Jan 29, 2026
You have been researching BPC-157 for weeks. Maybe months. The studies look promising. The mechanism makes sense. Tissue repair. Gut healing. Accelerated recovery. But then you hit the wall that stops most people cold.
Injections.
The thought of reconstituting peptides with bacteriostatic water, calculating doses, and self-administering subcutaneous injections feels overwhelming. You start looking for alternatives. And that is when Integrative Peptides appears in your search results.
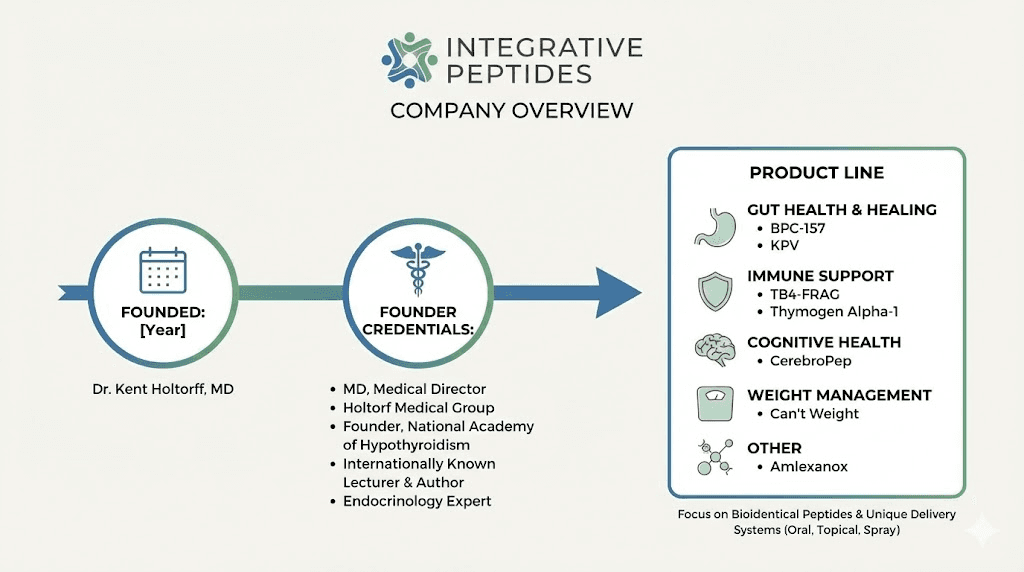
Founded in 2018 by Dr. Kent Holtorf, Integrative Peptides emerged from a simple observation. People needed access to quality peptides without the complexity, cost, and discomfort of injectable protocols. Before this company existed, your options were limited. Pay hundreds for compounded prescriptions. Risk buying research chemicals with questionable purity. Or simply go without. None of those paths felt right.
This guide examines Integrative Peptides BPC-157 products in detail. What they contain. How they work. Whether oral peptides can actually deliver the benefits that injectable protocols provide. The science behind bioavailability. Real user experiences. And most importantly, whether these supplements represent a legitimate option for those exploring BPC-157 peptide therapy.
SeekPeptides members frequently ask about oral alternatives to injectable peptides. The convenience factor matters. So does the question of effectiveness. By the end of this guide, you will understand exactly what Integrative Peptides offers and whether it aligns with your research goals.
Who is Integrative Peptides
Understanding a supplement company requires looking beyond their marketing. Integrative Peptides was founded by Dr. Kent Holtorf, a physician who graduated from UCLA School of Medicine. He serves as medical director of the Holtorf Medical Group and founded the non-profit National Academy of Hypothyroidism. His background brings medical credibility to a supplement brand operating in a largely unregulated space.
The company positions itself as a bridge. On one side, you have compounding pharmacies producing prescription peptides that cost hundreds of dollars and require physician oversight. On the other side, you have the gray market of research chemical suppliers selling products explicitly labeled not for human consumption. Integrative Peptides occupies the middle ground. Dietary supplements. Oral formulations. Quality testing claims. Accessible pricing.
Their flagship product remains BPC-157 Pure, but the product line has expanded significantly. TB4-Frag offers an oral form of the Thymosin Beta 4 active fragment. KPV provides the anti-inflammatory peptide in capsule and spray formats. CerebroPep delivers a porcine-derived peptide blend for cognitive support. Thymogen Alpha-1 targets immune function. Each product follows the same philosophy. Take peptides that traditionally required injection and make them accessible through oral delivery.
Manufacturing occurs in the United States under FDA-compliant conditions. The company claims each batch undergoes testing to confirm 99% or greater purity. Products are screened for heavy metals, microbials, and common contaminants. GMP certification applies to their facilities. These quality claims matter because peptide purity directly affects both safety and efficacy.
Understanding BPC-157 before evaluating products
BPC-157 is a pentadecapeptide. Fifteen amino acids arranged in a specific sequence derived from human gastric juice. The body produces a larger protein called Body Protection Compound naturally. Researchers isolated this fragment and discovered it retained remarkable regenerative properties when administered externally.
The peptide accelerates healing through multiple mechanisms. It promotes angiogenesis, the formation of new blood vessels that deliver nutrients to damaged tissue. It upregulates growth factors including VEGF and EGF. It modulates the nitric oxide system. It influences the dopamine and serotonin pathways. The peptide mechanism works at fundamental levels of tissue repair and systemic regulation.
Research in animal models shows impressive results. Tendons heal faster. Ligaments regenerate more completely. Gut lining repairs. Muscle tissue recovers. Bone fractures mend more quickly. The studies span decades and cover diverse injury types. While human clinical trials remain limited, the preclinical evidence creates strong theoretical support for therapeutic applications.
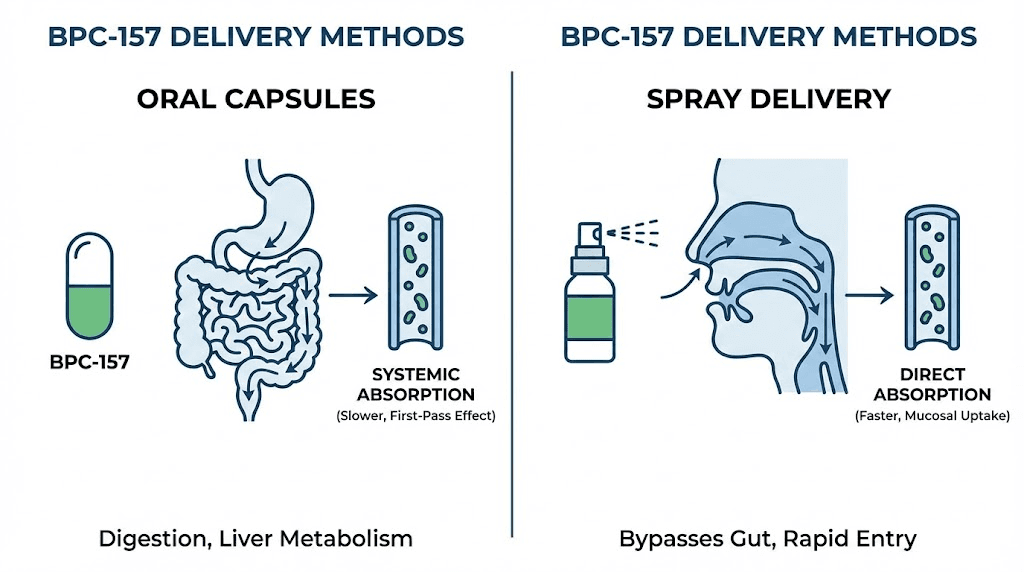
Traditional BPC-157 administration uses subcutaneous injection. The peptide enters the bloodstream directly, bypassing the digestive system. Bioavailability approaches 100%. The active compound reaches target tissues intact. This route of administration represents the gold standard for peptide delivery.
Oral administration introduces complications. Peptides are proteins. The digestive system exists specifically to break down proteins into individual amino acids for absorption. Stomach acid denatures protein structures. Digestive enzymes cleave peptide bonds. By the time an orally consumed peptide reaches the small intestine, most of it has been destroyed.
This is the fundamental challenge Integrative Peptides must address. Can their formulations protect BPC-157 through the digestive tract and deliver meaningful amounts to target tissues? The answer determines whether their products offer genuine therapeutic potential or simply expensive amino acids.
Integrative Peptides BPC-157 product line
The company offers BPC-157 in multiple formulations, each designed to address the bioavailability challenge through different mechanisms. Understanding these options helps you choose the right product for your specific goals.
BPC-157 Pure delayed release capsules
The flagship product contains 500 mcg of BPC-157 per capsule. The delayed release formulation uses a specialized vegetable capsule designed to resist stomach acid. Rather than dissolving immediately upon contact with gastric fluid, the capsule protects its contents until reaching the small intestine.
This approach makes theoretical sense. The small intestine has lower acidity than the stomach. Enzymatic activity differs. Some peptide absorption can occur through intestinal epithelial cells. By bypassing the harsh stomach environment, more intact peptide molecules theoretically survive to reach absorptive tissues.
The formulation includes D-Ribose at 25 mg per capsule. This simple sugar supports cellular energy production and may enhance absorption. Other ingredients include microcrystalline cellulose and silicon dioxide as inactive components.
Standard dosing suggests one capsule twice daily on an empty stomach. The empty stomach requirement matters because food presence affects gastric pH and transit time. Taking the capsule between meals optimizes the conditions for successful delivery past the stomach.
Pricing runs approximately $70-85 for 60 capsules, translating to roughly $2.50-3.00 per day at the standard dose. This compares favorably to compounded injectable BPC-157 from pharmacies, which often costs $200-400 for similar durations of therapy.
BPC-157 Pure oral spray
The oral spray represents a different delivery strategy. Rather than protecting the peptide through the stomach, this formulation aims for sublingual and buccal absorption. The thin tissues under the tongue and inside the cheeks allow certain compounds to enter the bloodstream directly.
Each spray delivers 500 mcg of BPC-157 in a peppermint-flavored solution. Users hold the liquid under their tongue for 30-60 seconds before swallowing. During this contact time, some peptide molecules may cross the mucosal membrane and enter systemic circulation without passing through the digestive tract.
This absorption route has precedent. Certain medications use sublingual delivery specifically to bypass first-pass metabolism in the liver. Nitroglycerin for chest pain. Some hormone preparations. The question is whether BPC-157 molecular characteristics allow meaningful absorption through this pathway.
The spray format offers convenience advantages. No swallowing large capsules. No timing around meals. The product travels easily. The peppermint flavoring makes the experience more pleasant than unflavored peptide solutions.
Pricing sits higher at approximately $140 per bottle, reflecting the more complex formulation and delivery system. Each bottle contains 30 servings at the standard dose.
BPC-157 immediate release capsules
For those who prefer simpler formulations, Integrative Peptides offers immediate release capsules. These dissolve normally in the stomach rather than using delayed release technology. The rationale focuses on local gut effects rather than systemic distribution.
BPC-157 shows particular promise for gastrointestinal healing. It protects stomach lining. It accelerates repair of intestinal epithelium. It modulates inflammatory responses in the gut. For these localized applications, systemic absorption may matter less than direct contact with digestive tissues.
Users dealing with gut issues including leaky gut syndrome, gastritis, or intestinal inflammation might find immediate release formulations appropriate. The peptide releases directly into the gastric environment where it can exert local protective effects before degradation occurs.
Pricing matches the delayed release version at approximately $70-85 for 30 capsules, though the serving count differs between products.
The oral bioavailability question
This is the central issue. Does oral BPC-157 actually work? The answer is more nuanced than a simple yes or no.
Studies specifically examining oral BPC-157 bioavailability in humans do not exist in peer-reviewed literature. The clinical research that established BPC-157 therapeutic potential used injectable administration. This creates a significant gap between what we know works and what these oral products claim to deliver.
However, several factors suggest oral BPC-157 may retain meaningful activity.
First, the peptide demonstrates unusual stability compared to most proteins. Its amino acid sequence and structural characteristics provide some resistance to degradation. While not immune to digestive breakdown, BPC-157 survives longer than many peptides would under similar conditions.
Second, some absorption of intact peptides does occur through the intestinal barrier. The extent varies dramatically between different peptide sequences, but the phenomenon exists. Certain proteins and peptides cross intestinal epithelium through transcytosis and other transport mechanisms.
Third, local effects in the gut may not require systemic absorption at all. If your goal involves gastrointestinal healing, the peptide contacting intestinal tissues directly could provide benefit even if it never reaches the bloodstream.
Fourth, the delayed release and sublingual formulations specifically address the degradation problem. Whether these approaches work as intended remains uncertain, but the theoretical basis makes sense.
The honest answer is that oral BPC-157 probably delivers less systemic peptide than equivalent injectable doses. How much less remains unknown. Whether the delivered amount crosses the threshold for therapeutic effect in any given individual also remains unknown. These products exist in a zone of scientific uncertainty.
What user experiences suggest
Anecdotal reports from Integrative Peptides customers show mixed results, as you would expect given the bioavailability uncertainty.
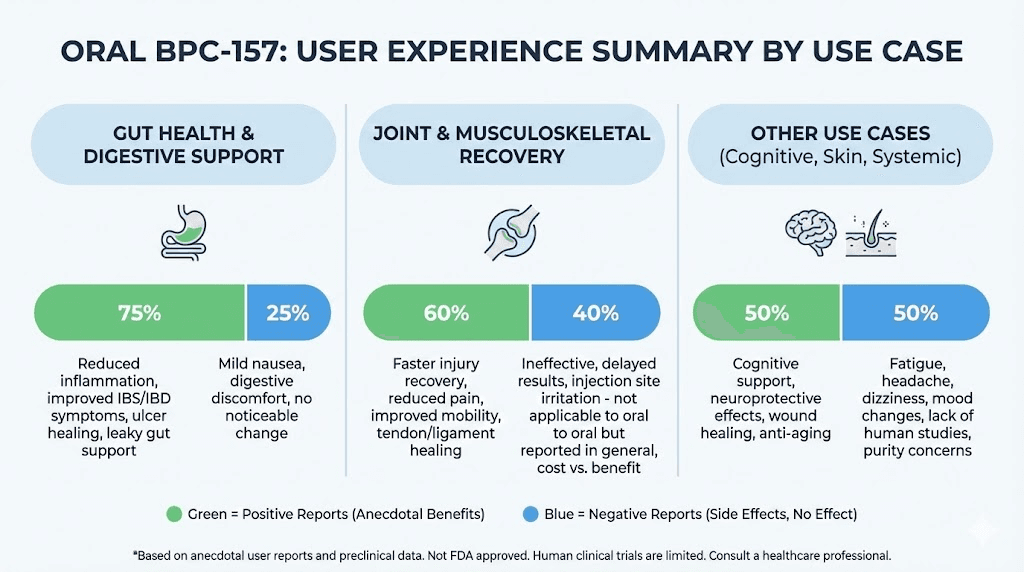
Positive experiences cluster around specific use cases. Gut healing receives the most favorable reports. Users describe improvements in digestive symptoms, reduced inflammation markers, and resolution of chronic gastrointestinal issues. These outcomes align with the local effect theory. The peptide contacting gut tissues directly may produce benefits regardless of systemic absorption levels.
One reviewer stated that BPC-157 Pure healed esophageal damage from acid reflux when conventional treatments had failed. Another described resolution of chronic H. pylori symptoms. A third reported reduced inflammation from inflammatory bowel conditions. These testimonials focus on digestive applications where oral delivery makes intuitive sense.
Reports on systemic healing show more variability. Some users describe reduced joint pain, faster recovery from injury, and improved tissue healing. Others report no noticeable effects despite weeks of consistent use. This pattern suggests that oral bioavailability may vary significantly between individuals and that systemic effects require adequate absorption that not everyone achieves.
Negative experiences include occasional reports of gastrointestinal upset, particularly nausea and dizziness. One reviewer described severe symptoms including vomiting and headache after taking a single capsule. These reactions appear rare based on the overall review distribution but warrant mention.
The price point generates complaints from some users who feel the products are expensive relative to research peptides available elsewhere. However, those comparison products lack the quality testing and manufacturing standards that Integrative Peptides claims to maintain.
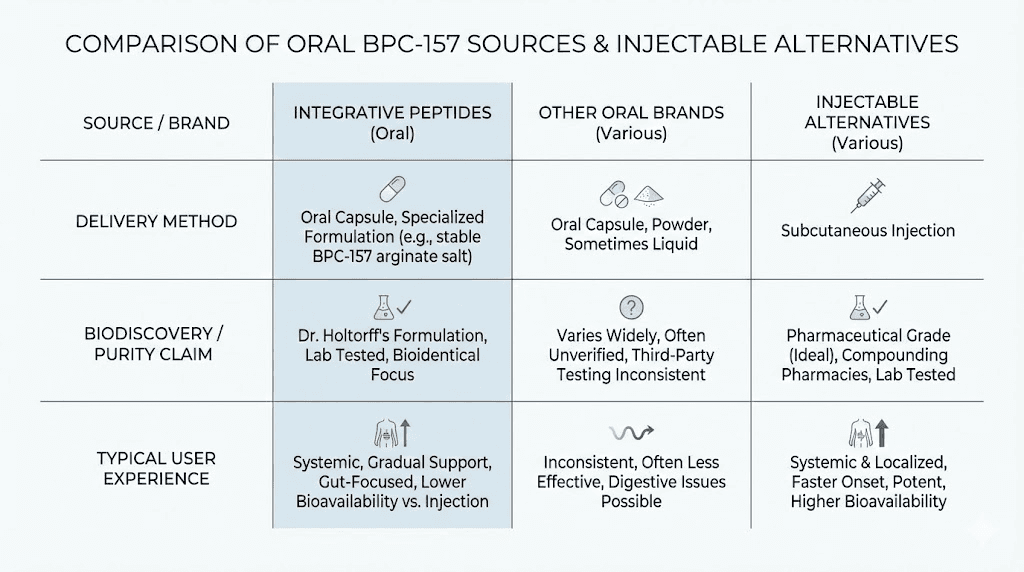
Comparing oral to injectable BPC-157
For researchers accustomed to injectable peptide protocols, understanding the trade-offs between oral and injectable BPC-157 helps inform product selection.
Bioavailability differences
Injectable BPC-157 delivers essentially 100% of the administered dose to systemic circulation. The peptide enters the bloodstream intact and distributes throughout the body. Dosing calculations translate directly to therapeutic exposure.
Oral BPC-157 delivers an unknown percentage of the administered dose. Estimates based on peptide pharmacology research suggest perhaps 1-10% survives to reach systemic circulation, though this number represents speculation rather than measured data for BPC-157 specifically. The delayed release and sublingual formulations may improve this percentage, but by how much remains uncertain.
This bioavailability gap means oral dosing requires higher amounts to achieve equivalent systemic exposure. A 500 mcg oral dose might deliver the biological equivalent of 50 mcg or less via injection. Or it might deliver more. Without direct comparison studies, precise translation remains impossible.
Convenience factors
Oral administration wins decisively on convenience. Swallow a capsule or spray under your tongue. No refrigeration of reconstituted peptides. No syringes. No injection site rotation. No concerns about sterile technique. No calculating reconstitution volumes.
For many people, this convenience factor determines product choice regardless of theoretical bioavailability differences. The psychological barrier to self-injection stops countless individuals from ever trying peptide therapy. Oral products remove that barrier entirely.
Travel becomes simpler with oral products. Capsules look like any other supplement. Sprays appear as breath freshener. No one questions why you carry these items. Injectable peptides require refrigeration during extended travel and raise questions if discovered.
Use case alignment
Certain applications favor oral delivery. Gut healing and gastrointestinal repair benefit from direct peptide contact with digestive tissues. The local effect theory suggests oral administration may actually work better than injection for these purposes. The peptide reaches the tissues that need it most without requiring systemic distribution.
Other applications favor injection. Tendon and ligament repair in specific locations. Joint healing. Localized injury recovery. These scenarios benefit from higher systemic peptide levels and potentially from injection near the injury site.
Systemic effects including cognitive benefits, mood regulation, and general tissue maintenance could work with either route, though injection delivers more predictable exposure levels.
Cost analysis
Compounded injectable BPC-157 from pharmacies typically costs $200-400 for a 4-6 week supply at standard dosing. Research peptides cost significantly less but come with purity concerns and legal gray areas.
Integrative Peptides BPC-157 costs approximately $140-170 per month at standard dosing. This positions the product between pharmacy compounding and research suppliers. For those who value quality assurance but want to avoid injections, the pricing represents reasonable value.
If you factor in the bioavailability discount, the effective cost per equivalent systemic dose increases substantially for oral products. A month of oral BPC-157 might deliver the therapeutic equivalent of a week or two of injectable BPC-157. This calculus matters for conditions requiring strong systemic effects.
Other Integrative Peptides products worth knowing
The BPC-157 products represent the company flagship offerings, but the full product line includes several other formulations relevant to peptide researchers.
TB4-Frag
Thymosin Beta 4 is another regenerative peptide with substantial research support. It promotes tissue repair, cell migration, and angiogenesis. TB-500 represents the most commonly studied fragment. Integrative Peptides offers their TB4-Frag product containing 250 mcg of the active fragment per capsule.
Like BPC-157, this peptide traditionally requires injection. The oral formulation uses similar delayed release technology. Some researchers combine TB4-Frag with BPC-157 for synergistic effects, as these peptides work through complementary mechanisms. The BPC-157 and TB-500 stacking guide covers the rationale for combination protocols.
TB4-Frag Max provides a higher potency formulation with additional ingredients designed to enhance absorption and activity. Pricing runs higher than the standard version but may deliver better value for those seeking maximum effect.
KPV capsules and spray
KPV is a tripeptide derived from alpha-melanocyte stimulating hormone. It demonstrates powerful anti-inflammatory properties through melanocortin receptor modulation. Research shows particular promise for gut inflammation, skin conditions, and systemic inflammatory states.
Integrative Peptides offers KPV in both capsule and oral spray formats. The 500 mcg dosing matches the BPC-157 products. For those dealing with inflammatory conditions, particularly in the gut, KPV represents an interesting option that may complement or substitute for BPC-157 depending on the specific situation.
The KPV peptide benefits extend beyond inflammation to include antimicrobial activity and wound healing support. Some researchers combine KPV with BPC-157 for comprehensive gut healing protocols.
CerebroPep
This unique formulation contains a porcine-derived peptide blend targeting cognitive function. The product aims to support brain health, mental clarity, and neurological recovery. While less extensively researched than BPC-157, the formulation represents an interesting option for those exploring peptides for brain function.
Pricing sits around $100-109 for 30 capsules, reflecting the specialized formulation and sourcing requirements.
Thymogen Alpha-1
Thymosin Alpha-1 is an immune modulating peptide with clinical applications in viral infections, cancer support, and immune deficiency conditions. Unlike the research peptides most people associate with performance enhancement, Thymosin Alpha-1 has actually received regulatory approval in some countries for specific medical indications.
The Integrative Peptides version offers 60 capsules at approximately $140-150. For those dealing with immune challenges or seeking to optimize immune function, this product provides oral access to a peptide with substantial clinical backing.

Quality and testing considerations
In the supplement industry, quality claims require scrutiny. Integrative Peptides makes specific assertions about their manufacturing and testing standards. Understanding what these claims mean helps assess product reliability.
Purity testing
The company states each batch undergoes testing to confirm 99% or greater purity. This standard matches what you would expect from reputable peptide suppliers. Purity testing typically uses High Performance Liquid Chromatography to identify the peptide and assess the concentration of the target compound versus impurities.
What the company does not prominently display is independent third-party testing from laboratories like Janoshik or Colmaric Analyticals that the peptide research community trusts. Many research peptide suppliers now provide Certificates of Analysis from these independent labs. Whether Integrative Peptides uses similar external verification or relies on internal or contract lab testing remains unclear from their public materials.
Contaminant screening
The company claims screening for heavy metals, microbials, and common contaminants. This testing matters because manufacturing processes can introduce harmful substances. Lead, arsenic, and other heavy metals appear in supplements more often than consumers realize. Bacterial contamination poses acute health risks.
Proper contaminant screening should cover at minimum heavy metals, microbial counts, endotoxins, and residual solvents. The specific panels Integrative Peptides uses are not detailed on their website, though their FDA-compliant manufacturing claims suggest adherence to current Good Manufacturing Practices that require such testing.
Manufacturing standards
Production in FDA-registered, GMP-certified facilities provides meaningful quality assurance. These certifications require documented procedures, quality control checks, and regulatory oversight. The manufacturing environment must meet cleanliness and contamination prevention standards.
However, GMP certification covers manufacturing process rather than product efficacy. A facility can be GMP certified while producing products that do not work as claimed. The certification means the pills contain what the label says in a clean manufacturing environment. It does not mean the product delivers therapeutic benefits.
Professional pricing program
Integrative Peptides offers qualified healthcare professionals preferred pricing with discounts up to 40% off retail rates. This program suggests the company positions itself as a practitioner brand rather than purely direct-to-consumer. Professional recommendation from physicians and healthcare providers adds credibility, though it does not substitute for clinical evidence.
How to use Integrative Peptides BPC-157
For those who decide to try these products, following optimal usage protocols maximizes the potential for benefit.
Dosing recommendations
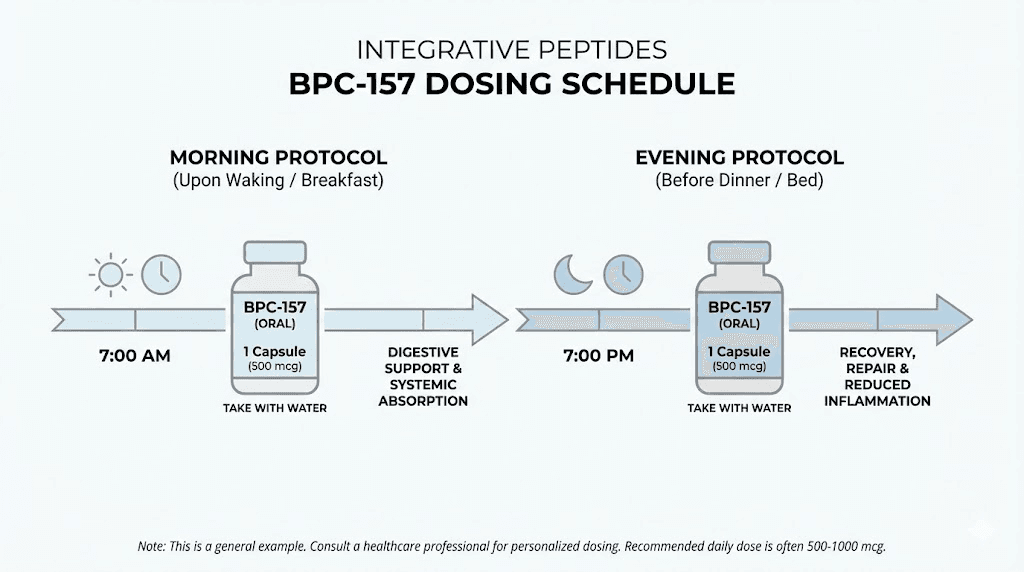
The standard recommendation is one capsule twice daily for the delayed release formulation. This delivers 1000 mcg total daily BPC-157. Taking doses on an empty stomach, at least 30 minutes before meals or 2 hours after, optimizes gastric conditions for the delayed release mechanism.
For the oral spray, the recommended protocol involves one spray twice daily. Hold the solution under the tongue for 30-60 seconds before swallowing. This contact time allows sublingual absorption to occur.
Some users take higher doses, particularly for acute healing situations. The products appear well tolerated at doses up to 2000 mcg daily based on available reports. However, higher doses increase cost without established evidence of proportionally increased benefit.
Timing considerations
Morning and evening dosing spreads peptide exposure throughout the day. Consistent timing helps establish steady-state levels. Taking the product at random times or inconsistently may reduce effectiveness.
For gut healing applications, some practitioners recommend dosing around meals in specific patterns. Taking the peptide 30 minutes before meals may coat the stomach before food-induced acid secretion peaks. Taking it on an empty stomach in the morning exploits the lower gastric pH present after overnight fasting.
Duration of use
Most peptide protocols run 4-12 weeks depending on the condition being addressed. Injectable BPC-157 research suggests 4 weeks produces measurable healing acceleration in many studies. Oral formulations may require longer durations given the bioavailability uncertainty.
Some users take BPC-157 products on an ongoing maintenance basis for gut health support. The safety profile appears favorable for extended use based on available data, though long-term studies specifically on oral formulations do not exist.
Cycling off periodically may help maintain sensitivity and response. A common approach uses 4-8 weeks on followed by 2-4 weeks off. The peptide cycle planning guide covers general principles applicable to these products.
Combining with other products
Stacking Integrative Peptides BPC-157 with their TB4-Frag product follows the same rationale as combining injectable BPC-157 with TB-500. The peptides work through complementary mechanisms and may produce synergistic healing effects.
Adding KPV to a BPC-157 protocol makes sense for inflammatory conditions, particularly in the gut. The anti-inflammatory effects of KPV complement the regenerative effects of BPC-157.
For cognitive goals, combining CerebroPep with BPC-157 addresses both structural brain health and regenerative support. The protocols for peptide stacking apply similarly to oral formulations.
Who should consider Integrative Peptides BPC-157
These products suit certain users better than others. Understanding the ideal use cases helps you determine whether Integrative Peptides aligns with your goals.
Best candidates
Those with gut healing goals represent the strongest candidate pool. The local effect theory provides the most solid rationale for oral BPC-157 efficacy. If your primary concern involves gastritis, leaky gut, intestinal inflammation, or other digestive issues, oral BPC-157 makes theoretical sense and receives the most favorable user reports.
People who will not self-inject represent another logical audience. If the choice is between oral BPC-157 and no BPC-157 at all, the oral version provides at least some exposure to the peptide. The convenience factor enables therapy that would otherwise never happen.
Those seeking general wellness support rather than treating specific acute injuries may find oral products adequate. If you want modest regenerative support as part of a broader health optimization protocol, the lower effective dose from oral administration might provide sufficient benefit.
Travelers and those with lifestyle constraints benefit from the portability and simplicity. No refrigeration requirements. No syringes to dispose of. No reconstitution calculations. Just take your supplements.
Less ideal candidates
Serious acute injuries requiring maximum healing support probably need injectable protocols. If you have a torn tendon, significant ligament damage, or major tissue injury, the bioavailability limitation of oral products may leave you undersupported.
Those comfortable with injection who prioritize efficacy over convenience should probably choose injectable BPC-157. The higher bioavailability translates to more predictable therapeutic exposure. Research supporting BPC-157 used injectable administration.
Budget-constrained individuals may find better value in research peptides for injection, though this option requires accepting lower quality assurance and navigating legal gray areas. The effective cost per therapeutic dose likely favors injection when bioavailability discounting is applied.
Regulatory and legal context
Understanding the regulatory landscape helps you make informed decisions about these products.
BPC-157 is not FDA approved for any medical indication. It cannot legally be marketed as a drug or medicine. The compound remains in research status, lacking the clinical trial data required for regulatory approval.
Integrative Peptides sells their products as dietary supplements. This classification allows legal sale without FDA approval for therapeutic claims. The company can describe what the peptide is and what research suggests but cannot claim the product treats, cures, or prevents any disease.
In September 2023, the FDA listed BPC-157 as a Category 2 bulk drug substance. This means compounding pharmacies cannot include it in customized medications. The designation acknowledges safety data gaps that preclude traditional drug compounding.
This regulatory action specifically targets injectable compounded formulations. It does not directly address dietary supplements containing BPC-157. The supplement industry operates under different rules that allow sale of substances not approved as drugs.
The World Anti-Doping Agency bans BPC-157 in competitive sports. Athletes subject to drug testing cannot use these products regardless of the format. The substance appears on the prohibited list under substances without current approval for human therapeutic use.
This regulatory environment creates uncertainty. The products exist in a space where they are legal to sell as supplements but lack regulatory blessing for therapeutic use. Users make personal decisions about a compound that research supports but regulators have not approved.
Integrative Peptides compared to alternatives
Several other companies now offer oral BPC-157 products. Understanding how Integrative Peptides compares helps with product selection.
Other oral BPC-157 brands
ProHealth Longevity offers a BPC-157 product with third-party testing claims and delayed release delivery. Healthletic markets an oral BPC-157 with similar quality assurances. Numerous smaller supplement brands have entered the market with their own formulations.
Integrative Peptides differentiates through physician founding, multiple formulation options, and a broader product line. The company has operated since 2018, giving them more track record than newer entrants. Their professional practitioner program suggests targeting healthcare providers as distribution channels.
Pricing varies across brands. Integrative Peptides sits at the higher end, reflecting their quality claims and marketing positioning. Lower-priced alternatives exist but may lack equivalent quality documentation.
Research peptides for injection
Traditional research peptide suppliers sell BPC-157 in lyophilized powder form for reconstitution and injection. These products cost significantly less per milligram than oral supplements. Quality varies dramatically between suppliers, with some providing COAs from independent labs and others offering minimal documentation.
The best peptide vendors guide covers what to look for when sourcing research peptides. For those comfortable with injection protocols, this route provides higher bioavailability and lower cost but requires more effort and risk assessment.
Compounding pharmacies
Until recent regulatory changes, compounding pharmacies produced prescription BPC-157 formulations. These products offered medical-grade quality with physician oversight. The Category 2 designation has limited this option, though some pharmacies may still produce BPC-157 under specific conditions or have remaining inventory.
Compounded products cost more than either oral supplements or research peptides. They provide the highest quality assurance and legal clarity but come with access limitations and physician gatekeeping.
Making your decision
Choosing whether Integrative Peptides BPC-157 products suit your needs requires weighing several factors.
Start with your primary goal. Gut healing applications align best with oral delivery. Systemic healing for injuries favors injection. General wellness falls somewhere in between.
Consider your willingness to inject. If subcutaneous injection is completely off the table for you, oral products become the obvious choice by default. Some peptide exposure beats none.
Evaluate your budget relative to your goals. If you need maximum healing support for a significant injury, investing in higher bioavailability makes sense. If you seek modest wellness support, the cost-effectiveness calculation differs.
Think about quality assurance requirements. Integrative Peptides provides more documentation and credibility signals than typical research suppliers. If this matters to you, the premium pricing may represent good value.
Assess your timeline. Oral formulations may require longer use to achieve comparable effects. If you need rapid healing, injectable protocols deliver faster results. If you have time for gradual improvement, oral products become more viable.
For those who decide to try Integrative Peptides, starting with the delayed release BPC-157 Pure capsules makes sense for most applications. The spray format may offer advantages for those who prefer sublingual delivery or have difficulty swallowing capsules. The immediate release version suits those focused purely on gut healing where local effects matter most.
SeekPeptides members have access to personalized protocol guidance that accounts for individual factors most general information sources overlook. The decision to use oral versus injectable peptides depends on circumstances that vary person to person.
Frequently asked questions
Does Integrative Peptides BPC-157 actually work?
User experiences suggest meaningful benefits for gut healing applications where local peptide effects matter. Systemic effects show more variability. The oral formulation almost certainly delivers less bioavailable peptide than equivalent injectable doses, but the delivered amount may still cross therapeutic thresholds for some individuals and conditions.
How does oral BPC-157 compare to injectable BPC-157?
Injectable BPC-157 delivers nearly 100% bioavailability while oral formulations likely deliver 1-10% or less. However, oral products work better for gut healing where direct tissue contact matters. The choice depends on your goals, with systemic healing favoring injection and gastrointestinal healing favoring oral delivery. See the BPC-157 benefits guide for more on what this peptide can do.
What is the proper dosage for Integrative Peptides BPC-157?
The standard recommendation is one 500 mcg capsule twice daily on an empty stomach. Some users take higher doses up to 2000 mcg daily without reported issues. For the spray, one dose twice daily with 30-60 seconds of sublingual holding is recommended. Consult the BPC-157 dosing guide for protocol details.
Are there side effects from Integrative Peptides BPC-157?
Most users report no side effects. Occasional reports mention gastrointestinal upset including nausea. Rare reports describe more significant reactions. The overall safety profile appears favorable based on available data, though individual responses vary. Review peptide safety considerations before starting any protocol.
Is Integrative Peptides a legitimate company?
The company was founded in 2018 by Dr. Kent Holtorf, a physician with medical credentials from UCLA. They manufacture in FDA-registered, GMP-certified facilities and claim 99%+ purity testing. Their professional practitioner program and product line expansion suggest business stability. They are not a fly-by-night operation.
Can I take Integrative Peptides BPC-157 with other supplements?
No known interactions prevent combining BPC-157 with other supplements. Many users take it alongside the company own TB4-Frag or KPV products for synergistic effects. Standard peptide stacking principles apply. The peptide stacking calculator helps plan combination protocols.
How long should I take Integrative Peptides BPC-157?
Most protocols run 4-12 weeks depending on goals. Gut healing may show results within 2-4 weeks. More significant healing needs may require 8-12 weeks. Some users take the product ongoing for maintenance. Cycling with breaks of 2-4 weeks between protocols may help maintain response.
Does Integrative Peptides provide third-party testing documentation?
The company claims each batch undergoes purity and contaminant testing. Their materials reference 99%+ purity verification and screening for heavy metals and microbials. Detailed Certificates of Analysis from named independent laboratories are not prominently displayed on their website. Contact the company directly for specific batch documentation.
For researchers serious about understanding peptide therapy options, SeekPeptides provides comprehensive guides, protocol resources, and personalized guidance that accounts for individual circumstances.



