Jan 20, 2026

You typed "biomax peptides" into your search bar for a reason. Maybe you saw the name mentioned somewhere. Maybe a friend recommended it. Maybe you stumbled across one of the several different companies that share variations of this name. Now you're trying to figure out which one is legitimate, whether any of them are trustworthy, and how to tell the difference between a reliable peptide vendor and one that might waste your money or compromise your research.
Here's what makes this particular search tricky. There isn't just one "Biomax Peptides." There are multiple companies operating under similar names, each with different histories, different testing standards, and different reputations. Some have been around for years. Others launched within the past few months. The confusion is real, and it matters, because choosing the wrong vendor doesn't just mean wasted money. It means unreliable peptides, inconsistent results, and potentially dangerous products entering your research.
This guide breaks down everything researchers need to know about the various Biomax-related peptide suppliers. We'll examine what each company offers, how to verify peptide quality through certificates of analysis, and the specific red flags that separate legitimate vendors from questionable ones. By the end, you'll have a complete framework for evaluating not just Biomax options but any peptide supplier you encounter.
SeekPeptides has built comprehensive resources for researchers navigating the complex peptide landscape, and vendor evaluation represents one of the most critical skills any researcher can develop.
Understanding the biomax peptide landscape
The name "Biomax" appears across multiple peptide-related businesses. This creates immediate confusion for researchers trying to find reliable sources. Let's clarify exactly who operates under variations of this name and what distinguishes each entity.
Biomaxpeptides.com
This domain registered in December 2024, making it extremely new to the market. The company claims to be based in California and describes itself as a provider of premium-grade research peptides for scientific applications. Their website states they were founded by "peptide research pioneers with over 25 years of experience."
Here's where researchers should pause.
A domain that's only months old paired with claims of decades-long experience creates an immediate credibility question. The company mentions stringent quality control protocols, maximum purity, and stable compounds. Standard marketing language. Nothing unusual there. But the domain age combined with private registration through a proxy service means verification becomes difficult.
Independent analysis tools give biomaxpeptides.com a trust score around 27.6 out of 100. That's considerably below the threshold most researchers would consider safe. The low score doesn't automatically mean the company is fraudulent, but it does indicate limited operational history and verifiable track record.
Before ordering from any new vendor, researchers should understand peptide safety considerations and establish baseline verification protocols.
BioMaxx Research (biomaxx.shop)
Notice the double "x" in this company's name. This is a completely different entity from biomaxpeptides.com. BioMaxx Research operates its own storefront and claims to provide third-party tested peptides with certificates of analysis available on product pages and through a dedicated COA section.
The company markets itself with the tagline "High Purity, Fast Shipping, Superb Service." They specifically mention that all peptides undergo third-party testing, which represents a significant distinction from vendors who only provide in-house testing documentation.
Third-party testing matters because it removes potential conflicts of interest. When a company tests its own products, there's inherent motivation to report favorable results. External laboratories have no stake in the outcome.
Researchers working with compounds like BPC-157 or TB-500 need reliable purity data to ensure experimental consistency.
Biomax.ca
This Canadian vendor operates entirely separately from the other Biomax entities. They offer both SARMs and peptides, claiming unmatched quality through rigorous testing and quality control processes.
One specific claim that stands out: they state peptides are stored in medical-grade refrigeration within five minutes of production, with every batch made within two weeks of order. Fresh synthesis and proper peptide storage significantly impact compound stability.
Geographic location matters for researchers. Canadian vendors may operate under different regulatory frameworks than US-based suppliers. Import considerations, shipping times, and customs procedures all factor into the purchasing decision.
Biomax Scientific (biomaxsci.com)
Yet another company sharing the Biomax name. This entity appears focused on broader scientific products beyond just peptides. Their product catalog suggests a different business model than the dedicated peptide vendors.
The proliferation of similar names isn't accidental. "Biomax" combines recognizable elements: "bio" suggesting biological or scientific credibility, and "max" implying maximum quality or potency. It's effective marketing language, which is exactly why multiple companies have adopted variations.
Why vendor verification matters for peptide research
Choosing the wrong peptide vendor doesn't just waste money. The consequences ripple through every aspect of research.
Research integrity
Unreliable peptides produce unreliable results. Simple as that.
Imagine running a multi-week protocol with what you believe is sermorelin or ipamorelin. You're tracking responses, adjusting variables, documenting everything carefully. But if the compound in your vial isn't what the label claims, or if purity falls significantly below stated levels, your entire dataset becomes questionable.
Peer-reviewed research depends on reproducibility. Others need to replicate your methods and achieve similar outcomes. When peptide quality varies between vendors, or even between batches from the same vendor, reproducibility suffers.
Safety considerations
Contaminated or degraded peptides introduce unknown variables into any protocol. Heavy metal contamination, bacterial endotoxins, residual solvents from synthesis, incomplete sequences, oxidation products, all of these can appear in low-quality peptides.
Understanding common peptide mistakes helps researchers avoid preventable problems. Quality verification represents the first line of defense.
Some contaminants produce no obvious signs. A peptide solution might look perfectly normal, reconstitute cleanly, and appear stable in refrigerated storage.
Yet mass spectrometry could reveal significant impurities invisible to visual inspection.
Financial waste
Research-grade peptides aren't cheap. A single vial might represent a significant investment depending on the compound and quantity. Multiply that across a full protocol lasting weeks or months, and the financial stakes become substantial.
Purchasing from an unreliable vendor means potentially throwing away that investment. Worse, you might not realize the problem until weeks into a protocol when results diverge from expectations.
The cost calculation shifts when you factor in wasted time, unusable data, and the need to repeat experiments with verified compounds. Saving a few dollars per vial through a questionable vendor often costs far more in the long run.
Legal and regulatory considerations
The regulatory landscape for peptides continues evolving. Enforcement actions increased significantly through late 2024 and into 2025. Researchers need to understand where their compounds originate and ensure proper documentation exists for every purchase.
Legitimate vendors maintain detailed records. Batch numbers link to specific synthesis runs. Certificates of analysis trace back to identified testing laboratories. This documentation matters if questions ever arise about compound origin or quality.
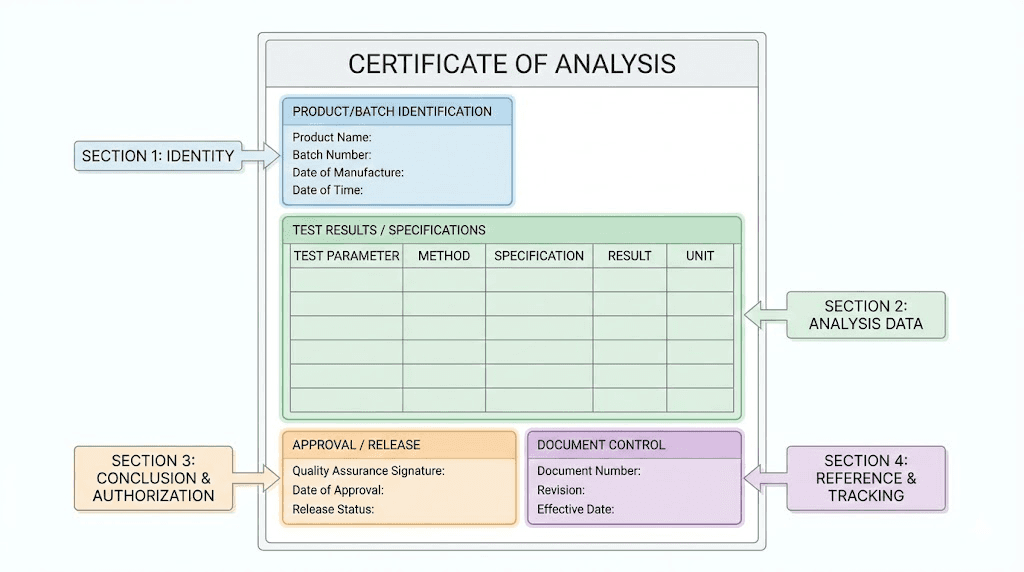
Certificates of analysis explained
A Certificate of Analysis represents the primary documentation for peptide quality verification. Understanding how to read and validate these documents separates informed researchers from those vulnerable to misrepresentation.
What a legitimate COA contains
Every credible certificate of analysis should include specific elements. Missing any of these raises immediate concerns.
Batch or lot number. This identifier links the tested sample to specific production runs. The number on your COA must match the label on your vial exactly. Any mismatch invalidates the document entirely, because you can't confirm the tested material matches what you received.
Testing date. Quality degrades over time, even under proper storage. A COA dated three years ago has limited relevance for a vial you're purchasing today. Most quality-focused vendors ensure testing occurs within three to six months of sale.
Purity percentage. This tells you what proportion of the sample consists of the target peptide versus impurities. Research-grade peptides typically show 95% purity or higher. Premium compounds often exceed 98% or 99%.
Analytical methods used. The document should specify exactly how testing occurred. High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) represent the standard methods for peptide analysis.
Actual chromatograms and spectra. Raw data matters. A COA stating "99% purity by HPLC" without showing the actual chromatogram provides no verification mechanism. The graph itself shows separation quality, potential co-eluting impurities, and peak characteristics that purity numbers alone can't capture.
Laboratory identification. Who performed the testing? A legitimate third-party laboratory will have a name, address, accreditation numbers, and contact information. ISO 17025:2017 certification indicates the lab meets international standards for analytical competence.
Observed versus expected molecular mass. Mass spectrometry confirms peptide identity by measuring molecular weight. The observed mass should match theoretical calculations within acceptable tolerances. Significant deviations suggest wrong sequences, modifications, or degradation products.
HPLC analysis in detail
High-Performance Liquid Chromatography serves as the primary method for determining peptide purity. Understanding what HPLC actually measures helps researchers interpret results correctly.
The technique separates compounds based on their chemical properties. A sample passes through a column containing stationary phase material, typically C18 reverse-phase media. Different components interact differently with this material, causing them to elute at different times.
The resulting chromatogram shows peaks representing detected compounds. The main peak corresponds to your target peptide. Additional peaks indicate impurities, whether synthesis byproducts, degradation products, or contaminants.
Purity calculation divides the main peak area by total peak area. A large, sharp main peak with minimal secondary peaks indicates high purity. Multiple significant peaks suggest problems.
However, HPLC has limitations. Compounds with similar chemical properties might co-elute, appearing as a single peak despite being different molecules. This is why mass spectrometry provides essential complementary data.
Researchers working with specific compounds like GHK-Cu or Epitalon should look for method details on their COAs. Different peptides may require modified analytical conditions for optimal separation.
Mass spectrometry confirmation
Where HPLC quantifies purity, mass spectrometry confirms identity. The technique measures molecular weight with extreme precision, often to multiple decimal places.
For peptides, expected mass comes from summing amino acid residue weights plus terminal modifications and counterions. The observed mass from MS testing should match this calculation.
Mass spectrometry also detects modifications that HPLC might miss. Oxidation adds 16 mass units. Deamidation adds approximately 1 mass unit. These changes can significantly affect biological activity even when the peptide appears pure by HPLC.
A complete quality assessment requires both methods. HPLC shows how much target peptide exists relative to impurities. MS confirms the target peptide actually is what it claims to be.
When evaluating vendors for compounds like MOTS-c or SS-31, look specifically for MS data showing correct molecular weight confirmation.
Third-party versus in-house testing
Not all COAs carry equal weight. The distinction between third-party and in-house testing significantly impacts reliability.
In-house testing occurs when the vendor tests their own products using their own equipment and personnel. While this can produce valid results, inherent conflict of interest exists. The company benefits financially from favorable test outcomes.
Third-party testing uses independent laboratories with no stake in the results. These labs risk their accreditation and reputation on accurate reporting. Falsifying results would destroy their business across all clients, not just peptide vendors.
Legitimate third-party laboratories include names like Janoshik, MZ Biolabs, and Colmaric. These facilities specialize in analytical testing and maintain detailed quality systems.
When a vendor claims third-party testing, verification should be possible. Contact information for the laboratory should be available. Many labs maintain verification portals where you can confirm specific reports actually came from their facility.
SeekPeptides emphasizes the importance of understanding testing methods when evaluating any peptide source. Members access detailed guidance on interpreting analytical data correctly.
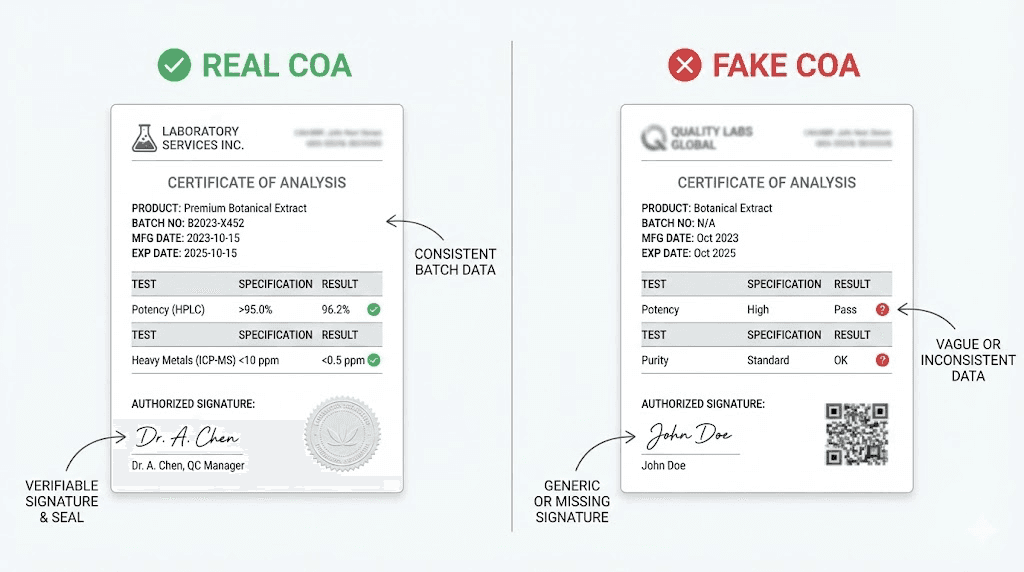
Spotting fake or manipulated COAs
Unfortunately, fraudulent documentation exists in the peptide market. Some vendors create entirely fabricated COAs. Others manipulate legitimate documents to misrepresent their products. Knowing what to look for helps researchers avoid these traps.
Common manipulation tactics
Recycled documents. A vendor might obtain a legitimate COA from one batch, then reuse it indefinitely. Every vial sold includes a copy of the same document regardless of actual production date or testing status. The batch numbers on labels may change while the COA stays identical.
Check dates carefully. If a document shows testing from two years ago for a "freshly synthesized" peptide, something doesn't align.
Digital alteration. PDF editing tools make document manipulation straightforward. A dishonest vendor might take a legitimate COA and change batch numbers, purity values, or dates to match their inventory.
Look for signs of editing: font inconsistencies, misaligned text, compression artifacts around modified areas, or text that doesn't quite match the document's overall style.
Generic templates. Some fake COAs use obviously templated formats with placeholder data filled in. Real analytical laboratories have distinct document styles. Generic-looking reports lacking specific laboratory branding raise concerns.
Missing raw data. A "certificate" that lists only purity percentages without actual chromatograms or spectra provides no verification mechanism. Anyone can type "99.2% purity" into a document. The supporting analytical data is what makes the claim credible.
Verification strategies
Contact the laboratory directly. If a COA names a specific testing facility, reach out to them. Provide the batch number and testing date from the document. Ask them to confirm they actually performed that analysis. Legitimate labs will verify their own reports.
Check laboratory accreditation. Real analytical laboratories maintain accreditation through recognized bodies. ISO 17025 certification can be verified through accreditation body databases. A laboratory claiming certifications should be findable in these systems.
Compare multiple COAs from the same vendor. If you've purchased from a vendor before, compare new COAs to previous ones. Legitimate testing produces variation between batches. Documents that look identical across different lot numbers suggest recycling.
Assess professional quality. Real laboratory reports have consistent formatting, proper units, clear methodology descriptions, and professional presentation. Sloppy documents with grammatical errors, inconsistent units, or missing information rarely come from accredited facilities.
Researchers buying compounds for weight loss research or muscle growth studies should be especially vigilant about COA verification given the popularity and corresponding fraud risk with these categories.
Red flags that indicate problems
Certain patterns strongly suggest a vendor should be avoided entirely.
No COA available. Any legitimate vendor provides certificates of analysis. "We don't have testing documents" or "COAs available only after purchase" are unacceptable responses. Walk away.
COA provided only as an image. Low-resolution images or screenshots of COAs, rather than actual PDF documents, often indicate manipulation. The lower quality makes alterations harder to detect.
Perfect round numbers. Real analytical results rarely land on exact values. "98.00% purity" is suspicious. "97.84%" reflects actual measurement variation. Perfectly round numbers across multiple metrics suggest fabrication.
No batch-specific documentation. Generic COAs that don't tie to specific production runs provide no actual verification. Every vial you receive should link to testing of that specific material.
Refusal to verify. If you ask for additional documentation or laboratory contact information and the vendor becomes evasive or hostile, that tells you something important.
Complete vendor evaluation framework
Beyond COA analysis, comprehensive vendor evaluation considers multiple factors. Use this framework whether assessing Biomax entities or any other peptide supplier.
Domain and business history
How long has the company operated? Newer isn't automatically worse, but established vendors have track records to examine. Check domain registration dates through WHOIS lookup tools.
A company claiming decades of experience should have operational history matching that claim. Mismatches between stated history and verifiable facts indicate potential dishonesty.
Look for physical address information. Legitimate businesses operate from real locations. Post office boxes or virtual office addresses provide less accountability than actual facilities.
Payment and security
What payment methods does the vendor accept? Reputable companies use secure payment processors with buyer protection.
Cryptocurrency-only or wire-transfer-only payment options offer no recourse if problems arise.
Check for HTTPS encryption on checkout pages. Look for security certifications from recognized payment processors. Basic website security indicates baseline professionalism.
Communication quality
Contact customer service before ordering. Ask specific questions about their products, testing methods, or shipping procedures. Response time and quality reveal how they'll handle actual problems.
Professional vendors respond within reasonable timeframes with knowledgeable answers. Delayed responses, form-letter replies, or inability to answer basic questions suggest inadequate operations.
Community reputation
Search for discussions on forums like Reddit, particularly r/Peptides and related communities. Look for patterns across multiple reviews rather than isolated opinions.
Be aware that fake reviews exist. New accounts posting suspiciously positive reviews, or multiple glowing testimonials appearing simultaneously, may indicate manipulation. Genuine community feedback develops organically over time.
Trustpilot, BBB ratings, and similar platforms provide additional data points. No single source should drive decisions, but consistent patterns across multiple platforms are informative.
Pricing reality
Peptide synthesis requires specialized equipment, skilled technicians, quality raw materials, and rigorous testing. This has real costs that legitimate vendors must recover through pricing.
Dramatically lower prices than competitors often indicate compromises somewhere. Maybe testing is skipped. Maybe raw materials are sourced cheaply without verification. Maybe the product simply isn't what it claims to be.
Conversely, high prices don't guarantee quality. Some vendors charge premium rates while delivering mediocre products. Price serves as one data point among many, not a sole indicator.
Researchers calculating costs for compounds like semaglutide or tirzepatide should use the peptide cost calculator to establish realistic benchmarks.
Product range and specialization
Some vendors specialize in specific peptide categories. Others offer broad catalogs covering everything from nootropic peptides to weight loss compounds to healing peptides.
Neither approach is inherently better. Specialists may have deeper expertise in their focus area. Generalists offer convenience for researchers needing multiple compound types.
What matters is whether the vendor demonstrates actual knowledge about their products. Generic descriptions copied from Wikipedia suggest limited understanding. Detailed technical information about synthesis, storage, and handling indicates expertise.
Shipping and handling
Peptides require specific conditions to maintain stability. How does the vendor ship? What packaging protects products during transit?
Cold chain shipping with ice packs or dry ice helps maintain temperature stability. Insulated packaging provides protection. Light-blocking containers prevent photodegradation.
Ask about shipping methods before ordering. A vendor who can't explain their cold chain procedures may not actually implement them.
Understanding how long peptides last at room temperature and proper reconstitution procedures helps researchers assess whether shipping methods are adequate.
Evaluating biomax options specifically
Applying this framework to the Biomax-named vendors reveals important distinctions.
Biomaxpeptides.com assessment
The extremely recent domain registration represents the primary concern. December 2024 means limited operational history exists for evaluation. The company literally cannot have a track record because they haven't existed long enough.
Claims of "25+ years of experience" paired with a new domain require explanation. Perhaps founders have extensive backgrounds from other organizations. Perhaps the company operated under different names previously. Or perhaps the claim is simply marketing language without substance.
Private domain registration through proxy services isn't unusual, but it does reduce transparency. Combined with the low trust scores from independent analysis tools, researchers should exercise significant caution.
This doesn't mean biomaxpeptides.com is necessarily fraudulent. New companies can be legitimate. But the burden of proof shifts to the vendor to demonstrate credibility through verifiable means like third-party tested COAs, responsive customer service, and transparent operations.
BioMaxx Research assessment
The explicit claim of third-party testing with publicly available COAs represents a significant positive indicator. If accurate, this demonstrates quality commitment beyond minimum standards.
Researchers should verify these claims directly. Request a COA for any product under consideration. Contact the testing laboratory named on the document to confirm authenticity. Check whether batch numbers align properly.
The double-x naming helps distinguish this vendor from similarly named competitors. Ensuring you're actually on biomaxx.shop rather than a look-alike domain matters for avoiding confusion.
Biomax.ca assessment
Canadian operation introduces specific considerations for US-based researchers. Import regulations, shipping logistics, and customs procedures differ from domestic purchases.
The claimed production and storage protocols, if accurate, suggest attention to compound stability. Fresh synthesis and immediate refrigeration would preserve peptide quality better than products sitting in warehouses for extended periods.
Geographic verification is possible for Canadian businesses through provincial registration databases. This provides another layer of accountability that fully anonymous vendors lack.
Researchers working with peptide stacks often order from multiple vendors to diversify risk. International and domestic options both have places in a comprehensive sourcing strategy.
Quality standards in peptide manufacturing
Understanding how legitimate peptide production works helps researchers recognize when vendors cut corners.
Synthesis methods
Modern peptide synthesis typically uses solid-phase methodology. Amino acids attach sequentially to a resin support, building the peptide chain one residue at a time. Each coupling step requires specific conditions for optimal efficiency.
Synthesis errors accumulate. Each coupling cycle might achieve 99% efficiency, but over a 30-residue peptide, impurities compound. Quality manufacturers monitor each step and implement purification to remove failed sequences.
Lower-cost production often skips monitoring steps or uses less rigorous purification. The result: products that technically contain the target peptide but also significant impurity levels.
Purification processes
Crude synthesis product undergoes purification, typically through preparative HPLC. This scaled-up version of analytical HPLC separates target peptide from synthesis byproducts.
Multiple purification cycles increase purity but also increase cost and reduce yield. Vendors balance these factors based on their target market. Research-grade products may undergo more extensive purification than bulk materials intended for different applications.
Asking vendors about their purification methods reveals sophistication level.
"We purify to 98%+" provides less confidence than detailed methodology descriptions.
Quality control testing
Beyond final product COAs, quality manufacturers test at multiple production stages. Raw material verification, in-process monitoring, and release testing create multiple checkpoints.
This level of quality system increases costs but dramatically improves consistency. Researchers working on sensitive applications benefit from multi-stage quality verification.
Good Manufacturing Practice (GMP) certification indicates facility compliance with pharmaceutical-grade quality systems. While not all research peptides require GMP production, the certification demonstrates operational capability.
Storage and stability
Proper storage begins immediately after synthesis. Lyophilized (freeze-dried) peptides offer better stability than solutions. Cold storage slows degradation. Protection from light prevents photosensitive compounds from breaking down.
How vendors handle post-production storage affects what reaches researchers. Products sitting at room temperature for weeks before shipping may have degraded regardless of initial quality.
The peptide storage guide covers optimal conditions for maintaining compound integrity after purchase. But researchers can only preserve what they receive, storage damage at the vendor level can't be reversed.
Understanding how long reconstituted peptides last and proper handling through the reconstitution calculator helps maximize useful life.
Specific peptide considerations
Different peptide types present unique quality challenges. Vendor selection should account for specific compound characteristics.
Growth hormone releasing peptides
Compounds like CJC-1295, ipamorelin, and sermorelin require careful handling. These secretagogues have specific stability requirements and degradation patterns.
Potency loss manifests as reduced biological activity without obvious visual changes. A degraded GHRP solution might look normal but produce diminished responses. Only proper testing confirms expected potency.
Vendors specializing in these categories should understand stability characteristics and implement appropriate handling throughout their supply chain.
Healing and repair peptides
Research compounds like BPC-157 and TB-500 have become extremely popular. This popularity attracts both legitimate vendors and those looking to profit from demand without quality commitment.
The BPC-157 vs TB-500 comparison helps researchers understand each compound's characteristics. But understanding what you should receive is only half the equation. Verifying that you actually received it requires the testing and documentation standards described throughout this guide.
For researchers exploring combination approaches, the BPC-157 and TB-500 stacking guide provides protocol details.
Weight management peptides
GLP-1 agonists and related compounds represent a particularly high-risk category for quality issues. The massive commercial demand for semaglutide, tirzepatide, and retatrutide creates strong incentives for fraud.
These complex molecules require sophisticated synthesis capabilities. Lower-cost knockoffs may contain incorrect sequences, insufficient purity, or entirely different compounds than labeled.
The best peptides for weight loss guide covers compound selection. But compound selection means nothing if what arrives in your vial doesn't match what you ordered.
Copper peptides and skincare compounds
GHK-Cu and related copper peptides present specific considerations. Metal-bound peptides have unique stability characteristics. Copper can dissociate from the peptide backbone under certain conditions, potentially creating both peptide and metal-related issues.
COAs for copper peptides should specifically address the metal content and binding characteristics, not just peptide purity. Some testing methods may not adequately assess metal-peptide complexes.
Researchers interested in topical applications can find detailed information in the copper peptide serum guide and skincare routine guide.
Research peptides and novel compounds
Newer compounds and research peptides may lack established testing standards. Vendors selling cutting-edge materials should demonstrate how they verify identity and purity when reference standards may not be commercially available.
Custom synthesis for research applications requires different quality documentation than catalog products. Researchers ordering novel sequences should understand what verification is possible and realistic.
The peptide research guide provides context for understanding how these compounds fit into broader scientific investigation.
Building a vendor verification workflow
Rather than evaluating each vendor from scratch, develop a systematic process you apply consistently.
Initial screening
Before spending significant time on any vendor, conduct quick checks that eliminate obviously problematic options.
Search the company name plus "scam" or "review" to surface any widespread complaints. Check domain age through WHOIS. Look for basic contact information on their website. Verify they provide COAs for products.
Vendors failing any of these basic checks likely don't deserve further investigation. Time spent here is time not spent on legitimate options.
Documentation request
For vendors passing initial screening, request specific documentation for products you're considering. Ask for the actual COA, not a generic example. Note response time and document quality.
A vendor unable or unwilling to provide batch-specific testing documentation before purchase raises immediate concerns. Legitimate companies understand that informed buyers want verification.
Verification checks
Examine received documentation carefully using the COA analysis framework discussed earlier. Check for required elements. Verify laboratory information. Compare batch numbers across documents and actual product labels.
If the vendor claims third-party testing, contact the named laboratory to confirm. This single step catches many fraudulent COAs.
Test orders
For vendors passing documentation review, consider small initial orders before committing to larger quantities. This allows evaluation of actual products, shipping quality, and customer service with limited risk.
Examine received products immediately. Does the vial label match COA batch numbers? Is packaging appropriate for temperature-sensitive compounds? Does product appearance match expectations?
Some researchers send samples from initial orders for independent testing verification. While this adds cost, it provides definitive quality confirmation beyond vendor documentation.
Ongoing monitoring
Vendor quality can change over time. Companies under financial pressure may reduce quality investments. Ownership changes might alter priorities. Supply chain disruptions can affect product consistency.
Regularly reassess even established vendors. Request updated COAs for repeat orders rather than relying on historical documents. Monitor community feedback for emerging concerns. Maintain relationships with multiple verified vendors rather than depending entirely on single sources.
SeekPeptides members access updated vendor analysis and community feedback to support ongoing evaluation processes.
Advanced quality verification methods
Beyond standard COA analysis, additional techniques help researchers verify peptide quality.
Independent testing
Sending samples to third-party laboratories provides definitive verification independent of vendor claims. Several analytical laboratories specialize in peptide testing for individual researchers.
Cost varies by compound complexity and testing thoroughness. Basic identity and purity confirmation runs less than comprehensive characterization including sequence verification and impurity profiling.
For high-value research or when vendor reliability is uncertain, independent testing investment often makes sense. The cost of a test is typically less than the cost of a failed research protocol.
Visual and physical inspection
While limited in scope, visual examination provides initial quality indicators. Lyophilized peptides should form clean, dry cakes or powders. Clumping, discoloration, or visible moisture suggests handling problems.
Reconstitution behavior offers additional data. Most peptides dissolve cleanly in appropriate solvents like bacteriostatic water. Unusual cloudiness, precipitation, or foaming might indicate degradation or contamination.
These observations can't replace analytical testing but can prompt additional investigation before using questionable materials.
Functional testing
In appropriate research contexts, bioactivity assessment provides quality information that chemical analysis alone doesn't capture. A peptide might show high purity by HPLC while having reduced biological activity due to subtle modifications or degradation.
Functional assays require appropriate research systems and aren't practical for all situations. But when available, they represent the ultimate quality metric, whether the compound actually does what it should do.
Batch-to-batch comparison
For researchers using peptides regularly, comparing results between batches reveals quality consistency. Significant performance variations between orders from the same vendor, with identical protocols, suggests quality control problems.
Documenting results systematically enables this kind of comparative analysis. Research notebooks should note vendor, batch numbers, reconstitution dates, and storage conditions alongside experimental data.
Handling quality issues
Sometimes despite careful verification, problems occur. Knowing how to respond protects both current research and future purchases.
Documentation
Preserve all evidence when quality concerns arise. Keep original packaging, vial labels, COAs, order confirmations, and payment records. Photograph any visual abnormalities. Record specific issues with dates and details.
This documentation supports vendor communication, payment disputes if necessary, and decisions about future purchases.
Vendor communication
Contact the vendor promptly with specific concerns. Provide clear descriptions of problems along with supporting documentation. Note their response time and quality.
Legitimate vendors take quality complaints seriously. They may request product returns for testing, offer replacements, or issue refunds. Dismissive or hostile responses to documented concerns indicate problematic operations.
Payment protection
Credit card purchases provide chargeback options when vendors fail to deliver acceptable products. This buyer protection is one reason reputable vendors accept card payments rather than requiring alternative methods.
Document everything thoroughly if pursuing chargebacks.
Card companies require evidence supporting claims of undelivered or misrepresented products.
Community reporting
Sharing experiences, both positive and negative, helps the broader research community make informed decisions. Detailed reviews that describe specific issues serve researchers better than vague complaints.
Be factual and specific in public comments. State what happened, what documentation exists, and how the vendor responded. This helps others while avoiding potential defamation concerns.
Building research-grade protocols
Quality vendors are just one component of reliable peptide research. Proper handling after purchase matters equally.
Storage optimization
Implement appropriate storage immediately upon receipt. Most lyophilized peptides benefit from frozen storage at -20°C or colder. Reconstituted solutions typically require refrigeration at 2-8°C with light protection.
The comprehensive storage guide details optimal conditions for various peptide types. Following these guidelines preserves the quality you verified during vendor selection.
Reconstitution best practices
Proper reconstitution technique prevents damage during solution preparation.
Use appropriate solvents, add liquid gently, avoid vigorous shaking, and allow full dissolution before use.
The reconstitution calculator helps determine correct volumes for desired concentrations. Accurate reconstitution ensures consistent dosing throughout protocols.
Protocol documentation
Maintain detailed records throughout research. Note compound sources, batch numbers, reconstitution dates, storage conditions, and handling procedures. This documentation enables troubleshooting if results deviate from expectations.
Consistent protocols allow meaningful comparison between experiments. Varying procedures introduce uncontrolled variables that complicate interpretation.
Multiple sourcing strategy
Relying on single vendors creates vulnerability. Supply disruptions, quality changes, or business failures can interrupt research. Maintaining relationships with multiple verified vendors provides backup options.
Periodic comparison testing between vendors helps confirm consistent quality across sources. Significant differences in results using supposedly identical compounds from different vendors reveals important information about actual quality.
Frequently asked questions
Is biomaxpeptides.com a legitimate peptide vendor?
The extremely recent domain registration (December 2024) combined with low trust scores from independent analysis tools suggests researchers should exercise significant caution. While new companies can be legitimate, the lack of verifiable track record makes quality confirmation difficult. Request specific COA documentation and verify through third-party laboratory contact before purchasing.
What distinguishes BioMaxx Research from other Biomax vendors?
BioMaxx Research (biomaxx.shop) specifically claims third-party testing with publicly available certificates of analysis. The double-x naming distinguishes them from similarly named competitors. Verify these claims by requesting batch-specific COAs and contacting the testing laboratory to confirm document authenticity.
How can I verify if a peptide COA is real or fake?
Check for required elements including batch numbers, testing dates, laboratory identification, analytical methods, and actual chromatograms or spectra. Contact the named laboratory directly to confirm they issued the document. Compare batch numbers between COA and vial labels. Watch for signs of digital manipulation like font inconsistencies or round number percentages that suggest fabrication.
What purity level should I expect from research-grade peptides?
Research-grade peptides typically show 95% purity or higher by HPLC analysis. Premium compounds often exceed 98-99% purity. Lower purity levels may be acceptable for some applications but increase the proportion of impurities that could affect results. Mass spectrometry confirmation of molecular identity complements purity percentage in comprehensive quality assessment.
Why do multiple companies use the Biomax name?
"Biomax" combines recognizable elements suggesting biological credibility and maximum quality. This effective marketing language attracts multiple businesses to adopt variations. The result is marketplace confusion where researchers must carefully distinguish between entirely separate companies sharing similar branding.
Should I avoid new peptide vendors entirely?
Not necessarily, but new vendors require more rigorous verification than established ones. Without operational history, researchers cannot rely on track record for quality assessment. Demand verifiable third-party COAs, confirm laboratory documentation independently, start with small test orders, and maintain healthy skepticism about marketing claims until supported by actual evidence.
What testing methods should a legitimate COA include?
Comprehensive COAs include High-Performance Liquid Chromatography (HPLC) for purity determination with actual chromatograms showing peak separation. Mass spectrometry (MS) confirms molecular identity by verifying expected molecular weight. Both methods together provide complementary information that neither alone can fully establish.
How often should I request new COAs from regular vendors?
Request batch-specific COAs for each order rather than relying on historical documents. Quality can vary between production runs, and testing dates should be reasonably recent, typically within three to six months of purchase. Vendors maintaining current documentation demonstrate ongoing quality commitment.
For researchers serious about optimizing their peptide protocols with verified compounds and evidence-based guidance, SeekPeptides provides comprehensive resources including vendor evaluation frameworks, quality verification guides, and community insights from thousands of experienced researchers.