Jan 27, 2026
Ninety-eight percent purity. That number appears on countless peptide vials. It looks impressive. Professional. Trustworthy.
But here is the uncomfortable truth that most researchers discover too late. That 98% figure can mean almost anything. Or nothing at all.
A peptide can test at 98% purity while containing dangerous levels of endotoxins. It can pass basic quality checks while harboring heavy metal contamination invisible to standard testing. The chromatogram might look clean while the actual peptide formula contains synthesis byproducts that sabotage your research results. Understanding what separates a legitimate pure peptides lab from operations cutting corners requires diving deep into analytical chemistry, manufacturing processes, and quality verification systems that most vendors never explain.
The difference between a genuinely pure peptide and one that merely appears pure on paper can mean the difference between breakthrough research findings and months of wasted effort chasing artifacts. SeekPeptides members consistently report that understanding these quality distinctions transformed their research outcomes, turning inconsistent results into reproducible findings.
This guide breaks down everything you need to know about evaluating peptide lab quality. You will learn to read certificates of analysis like a trained analytical chemist. You will understand why certain testing methods matter more than others. And you will develop the expertise to separate marketing claims from genuine quality indicators, so every peptide you acquire meets the standards your research demands.
What defines a pure peptides lab
The term "pure peptides lab" gets thrown around freely in vendor marketing. Understanding what genuine purity means at the manufacturing level helps you evaluate these claims critically.
Purity in peptide chemistry refers specifically to the percentage of target compound present relative to other substances in the sample. A 99% pure BPC-157 sample contains 99% of the intended BPC-157 sequence, with the remaining 1% consisting of related impurities, typically truncated sequences, deletion sequences, or oxidized variants from the synthesis process.
But purity tells only part of the story. A peptide can achieve impressive purity numbers while still containing problematic contaminants that standard purity testing does not detect.
The distinction between purity and quality
High-performance liquid chromatography determines peptide purity by measuring the target compound against peptide-related impurities. The technique excels at detecting synthesis byproducts, incomplete sequences, and chemical modifications of the peptide backbone.
What HPLC purity testing does not measure includes bacterial endotoxins from manufacturing equipment, heavy metals that accumulate during reconstitution or storage, residual organic solvents from purification steps, and counterion content that affects actual peptide concentration per milligram.
A pure peptides lab addresses all these quality dimensions, not just the HPLC purity percentage that appears prominently in marketing materials. The comprehensive approach requires multiple analytical methods, specialized equipment, and quality systems that add significant cost to manufacturing operations.
Manufacturing environment requirements
Genuine peptide testing labs and synthesis facilities operate under controlled environmental conditions. These standards exist because peptide quality depends heavily on manufacturing environment.
ISO 17025 accreditation represents the gold standard for analytical testing laboratories. This certification requires documented quality management systems, validated testing methods, and regular proficiency testing to verify accuracy.
GMP compliance matters for peptides intended for pharmaceutical development. Good Manufacturing Practice standards specify everything from facility design to personnel training, documentation requirements, and process controls. Research-grade peptides may not require full GMP compliance, but the underlying principles of contamination control and process consistency apply regardless of intended use.
Environmental monitoring tracks particulate counts, microbial contamination, temperature, and humidity throughout production areas. Pure peptides labs document these parameters continuously, not just during inspections.
How peptide synthesis affects purity
Understanding the synthesis process reveals why purity varies so dramatically between vendors and why certain impurity profiles indicate manufacturing problems.
Solid phase peptide synthesis remains the dominant manufacturing method for research and therapeutic peptides. The process involves building the peptide chain one amino acid at a time on an insoluble resin support, with each addition cycle requiring deprotection, coupling, and washing steps.
The SPPS process explained
Each synthesis cycle begins with removing the protecting group from the growing peptide chain. Fmoc chemistry, the most common approach, uses piperidine to cleave the fluorenylmethyloxycarbonyl group from the terminal amine. This deprotection must be complete. Incomplete removal leaves protected amino groups that cannot participate in the next coupling reaction.
The coupling step attaches the next amino acid to the deprotected chain. Activation reagents convert the incoming amino acid into a reactive species capable of forming the amide bond. Coupling efficiency depends on reagent quality, reaction conditions, and peptide sequence characteristics.
Certain sequences present notorious coupling difficulties. Aggregation-prone sequences can fold during synthesis, burying reactive sites. Consecutive bulky amino acids create steric hindrance. Aspartimide formation introduces racemization at aspartic acid residues. Experienced synthesis chemists anticipate these problems and adjust protocols accordingly.
After coupling, washing removes excess reagents, byproducts, and unreacted amino acids. Inadequate washing carries impurities forward to subsequent cycles, compounding purity losses.
Why synthesis impurities accumulate
Consider a 20-amino acid peptide synthesized with 99% coupling efficiency at each step. The mathematics seem favorable. But compounded over 19 coupling cycles, final yield of the correct sequence drops to approximately 83%. The remaining 17% consists of deletion sequences, truncated peptides, and other impurities that must be removed during purification.
Longer peptides face exponentially greater challenges. A 40-residue sequence with the same 99% coupling efficiency yields only about 67% correct product before purification. This explains why longer peptide stacks often cost more per milligram, as synthesis becomes progressively more difficult.
Pure peptides labs invest in optimized synthesis protocols, premium reagents, and experienced chemists to maximize crude purity before purification begins. Starting with cleaner crude product simplifies purification and improves final yield.
Purification methods and their limitations
Reverse-phase HPLC serves as the primary purification method for research peptides. The technique separates compounds based on hydrophobicity differences, allowing isolation of the target peptide from synthesis impurities.
Purification does not eliminate all problems. Co-eluting impurities with similar hydrophobicity to the target peptide may not separate cleanly. Harsh purification conditions can introduce new impurities through oxidation or degradation. Residual TFA from mobile phase creates counterion variability that affects peptide dosage calculations.
Understanding these limitations helps explain why purity specifications alone do not guarantee quality. A peptide might achieve 98% purity by HPLC while still containing problematic counterion levels or oxidized variants that affect biological activity.
Understanding certificates of analysis
The certificate of analysis represents your primary documentation of peptide quality. Learning to read COAs critically separates informed researchers from those who accept marketing claims at face value.
Essential COA components
A legitimate COA from a pure peptides lab includes specific elements that allow independent verification of quality claims.
Batch or lot number connects the documentation to your specific vial. Without this, the COA might describe a different production run entirely. Verifiable batch tracking allows you to confirm that testing results apply to the product you received.
Testing date matters because peptides degrade over time. A COA from two years ago does not reflect current product quality even if storage conditions remained optimal. Recent testing, ideally within the past 12 months, provides relevant quality information.
Laboratory identification allows verification of testing credentials. Reputable labs provide contact information and credentials on their reports. Anonymous or unverifiable testing laboratories represent a red flag.
Method details specify how testing was performed. HPLC purity reported without method information cannot be interpreted meaningfully. Column type, mobile phase composition, gradient conditions, and detection wavelength all affect results. Standardized methods referenced by USP chapter numbers or equivalent standards indicate professional testing practices.
HPLC purity analysis decoded
HPLC purity appears as a percentage, typically ranging from 95% to 99% for research-grade peptides. The number represents the area of the target peptide peak divided by total peak area in the chromatogram.
Higher purity is not always necessary or cost-effective. For initial screening experiments, 95% purity may suffice. For quantitative studies requiring precise dosage calculations, 98% or higher provides better consistency. Therapeutic development typically requires greater than 99% purity with comprehensive impurity characterization.
A chromatogram should accompany purity data. The visual representation shows peak shape, baseline quality, and separation from impurities. A clean, symmetrical main peak with flat baseline indicates good purification. Multiple overlapping peaks suggest co-eluting impurities that may not be fully resolved.
Retention time identifies the compound by its characteristic elution behavior. Significant deviation from expected retention time raises questions about identity or sample preparation.
Mass spectrometry for identity confirmation
HPLC tells you how pure the sample is. Mass spectrometry tells you what the sample is. Both are essential for complete quality characterization.
Mass spec measures the mass-to-charge ratio of ionized molecules. For peptides, the observed mass should match the theoretical molecular weight of the target sequence. Small variations of 1-2 Daltons are acceptable due to instrument calibration and isotope effects. Larger discrepancies indicate identity problems.
Electrospray ionization and MALDI represent the two common ionization methods for peptide analysis. ESI-MS produces multiply charged ions, creating characteristic charge state distributions that allow molecular weight calculation. MALDI typically produces singly charged ions, providing direct mass measurement.
Mass spectrometry data on a legitimate COA includes the observed mass, theoretical mass, and often a spectrum image showing the mass peak. Verification of identity protects against mislabeled products, synthesis failures, and cross-contamination between production batches.
Beyond basic purity testing
Standard purity and identity testing represents the minimum acceptable quality control. Pure peptides labs committed to genuine quality go further with additional testing that addresses contamination concerns invisible to basic analysis.
Endotoxin testing requirements
Bacterial endotoxins represent perhaps the most dangerous contamination risk in peptide research. These lipopolysaccharide molecules from gram-negative bacteria cell walls trigger severe inflammatory responses at extraordinarily low concentrations.
The danger compounds because endotoxins resist standard sterilization. Heat treatment that kills bacteria does not destroy endotoxins. Autoclaving, filtration through standard membrane filters, and even exposure to harsh chemicals may leave endotoxins intact while eliminating the bacteria that produced them.
Endotoxin contamination manifests in research through unexpected inflammatory responses, fever-like effects in animal models, and skewed results in any study involving immune function. A researcher studying inflammation peptides or immune modulation cannot obtain valid results with endotoxin-contaminated samples, regardless of purity percentage.
The LAL test, derived from horseshoe crab blood cells, provides sensitive detection of bacterial endotoxins. FDA standards specify maximum permissible endotoxin levels of 5 EU/kg for injectable drugs and 0.25-0.5 EU/mL for sterile water depending on intended use.
Most peptide vendors skip endotoxin testing entirely. The specialized testing adds cost and requires certified laboratories. A pure peptides lab committed to quality includes endotoxin testing for products intended for in vivo research applications.
Heavy metal analysis
Heavy metals accumulate during synthesis and purification from reagents, equipment, and water sources. Lead, arsenic, mercury, and cadmium represent particular concerns due to biological toxicity at trace levels.
Inductively coupled plasma mass spectrometry provides the sensitivity required for detecting trace metal contamination. The technique quantifies individual elements at parts-per-billion concentrations, identifying contamination invisible to other analytical methods.
Heavy metal contamination affects research in multiple ways. Direct toxicity confounds studies of peptide safety. Metal ions catalyze oxidation and degradation of peptides during storage. Certain metals interfere with binding assays and enzymatic studies, creating artifacts that mimic or mask true peptide effects.
Regulatory guidance from ICH Q3D specifies permissible daily exposure limits for elemental impurities in pharmaceutical products. Research-grade peptides may not face identical requirements, but the underlying toxicity concerns apply regardless of regulatory classification.
Sterility and microbial testing
Microbial contamination degrades peptides during storage and introduces variables into biological studies. Bacteria and fungi consume peptides as nutrient sources, reducing active concentration over time. Metabolic byproducts contaminate samples even after microbial death.
Sterility testing confirms absence of viable microorganisms in the final product. The testing involves incubating samples in growth media and monitoring for microbial proliferation. Negative results indicate the product meets sterility specifications at the time of testing.
Bioburden testing quantifies microbial load in products not claimed as sterile. Lower bioburden indicates cleaner manufacturing processes and reduced contamination risk during handling and storage.
Residual solvent analysis
Organic solvents used during synthesis and purification can persist in final products. Acetonitrile, methanol, dichloromethane, and dimethylformamide represent common residual solvents with varying toxicity profiles.
Gas chromatography identifies and quantifies residual solvents with high sensitivity. ICH Q3C provides classification of solvents by toxicity and specifies permissible residual limits. Class 1 solvents like benzene face strict limits due to known carcinogenicity. Class 2 solvents like acetonitrile allow higher residual levels but still require monitoring. Class 3 solvents present low toxicity concerns at typical residual concentrations.
Residual solvent content affects researchers working with injectable peptides or performing studies where solvent interference could affect results. Knowledge of residual solvent profile allows appropriate protocol adjustments.
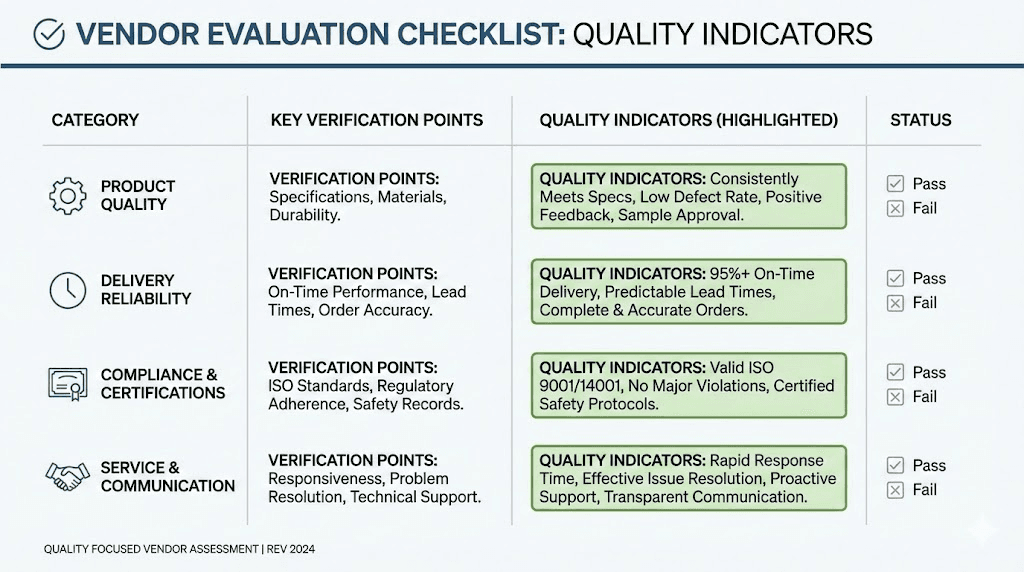
Evaluating peptide vendors critically
Armed with understanding of quality parameters, you can evaluate vendors systematically rather than relying on marketing claims or price comparisons alone.
Documentation availability
Request batch-specific COAs before purchasing. Legitimate vendors provide documentation readily because quality control exists as a normal business function, not an exceptional request. Reluctance to provide documentation raises immediate concerns.
Verify that COAs include all essential elements discussed earlier. Lot numbers, testing dates, method details, and laboratory identification should appear on every certificate. Generic templates lacking specific batch information suggest documentation exists primarily for marketing purposes rather than actual quality control.
Compare COA format and content across vendors. Professional laboratories produce consistent, detailed documentation. Varying formats, missing information, or inconsistent detail levels suggest quality control processes that change based on convenience rather than systematic protocols.
Third-party testing verification
Independent testing by accredited laboratories provides the strongest quality assurance. In-house testing, while necessary for production quality control, lacks the independence that prevents bias or pressure to release substandard material.
Janoshik Analytical has emerged as a trusted independent testing laboratory in the peptide community. Their testing methodology follows pharmaceutical standards, and results can be verified online through QR codes or verification links. Other reputable laboratories include Chromate, MZ Biolabs, and various ISO 17025 accredited facilities.
Finnrick Analytics provides a vendor rating database based on independent testing across thousands of samples. Their data shows significant quality variation between vendors, with average scores ranging from below 4 to above 9 on a 10-point scale. Such resources allow evidence-based vendor selection rather than reliance on self-reported quality claims.
SeekPeptides members gain access to curated vendor information and quality verification resources that simplify the selection process. The platform aggregates testing data and user experiences to identify consistently reliable sources.
Vendor transparency indicators
Certain characteristics correlate with quality commitment beyond specific documentation.
Contact information accessibility matters. A business phone number, physical address, and professional email domain indicate legitimate operations. Anonymous vendors using only generic email addresses present elevated risk.
Response quality provides information about operations. Vendors who answer technical questions accurately and provide requested documentation promptly demonstrate operational competence. Vague responses, inability to explain testing procedures, or reluctance to provide information suggest potential problems.
Pricing patterns offer indirect quality signals. Peptide synthesis has real costs. Equipment, reagents, skilled personnel, analytical testing, and facility operations require investment. Prices dramatically below market rates raise questions about where costs were cut. Conversely, premium pricing alone does not guarantee quality, as marketing budgets and profit margins vary independently of product quality.
Customer review patterns help when interpreted carefully. Consistent feedback about product quality, accurate delivery, and professional communication suggests reliable operations. Patterns of complaints about inconsistent results, documentation problems, or communication difficulties indicate systemic issues.
Understanding net peptide content
Net peptide content represents one of the most misunderstood aspects of peptide quality, yet it directly affects dosing accuracy in ways that purity percentage does not.
The difference from purity
Purity measures target peptide relative to peptide impurities. Net peptide content measures actual peptide mass relative to total sample weight.
The distinction matters because lyophilized peptide powder contains more than just peptide molecules. Water content varies based on drying conditions and storage humidity. Counterions, typically trifluoroacetate from HPLC purification, contribute significant mass without biological activity. Residual solvents and salts add further to total weight.
A peptide might test at 99% purity while containing only 70% net peptide content. That means 300 micrograms of actual peptide in what appears to be a 1 milligram sample. Researchers assuming 100% content will systematically underdose by 30%.
Impact on research accuracy
Consider a study comparing peptide effectiveness at different concentrations. The researcher prepares solutions based on label weight, assuming the powder contains 100% peptide. Unknown to them, net peptide content is actually 75%.
Every concentration prepared is 25% lower than intended. The dose-response curve shifts. IC50 or EC50 values appear higher than reality. Comparisons with literature values diverge without explanation. The research produces valid data relative to itself but cannot be accurately compared with other studies using peptides of different net content.
For muscle growth peptides, fat loss peptides, or any application where dosing precision affects outcomes, net peptide content becomes critical information.
Methods for determining net content
Amino acid analysis provides the most accurate net peptide content determination. The method involves complete hydrolysis of the peptide into constituent amino acids, followed by quantification against standards. The known amino acid composition of the target peptide allows calculation of original peptide mass.
Nitrogen content analysis offers an alternative approach. Peptides contain predictable nitrogen percentages based on their sequences. Measuring total nitrogen and comparing to theoretical values indicates net peptide content.
UV spectrophotometry at 205 or 280 nanometers provides rapid estimation of peptide concentration in solution. The method requires extinction coefficient data for the specific peptide and assumes consistent aromatic amino acid content across batches.
Quality-focused vendors report net peptide content alongside purity data. This information allows accurate solution preparation and meaningful comparison of results across different peptide sources.
Storage and stability considerations
Even peptides from the purest labs degrade if handled or stored improperly. Understanding stability factors protects your investment and ensures consistent research results over time.
Degradation mechanisms
Peptides degrade through several chemical pathways, each presenting different risk factors and prevention strategies.
Hydrolysis cleaves peptide bonds, fragmenting the molecule into smaller, inactive pieces. Water serves as the reaction medium, making moisture the primary enemy of peptide stability. Lyophilized peptides in sealed vials under inert atmosphere resist hydrolysis effectively. Once reconstituted, the clock starts ticking.
Oxidation modifies susceptible amino acids, particularly methionine, cysteine, tryptophan, and histidine. Oxidized peptides may retain partial activity or become completely inactive depending on the modification site and peptide function. Oxygen exclusion during storage and handling minimizes oxidation risk.
Aggregation occurs when peptide molecules associate inappropriately, forming inactive clusters. Some sequences aggregate readily, particularly those with hydrophobic regions that favor intermolecular association. Aggregated peptides may precipitate from solution or remain soluble but inactive.
Deamidation converts asparagine and glutamine residues to aspartic and glutamic acid, respectively. The modification introduces negative charge and can dramatically affect biological activity. Elevated pH and temperature accelerate deamidation.
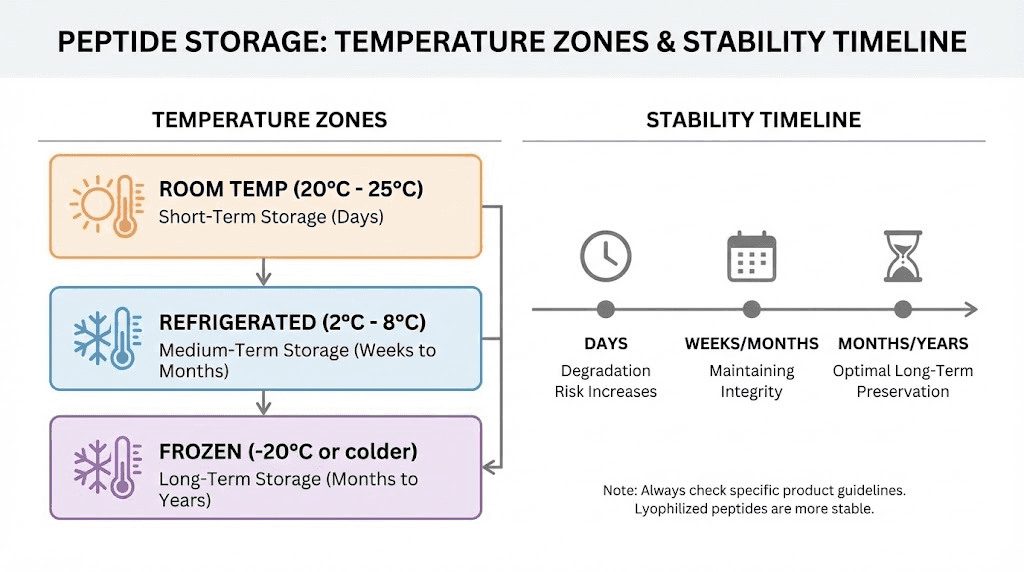
Optimal storage conditions
Lyophilized peptides stored at -20C or colder in sealed vials under dry conditions maintain stability for years. The frozen state eliminates molecular mobility required for degradation reactions. Absence of water prevents hydrolysis. Sealed containers exclude oxygen and humidity.
Room temperature storage, while sometimes unavoidable during shipping, accelerates all degradation pathways. Minimize exposure to ambient conditions. Transfer received peptides to freezer storage promptly.
Reconstituted peptides require different considerations. Aqueous solutions should be stored frozen in single-use aliquots to avoid repeated freeze-thaw cycles. Sterile bacteriostatic water prevents microbial growth that would otherwise consume peptides rapidly at refrigerator temperatures.
The question of how long reconstituted peptides last depends on the specific peptide, storage temperature, and reconstitution medium. General guidelines suggest using refrigerated reconstituted peptides within 4-8 weeks, but stability varies significantly between sequences.
Shipping and handling impacts
Temperature excursions during shipping stress peptides even in lyophilized form. Hot warehouses, unrefrigerated delivery trucks, and mailboxes exposed to summer sun all present risks.
Quality vendors use cold-chain shipping with appropriate packaging for temperature-sensitive products. Insulated containers with gel packs or dry ice maintain acceptable temperatures during transit. Tracking information allows monitoring of delivery timing to minimize exposure after arrival.
Document condition upon receipt. Inspect packaging integrity, verify cold pack status, and photograph any concerns before opening vials. This documentation supports quality claims if products underperform due to shipping damage.
Common purity misconceptions
Several persistent misconceptions about peptide purity lead researchers astray. Correcting these misunderstandings improves decision-making and research outcomes.
Higher purity always means better quality
This seems intuitive but oversimplifies reality. A 99% pure peptide with uncharacterized impurities, no endotoxin testing, and unknown net peptide content may perform worse in research than a 95% pure peptide with comprehensive quality characterization.
Purity matters most when impurities interfere with your specific application. For cell-based assays sensitive to endotoxins, endotoxin-free 95% purity beats contaminated 99% purity. For quantitative studies requiring precise concentrations, known net peptide content matters more than small purity differences.
Match purity specifications to application requirements rather than defaulting to highest available. This approach optimizes both cost and actual quality for your purposes.
All purity testing methods are equivalent
HPLC purity percentages from different laboratories using different methods cannot be directly compared. Column chemistry, mobile phase composition, gradient profiles, and detection wavelengths all affect resolution and quantification.
A peptide testing at 98% purity on one system might show 95% or 99% on a different system, depending on whether the alternative method better or worse resolves specific impurities. Standardized methods referenced to USP chapters provide comparability. Proprietary methods without detailed documentation do not.
When comparing vendors, compare testing methodologies as well as purity values. Higher numbers from poorly characterized testing may indicate less stringent standards rather than better products.
Research grade means lower quality
The distinction between research-grade and pharmaceutical-grade peptides concerns regulatory compliance and documentation requirements, not necessarily molecular quality.
Research-grade peptides from quality suppliers may achieve pharmaceutical-grade purity levels. They lack the extensive documentation, stability testing, and regulatory filings required for human therapeutic use. But the molecules themselves can be equally pure.
Pharmaceutical-grade designation adds cost for regulatory compliance infrastructure rather than necessarily improving the peptide itself. Research applications rarely require this additional overhead.
Evaluate actual quality specifications rather than grade designations when selecting peptides for research use.
Specific peptide quality considerations
Different peptide classes present unique quality challenges that affect purity testing and vendor evaluation.
Oxidation-prone peptides
Peptides containing methionine, cysteine, or tryptophan oxidize readily during synthesis, purification, and storage. BPC-157 contains multiple oxidation-susceptible residues, making oxidation state a critical quality parameter.
Mass spectrometry detects oxidation through characteristic +16 Dalton mass shifts. High-resolution chromatography may resolve oxidized variants as distinct peaks. Quality COAs for oxidation-prone peptides should address oxidation specifically.
Vendors handling oxidation-prone sequences implement protective measures including inert atmosphere handling, antioxidant addition during processing, and enhanced packaging to exclude oxygen during storage.
Disulfide-containing peptides
Peptides with cysteine residues may form disulfide bonds, either within the molecule or between molecules. Correct disulfide connectivity is essential for proper folding and biological activity.
TB-500 and other disulfide-containing peptides require verification of correct disulfide pairing. Misfolded variants may show identical mass and similar purity but lack activity due to improper structure.
Peptide mapping through enzymatic digestion followed by mass spectrometry can verify disulfide connectivity. This advanced characterization goes beyond standard COA parameters but matters for peptides where structure determines function.
Modified peptides
Acetylation, amidation, PEGylation, and other modifications introduce additional quality considerations. The modification itself must be complete and correctly positioned.
Modified peptides require mass spectrometry confirmation of correct modification mass. HPLC should resolve modified from unmodified species if both are present. Incomplete modification represents a distinct impurity type that standard synthesis impurities do not capture.
Long peptides and proteins
Peptides exceeding 50 amino acids present escalating synthesis challenges. Coupling efficiencies compound over the extended chain, reducing crude purity. Purification becomes more difficult as molecular complexity increases.
IGF peptides and other longer sequences require specialized synthesis expertise. Native chemical ligation, fragment condensation, and other advanced techniques address length limitations of standard SPPS.
Expect lower purity specifications for long peptides and verify that vendors have demonstrated capability with sequences of comparable length and complexity.
Building your quality verification system
Systematic approaches to quality verification protect research investments and ensure reproducible results. Developing consistent practices improves efficiency and reduces risk of quality-related problems.
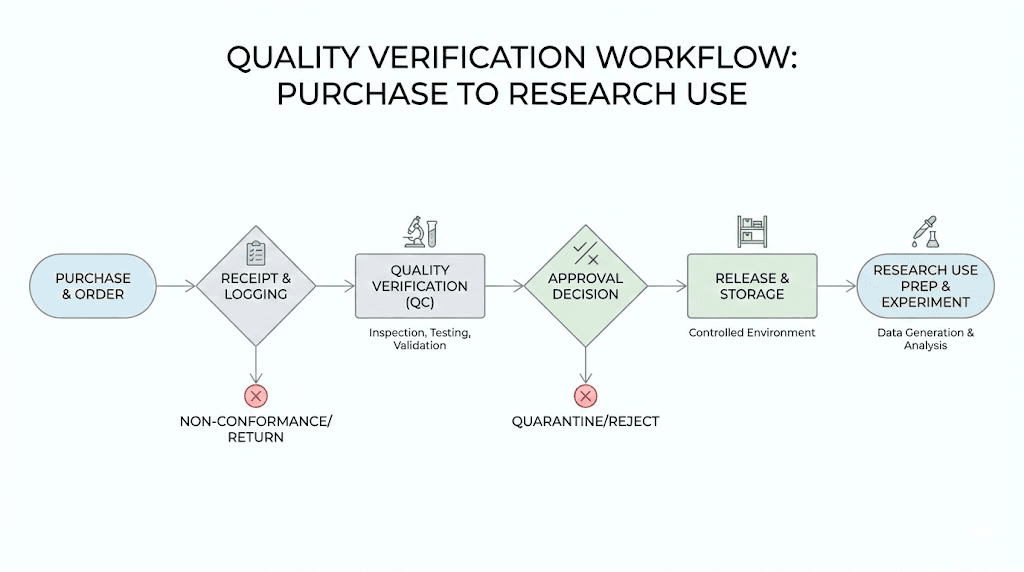
Pre-purchase verification
Before committing to a vendor or specific batch, gather and evaluate quality documentation.
Request batch-specific COAs for the product you intend to purchase. Review documentation for completeness using the criteria established earlier. Note any missing information and request clarification.
Verify testing laboratory credentials independently when possible. ISO accreditation databases, laboratory websites, and industry references help confirm that testing sources are legitimate.
For new vendors, consider ordering small quantities initially to verify that delivered quality matches documentation. Establishing quality track record with limited financial exposure makes sense before larger commitments.
Receipt inspection protocols
Develop consistent practices for inspecting received peptides.
Document shipment condition including packaging integrity, temperature indicator status, and delivery timing. Photograph any concerns before handling products.
Verify that received products match orders. Check that vial labels correspond to ordered items and that batch numbers match provided COAs.
Inspect vials visually for appropriate appearance. Lyophilized peptides typically appear as white to off-white powder or solid cake. Unusual coloration, sticky residue, or visible particulates may indicate quality problems.
In-house verification options
Researchers with analytical capabilities can verify vendor quality claims independently.
HPLC systems common in research laboratories can run peptide purity analyses using published methods. Comparing in-house results to vendor COAs identifies discrepancies that warrant investigation.
Mass spectrometry verification confirms identity and detects obvious problems like incorrect products or major degradation.
Simple solubility and appearance testing provides quick quality screening. Peptides that should dissolve readily in specified solvents but do not may have purity or composition problems.
For critical applications, consider sending samples to independent testing laboratories before beginning experiments. The cost of confirmation testing often saves more in avoided wasted research time.
The economics of peptide quality
Quality costs money. Understanding the economics helps evaluate whether premium quality justifies premium pricing and where cost savings create unacceptable risk.
Where quality costs originate
Peptide manufacturing involves multiple cost drivers that directly affect quality potential.
Reagent quality significantly impacts synthesis outcomes. Premium amino acids, coupling reagents, and solvents produce cleaner crude products. Cutting reagent costs increases impurity burden before purification begins.
Equipment sophistication affects achievable purity. Modern synthesizers with precise temperature control, efficient mixing, and automated protocols outperform older or simplified equipment. HPLC systems with better resolution separate impurities more effectively.
Personnel expertise determines how well equipment and reagents are utilized. Experienced synthesis chemists recognize problems early, adjust protocols appropriately, and achieve higher yields of quality product. Training, retention, and staffing levels all cost money.
Quality control testing adds direct cost to each batch. Comprehensive testing including HPLC, MS, endotoxin, sterility, heavy metals, and residual solvents significantly exceeds basic purity testing alone. Third-party verification adds further expense.
Facility operations including clean rooms, environmental monitoring, and quality management systems require ongoing investment. GMP or GMP-like environments cost substantially more than minimal manufacturing facilities.
Cost-quality tradeoffs
Recognizing legitimate cost drivers helps distinguish quality-based pricing from mere margin extraction.
Vendors cannot provide comprehensive quality testing, premium reagents, experienced personnel, and proper facilities while dramatically undercutting competitor pricing. Something must give. Dramatically lower prices indicate reduced quality investment somewhere in the production chain.
Conversely, premium pricing alone does not guarantee quality. Marketing costs, profit margins, and operational inefficiencies can inflate prices without corresponding quality benefits. Price indicates cost recovery requirements, not necessarily quality levels.
Evaluate quality evidence directly rather than inferring quality from price. Documentation, testing comprehensiveness, third-party verification, and vendor transparency matter more than pricing tier for predicting actual quality.
False economy in peptide purchasing
The cheapest peptide is rarely the most economical choice when total research costs are considered.
Consider a researcher who saves $100 by purchasing lower-quality peptide for an experiment that costs $5000 in total resources including time, reagents, and equipment. If peptide quality problems invalidate results, requiring experiment repetition, the $100 savings costs $5000 in wasted effort.
Research time represents the largest hidden cost of quality problems. Troubleshooting inconsistent results, repeating failed experiments, and investigating artifacts all consume time that could produce productive outcomes with quality materials.
Publication and career implications extend the cost horizon further. Research based on contaminated or degraded peptides may produce misleading results that affect scientific literature and researcher reputation.
SeekPeptides members report that investing in quality verification upfront consistently saves money over time by avoiding repeat purchases and failed experiments. The platform provides resources to identify reliable quality at appropriate price points.
Troubleshooting quality-related problems
Even with careful vendor selection and verification, quality problems occasionally occur. Systematic troubleshooting helps identify whether peptide quality contributed to unexpected results.
Recognizing quality-related issues
Several patterns suggest peptide quality may contribute to research problems.
Inconsistent results between experiments using the same protocol and new peptide aliquots may indicate quality variation between vials or batches. If results were consistent until a new peptide lot was opened, batch quality differences warrant investigation.
Results that diverge from literature values using established protocols suggest possible peptide quality issues. While protocol differences explain some variation, dramatic divergence from expected outcomes may indicate quality problems.
Unexpected biological effects unrelated to known peptide actions might result from contaminants rather than the peptide itself. Endotoxin contamination commonly causes inflammatory responses that confound research involving immune function or cell viability.
Degradation during storage manifests as progressive loss of activity over time even with consistent handling. If a peptide worked initially but loses effectiveness over weeks or months, stability problems may be occurring.
Investigation approaches
When quality-related problems are suspected, systematic investigation helps identify causes.
Review storage and handling history. Did the peptide experience temperature excursions? Was reconstituted peptide stored appropriately? Have multiple freeze-thaw cycles occurred?
Compare COA specifications with observed behavior. If a peptide should be highly soluble but dissolves poorly, or if solution appearance differs from expectations, quality deviation has occurred.
Test with alternative peptide sources if possible. Using peptide from a different vendor or batch helps isolate whether problems originate with specific material or with experimental protocols.
Consider independent testing for critical questions. If significant resources depend on peptide quality conclusions, laboratory verification provides definitive answers.
Communication with vendors
Quality problems should be communicated to vendors promptly and professionally.
Document issues thoroughly before contacting vendors. Specific observations, dates, lot numbers, and any testing data strengthen quality claims and facilitate investigation.
Professional vendors appreciate quality feedback and work to resolve legitimate concerns. Replacement products, refunds, or investigation results typically follow reasonable complaints about documented quality problems.
Vendor response to quality concerns provides valuable information about their operations. Responsive, thorough investigation suggests quality commitment. Dismissive responses or refusal to investigate raise concerns about quality priorities.
Regulatory landscape for peptide quality
Understanding regulatory frameworks helps contextualize quality requirements and vendor claims about compliance.
Research vs. pharmaceutical standards
Research peptides exist in regulatory space distinct from therapeutic pharmaceuticals. This distinction affects quality requirements and documentation standards.
Pharmaceutical peptides for human therapeutic use must meet FDA requirements including GMP manufacturing, stability testing, impurity characterization, and extensive documentation. Regulatory approval requires demonstrating safety and efficacy through clinical trials.
Research peptides sold for in vitro or non-human research face fewer regulatory requirements. The "for research only" label indicates that products have not undergone pharmaceutical regulatory review. Quality depends on voluntary vendor standards rather than regulatory enforcement.
This regulatory gap means research peptide quality varies widely between vendors. Without regulatory oversight ensuring minimum standards, responsibility falls on researchers to verify quality independently.
International variation
Regulatory frameworks differ significantly between countries, affecting peptide availability and quality standards in different markets.
China produces a large proportion of global research peptides. Chinese manufacturing operates under different regulatory oversight than US or European production. Quality ranges from excellent to problematic depending on specific manufacturers. Chinese peptide sourcing requires careful vendor verification given the variable quality landscape.
European regulations differ from US frameworks in certain respects. UK peptide regulations changed following Brexit. Researchers sourcing internationally should understand relevant import regulations and quality implications.
Quality system standards
Several quality system standards provide frameworks for peptide manufacturing and testing regardless of regulatory jurisdiction.
ISO 9001 establishes general quality management system requirements applicable across industries. Certification indicates documented processes, quality objectives, and continuous improvement commitment.
ISO 17025 specifically addresses testing laboratory competence. Accreditation requires demonstrated technical capability, quality management, and proficiency testing. Analytical results from ISO 17025 accredited laboratories carry greater credibility than non-accredited testing.
GMP principles, while legally required only for pharmaceuticals, provide useful quality frameworks for research peptide manufacturing. Vendors implementing GMP-like controls typically achieve higher and more consistent quality than those without systematic quality management.
Future trends in peptide quality
The peptide industry continues evolving, with several trends affecting quality standards and verification practices.
Increased transparency demands
Researchers increasingly demand comprehensive quality documentation rather than accepting minimal testing. Vendor rating platforms, independent testing databases, and community information sharing enable informed purchasing decisions that reward quality commitment.
This market pressure drives quality investment as vendors recognize that transparency correlates with customer preference. Companies that once provided minimal documentation now compete on testing comprehensiveness and verification accessibility.
Testing technology advances
Analytical methods continue improving in sensitivity, speed, and cost-effectiveness. Mass spectrometry techniques enable detailed characterization previously available only to specialized laboratories. Portable testing devices may eventually allow on-site quality verification.
Improved testing accessibility should raise baseline quality expectations as researchers gain capability to verify vendor claims independently.
Manufacturing improvements
Synthesis technology advances improve achievable purity and reduce manufacturing costs. Microwave-assisted synthesis, flow chemistry, and automated protocols increase efficiency while maintaining quality. Ultra-efficient synthesis methods dramatically reduce solvent waste while improving throughput.
These improvements may eventually lower the cost premium for quality peptides, making comprehensive quality control economically accessible to more vendors.
Standardization efforts
Industry groups and academic organizations work toward standardized testing protocols and quality specifications for research peptides. Standardization would enable meaningful quality comparisons between vendors and establish minimum acceptable standards.
Progress remains gradual given the fragmented industry structure and limited regulatory pressure. Researcher demand for standardization provides the primary motivation for voluntary adoption of consistent quality frameworks.
Frequently asked questions
What purity level should I require for research peptides?
Purity requirements depend on your application. Screening experiments may accept 95% purity. Quantitative studies benefit from 98% or higher with known net peptide content. In vivo research requires endotoxin-free material regardless of purity percentage. Match specifications to your specific needs rather than defaulting to maximum available purity.
How can I verify that a COA is legitimate?
Legitimate COAs include batch-specific lot numbers, recent testing dates, laboratory identification with contact information, method details, and raw data like chromatograms. Verify laboratory credentials independently when possible. Some testing services like Janoshik provide online verification through QR codes. Generic templates lacking specific batch information raise concerns.
Does higher price guarantee better peptide quality?
Not necessarily. Price reflects production costs, marketing expenses, and profit margins. Quality vendors may command premium prices, but premium prices alone do not guarantee quality. Evaluate quality evidence directly through documentation, testing comprehensiveness, and third-party verification rather than inferring quality from price tier.
What is the difference between research grade and pharmaceutical grade peptides?
The distinction primarily concerns regulatory compliance and documentation rather than molecular quality. Pharmaceutical-grade peptides meet FDA requirements for human therapeutic use including GMP manufacturing and stability testing. Research-grade peptides may achieve equivalent purity but lack regulatory documentation. For non-therapeutic research, research grade typically suffices at lower cost.
How long do peptides remain stable in storage?
Lyophilized peptides stored at -20C or colder in sealed vials maintain stability for years. Reconstituted peptides degrade faster. Refrigerated solutions in bacteriostatic water typically remain usable for 4-8 weeks depending on specific peptide stability. Frozen aliquots extend stability but should avoid repeated freeze-thaw cycles.
Why would a peptide with high purity still not work in my experiments?
Several factors beyond purity can cause experimental failure. Incorrect identity despite passing purity testing, improper storage causing degradation, endotoxin contamination affecting cell-based assays, incorrect reconstitution, and unknown net peptide content causing dosing errors all produce problems unrelated to HPLC purity percentage. Comprehensive quality characterization addresses these concerns.
What testing should I request beyond standard HPLC purity?
Mass spectrometry for identity confirmation is essential. For injectable applications, request endotoxin testing. Heavy metal analysis matters for in vivo research. Net peptide content enables accurate dosing. Residual solvent data may be relevant for specific applications. Match testing requests to your research requirements and risk tolerance.
Can I trust peptide quality reviews online?
Approach reviews critically. Patterns of consistent feedback from multiple sources carry more weight than isolated testimonials. Independent testing databases like Finnrick provide objective quality data. Peptide forums and research communities share experiences that help identify reliable vendors. Vendor-controlled review platforms may lack negative feedback that would appear on independent sites.
For researchers committed to building reliable peptide research protocols, SeekPeptides provides comprehensive resources including vendor verification guides, quality assessment frameworks, and a community of experienced researchers who share knowledge about sourcing and evaluating peptide quality.
In case I do not see you, good afternoon, good evening, and good night. May your peptides stay pure, your research stay reproducible, and your vendors stay transparent. Join here.



