Jan 27, 2026
The letters SIINFEKL might look like a random string of characters. They are not. These eight letters represent the most extensively studied peptide sequence in immunology research. Period.
If you have ever read a paper about CD8+ T cell responses, you have almost certainly encountered SIINFEKL. If you have explored vaccine development research, this sequence has appeared. And if you are interested in understanding how the immune system recognizes and destroys infected cells, SIINFEKL provides the clearest window into that process.
This is not hyperbole. The combination of SIINFEKL with its binding partner H-2Kb has become the single most examined T cell receptor-peptide-MHC complex in scientific literature. Thousands of studies. Decades of research. One eight-amino-acid sequence that has taught us more about adaptive immunity than perhaps any other tool in the immunologist's arsenal.
Why does this matter to peptide researchers? Because understanding how the immune system sees peptides transforms how we think about everything from peptide safety to cancer immunotherapy. The principles discovered through SIINFEKL research apply far beyond this single sequence. They reveal fundamental truths about peptide recognition, presentation, and immune activation that inform bioregulator peptide development, anti-inflammatory peptide research, and longevity peptide applications.
This guide covers everything researchers need to know about SIINFEKL peptide. We will examine its molecular structure, explain why it became the gold standard model antigen, explore its applications in cancer and vaccine research, and discuss practical considerations for working with this sequence. Whether you are new to immunological research or looking to deepen your understanding of peptide mechanisms, this comprehensive resource provides the foundation you need.
What is SIINFEKL peptide?
SIINFEKL is an eight-amino-acid peptide derived from chicken ovalbumin, specifically the sequence spanning positions 257-264 of the full protein. The name comes from the single-letter codes for each amino acid: serine-isoleucine-isoleucine-asparagine-phenylalanine-glutamic acid-lysine-leucine.
That sequence matters.
The molecular formula is C45H74N10O13 with a molecular weight of 963.12 daltons. Not particularly large for a research peptide. Not particularly small either. Just the right size to fit perfectly into the binding groove of MHC class I molecules, specifically the mouse H-2Kb allele.
This binding affinity is extraordinary. Among the countless peptide sequences that researchers have tested for MHC binding, SIINFEKL demonstrates one of the strongest and most consistent interactions with H-2Kb. This reliability made it invaluable for research. When scientists needed to study peptide presentation to CD8+ T cells, they needed a system that worked every time. SIINFEKL delivered.
The structural basis of SIINFEKL function
Understanding why SIINFEKL works so well requires examining its structure. The N-terminal half contains two hydrophobic isoleucine residues. The C-terminal half contains two adjacent hydrophilic amino acids, glutamic acid and lysine. This amphipathic arrangement allows the peptide to interact with both the hydrophobic floor and hydrophilic residues of the MHC binding groove.
Interestingly, this structural arrangement also allows SIINFEKL to self-assemble into fibrillar structures similar to other peptide hydrogels. While this property is not relevant for most immunological applications, it highlights the unique biophysical characteristics of this sequence.
The peptide anchors into the MHC groove through specific residue interactions. The phenylalanine at position 5 and the leucine at position 8 serve as primary anchor residues, inserting into pockets within the H-2Kb molecule. The remaining residues are exposed on the surface, creating the molecular signature that T cells recognize.
Historical development
Chicken ovalbumin became a model antigen almost by accident. In the early days of immunology research, scientists needed readily available proteins to study immune responses. Chicken eggs provided an abundant, inexpensive, and easily purified protein source. Ovalbumin emerged as a practical choice.
As technology advanced and researchers began mapping T cell epitopes, SIINFEKL was identified as the immunodominant epitope within ovalbumin for H-2Kb-expressing mice. This meant that when mice with this MHC type encountered ovalbumin, the majority of their CD8+ T cell response targeted this specific eight-amino-acid sequence.
The significance cannot be overstated. Having a dominant epitope simplified experimental design enormously. Researchers could predict which peptide T cells would recognize, monitor that response precisely, and manipulate it systematically. The development of transgenic mice expressing T cell receptors specific for SIINFEKL, known as OT-I mice, amplified this utility exponentially.
The OT-I system: engineering precision into immunology
OT-I transgenic mice represent one of the most powerful tools in modern immunology. Every CD8+ T cell in these mice expresses an identical T cell receptor that recognizes SIINFEKL presented on H-2Kb. This uniformity eliminates the heterogeneity that normally complicates immune response studies.
Think about what this means.
In a normal mouse, millions of different T cell receptors exist, each recognizing different peptide-MHC combinations. Studying the response to any given antigen requires finding the rare cells that respond, tracking them through complex mixtures, and accounting for the diversity in their behavior. With OT-I mice, every CD8+ T cell is functionally identical. The response to SIINFEKL is predictable, measurable, and reproducible.
Applications of OT-I cells
Researchers use OT-I cells in several key experimental approaches. Adoptive transfer involves isolating OT-I T cells and injecting them into wild-type recipient mice. This allows scientists to track a known population of antigen-specific cells through an immune response without the genetic modifications of the donor mice complicating interpretation.
The standard protocol transfers approximately 1 to 2 million OT-I cells into a recipient mouse, which then receives an immunization containing SIINFEKL peptide or ovalbumin protein. Researchers can label the transferred cells with fluorescent dyes to track their proliferation and movement through tissues.
This system has revealed fundamental principles about T cell biology. How do T cells expand after encountering their cognate antigen? How long do they survive? Where do they go? What signals do they need? The OT-I system provided answers to these questions that were impossible to obtain through other methods.
The importance of adjuvants
Simply injecting SIINFEKL peptide does not guarantee a productive immune response. In fact, peptide alone often induces tolerance rather than immunity. This seemingly counterintuitive finding has major implications for peptide therapy development and vaccine design.
Effective SIINFEKL immunization requires adjuvants, substances that activate the innate immune system and provide danger signals. Common choices include lipopolysaccharide (LPS), CpG oligonucleotides, or poly I:C. These molecules signal to the immune system that the peptide represents a threat worth responding to, not just an innocuous environmental protein.
Without adjuvant, T cells that encounter SIINFEKL may become anergic, a state of functional unresponsiveness. This anergy mechanism normally prevents autoimmune responses to self-proteins but presents challenges for peptide-based therapeutics.
Safety considerations with OT-I mice
A critical warning for researchers: injecting SIINFEKL peptide directly into OT-I mice can be lethal. Because virtually every T cell in these animals recognizes SIINFEKL, the peptide triggers massive, simultaneous activation of the entire T cell compartment. The resulting cytokine storm can kill the mouse within minutes.
This extreme response highlights the power of antigen-specific T cell activation and underscores why controlled experimental conditions matter. It also provides insight into certain rare but severe immune reactions that can occur when the immune system mounts an overwhelming response to an antigen.
MHC class I antigen presentation
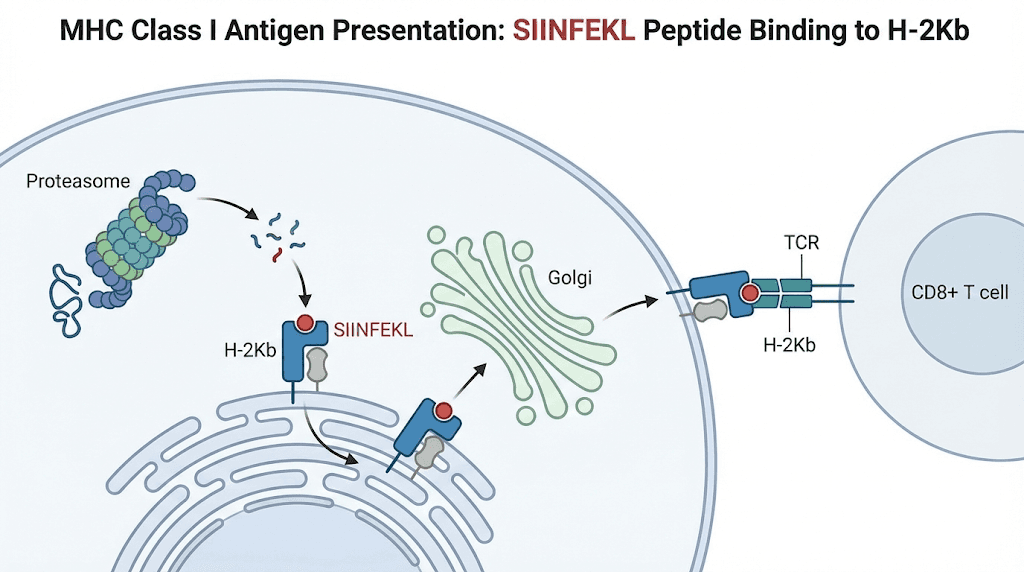
Understanding SIINFEKL requires understanding MHC class I antigen presentation, the process by which cells display peptide fragments on their surface for T cell inspection. This surveillance system allows the immune system to detect intracellular infections and malignancies.
The pathway from protein to presented peptide
When cells synthesize proteins, a fraction of those proteins gets diverted to the proteasome, a large protein complex that chops proteins into short peptide fragments. Some of these fragments have the right properties to bind MHC class I molecules.
Suitable peptides get transported into the endoplasmic reticulum by a dedicated transporter called TAP. Inside the ER, they encounter newly synthesized MHC class I heavy chains bound to beta-2-microglobulin. Peptides with appropriate binding characteristics stabilize the MHC complex, allowing it to traffic through the Golgi apparatus to the cell surface.
On the cell surface, the peptide-MHC complex awaits inspection by CD8+ T cells. If a T cell bearing a receptor specific for that peptide-MHC combination encounters the complex, it can become activated and mount an immune response.
Using SIINFEKL to study antigen presentation
SIINFEKL has become the standard tool for dissecting this pathway. Researchers can incorporate the SIINFEKL sequence into larger proteins, viral vectors, or cellular constructs, then monitor when and how efficiently the peptide appears on the cell surface.
The 25D1.16 monoclonal antibody revolutionized this work. This antibody specifically recognizes SIINFEKL only when bound to H-2Kb. It does not react with empty H-2Kb molecules or H-2Kb presenting different peptides. This specificity allows researchers to directly visualize and quantify SIINFEKL presentation using flow cytometry or microscopy.
Studies using this system have revealed several important principles. Antigen presentation efficiency varies dramatically depending on how the antigen enters the cell. Cytoplasmic expression leads to different presentation kinetics than secreted proteins. Viral infection patterns influence which peptides dominate the response. Pharmacological manipulation of different pathway components affects presentation in distinct ways.
Cross-presentation and dendritic cells
Most cells present peptides derived from their own intracellular proteins. However, certain specialized immune cells, particularly dendritic cells, can present peptides from proteins they acquire externally. This process, called cross-presentation, is essential for initiating immune responses against viruses that do not infect antigen-presenting cells and against tumors.
SIINFEKL has been crucial for understanding cross-presentation. Researchers can feed dendritic cells ovalbumin protein or beads coated with ovalbumin and measure how efficiently SIINFEKL appears on the cell surface. This approach has identified distinct pathways for cross-presentation and clarified the conditions that favor efficient responses.
These findings have direct relevance for peptide vaccine development. Understanding how to optimize cross-presentation could improve vaccine efficacy by ensuring that the peptides reach the surface of antigen-presenting cells in immunogenic contexts.
SIINFEKL in cancer immunotherapy research
Cancer immunotherapy represents one of the most exciting frontiers in medicine, and SIINFEKL has played a starring role in developing our understanding of tumor immunology. The E.G7 tumor model, a lymphoma cell line engineered to express ovalbumin, has become a standard system for testing cancer immunotherapy approaches.
The E.G7 model system
E.G7 cells express ovalbumin as a model tumor antigen, presenting SIINFEKL on their surface. This allows researchers to use OT-I T cells as a defined population of tumor-specific effector cells. The system provides unprecedented control over both the tumor antigen and the responding T cell population.
Early studies established proof of principle. Immunizing mice with SIINFEKL before tumor challenge provided protection against E.G7 growth. This demonstrated that antigen-specific CD8+ T cells could mediate tumor rejection when appropriately activated.
However, therapeutic immunization, treating mice with established tumors, proved more challenging. This difficulty mirrors the clinical situation in cancer patients and has driven extensive research into overcoming tumor-associated immune suppression.
Combination therapy approaches
Modern cancer immunotherapy often combines multiple interventions. SIINFEKL-based studies have contributed significantly to developing these combinations. One particularly promising approach combines SIINFEKL-loaded nanoparticles with checkpoint inhibitors like anti-PD-1 antibodies.
The rationale is elegant. Peptide vaccines activate tumor-specific T cells, while checkpoint inhibitors remove the brakes that tumors use to suppress those T cells. Together, these interventions can achieve therapeutic effects that neither accomplishes alone.
Preclinical studies using PLGA nanoparticles loaded with SIINFEKL, combined with the TLR3 agonist poly-IC and anti-PD-1 antibodies, showed dramatic reductions in tumor growth and improved survival in treated mice. These findings are now informing clinical trial design for human cancer vaccines.
Intratumoral injection strategies
An innovative approach involves injecting peptides directly into tumors. When researchers injected SIINFEKL into ovalbumin-negative tumors, they observed tumor growth inhibition without significant side effects. The mechanism involves loading the peptide onto MHC class I molecules already present on tumor cells, making those cells visible to antigen-specific T cells.
This approach, called epitope spreading or peptide decoration, could allow immunotherapy to target tumors that do not naturally express the vaccine antigen. The SIINFEKL studies provided proof of concept that is now being explored with tumor-specific neoantigens.
Limitations discovered through SIINFEKL research
Not all lessons from SIINFEKL research are optimistic. Studies have revealed significant challenges in translating peptide vaccines from prevention to therapy.
Immunization with SIINFEKL effectively protects tumor-free mice from subsequent E.G7 challenge. However, therapeutic immunization of tumor-bearing mice shows much weaker effects. The tumor microenvironment suppresses T cell function, and tumor-induced tolerance limits the magnitude of vaccine responses.
These findings have redirected the field toward combination approaches and understanding tumor-intrinsic immune evasion mechanisms. While sobering, this knowledge is essential for developing effective cancer immunotherapies.
Vaccine development applications
Beyond cancer, SIINFEKL has informed vaccine development for infectious diseases. The principles discovered using this model antigen apply broadly to designing peptide-based vaccines against pathogens.
Adjuvant development
Peptide vaccines face a fundamental challenge: peptides alone are poor immunogens. They are rapidly degraded by enzymes in the body, fail to trigger innate immune activation, and often induce tolerance rather than immunity. Overcoming these limitations requires sophisticated adjuvant strategies.
SIINFEKL immunization studies have tested countless adjuvant formulations. Toll-like receptor agonists, emulsions, nanoparticles, and other delivery systems have all been evaluated for their ability to enhance SIINFEKL-specific T cell responses. This systematic comparison has established principles for adjuvant selection that now guide clinical vaccine development.
Key findings include the importance of depot effects, where slow-release formulations extend antigen availability. The value of targeting specific immune cell populations, particularly dendritic cells, has been established. And the timing of adjuvant administration relative to antigen exposure matters critically for response quality.
Memory T cell generation
Effective vaccines must generate long-lasting immune memory. OT-I transfer experiments have mapped the requirements for memory T cell formation with remarkable precision.
Initial activation requires strong T cell receptor signaling plus co-stimulation. The duration and intensity of this signal affects whether cells become short-lived effectors or long-lived memory cells. Cytokine signals, particularly from IL-7 and IL-15, support memory cell survival. And the location of memory cells, whether circulating or tissue-resident, depends on signals received during the response.
These principles, established using SIINFEKL and OT-I cells, now inform the design of vaccines against HIV, tuberculosis, malaria, and emerging infectious diseases. The fundamental biology is the same even when the target antigen differs.
Peptide stability challenges
Free peptides are notoriously unstable in vivo. Proteases degrade them within minutes, limiting their ability to reach antigen-presenting cells in intact form. Peptide stability has emerged as a major focus of vaccine development.
SIINFEKL research has tested various approaches to enhance peptide stability. Formulation in protective nanoparticles can shield peptides from degradation. Chemical modifications like amino acid substitutions or backbone alterations can reduce protease sensitivity. Conjugation to carrier proteins or polymers extends peptide half-life.
One particularly interesting approach involves beta-amino acid substitutions, where the normal alpha-amino acids are replaced with isomers that proteases do not recognize. These modified peptides resist degradation but may alter immune recognition. Studies with SIINFEKL analogs containing beta-amino acids have shown complex effects on T cell responses, highlighting the need for careful optimization.
Flow cytometry and tetramer technology
Detecting and tracking antigen-specific T cells requires specialized tools. MHC tetramers, developed in the 1990s, revolutionized this field, and SIINFEKL-loaded tetramers were among the first and most widely used reagents.
How tetramers work
MHC tetramers consist of four MHC-peptide complexes linked together via a streptavidin backbone. Individual T cell receptors bind MHC-peptide with relatively low affinity, but the tetramer configuration provides avidity, allowing stable binding to T cells expressing the appropriate receptor.
Fluorescently labeled tetramers can be mixed with cell samples and analyzed by flow cytometry. Cells that bind the tetramer, indicating they express a receptor specific for that peptide-MHC combination, appear as a distinct positive population.
H-2Kb-SIINFEKL tetramers detect OT-I T cells with exquisite sensitivity and specificity. They have been used in thousands of studies to track antigen-specific T cell responses in infection, vaccination, autoimmunity, and cancer models.
Technical considerations
Successful tetramer staining requires attention to several technical details. Cell viability matters critically since dead cells often show non-specific tetramer binding. Temperature affects binding kinetics, with room temperature typically providing optimal staining. And proper controls are essential for distinguishing true positive signals from background.
The standard protocol involves incubating cells with tetramer for 10 to 30 minutes, followed by antibody staining for surface markers like CD8 to identify the T cell population. Analysis gates on viable lymphocytes, then identifies tetramer-positive cells within the CD8+ population.
For detecting very rare populations, additional enrichment steps may be necessary. Magnetic bead selection or fluorescence-activated cell sorting can concentrate tetramer-positive cells before analysis. The detection limit approaches 0.02 percent of total T cells with optimized protocols.
Dextramer and other multimer formats
Beyond tetramers, other multimer formats provide advantages for certain applications. Dextramers use a dextran backbone to display multiple MHC-peptide complexes, providing even higher avidity than tetramers. Pentamers and streptamers offer different trade-offs between avidity and reversibility.
SIINFEKL has been produced in all these formats, allowing direct comparison of their properties. Generally, higher-order multimers provide stronger signals but may detect lower-affinity T cells that tetramers miss. The choice depends on the experimental question and the T cell population being studied.
Cross-reactivity and molecular mimicry
One of the most surprising discoveries from SIINFEKL research involves cross-reactivity. T cells that recognize SIINFEKL can sometimes respond to completely different peptides, a phenomenon with important implications for understanding autoimmunity and infection.
Brucella cross-reactivity
Researchers engineering Brucella bacteria to express ovalbumin made an unexpected observation. OT-I T cells, which should only recognize SIINFEKL, also responded to Brucella bacteria that did not express ovalbumin at all. Something in native Brucella was activating these supposedly SIINFEKL-specific T cells.
Further investigation revealed that Brucella presents peptides with sufficient similarity to SIINFEKL to trigger OT-I T cell activation. This molecular mimicry, where pathogen-derived peptides resemble self-peptides or model antigens, is thought to contribute to autoimmune diseases when immune responses against pathogens inadvertently target self tissues.
The OT-I-Brucella system now provides a model for studying molecular mimicry in a controlled setting. Researchers can identify the mimicking peptides, characterize the T cell response, and test interventions that might distinguish beneficial antimicrobial immunity from harmful cross-reactive responses.
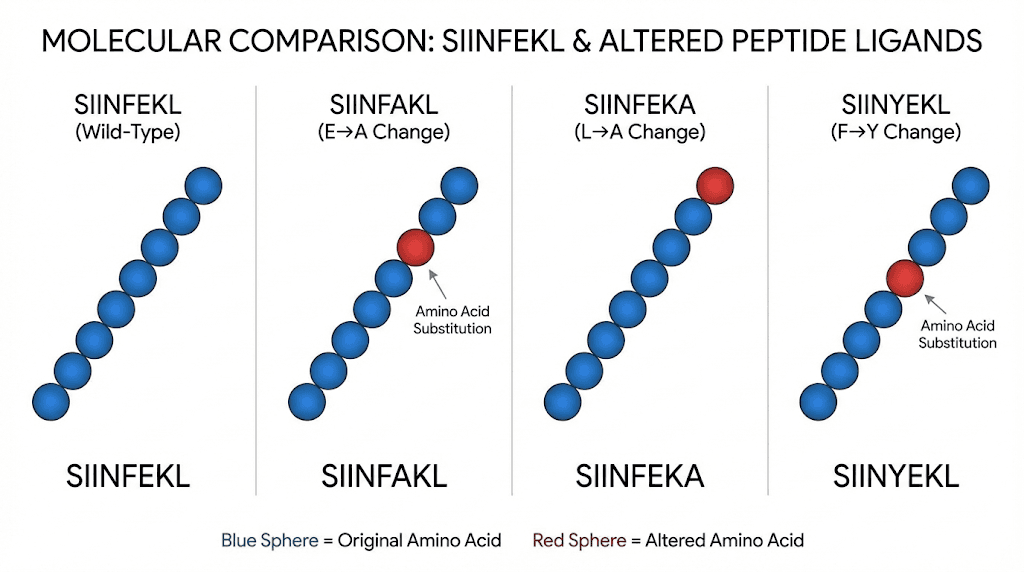
Altered peptide ligands
Systematic studies with altered peptide ligands, variants of SIINFEKL with single amino acid substitutions, have revealed how small changes affect T cell recognition. The OT-I T cell receptor tolerates some substitutions but not others, with effects ranging from unchanged activation to complete loss of recognition to antagonism.
Five well-characterized variants differing in their binding affinity to the OT-I receptor serve as a standard panel for studying how T cell receptor affinity affects response characteristics. Low-affinity variants activate T cells but induce different functional programs than high-affinity variants. These studies have implications for understanding T cell responses to tumor neoantigens, which often differ by single amino acids from self-peptides.
Implications for immunotherapy
Cross-reactivity can be both helpful and harmful for immunotherapy. On one hand, T cells activated against one tumor antigen might cross-react with other tumor cells, broadening the therapeutic effect. On the other hand, cross-reactivity with self-peptides could cause autoimmune side effects.
SIINFEKL research has provided tools for studying these phenomena systematically. Researchers can test whether T cells activated against specific tumor antigens show concerning cross-reactivity profiles before advancing therapies to clinical trials.
Practical considerations for researchers
Working with SIINFEKL requires attention to practical details that can determine experimental success or failure. This section covers key considerations for obtaining, handling, and using SIINFEKL in research.
Sources and specifications
Multiple commercial suppliers offer synthesized SIINFEKL peptide. Quality specifications typically include purity greater than 85 percent determined by HPLC, with endotoxin levels below acceptable thresholds for immunological applications. Pricing varies considerably, from approximately $250 for 5 mg quantities to lower per-milligram costs for larger orders.
Major suppliers include JPT Peptide Technologies, LifeTein, NovoPro Labs, and IBA Life Sciences. Each offers the standard sequence and various modified versions, including biotinylated forms for streptavidin-based applications and fluorophore-conjugated versions for microscopy or flow cytometry.
Custom synthesis services allow researchers to obtain SIINFEKL variants or larger quantities tailored to specific needs. When ordering custom peptides, specifying the purity requirements, endotoxin testing, and any required modifications upfront avoids delays and ensures the product meets experimental needs.
Storage and handling
Lyophilized SIINFEKL peptide should be stored at or below minus 20 degrees Celsius. Under proper storage conditions, the peptide remains stable for at least 12 months. Repeated freeze-thaw cycles should be avoided by aliquoting the peptide into single-use portions after initial reconstitution.
Reconstitution typically uses sterile water, phosphate-buffered saline, or DMSO depending on the application. For in vivo use, sterile, endotoxin-free solutions are essential. Working concentrations for most applications range from micrograms per milliliter to milligrams per milliliter depending on the specific protocol.
Proper peptide storage principles apply to SIINFEKL as they do to other research peptides. Light exposure, temperature fluctuations, and microbial contamination can all degrade peptide quality and compromise experimental results.
Common applications and protocols
Standard SIINFEKL applications include T cell stimulation assays, antigen presentation studies, and in vivo immunization experiments. Each application has optimized protocols that have been refined over decades of use.
For in vitro T cell stimulation, SIINFEKL concentrations typically range from nanomolar to micromolar. Dose-response experiments are advisable when establishing new protocols since optimal concentrations vary depending on T cell source, culture conditions, and readout method.
In vivo immunization protocols typically use 25 to 100 micrograms of SIINFEKL per mouse, combined with appropriate adjuvant. The route of administration, whether subcutaneous, intradermal, or intraperitoneal, affects the character of the resulting immune response. Subcutaneous immunization generally favors systemic responses, while intradermal administration can enhance dendritic cell targeting.
Controls and validation
Rigorous experimental design requires appropriate controls. Negative controls typically include irrelevant peptides of similar characteristics that do not bind H-2Kb or bind but are not recognized by the T cells being studied. The SIYRYYGL peptide from vesicular stomatitis virus is a common choice as an irrelevant H-2Kb-binding peptide.
Positive controls depend on the specific application but might include purified OT-I T cells for stimulation assays or validated tetramer-positive cell populations for flow cytometry. Running both positive and negative controls with each experiment ensures that observed results reflect the intended biology rather than technical artifacts.
Beyond SIINFEKL: other model antigen systems
While SIINFEKL dominates the literature, other model antigen systems provide complementary tools for immunological research. Understanding these alternatives helps researchers choose the right system for their specific questions.
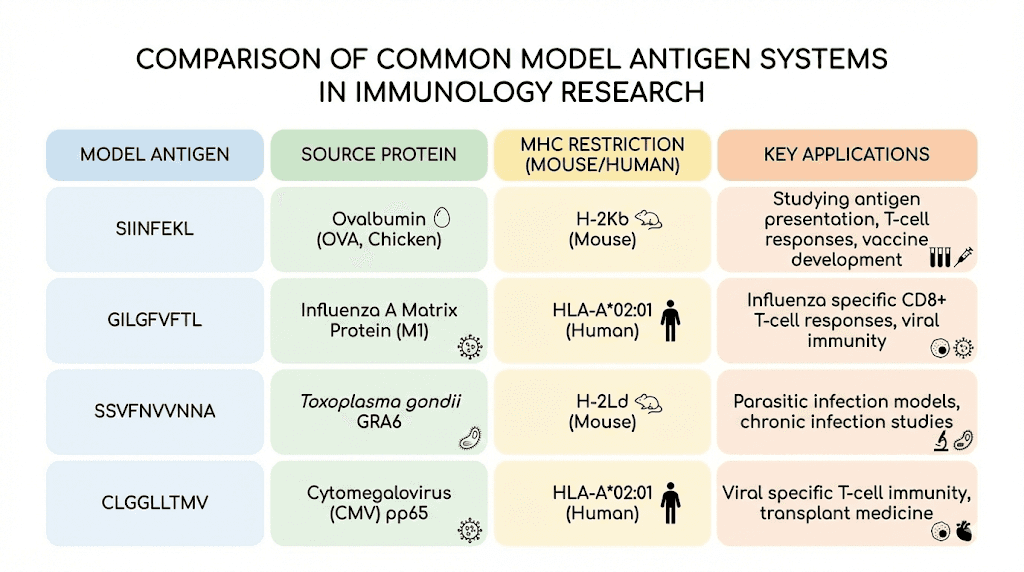
Human HLA systems
SIINFEKL binds mouse MHC molecules, limiting its direct relevance to human immunology. For studies requiring human-relevant systems, viral epitopes like those from influenza, HIV, or cytomegalovirus provide well-characterized alternatives.
The influenza matrix protein M1 58-66 peptide (GILGFVFTL) presented on HLA-A2 is among the most extensively studied human epitopes. Like SIINFEKL in the mouse system, it serves as a standard for developing and validating reagents and protocols.
Humanized mouse models expressing human HLA molecules can sometimes use human epitopes directly, bridging the gap between mouse model systems and human relevance. These systems are increasingly important for preclinical development of therapeutic peptides.
Other mouse model antigens
Beyond ovalbumin, other model antigens provide different advantages. Lymphocytic choriomeningitis virus (LCMV) epitopes offer well-defined systems for studying viral infection and memory T cell formation. Listeria monocytogenes antigens model bacterial infection. And tumor-associated antigens from melanoma or other cancers provide systems closer to clinical cancer immunotherapy.
Each system has accumulated its own body of literature, reagents, and protocols. The choice among them depends on the biological question being addressed and the resources available in a given laboratory.
Neoantigen discovery platforms
Modern cancer immunotherapy increasingly focuses on neoantigens, peptides derived from tumor-specific mutations that the immune system can distinguish from self. While these are not model antigens in the traditional sense, the principles established through SIINFEKL research underpin neoantigen identification and validation.
Mass spectrometry-based identification of MHC-presented peptides, algorithms predicting peptide-MHC binding, and T cell assays for validating immunogenicity all build on foundations established using SIINFEKL. The model antigen paved the way for personalized cancer vaccines targeting patient-specific neoantigens.
Emerging research directions
SIINFEKL research continues to yield new insights despite decades of study. Several emerging areas are expanding our understanding of fundamental immunology and pointing toward novel therapeutic applications.
Single-cell technologies
Single-cell RNA sequencing and related technologies are revolutionizing immunology by revealing heterogeneity within what were previously considered uniform cell populations. Studies using OT-I T cells are mapping the transcriptional programs that distinguish different T cell fates with unprecedented resolution.
Even within a clonal T cell population responding to a single antigen, individual cells diverge into distinct functional states. Some become effector cells optimized for immediate pathogen killing. Others differentiate into memory precursors that will provide long-term protection. Understanding what determines these fate decisions could improve vaccine design and adoptive cell therapy.
Metabolic immunology
The metabolic requirements of T cells during activation, effector function, and memory formation have become a major research focus. SIINFEKL-based studies have contributed to understanding how nutrients and metabolic pathways regulate immune responses.
Activated T cells require massive increases in glucose uptake and glycolysis to fuel proliferation and effector function. Memory T cells, in contrast, rely more heavily on fatty acid oxidation. Manipulating these metabolic programs could enhance vaccine responses or improve the persistence of adoptively transferred T cells.
Spatial immunology
Where T cells encounter antigen profoundly affects the resulting response. SIINFEKL studies using intravital microscopy and tissue imaging have mapped T cell-dendritic cell interactions with remarkable spatial resolution.
The duration and location of these interactions influence T cell differentiation. Brief contacts may be sufficient for activation but longer interactions appear necessary for optimal memory formation. The tissue context, whether lymph node, spleen, or peripheral tissue, also shapes T cell fate through local cytokine and metabolic cues.
Engineering improved responses
Armed with detailed understanding of T cell activation requirements, researchers are engineering systems to optimize immune responses. Synthetic biology approaches create artificial antigen-presenting cells displaying SIINFEKL along with precisely controlled co-stimulatory signals.
These systems test hypotheses about which signals are necessary and sufficient for specific T cell responses. They also provide manufacturing platforms for expanding therapeutic T cells under defined conditions, potentially improving consistency and efficacy of cell-based therapies.
Applications across peptide research
The principles discovered through SIINFEKL research extend far beyond this single peptide sequence. Understanding how the immune system recognizes, responds to, and remembers peptides has implications for diverse areas of peptide science.
Peptide drug development
Any peptide introduced into the body faces potential recognition by the immune system. Immunogenicity can limit therapeutic efficacy through neutralizing antibodies or cause dangerous hypersensitivity reactions. SIINFEKL research has established principles for predicting and minimizing these risks.
Peptides containing sequences that bind efficiently to MHC molecules and resemble foreign proteins carry higher immunogenicity risk. Computational tools can scan candidate therapeutic peptides for potential T cell epitopes, allowing problematic sequences to be modified during drug development. These principles apply whether developing bioregulator peptides, nutritional peptides, or therapeutic compounds.
Understanding peptide mechanisms
The detailed molecular understanding of SIINFEKL-MHC-T cell receptor interactions provides a template for understanding how other peptides exert their effects. While most therapeutic peptides do not directly engage T cells, the principles of molecular recognition, binding kinetics, and structural requirements apply broadly.
Peptide mechanisms often involve binding to cellular receptors, and the SIINFEKL system illustrates how small changes in peptide sequence can dramatically alter binding affinity and downstream effects. These lessons inform structure-activity relationship studies across peptide classes.
Quality control and validation
SIINFEKL provides a gold-standard control for peptide quality assessment. Laboratories can use SIINFEKL-based assays to validate their immunological techniques, ensuring that equipment and reagents perform as expected before testing novel peptides.
The extensive literature on SIINFEKL provides benchmark data for comparison. If a new assay gives expected results with SIINFEKL, researchers can have confidence in results obtained with other peptides using the same system.
The bigger picture: why model antigens matter
SIINFEKL is more than a convenient experimental tool. It represents an approach to science that has proven remarkably productive, the use of simplified model systems to understand complex biological processes.
Reductionism in immunology
The immune system is staggeringly complex. Billions of cells, thousands of receptors, countless interactions, and emergent behaviors that resist simple explanation. Studying this system in its full complexity would be impossible without simplifying strategies.
Model antigens like SIINFEKL provide controlled variables in an otherwise variable system. When researchers use SIINFEKL and OT-I cells, they know exactly which peptide the T cells will recognize and can predict with confidence how they will respond. This predictability enables precise questions about specific aspects of immune function.
The knowledge gained from simplified systems then informs understanding of more complex situations. Principles of T cell activation, memory formation, and effector function discovered using SIINFEKL apply to responses against viruses, bacteria, and tumors even though those systems involve vastly more complexity.
Translation to human health
Model systems face criticism that findings may not translate to clinical relevance. This concern is legitimate but often overstated. The fundamental biology of T cell activation is conserved between mice and humans. While specific details differ, the general principles hold.
Checkpoint inhibitor therapy, one of the most important advances in cancer treatment, emerged from basic research that included extensive SIINFEKL-based studies. Understanding how T cells could be suppressed or activated in controlled systems paved the way for manipulating these pathways therapeutically.
Similarly, vaccine development benefits from principles established using model antigens. The requirements for effective immunization, including antigen stability, adjuvant co-administration, delivery to appropriate immune cells, and generation of durable memory, were systematically characterized using systems like SIINFEKL. These principles now guide development of vaccines against COVID-19, cancer, and other diseases.
Continuing relevance
After decades of study, SIINFEKL might seem played out. Every obvious question has been asked. Every basic principle has been established. Yet new technologies continue to reveal aspects of SIINFEKL biology that were previously invisible.
Single-cell technologies are showing that T cell populations once considered homogeneous actually contain distinct subsets with different fates and functions. Spatial technologies are mapping interactions at the subcellular level. Computational approaches are extracting new insights from accumulated data. SIINFEKL remains the substrate for discoveries we cannot yet anticipate.
Frequently asked questions
What does SIINFEKL stand for?
SIINFEKL is the single-letter amino acid code for the peptide sequence serine-isoleucine-isoleucine-asparagine-phenylalanine-glutamic acid-lysine-leucine. It is derived from chicken ovalbumin, spanning residues 257-264 of the full protein. The sequence is recognized by cytotoxic T lymphocytes when presented on mouse MHC class I H-2Kb molecules.
Why is SIINFEKL used in immunology research?
SIINFEKL became the dominant model antigen in immunology because it binds H-2Kb MHC molecules with exceptional reliability and was the first well-characterized dominant epitope with transgenic T cell receptor mice available. The combination of defined peptide, defined MHC, and defined T cells created an unparalleled system for studying antigen-specific immune responses.
Can SIINFEKL be used in human studies?
SIINFEKL binds mouse MHC class I molecules, specifically H-2Kb, and is not directly applicable to human studies. Human immunology research uses epitopes that bind human HLA molecules, such as influenza or CMV-derived peptides. However, the principles established using SIINFEKL inform human vaccine and immunotherapy development.
What are OT-I mice?
OT-I mice are transgenic animals in which all CD8+ T cells express a single T cell receptor specific for SIINFEKL presented on H-2Kb. This uniformity eliminates the diversity that complicates immune response studies, allowing precise tracking and manipulation of antigen-specific T cells.
How stable is SIINFEKL peptide?
Lyophilized SIINFEKL peptide is stable for at least 12 months when stored at minus 20 degrees Celsius. Reconstituted peptide should be aliquoted to avoid repeated freeze-thaw cycles and used within recommended timeframes. In vivo, free peptide is rapidly degraded by proteases, which is why formulation strategies are important for peptide-based therapeutics.
What is a SIINFEKL tetramer?
A SIINFEKL tetramer consists of four H-2Kb-SIINFEKL complexes linked through streptavidin. This configuration provides sufficient binding avidity to stably label T cells expressing receptors specific for the peptide-MHC combination. Fluorescently labeled tetramers allow detection and quantification of antigen-specific T cells by flow cytometry.
Can SIINFEKL cause an immune response in humans?
While SIINFEKL does not efficiently bind human MHC molecules, any foreign peptide could potentially be processed and presented. However, the specific immune dominance seen in H-2Kb mice would not apply. Human immune responses to chicken ovalbumin involve different epitopes presented on human HLA molecules.
What concentration of SIINFEKL should I use for T cell assays?
Optimal SIINFEKL concentrations depend on the specific application. In vitro T cell stimulation typically uses nanomolar to low micromolar concentrations, with dose-response experiments recommended for new protocols. In vivo immunization protocols typically employ 25 to 100 micrograms per mouse combined with appropriate adjuvant.
For researchers seeking to understand the foundations of peptide immunology or exploring how the immune system interacts with peptides, SeekPeptides provides comprehensive resources covering everything from basic peptide concepts to advanced protocol optimization. Our growing peptide database helps researchers navigate the expanding landscape of bioactive sequences.



