Jan 20, 2026

For decades, men with low testosterone faced a frustrating choice. Accept the symptoms, fatigue, muscle loss, declining libido, or commit to lifelong hormone replacement. Neither option felt right. Traditional testosterone replacement therapy works, but it comes with baggage. Suppressed natural production. Fertility concerns. FDA black-box warnings about cardiovascular risks. The treatment becomes a permanent crutch rather than a solution.
Then came peptides.
Researchers began exploring ways to coax the body into producing more testosterone on its own. Peptides for testosterone support emerged as a promising field, with compounds like kisspeptin and gonadorelin showing the potential to stimulate the hypothalamic-pituitary-gonadal axis. But these approaches still relied on injectable delivery and worked through indirect pathways. What if there was a peptide that could directly activate testosterone synthesis at the cellular level, taken orally, without suppressing natural production?
This is where ACE-167 enters the conversation.
Developed by Acesis BioMed, ACE-167 represents a fundamentally different approach to treating low testosterone. Rather than flooding the body with external hormones or stimulating upstream signals, this tetrapeptide works at the mitochondrial level within Leydig cells themselves. It targets a specific protein interaction that normally limits testosterone production. The result, at least in preclinical studies, is increased endogenous testosterone that self-regulates through natural feedback mechanisms.
The implications are significant. An oral, non-steroidal peptide therapy that restores testosterone production without the side effects of traditional TRT could transform how we approach hypogonadism. SeekPeptides has been tracking this development closely, and in this comprehensive guide, we'll examine everything researchers need to know about ACE-167, from its molecular mechanism to its potential applications and current development status.
Understanding testosterone and why production declines
Before diving into ACE-167's mechanism, we need to understand the problem it aims to solve. Testosterone isn't just about muscles and sex drive.
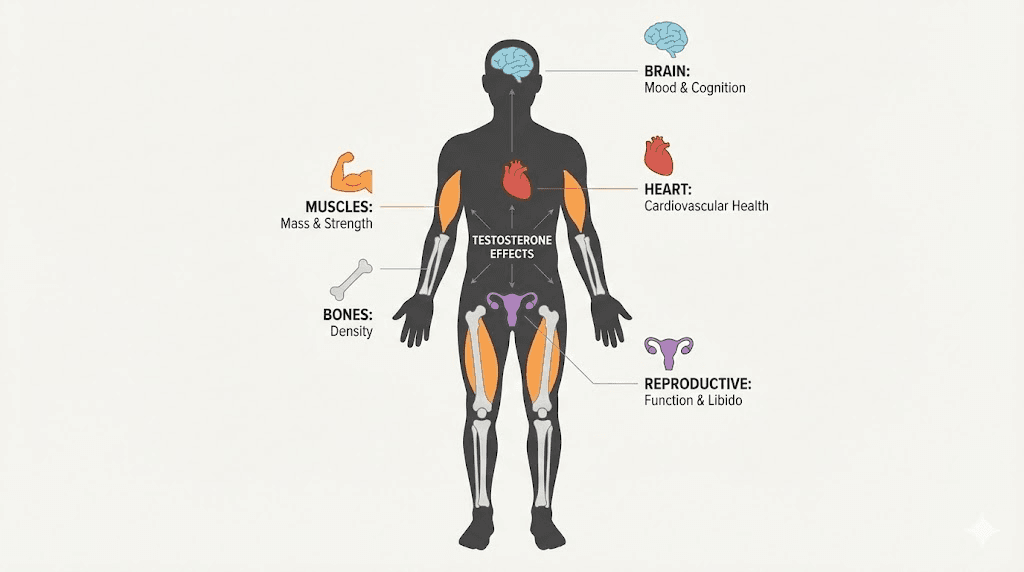
This hormone influences bone density, red blood cell production, fat distribution, mood regulation, cognitive function, and cardiovascular health.
When levels drop, the effects ripple through every system.
The decline begins earlier than most men realize.
Testosterone production peaks in early adulthood and gradually decreases by approximately 1-2% per year after age 30. By age 45, roughly 40% of men experience some degree of testosterone deficiency. By age 80, that number exceeds 50%. This isn't just aging, it's a clinical condition called hypogonadism that affects quality of life, metabolic health, and longevity.
Symptoms of low testosterone
The signs often get dismissed as "getting older." Fatigue that doesn't improve with rest. Mental fog that makes concentration difficult. Muscle mass that fades despite consistent training. Weight gain, particularly around the midsection. Declining interest in sex. Mood changes, irritability, even depression. Reduced bone density that increases fracture risk. These symptoms create a negative spiral where men feel less capable, exercise less, sleep worse, and experience further hormonal decline.
What many don't realize is that these changes aren't inevitable. They're treatable.
The challenge has always been finding treatment approaches that address the root cause without creating new problems. Traditional testosterone replacement helps the symptoms but often makes the underlying problem worse by shutting down natural production entirely. This is where understanding peptides for libido and testosterone support becomes valuable.
How testosterone is made: the Leydig cell story
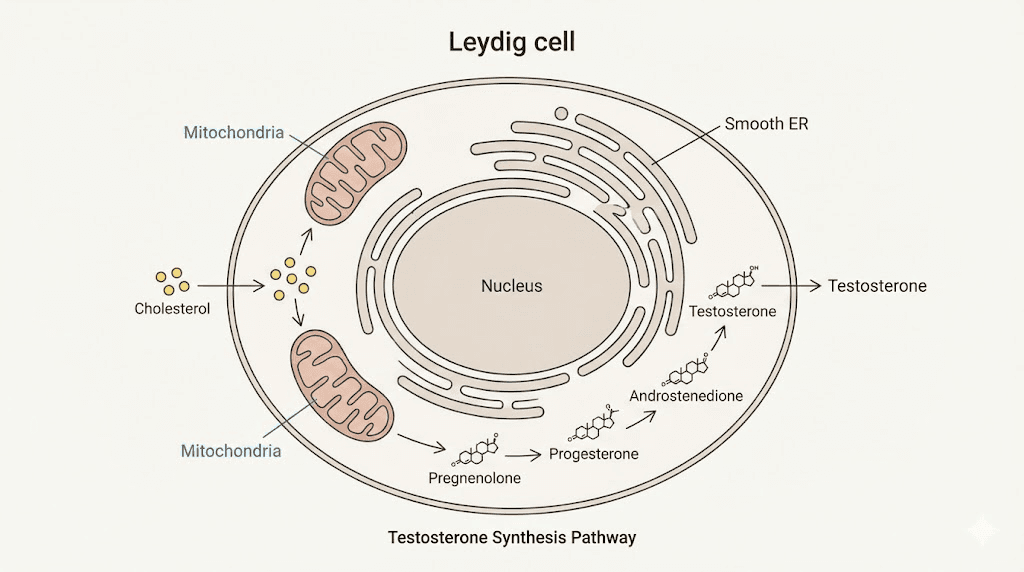
Testosterone production happens primarily in the Leydig cells of the testes. These specialized cells respond to luteinizing hormone (LH) signals from the pituitary gland. When LH binds to receptors on Leydig cells, it triggers a cascade that ultimately converts cholesterol into testosterone.
The process is elegant but complex.
Cholesterol serves as the raw material. It must be transported into the mitochondria, the powerhouses of the cell, where the conversion process begins. This transport step is rate-limiting, meaning it controls how fast testosterone can be produced regardless of how much cholesterol is available. The cholesterol crosses the mitochondrial membranes through a protein complex called the transduceosome, which includes several key players: STAR (steroidogenic acute regulatory protein), TSPO (translocator protein), and VDAC1 (voltage-dependent anion channel 1).
Once inside the mitochondria, the enzyme CYP11A1 converts cholesterol to pregnenolone. From there, additional enzymes in the smooth endoplasmic reticulum complete the transformation to testosterone. The entire pathway depends on proper signaling, adequate cholesterol supply, and critically, efficient transport into the mitochondria.
This is where aging causes problems. Research shows that age-related testosterone decline isn't primarily due to losing Leydig cells. Instead, it results from reduced efficiency in LH-stimulated signaling and impaired cholesterol transport into mitochondria. The machinery exists but works less effectively.
SeekPeptides members understand that targeting these specific bottlenecks represents a more sophisticated approach than simply adding external hormones. And this brings us to the discovery that led to ACE-167.
The science behind ACE-167: targeting the 14-3-3ε-VDAC1 interaction
In the early 2000s, Dr. Vassilios Papadopoulos and his research team at McGill University made a discovery that would eventually lead to ACE-167. They identified a protein called 14-3-3ε that acts as a negative regulator of steroidogenesis. Think of it as a brake pedal on testosterone production.
Here's what happens at the molecular level.
The 14-3-3ε protein binds to VDAC1 on the outer mitochondrial membrane.
When this happens, it blocks the efficient interaction between VDAC1 and TSPO, two proteins that normally work together to import cholesterol.
By inserting itself into this complex, 14-3-3ε effectively limits how much cholesterol can enter the mitochondria for conversion to testosterone. More 14-3-3ε binding means less cholesterol transport means lower testosterone output.
The research team reasoned that blocking this 14-3-3ε-VDAC1 interaction should increase testosterone production. If you remove the brake, the car goes faster.
From concept to peptide
The researchers identified that amino acids 159-172 of VDAC1, particularly the region around serine-167, mediated the interaction with 14-3-3ε. They created a 27-amino acid fusion peptide called TV159-172 that could compete with endogenous VDAC1 for 14-3-3ε binding. When this peptide bound to 14-3-3ε, it freed up the native VDAC1-TSPO complex to transport more cholesterol.
The results were remarkable.
When administered directly to rat testes, TV159-172 increased intratesticular testosterone by 12-fold and serum testosterone by 5-fold. Even more interesting, the peptide worked in chemically castrated rats, those treated with GnRH antagonists to suppress LH, demonstrating that it could stimulate testosterone production somewhat independently of upstream hormonal signals.
However, the original peptide had limitations. It required injection directly into the testes and degraded quickly. Not exactly practical for treating millions of men with low testosterone. The team needed to develop something more usable.
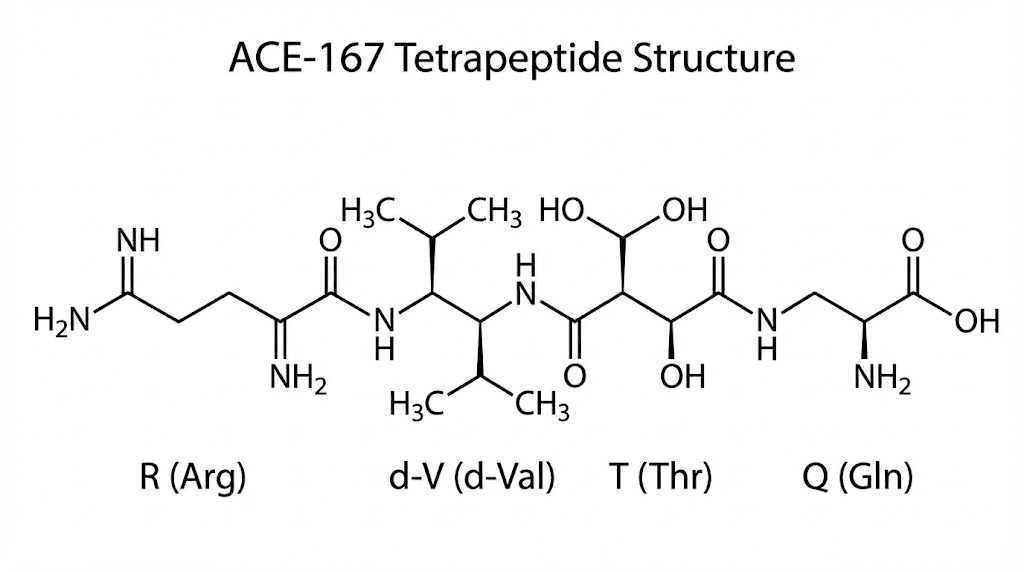
Discovering the core: RVTQ and RdVTQ
Through systematic testing, researchers identified the minimal bioactive sequence: a tetrapeptide called RVTQ (arginine-valine-threonine-glutamine) corresponding to amino acids 163-166 of VDAC1. At just 503 Daltons, this tiny molecule retained the ability to block 14-3-3ε-VDAC1 interactions while being much more practical to manufacture and potentially deliver.
But oral bioavailability presented challenges. The native RVTQ peptide would be rapidly degraded by enzymes in the digestive system. To solve this, researchers substituted the natural L-valine with its mirror-image D-valine, creating RdVTQ. This modification dramatically increased stability and allowed for oral administration.
RdVTQ became the lead candidate that evolved into ACE-167.
Understanding peptide solutions and delivery methods helps explain why this oral capability is so significant.
Most peptides require injection because they can't survive the harsh conditions of digestion. An oral peptide that can stimulate testosterone production at the cellular level represents a major therapeutic advancement for those researching peptide research.
How ACE-167 works: mechanism of action explained
ACE-167 functions as an LH-signaling amplifier rather than a replacement for natural hormonal control. This distinction matters enormously for safety and practicality.
When you take ACE-167 orally, the peptide survives digestion and enters circulation. Upon reaching Leydig cells, it competes with endogenous VDAC1 for binding to 14-3-3ε. By occupying 14-3-3ε, the peptide prevents this regulatory protein from blocking cholesterol transport. The result: more cholesterol enters the mitochondria, more pregnenolone is produced, and more testosterone synthesis occurs.
Critically, ACE-167 doesn't force testosterone production beyond what the body's own LH signaling supports. In animal studies, hypophysectomized rats (those with removed pituitary glands, thus no LH) showed no testosterone increase from ACE-167. The peptide amplifies existing signals rather than creating artificial ones.
The self-regulation advantage
This mechanism provides a built-in safety feature that traditional TRT lacks. When testosterone levels rise, the hypothalamic-pituitary-gonadal axis responds through negative feedback. Higher testosterone suppresses LH release, which reduces the signal for Leydig cells to produce more testosterone. Because ACE-167 works by amplifying LH signals rather than bypassing them, this feedback loop remains intact.
What does this mean practically?
According to preclinical data, ACE-167 cannot cause excessive testosterone increases. The ceiling depends on the individual's own hormonal machinery. If natural LH signaling is suppressed by rising testosterone, ACE-167's effects diminish accordingly. This self-regulation prevents the supraphysiological spikes that can occur with TRT, reducing risks of side effects and abuse.
Compare this to injectable testosterone, which bypasses all natural regulation. External testosterone floods the system regardless of what the body needs, suppresses natural production, and can create dependency. SeekPeptides emphasizes understanding these mechanistic differences when evaluating different testosterone support approaches.
Preclinical results: what the studies show
Multiple animal studies have examined ACE-167 and its precursor peptides. The results paint an encouraging picture.
In subcutaneous delivery studies with TV159-172, doses as low as 30-83 ng/kg/day produced significant testosterone increases in rats. Some animals achieved upwards of 10-fold increases above control levels. The RVTQ tetrapeptide at 309 ng/kg/day also significantly elevated testosterone.
More importantly for practical application, oral administration studies showed that RdVTQ and related derivatives maintained bioactivity when given by mouth. Peak plasma concentrations occurred within 15-30 minutes of administration, indicating rapid absorption. Dual-peptide formulations achieved significant effects at ultra-low doses, representing a 55-fold improvement in efficacy compared to single-peptide approaches.
Age-dependent responses were observed, with maximal effects in 5-month-old rats that gradually decreased with age. This pattern mirrors the natural decline in testosterone responsiveness seen in aging and suggests ACE-167 might be most effective in earlier stages of hypogonadism before Leydig cell function deteriorates significantly.
Safety assessments showed favorable results. At therapeutic doses, the peptide did not significantly affect corticosterone levels, indicating specificity for testosterone pathways. Estradiol and aldosterone levels remained comparable to controls. No obvious adverse effects were noted in the animal models studied.
ACE-167 vs traditional testosterone replacement therapy
To appreciate ACE-167's potential, we need to examine the limitations of current treatments. Testosterone replacement therapy has been used since the 1930s and remains the standard approach for hypogonadism. It works. Symptoms improve. Men feel better.
But the side effects create real concerns.
The problems with TRT
Suppressed natural production: External testosterone signals the brain to stop producing LH. Without LH stimulation, Leydig cells reduce their own testosterone output. Many men on TRT become permanently dependent because their natural production has atrophied. Stopping TRT often leads to worse symptoms than before starting.
Fertility impact: TRT suppresses the hormonal signals necessary for sperm production. Men on testosterone replacement commonly experience reduced sperm counts or complete infertility. For younger men who might want children, this creates a significant barrier. While alternatives like hCG or clomiphene can help maintain fertility during TRT, they add complexity and cost.
Cardiovascular concerns: TRT can increase red blood cell counts, thickening blood and raising cardiovascular risks. The FDA mandated black-box warnings about heart attack and stroke risks. While recent large-scale trials have been more reassuring (showing non-inferiority to placebo for major cardiovascular events), the concern remains for certain populations.
Prostate considerations: Though evidence doesn't clearly link TRT to increased prostate cancer risk, the therapy is contraindicated in men with existing prostate cancer. Regular PSA monitoring is required, and many men with prostate concerns avoid TRT altogether.
Administration burden: Options include daily gels (messy, risk of transfer to partners or children), weekly to biweekly injections, or surgical pellet implants every few months. None are ideal for long-term compliance.
These limitations have driven interest in alternatives. Peptides for erectile dysfunction and hormonal support offer different mechanisms that may avoid some of TRT's drawbacks.
How ACE-167 addresses these limitations
ACE-167's mechanism directly solves several TRT problems:
Maintains natural production: Rather than replacing endogenous testosterone, ACE-167 enhances the body's own synthesis. The Leydig cells continue functioning, they just work more efficiently. Natural feedback loops remain intact, preventing dependency.
Preserves fertility potential: Because ACE-167 doesn't suppress LH (and actually requires LH for its effects), the hormonal signals for sperm production should remain intact. Preclinical data suggests normal HPG axis function during treatment.
Self-regulating safety: The inability to cause supraphysiological testosterone levels reduces cardiovascular and other risks associated with excessive hormone exposure. The body's own feedback mechanisms prevent overshooting.
Oral administration: No injections, no messy gels, no surgical implants. A simple oral peptide taken daily could dramatically improve compliance and accessibility.
Non-steroidal: Because ACE-167 is a peptide rather than a steroid hormone, it lacks the direct androgenic effects that cause some TRT side effects. It promotes testosterone production rather than being testosterone itself.
The comparison isn't meant to suggest TRT is bad. For many men, it remains an effective option. But ACE-167 represents what could be a fundamental advance, treating the cause of low testosterone rather than just supplementing the missing hormone. Peptides for men are opening new therapeutic possibilities across multiple conditions.
Current development status and clinical pathway
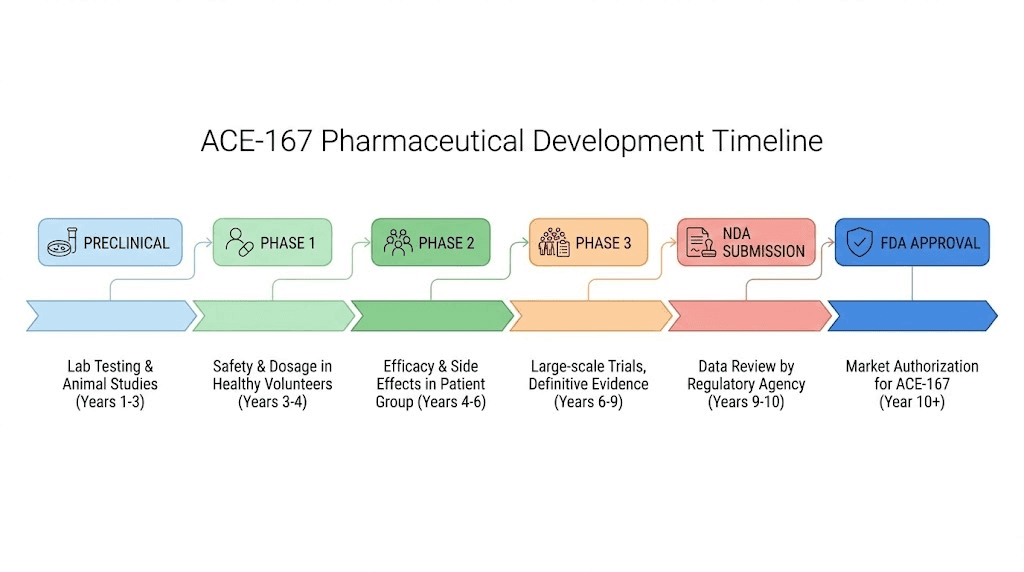
As of the latest available information, ACE-167 remains in preclinical development. Acesis BioMed has completed lead peptide identification, extensive testing, and is conducting pre-IND (Investigational New Drug) and IND-enabling studies.
These are the preparatory stages before requesting FDA permission to begin human clinical trials.
What must happen before approval
The path from preclinical success to approved therapy is long and uncertain. Many promising compounds fail in human trials. For ACE-167, the following milestones remain:
IND submission: Acesis BioMed must compile all preclinical safety and efficacy data, proposed clinical trial protocols, and manufacturing information for FDA review. This package demonstrates sufficient evidence of safety to justify testing in humans.
Phase 1 trials: First-in-human studies will test safety, tolerability, and pharmacokinetics in a small group of healthy volunteers. These trials determine safe dosing ranges and identify any unexpected side effects.
Phase 2 trials: Larger studies in men with hypogonadism will evaluate efficacy, optimal dosing, and safety over longer treatment periods. These trials provide preliminary evidence that the peptide actually works in the target population.
Phase 3 trials: Large, randomized, controlled trials comparing ACE-167 to placebo (and potentially to TRT) will establish definitive efficacy and safety. These studies typically involve hundreds to thousands of patients over extended periods.
FDA review and approval: If trials succeed, Acesis BioMed will submit a New Drug Application. The FDA review process typically takes 10-12 months for standard applications.
The entire process commonly takes 10-15 years from initial discovery to approval. Given that ACE-167's foundational research began in the mid-2010s and remains in preclinical stages, realistic availability, if development succeeds, is likely years away.
Patent protection and company background
Acesis BioMed holds two patent families covering the peptide compounds and steroidogenesis modulation methods. This intellectual property protection provides the commercial incentive necessary to fund expensive clinical development.
The company has positioned ACE-167 for multiple potential indications beyond male hypogonadism, including metabolic syndrome, type 2 diabetes (where testosterone deficiency is common), obesity, osteoporosis, Klinefelter syndrome, and opioid-induced testosterone deficiency. This broader potential market may attract the investment needed to complete clinical development.
For researchers following peptide regulation news, ACE-167 represents an interesting case study in how novel peptide therapies progress through the regulatory system.
Comparing ACE-167 to other testosterone-supporting peptides
ACE-167 isn't the only peptide approach to testosterone support. Several other compounds work through different mechanisms, each with distinct advantages and limitations. Understanding these alternatives helps contextualize ACE-167's unique value proposition.
Kisspeptin
Kisspeptin is a naturally occurring peptide that regulates the HPG axis at the hypothalamic level. It triggers the release of gonadotropin-releasing hormone (GnRH), which stimulates LH and FSH release from the pituitary, ultimately increasing testosterone production.
Clinical studies show kisspeptin-10 can significantly increase testosterone levels within hours of administration.
One trial reported testosterone increases from 479 ng/dL to 692 ng/dL after less than 24 hours of infusion. The peptide also increases LH pulse frequency and amplitude.
Advantages include maintaining fertility and working through natural pathways. However, kisspeptin requires injection, has a short half-life requiring frequent dosing, and works upstream in the HPG axis rather than directly at Leydig cells.
Gonadorelin
Gonadorelin is a synthetic version of GnRH. By mimicking the body's natural releasing hormone, it stimulates the pituitary to produce LH and FSH. Trials have shown it can increase testosterone up to 1560 ng/dL in some subjects with tertiary hypogonadism.
Doctors often use gonadorelin alongside TRT to maintain fertility or as a standalone therapy. Like kisspeptin, it preserves natural hormonal architecture but requires injection and works indirectly.
Growth hormone secretagogues
Compounds like sermorelin and MK-677 stimulate growth hormone release, which indirectly supports testosterone through metabolic improvements and enhanced Leydig cell function. While not direct testosterone therapies, they may provide complementary benefits.
For those researching IGF peptides and growth hormone pathways, understanding the interconnection between GH and testosterone systems reveals potential synergistic approaches.
Where ACE-167 differs
ACE-167's unique position comes from several factors:
Direct cellular action: Rather than working through hypothalamus or pituitary signals, ACE-167 acts at the Leydig cell mitochondria where testosterone synthesis actually occurs. This targeted approach may be more efficient.
Oral bioavailability: Most testosterone-supporting peptides require injection. ACE-167's oral delivery could dramatically improve accessibility and compliance.
Novel mechanism: By targeting the 14-3-3ε-VDAC1 interaction, ACE-167 addresses a previously untreatable bottleneck in testosterone synthesis. This represents a genuinely new therapeutic approach.
Self-regulation: The dependence on natural LH signaling provides inherent safety that other approaches may lack.
The comparison table below summarizes key differences:
Peptide | Mechanism | Delivery | Direct/Indirect | Fertility Impact |
|---|---|---|---|---|
ACE-167 | 14-3-3ε-VDAC1 blocker | Oral | Direct (Leydig cells) | Preserves |
Kisspeptin | GnRH stimulator | Injection | Indirect (hypothalamus) | Preserves |
Gonadorelin | GnRH mimetic | Injection | Indirect (pituitary) | Preserves |
Sermorelin | GHRH mimetic | Injection | Indirect (GH pathway) | Neutral |
TRT | Hormone replacement | Various | Replacement (external) | Suppresses |
SeekPeptides provides comprehensive comparisons and protocol guidance for members researching these different approaches to testosterone optimization.
Potential benefits of ACE-167 beyond testosterone
While testosterone restoration is ACE-167's primary target, the implications extend further. Testosterone influences multiple body systems, and restoring optimal levels could provide cascading benefits.
Body composition
Testosterone promotes muscle protein synthesis and inhibits fat storage. Men with low testosterone typically experience muscle loss and fat gain, particularly visceral fat around the abdomen. By restoring testosterone levels, ACE-167 could help reverse these changes.
Research on peptides for fat loss shows that hormonal optimization often produces better results than direct fat-burning approaches. Similarly, fat burning peptides may work synergistically with testosterone support.
Bone health
Testosterone plays a crucial role in maintaining bone mineral density. Hypogonadal men have significantly increased osteoporosis and fracture risk. The preclinical studies on ACE-031 (a different compound from the same research program but focused on myostatin inhibition) showed improved bone mineral density markers.
While ACE-167 works through a different mechanism, testosterone restoration should similarly benefit bone health.
Metabolic function
Low testosterone is associated with insulin resistance, metabolic syndrome, and type 2 diabetes. Restoring testosterone levels often improves glucose metabolism and reduces cardiovascular risk factors. Acesis BioMed has specifically identified type 2 diabetes as a potential indication for ACE-167, recognizing this metabolic connection.
Cognitive function
Brain fog is a common complaint among men with low testosterone. The hormone influences neurotransmitter function, spatial reasoning, and memory. While research on testosterone's cognitive effects remains somewhat mixed, many men report improved mental clarity with testosterone optimization. Nootropic peptides and testosterone support may offer complementary benefits for cognitive enhancement.
Mood and energy
Testosterone deficiency commonly causes fatigue, irritability, and depressive symptoms. These effects significantly impact quality of life, often driving men to seek treatment in the first place. Restoring testosterone levels typically improves energy, motivation, and overall well-being. For those researching DSIP peptide benefits for sleep and mood, understanding testosterone's role in these systems provides important context.
Sexual function
Perhaps the most noticeable symptom of low testosterone is reduced libido and sexual function. Testosterone is essential for desire, arousal, and erectile function. ACE-167's ability to restore endogenous testosterone should improve these parameters without the complications of external hormone administration.
Who might benefit from ACE-167
Not everyone with low testosterone is the same candidate for ACE-167. Understanding the different types of hypogonadism helps clarify who might benefit most.
Primary hypogonadism
In primary hypogonadism, the problem lies in the testes themselves. Leydig cells don't produce adequate testosterone despite normal or elevated LH signals. Causes include genetic conditions like Klinefelter syndrome, testicular injury, infection, or chemotherapy damage.
ACE-167's effectiveness in primary hypogonadism depends on whether functional Leydig cells remain. If some Leydig cell capacity exists, even if impaired, the peptide might enhance their output. However, if Leydig cells are absent or completely non-functional, ACE-167 would have no substrate to work with.
Secondary hypogonadism
Secondary hypogonadism involves problems at the pituitary gland, resulting in inadequate LH production. Without sufficient LH, Leydig cells don't receive the signal to produce testosterone. Causes include pituitary tumors, medications (especially opioids), obesity, and chronic illness.
Because ACE-167 requires LH signaling to function, pure secondary hypogonadism might not respond well. The peptide amplifies LH signals but cannot create them.
However, many cases involve reduced rather than absent LH, and ACE-167 might benefit these partial deficiencies.
Late-onset hypogonadism
Also called age-related hypogonadism, this condition involves both central (reduced LH pulsatility) and gonadal (reduced Leydig cell responsiveness) components. It's the most common form of testosterone deficiency and represents ACE-167's primary target population.
Research suggests that age-related decline specifically involves impaired LH-stimulated cAMP production and cholesterol transport into mitochondria, exactly the bottleneck ACE-167 targets.
The preclinical studies showing age-dependent responses (with effects declining in older animals) suggest optimal benefit in earlier-stage decline before Leydig cell function deteriorates severely.
Opioid-induced testosterone deficiency
Chronic opioid use suppresses the HPG axis, causing low testosterone in up to 90% of men on long-term opioid therapy. This creates a challenging treatment situation since affected men often need ongoing pain management. ACE-167's mechanism might restore some testosterone production even with partially suppressed central signaling, though this would require clinical study to confirm.
Who might not benefit
Some populations likely wouldn't respond to ACE-167:
Men with complete Leydig cell failure from bilateral orchiectomy, severe testicular damage, or complete primary hypogonadism would have no cells for ACE-167 to act upon. Similarly, those with complete pituitary failure producing zero LH would lack the hormonal signal ACE-167 amplifies. These individuals would still require traditional testosterone replacement.
Understanding your specific type of testosterone deficiency through proper peptide testing and hormonal evaluation is essential before pursuing any therapy.
SeekPeptides emphasizes the importance of baseline testing and proper diagnosis in optimizing peptide research outcomes.
Safety considerations and what preclinical data suggests
Any new therapy raises safety questions. While ACE-167 hasn't been tested in humans yet, the preclinical data provides some reassurance, along with areas requiring further study.
Favorable preclinical safety signals
Animal studies showed no obvious adverse effects at therapeutic doses. Specifically:
Steroid selectivity: At appropriate doses, ACE-167 increased testosterone without significantly affecting corticosterone (the rat equivalent of cortisol). This suggests the peptide specifically targets the testosterone pathway rather than broadly stimulating all steroid production.
Hormone balance: Estradiol and aldosterone levels remained comparable to controls, indicating the peptide doesn't disrupt related hormonal systems.
Self-regulation: The HPG axis feedback system remained functional, preventing excessive testosterone increases. Prolonged infusion actually resulted in testosterone suppression as the negative feedback loop engaged, confirming the self-regulatory mechanism works.
No observed toxicity: Standard safety assessments in animal models didn't reveal obvious toxic effects, though comprehensive long-term safety studies would be needed for human application.
Questions requiring human data
Preclinical studies cannot fully predict human responses. Areas requiring clinical investigation include:
Efficacy in humans: Will the promising animal results translate to meaningful testosterone increases in men with hypogonadism? Human hormonal systems differ from rats, and clinical response must be demonstrated.
Optimal dosing: The animal studies used various doses and delivery methods. Human trials will need to establish the correct dose for efficacy while minimizing any risks.
Long-term safety: The preclinical studies involved relatively short treatment periods. Treating hypogonadism typically requires years to decades of therapy. Long-term effects on any body system remain unknown.
Drug interactions: How ACE-167 interacts with common medications, especially those affecting the HPG axis, needs investigation.
Special populations: Safety in older men, those with cardiac conditions, prostate issues, or other comorbidities requires specific study.
Theoretical safety advantages
Based on its mechanism, ACE-167 should theoretically avoid several TRT-related concerns:
No exogenous hormone: Because the body produces its own testosterone in response to ACE-167, there's no artificial hormone to cause direct androgenic effects beyond what the body naturally produces.
Cannot exceed natural limits: The HPG feedback system prevents testosterone from exceeding physiological levels, unlike TRT which can create supraphysiological exposure.
Maintains fertility: Without suppressing LH and FSH, the hormonal signals for spermatogenesis should remain intact.
Low abuse potential: The self-regulating mechanism prevents the excessive testosterone increases that might be sought for performance enhancement.
These theoretical advantages need confirmation in human studies, but they represent significant potential benefits over current options.
The broader context: peptides and the future of hormone therapy
ACE-167 represents part of a larger shift in how we approach hormone-related conditions. Traditional endocrinology focused on measuring deficiencies and replacing missing hormones directly. Peptide therapeutics enable more nuanced interventions.
From replacement to restoration
The philosophical difference matters. TRT replaces testosterone but doesn't restore the body's ability to make it. In fact, it typically impairs natural production through feedback suppression. Peptide approaches like ACE-167 aim to restore natural function, addressing root causes rather than masking symptoms.
This restoration paradigm applies across peptide therapeutics. BPC-157 and TB-500 promote tissue healing rather than just managing pain. KPV peptide for inflammation modulates immune responses rather than broadly suppressing them. DSIP peptide supports natural sleep architecture rather than forcing sedation.
Precision targeting
ACE-167's mechanism illustrates the precision possible with peptide therapeutics. Rather than flooding the body with a hormone that affects every androgen receptor, it targets one specific protein interaction in one specific cell type. This precision reduces off-target effects while achieving the desired outcome.
The research that led to ACE-167 involved detailed mapping of protein interactions, identification of regulatory mechanisms, and design of compounds to modulate specific pathways.
This approach reflects advances in molecular biology that enable therapeutics impossible even a decade ago.
Oral peptide delivery
ACE-167's oral bioavailability represents a technical achievement worth noting. Most peptides can't survive digestion because gastric acid and enzymes destroy them before absorption. ACE-167's D-amino acid modification provides stability while maintaining bioactivity.
This breakthrough could influence other peptide therapies. If one peptide can be made orally bioavailable through structural modifications, others might follow. The convenience of oral dosing dramatically improves patient compliance and accessibility compared to injections.
For researchers interested in peptide reconstitution and handling, understanding these stability considerations provides important context for working with various peptide compounds.
Practical considerations for researchers following ACE-167
Since ACE-167 isn't yet available outside clinical trials, what can researchers interested in testosterone optimization do now?
Monitor development progress
Stay informed about Acesis BioMed's clinical development progress. Key milestones to watch include:
IND application submission and FDA acceptance
Initiation of Phase 1 trials
Publication of any clinical trial results
Regulatory decisions at each development stage
Clinical trial registries like ClinicalTrials.gov will list any human studies once they begin. Peptide forums and communities often provide early information about research developments.
Understand alternative approaches
While waiting for ACE-167, other peptide and therapeutic approaches may provide benefit:
Kisspeptin and gonadorelin: Already available through compounding pharmacies under physician supervision, these peptides offer another way to support natural testosterone production.
Lifestyle optimization: Sleep, exercise, nutrition, and stress management significantly impact testosterone levels. These foundational factors should be optimized regardless of any therapeutic approach.
Current peptide research: Testagen peptide and other compounds in the testosterone support category may offer complementary benefits worth exploring.
Get proper testing
Understanding your specific hormonal profile is essential for any optimization strategy. Comprehensive testing should include:
Total testosterone
Free testosterone
LH and FSH
SHBG (sex hormone-binding globulin)
Estradiol
Prolactin
Thyroid function
These markers help identify whether testosterone deficiency is primary, secondary, or late-onset, and guide appropriate intervention strategies. Working with knowledgeable healthcare providers who understand both conventional and peptide approaches provides the best outcomes.
Consider the research context
ACE-167 remains a research compound. It hasn't been proven safe or effective in humans. The promising preclinical data must be validated through rigorous clinical trials. Many compounds that show benefit in animals fail in human studies.
This isn't reason for pessimism but for calibrated expectations. ACE-167 represents a promising development worth following, not a guaranteed solution. The scientific foundation is solid, the mechanism is novel, and the preclinical results are encouraging. Whether these translate to an approved therapy remains to be seen.
SeekPeptides provides ongoing updates on peptide development and helps members navigate the evolving landscape of research compounds and therapeutic options.
Understanding the limitations of current knowledge
Intellectual honesty requires acknowledging what we don't know about ACE-167. The enthusiasm generated by preclinical results must be tempered by the realities of drug development.
Translation uncertainty
Animal models imperfectly predict human responses. The rat studies provide proof-of-concept that the mechanism works and the peptide is bioactive. They don't prove ACE-167 will effectively treat human hypogonadism.
Differences in metabolism, dosing, response patterns, and side effect profiles between species mean human trials could reveal unexpected challenges. This is why clinical development exists, to systematically test whether promising compounds actually work in people.
Limited long-term data
The preclinical studies involved relatively short treatment periods. Hypogonadism treatment typically continues for years or decades.
We don't know how the body responds to long-term 14-3-3ε-VDAC1 modulation.
Could compensatory mechanisms develop that reduce effectiveness over time? Could unforeseen long-term effects emerge? Only extended human studies can answer these questions.
Competitive landscape
Other companies may be developing similar approaches. The pharmaceutical landscape shifts constantly. Whether ACE-167 specifically or a related compound eventually reaches market depends on many factors beyond scientific merit, including funding, regulatory decisions, and competitive dynamics.
Availability timeline
Even if everything goes well, ACE-167 is likely years away from FDA approval. The entire clinical development process commonly takes a decade or longer. Men seeking testosterone support today need to consider currently available options rather than waiting for a compound that may or may not materialize.
This realistic perspective doesn't diminish ACE-167's significance. It's potentially transformative. But managing expectations appropriately ensures researchers make informed decisions about their current health optimization strategies.
Frequently asked questions
What exactly is ACE-167?
ACE-167 is an oral tetrapeptide (four amino acids: RdVTQ) developed by Acesis BioMed to stimulate natural testosterone production. It works by blocking the 14-3-3ε-VDAC1 protein interaction in Leydig cells, enhancing cholesterol transport into mitochondria where testosterone synthesis begins. Unlike traditional testosterone replacement, it restores endogenous production rather than providing external hormone.
How is ACE-167 different from testosterone replacement therapy?
TRT provides external testosterone that suppresses natural production and can affect fertility. ACE-167 works by enhancing the body's own testosterone synthesis, maintaining natural HPG axis function. The peptide is oral rather than injectable, self-regulates through natural feedback mechanisms, and theoretically preserves fertility. For comprehensive comparison, review our peptides for testosterone guide.
Is ACE-167 available to purchase?
No. ACE-167 is currently in preclinical development and has not yet entered human clinical trials. It is not available for purchase from any legitimate source. Any product claiming to be ACE-167 should be treated with extreme caution. Peptide testing and verification is essential for any research compounds.
When will ACE-167 be available?
If clinical development succeeds, ACE-167 is realistically several years away from potential FDA approval. The compound must complete IND-enabling studies, Phase 1-3 clinical trials, and FDA review. The entire process commonly takes 10-15 years from initial discovery. Follow peptide regulation news for development updates.
Does ACE-167 have side effects?
Preclinical animal studies showed favorable safety profiles with no obvious adverse effects at therapeutic doses. However, human safety data doesn't exist yet. The self-regulating mechanism theoretically prevents excessive testosterone increases that could cause side effects, but this requires clinical confirmation.
Can ACE-167 be used for bodybuilding?
ACE-167 is being developed to treat hypogonadism, not enhance athletic performance. Its self-regulating mechanism prevents supraphysiological testosterone levels, limiting potential for abuse. The compound cannot force testosterone above what natural LH signaling supports. For performance optimization, consider our guides on peptides for cardio endurance and related topics.
How does ACE-167 compare to kisspeptin for testosterone?
Kisspeptin works at the hypothalamic level, stimulating GnRH release that triggers LH production and subsequently testosterone synthesis. ACE-167 works directly at Leydig cells, enhancing testosterone production at the cellular level. Kisspeptin requires injection while ACE-167 is oral. Both preserve fertility and natural hormonal function, but through different mechanisms.
Will ACE-167 affect fertility?
Preclinical evidence suggests ACE-167 preserves fertility because it works through natural LH signaling rather than suppressing it. The HPG axis feedback system remains intact. This contrasts with TRT, which commonly reduces sperm production. Clinical studies will need to confirm fertility preservation in humans.
In case I don't see you, good afternoon, good evening, and good night. May your testosterone stay optimal, your Leydig cells stay active, and your research stay rewarding.