains at least seven distinct families of bioactive peptides:
Caeruleins (phyllocaerulein): Potent stimulants of smooth muscle contraction and gastric acid secretion. Related to the mammalian peptide cholecystokinin.
Bradykinins (phyllokinin): Powerful vasodilators that cause pain and inflammation. These contribute to the intense discomfort that a predator would experience upon biting the frog.
Tachykinins (phyllomedusin): Excite neurons, evoke behavioral responses, contract smooth muscles, and cause vasodilation and secretion. These contribute to the nausea and gastrointestinal effects reported in Kambo ceremonies.
Bombesins (phyllolitorin): Affect gastric acid secretion, thermoregulation, and food intake regulation.
Sauvagine: A potent vasodilator and stress-hormone modulator related to corticotropin-releasing factor (CRF). Named after Phyllomedusa sauvagei, the same species that produces dermorphin.
Opioid peptides (dermorphins and deltorphins): The mu-selective and delta-selective compounds discussed throughout this guide.
Tryptophyllins: A more recently characterized family whose biological functions are still being investigated.
Additionally, Phyllomedusa frogs produce antimicrobial peptides called dermaseptins that are active against bacteria, fungi, and even some viruses. With over 200 peptide sequences cataloged from this frog genus alone, the diversity rivals that of a pharmaceutical compound library, except that evolution, not medicinal chemists, did the optimization.
Implications for drug discovery
The pharmaceutical value of amphibian skin peptides extends well beyond opioid research. Antimicrobial peptides from Phyllomedusa are being studied as potential alternatives to conventional antibiotics at a time when antimicrobial resistance threatens global health. Wound-healing peptides could find applications in surgical and trauma settings. Vasodilatory peptides offer potential in cardiovascular research.
For researchers exploring the world of bioactive peptides, Phyllomedusa frog secretions represent one of the richest natural libraries of novel pharmacologically active compounds. The conservation of these Amazonian frog species is therefore not just an environmental concern but a practical matter of preserving future pharmaceutical potential.
Frequently asked questions
What is dermorphin made from?
Dermorphin is a naturally occurring heptapeptide originally isolated from the skin secretions of South American tree frogs in the genus Phyllomedusa, particularly Phyllomedusa sauvagei. For research purposes, it is produced through chemical peptide synthesis rather than extracted from frogs. The synthetic version is identical in structure to the natural compound: H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2.
How potent is dermorphin compared to morphine?
Dermorphin is approximately 30 to 40 times more potent than morphine in standard pharmacological assays when comparing equivalent administration routes. When delivered directly to the brain (intracerebroventricularly), dermorphin can be 750 to 2,170 times more potent depending on the pain assessment method used. In the only human clinical trial, 20 micrograms of intrathecal dermorphin outperformed 500 micrograms of intrathecal morphine in both duration and degree of pain relief.
Is dermorphin approved for medical use?
No. Dermorphin has never been approved for medical use in humans or animals by any regulatory agency worldwide. It remains a research compound. While one clinical trial in 1985 showed promising results for postoperative pain, no follow-up clinical development occurred. The compound has no pharmaceutical registration, no marketing authorization, and no clinical guidelines for use.
What is the difference between dermorphin and Kambo?
Dermorphin is a single, purified peptide with a defined seven-amino-acid sequence. Kambo is the crude skin secretion of Phyllomedusa bicolor that contains dermorphin alongside dozens of other bioactive peptides. Using Kambo exposes someone to an uncontrolled mixture of compounds at unknown doses, which has been associated with serious adverse events including deaths. Isolated dermorphin used in research settings represents a completely different scenario from crude frog secretion application.
Why was dermorphin used in horse racing?
Dermorphin's combination of pain suppression, locomotor stimulation, and exhaustion suppression made it attractive for illicit use in horse racing. It was administered before races to enhance performance. The substance went undetected until 2011 because existing drug tests did not screen for naturally occurring frog peptides. It is now classified as a Class I banned substance in equine competition.
Can dermorphin cross the blood-brain barrier?
No. When administered peripherally (intravenously or subcutaneously), dermorphin cannot effectively cross the blood-brain barrier in its intact form. This was confirmed in 1982 and represents one of the main limitations that hindered its pharmaceutical development. Intrathecal (spinal) administration bypasses this barrier, which is why the clinical trial used that route. Recent research on nasal spray peptide delivery and modified analogs explores ways to overcome this limitation.
Does dermorphin cause addiction?
Dermorphin does produce tolerance and physical dependence with chronic exposure, as do all mu-opioid receptor agonists. However, research consistently shows that these effects are less severe with dermorphin than with morphine. After four days of continuous exposure in animal studies, dermorphin maintained analgesic efficacy in 65 percent of subjects versus only 10 percent for morphine. Withdrawal severity was also significantly lower.
What are dermorphin analogs?
Scientists have created nearly 30 synthetic variants of dermorphin by modifying its amino acid sequence. Notable analogs include DALDA (which targets peripheral opioid receptors without entering the brain), DMTP (which completed Phase I and Phase II clinical studies), and [Lys7]-dermorphin (which shows a 17-fold better ratio of analgesic to respiratory-depressant effects compared to morphine). These analogs aim to retain dermorphin's benefits while reducing its limitations. Exploring peptide combinations and analogs is a growing area of research.
External resources
For researchers serious about understanding the full spectrum of peptide science and navigating the complex landscape of opioid peptide research, SeekPeptides provides the most comprehensive resource available. Members access evidence-based guides, detailed dosing protocols, and a community of thousands of researchers who have navigated these exact questions.
From peptide calculators to safety guides, SeekPeptides offers the tools and knowledge that serious peptide researchers need.
In case I do not see you, good afternoon, good evening, and good night. Join SeekPeptides.



