Jan 24, 2026
You have tried the diets. The calorie counting. The hours of cardio that leave you exhausted but somehow still staring at the same number on the scale.
Nothing seems to move the needle in any meaningful way. And now you are hearing about bioactive precision peptides, these mysterious compounds that supposedly work with your body rather than against it, that target fat cells directly while preserving the muscle you have worked so hard to build.
But what are they really? Are they the same as those expensive weight loss peptides everyone seems to be talking about? Or something different entirely? The confusion is understandable. Bioactive peptides come from many sources. Some are naturally present in the foods you already eat. Others are engineered in laboratories with precision that borders on science fiction. Some require prescriptions. Others exist in regulatory gray zones. And the marketing claims range from the genuinely promising to the absurdly exaggerated.
Here is what makes this topic genuinely fascinating. Your body already uses peptides as signaling molecules every single day. They regulate your appetite. They tell your fat cells when to release stored energy. They communicate between your gut and your brain in ways scientists are only beginning to understand. Bioactive precision peptides work by amplifying or mimicking these natural signals, pushing your metabolism in directions it might struggle to reach on its own. The science is real. The mechanisms are understood. The results, when protocols are followed correctly, can be remarkable.
This guide will walk you through everything you need to know. We will examine food-derived bioactive peptides that you can obtain through diet and supplements. We will explore the synthetic and pharmaceutical options that dominate clinical research. We will discuss the mechanisms behind each approach, the protocols that produce results, and the considerations that separate effective use from wasted effort. SeekPeptides has compiled this research specifically for people who want to understand what actually works, not marketing fluff, but real science applied to real goals.
What are bioactive precision peptides and why do they matter for weight loss
The term bioactive precision peptides sounds complicated. It is not. Break it down. Peptides are simply short chains of amino acids, the same building blocks that make up proteins. Bioactive means they have biological activity, that they do something in your body beyond just providing raw materials for tissue building. Precision refers to their targeted effects, their ability to influence specific pathways rather than creating systemic chaos.
Your body contains thousands of different peptides. Some function as hormones. Others work as neurotransmitters. Many regulate metabolism directly. The peptides involved in weight management include those that control appetite, those that regulate how your cells process energy, those that determine whether calories get stored as fat or burned for fuel, and those that influence the very survival or death of fat cells themselves.
When researchers talk about bioactive precision peptides for weight loss, they mean compounds that can influence these pathways with specificity. Unlike stimulants that simply rev up your entire nervous system, or appetite suppressants that make you feel vaguely unwell around food, precision peptides work through defined molecular mechanisms. They bind to specific receptors. They activate particular signaling cascades. They produce predictable effects because their actions are targeted rather than diffuse.
The difference between food-derived and synthetic bioactive peptides
Food-derived bioactive peptides originate from the proteins in your diet. Dairy products. Fish. Eggs. Meat. Plant proteins. When your digestive system breaks down these proteins, it sometimes releases peptide fragments that have biological activity beyond simple nutrition. Some of these fragments influence cholesterol metabolism. Others affect blood pressure. And increasingly, researchers are discovering peptides from food sources that impact fat metabolism and appetite regulation directly.
Synthetic bioactive peptides are engineered compounds. Some are identical copies of peptides your body already produces, manufactured in laboratories for therapeutic use. Others are modified versions designed for improved stability, better absorption, or enhanced potency. Still others are entirely novel molecules that mimic natural signaling peptides without being chemically identical to anything found in nature.
The best peptides for weight loss often fall into this synthetic category. Compounds like semaglutide and tirzepatide represent decades of research into how natural gut hormones regulate appetite and metabolism, distilled into pharmaceutical forms that produce dramatic weight reduction in clinical trials. But food-derived peptides should not be dismissed. They offer more subtle effects, certainly, but they also come with established safety profiles and the advantage of being obtainable without prescriptions or regulatory complications.
The science behind bioactive peptides and fat metabolism
Understanding how bioactive peptides affect weight loss requires examining the mechanisms at the cellular level. Fat tissue is not just passive storage. It is an active endocrine organ that communicates with your brain, your liver, your muscles, and virtually every other system in your body. Peptides influence this communication network at multiple points.
Appetite regulation through the gut-brain axis
The gut-brain axis represents one of the most important targets for bioactive weight loss peptides. Your gastrointestinal tract produces numerous peptide hormones in response to food. Cholecystokinin, or CCK, gets released when fats and proteins enter your small intestine. Glucagon-like peptide-1, known as GLP-1, comes from specialized cells in your lower gut. Peptide YY, abbreviated PYY, follows a similar pattern. These hormones travel through your bloodstream to your brain, specifically to regions in your hypothalamus that regulate hunger and fullness.
What happens when these signals are amplified? You feel satisfied sooner. You stay full longer. The drive to eat diminishes not through willpower or discomfort, but through the same natural satiety mechanisms your body uses after every meal. This is why GLP-1 receptor agonists have proven so effective for weight loss. They do not fight against your biology. They work with it, enhancing signals that already exist.
Research from multiple clinical trials demonstrates just how powerful this approach can be. Participants taking semaglutide in the STEP trials lost an average of 15 to 20 percent of their body weight. Tirzepatide, which activates both GLP-1 and GIP receptors, produced even more dramatic results in the SURMOUNT trials, with some participants losing more than 22 percent of their starting weight. These are not marginal improvements. These are transformative changes achieved primarily through appetite regulation.
Lipolysis and fat cell metabolism
Beyond appetite, bioactive peptides can influence how fat cells handle stored energy. The process of breaking down stored triglycerides into free fatty acids and glycerol is called lipolysis. Several peptides enhance this process. AOD-9604, a modified fragment of human growth hormone, specifically promotes lipolysis without the broader metabolic effects associated with full growth hormone therapy. It binds to beta-adrenergic receptors in adipose tissue, triggering the same fat-burning cascade that exercise and fasting activate.
The HGH fragment 176-191 works through similar mechanisms. This peptide represents amino acids 176 through 191 of the human growth hormone molecule, the specific portion responsible for fat metabolism effects. Research shows it can reduce adipocyte size, meaning it literally shrinks fat cells, without affecting blood sugar levels or stimulating IGF-1 production the way complete growth hormone would.
Then there is 5-amino-1MQ, which takes a different approach entirely. This compound inhibits an enzyme called NNMT (nicotinamide N-methyltransferase) that plays a key role in fat cell metabolism. When NNMT activity is high, your cells tend to store more fat and burn less. By blocking this enzyme, 5-amino-1MQ essentially resets the metabolic thermostat of your fat tissue. Studies in mice showed a 30 percent reduction in adipocyte size and over 40 percent reduction in adipocyte volume without any change in food intake.
Mitochondrial function and energy expenditure
Every cell in your body contains mitochondria, the organelles responsible for converting nutrients into usable energy in the form of ATP. The efficiency and number of your mitochondria directly influence your metabolic rate. More mitochondria, working more efficiently, means more calories burned even at rest.
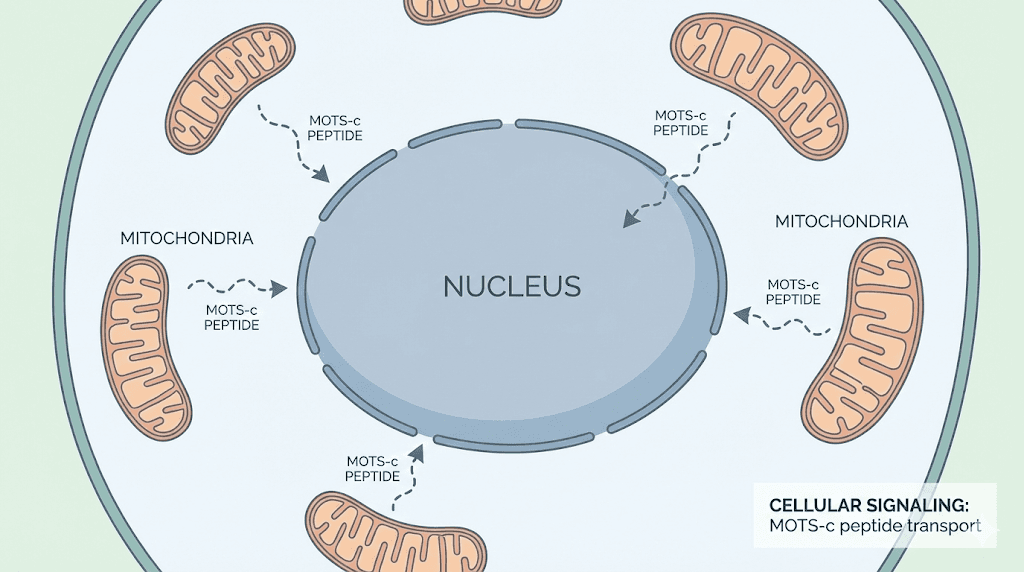
MOTS-c is a mitochondrial-derived peptide that has captured significant research attention. This 16-amino-acid peptide is encoded by your mitochondrial DNA and acts as a metabolic regulator throughout your body. In animal studies, MOTS-c treatment prevented both age-related and diet-induced obesity. It improved insulin sensitivity. It enhanced glucose uptake in muscle tissue. It increased energy expenditure. Perhaps most remarkably, it appears to do all this by traveling from mitochondria to the cell nucleus and directly regulating genes involved in metabolism.
The correlation between MOTS-c levels and metabolic health in humans is striking. Studies show that plasma MOTS-c levels are negatively correlated with fasting insulin, glycosylated hemoglobin, and body mass index. People with more circulating MOTS-c tend to be leaner and more metabolically healthy. This observation has driven interest in MOTS-c as a potential therapeutic peptide, though it remains in the research phase for now.
SS-31, also known as elamipretide, takes a different approach to mitochondrial optimization. Rather than acting as a signaling molecule like MOTS-c, SS-31 directly stabilizes the inner mitochondrial membrane by binding to cardiolipin. This interaction enhances ATP synthesis, reduces oxidative damage, and improves overall mitochondrial function. While SS-31 is being studied primarily for conditions like heart failure and kidney disease, its effects on cellular energy production have implications for metabolic health and weight management.
Food-derived bioactive peptides for weight management
Not all bioactive peptides come from injections or pharmaceutical manufacturing. Many originate in the foods you eat every day. When your digestive enzymes break down dietary proteins, they release peptide fragments. Most of these fragments are simply broken down further into individual amino acids. But some fragments remain intact long enough to exert biological effects, either within your gut or after absorption into your bloodstream.
Dairy-derived peptides
Milk proteins, both casein and whey, are rich sources of bioactive peptides. The fermentation process used to make cheese and yogurt can liberate even more of these compounds. Researchers have identified peptides from dairy that influence virtually every aspect of metabolic health, from blood pressure regulation to cholesterol management to appetite control.
Whey protein is particularly notable for its satiety effects. Studies consistently show that whey protein produces greater feelings of fullness compared to other protein sources or carbohydrates. This effect appears to be mediated by gut hormone release. When researchers measured CCK, GLP-1, and PYY levels after whey consumption, they found significantly greater increases compared to control beverages. The satiety effect begins within 30 minutes and persists for up to two hours.
Casein hydrolysates also show promise. In a study of obese patients, supplementation with 0.5 grams of beta-casein hydrolysate daily for eight weeks increased serum levels of fibroblast growth factor 21 (FGF-21), a hormone that improves insulin sensitivity and regulates glucose metabolism. The tripeptide Val-Pro-Pro, derived from casein, has been shown to reduce diet-induced adipose tissue inflammation in animal models.
Marine and fish-derived peptides
The fish processing industry generates enormous quantities of byproducts, including skins, bones, heads, and viscera. These waste streams are actually rich sources of bioactive peptides. Marine collagen peptides represent one of the most studied categories.
Research on marine collagen peptides for metabolic health is encouraging. In one study of Chinese patients with type 2 diabetes, 13 grams of marine collagen peptides daily for three months produced measurable improvements in glucose homeostasis. Fasting insulin decreased by nearly 20 percent. Insulin sensitivity improved by a similar margin. Fish collagen peptides obtained through subcritical water hydrolysis have been shown to inhibit lipid accumulation in laboratory adipocyte cultures and reduce adipocyte sizes in mice fed high-fat diets.
Fish protein hydrolysates more broadly demonstrate anti-obesity effects through multiple mechanisms. Some peptides from fish inhibit pancreatic lipase, the enzyme responsible for breaking down dietary fats for absorption. Others influence fat cell differentiation, reducing the formation of new adipocytes from precursor cells. The peptide AIPPHPYP, derived from fermented fish, has shown activity in regulating both appetite and fat metabolism.
Plant-derived peptides
Plant proteins also yield bioactive peptides with potential weight management applications. Soy protein hydrolysates contain peptides that interact with cholesterol metabolism and fat storage pathways. Pea protein has demonstrated satiety effects comparable to whey in some studies, with similar increases in CCK and GLP-1 after consumption.
The mechanism of plant peptide bioactivity often involves interaction with receptors in the gut. Enteroendocrine cells, the specialized cells that produce satiety hormones, have receptors on their surfaces that can detect specific peptide sequences. When the right peptides bind, these cells release hormones that signal fullness to your brain. Researchers are actively working to identify the most potent sequences and optimize production methods.
GLP-1 receptor agonists: the pharmaceutical precision peptides
No discussion of bioactive precision peptides for weight loss would be complete without addressing the GLP-1 receptor agonists. These compounds have transformed obesity treatment. They represent the culmination of decades of research into gut hormone biology, distilled into therapeutic agents that produce weight loss previously achievable only through bariatric surgery.
Understanding how GLP-1 works in your body
GLP-1 is a natural hormone produced by L-cells in your lower intestine. It gets released in response to food intake and produces several effects relevant to weight management. It enhances insulin secretion, but only when blood glucose is elevated. It slows gastric emptying, keeping food in your stomach longer. It reduces glucagon production, limiting the liver's release of stored glucose. And critically for weight loss, it acts on brain regions involved in appetite regulation, reducing hunger and promoting satiety.
The problem with natural GLP-1 is that it degrades rapidly. An enzyme called dipeptidyl peptidase-4 (DPP-4) breaks down circulating GLP-1 within minutes. This is where pharmaceutical engineering comes in. Semaglutide is a modified GLP-1 analog that resists DPP-4 degradation. A fatty acid chain attached to the peptide backbone allows it to bind to albumin in your blood, extending its half-life to approximately one week. This means once-weekly dosing produces continuous GLP-1 receptor activation.
Clinical evidence for semaglutide
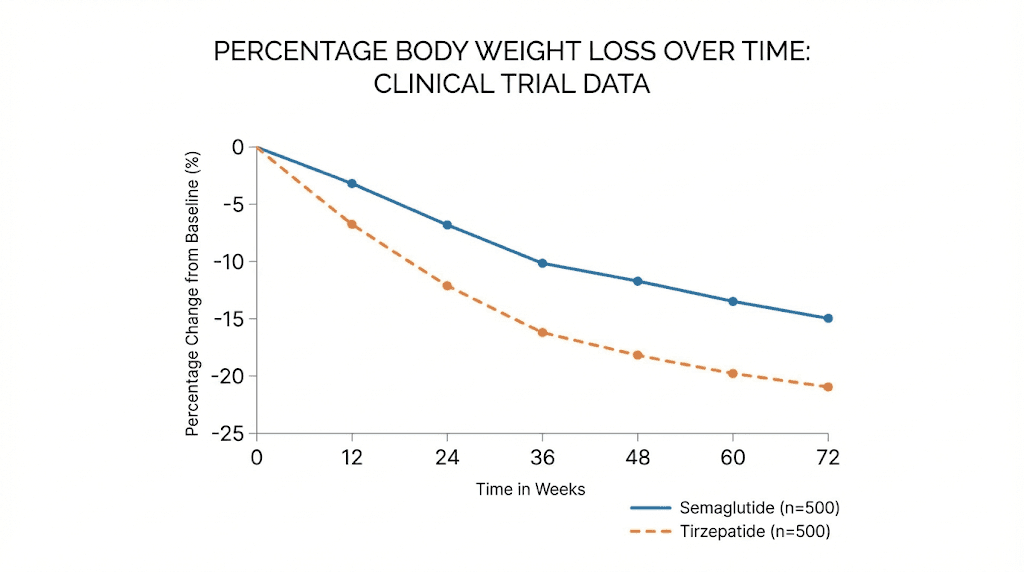
The STEP clinical trial program established semaglutide as a groundbreaking obesity treatment. In STEP 1, participants taking 2.4 mg semaglutide weekly lost an average of 14.9 percent of their body weight over 68 weeks, compared to 2.4 percent in the placebo group. More than a third of participants lost more than 20 percent of their starting weight. These results far exceeded anything achieved by previous pharmaceutical obesity treatments.
Subsequent trials confirmed and extended these findings. STEP 4 demonstrated that discontinuing semaglutide leads to weight regain, underscoring that the medication addresses the biology of obesity rather than providing a permanent cure. STEP 5 showed sustained weight loss through 104 weeks of treatment. Real-world results have generally confirmed the trial findings, though individual responses vary significantly.
Tirzepatide: dual receptor activation
Tirzepatide represents the next evolution in GLP-1 based therapy. Rather than activating only the GLP-1 receptor, tirzepatide also activates receptors for glucose-dependent insulinotropic polypeptide (GIP). This dual mechanism produces even greater weight loss than GLP-1 agonists alone.
The SURMOUNT trials demonstrated tirzepatide's remarkable efficacy. At the highest approved dose, participants lost an average of 22.5 percent of their body weight over 72 weeks. Many participants lost more than 25 percent. These results approach those seen after bariatric surgery, but achieved through weekly injection rather than surgical intervention. Comparing semaglutide to tirzepatide shows clear advantages for the dual agonist approach, though both represent major advances over previous treatments.
Emerging bioactive peptides for metabolic health
The GLP-1 agonists dominate current discussions of peptide-based weight loss, but they are not the only compounds under investigation. Several other bioactive peptides show promise for metabolic applications, each working through distinct mechanisms.
BRP: the naturally occurring peptide that rivals Ozempic
In early studies that generated significant excitement, Stanford researchers identified a naturally occurring peptide called BRP (BRINP2-related peptide) that suppressed appetite and promoted weight loss in animal models. Unlike GLP-1 agonists, BRP works through a completely different pathway involving central FOS signaling, a protein network involved in hunger regulation in the brain.
What makes BRP particularly interesting is its side effect profile. In the animal studies conducted so far, BRP reduced food intake without causing the nausea that commonly affects people taking GLP-1 medications. It also appeared to preserve muscle mass better than existing weight loss drugs, with fat making up nearly all of the weight lost. Mice treated with daily BRP injections for 14 days lost an average of 3 grams, almost entirely from fat, while control animals gained 3 grams.
A company has been formed to advance BRP toward human clinical trials. If these early results translate to humans, BRP could represent a significant addition to the obesity treatment toolkit, potentially offering similar efficacy to existing options with improved tolerability.
Tesamorelin and growth hormone releasing peptides
Growth hormone influences body composition significantly. It promotes fat breakdown while supporting lean tissue preservation. But administering growth hormone directly comes with significant risks and side effects. This is where growth hormone releasing peptides, or GHRPs, and growth hormone releasing hormone analogs like tesamorelin offer a more physiological approach.
Tesamorelin stimulates your pituitary gland to release its own growth hormone in natural pulsatile patterns. It is FDA-approved for reducing visceral fat in HIV-positive patients with lipodystrophy, but research suggests broader applications for metabolic health. Unlike direct growth hormone administration, tesamorelin maintains feedback mechanisms that prevent excessive hormone levels.
Ipamorelin works similarly but through different receptors. It stimulates growth hormone release without the pronounced effects on cortisol and prolactin seen with some other secretagogues. This selectivity makes it an attractive research compound for those interested in the body composition benefits of enhanced growth hormone signaling without broader hormonal disruption. The combination of ipamorelin with sermorelin or other releasing hormones represents a common approach in research protocols.
CJC-1295 and its variants
CJC-1295 is a synthetic analog of growth hormone releasing hormone (GHRH) with enhanced stability. By extending the half-life of the molecule, CJC-1295 produces more sustained elevation of growth hormone levels compared to natural GHRH, which degrades within minutes. The version known as CJC-1295 with DAC (Drug Affinity Complex) has a half-life of approximately one week, allowing for less frequent dosing.
Research interest in CJC-1295 centers on its potential to enhance fat loss while preserving or building lean tissue. Users in research contexts often combine it with GHRPs like ipamorelin for synergistic effects, as the two compounds work through complementary mechanisms. The comparison between ipamorelin and CJC-1295 highlights how different approaches to growth hormone optimization can serve different goals.
Practical protocols for bioactive peptides
Understanding which peptides exist and how they work is only part of the equation. Practical application requires knowledge of dosing, timing, administration, and integration with other lifestyle factors. The following protocols reflect current research understanding, though individual responses always vary.
Food-derived peptide protocols
Optimizing intake of food-derived bioactive peptides does not require exotic supplements. Strategic food choices can increase your exposure to these compounds significantly.
Dairy protocol for satiety enhancement:
Consume 20-30 grams of whey protein within 30 minutes of meals where appetite control is challenging
Include fermented dairy products like Greek yogurt or kefir daily, which contain predigested peptides
Consider a dedicated collagen peptide supplement of 10-15 grams daily for potential metabolic benefits
The timing matters. Consuming protein before or at the start of a meal produces greater satiety effects than consuming it afterward. The gut hormone response is most robust when peptides reach your intestine while you are still eating, allowing the satiety signals to influence how much you ultimately consume.
Marine collagen protocol:
Standard supplementation ranges from 5-15 grams of marine collagen peptides daily
Research showing metabolic benefits used 13 grams daily for three months
Take with vitamin C to enhance absorption and utilization
Morning consumption on an empty stomach may improve absorption
Marine collagen peptides are generally well-tolerated. The main consideration is source quality, as marine products can vary significantly in purity and peptide content depending on processing methods.
Pharmaceutical GLP-1 agonist considerations
Semaglutide and tirzepatide require prescriptions and medical oversight. They are powerful medications with significant effects and potential side effects. But for those who obtain them through appropriate medical channels, understanding the typical approach is valuable.
Semaglutide dosing typically starts at 0.25 mg weekly and increases gradually over several months to the maintenance dose of 2.4 mg weekly. This slow titration helps minimize gastrointestinal side effects, which are the most common complaint with GLP-1 therapy. Nausea, constipation, and reduced appetite are expected, especially during dose increases.
Tirzepatide follows a similar titration pattern, starting at 2.5 mg weekly and increasing to maximum doses of 10-15 mg depending on response and tolerability. The dual receptor activity may produce somewhat different side effect patterns compared to pure GLP-1 agonists, though gastrointestinal complaints remain common.
Critical considerations for GLP-1 therapy:
Muscle preservation requires adequate protein intake (at least 1.0-1.2 grams per kilogram of body weight daily)
Resistance training during weight loss helps maintain lean tissue
Hydration becomes especially important as appetite decreases
Some users experience changes in taste preferences, particularly decreased interest in fatty or fried foods
Research peptide protocols
Peptides like MOTS-c, 5-amino-1MQ, and AOD-9604 exist in a different regulatory category. They are not FDA-approved medications and are typically obtained through research chemical suppliers. Their use falls outside standard medical practice, though research interest remains high.
MOTS-c protocols in research contexts:
Typical dosing ranges from 5-10 mg administered subcutaneously
Frequency varies from daily to three times weekly
Some protocols involve cycling, with periods of use followed by breaks
Morning administration may align with natural circadian patterns of mitochondrial function
5-amino-1MQ protocols:
Research dosing typically ranges from 50-100 mg daily
Oral administration has been used in research settings
Duration in animal studies extended to several weeks
The compound is not approved for human use and lacks clinical trial data in humans
The absence of human clinical trial data for many research peptides means that protocols are extrapolated from animal studies and anecdotal reports. This introduces significant uncertainty about appropriate dosing, safety, and efficacy in humans. Anyone considering such compounds should understand these limitations clearly.
Combining bioactive peptides with lifestyle interventions
Bioactive peptides do not work in isolation. Their effects interact with your diet, exercise habits, sleep patterns, and stress levels. Understanding these interactions allows you to optimize outcomes and avoid common pitfalls.
Nutritional considerations
Protein intake becomes critically important when using weight loss peptides. The appetite suppression that makes these compounds effective for fat loss can also reduce protein consumption, potentially leading to muscle loss if not addressed consciously. Most experts recommend maintaining protein intake at 1.0 to 1.2 grams per kilogram of body weight even when total caloric intake decreases significantly.
The composition of your diet also matters. Gut health influences both the production of natural satiety peptides and the response to administered ones. A diet rich in fiber supports the gut microbiome populations that contribute to healthy metabolism. Fermented foods provide additional beneficial bacteria along with predigested peptides from their protein content.
Meal timing interacts with peptide effects. For those using food-derived peptides for satiety, consuming protein early in the meal maximizes the satiety signal before you have eaten too much. For those on GLP-1 agonists, the reduced appetite may make traditional meal timing less relevant, but ensuring adequate nutrition in whatever eating window remains comfortable becomes essential.
Exercise optimization
Resistance training during any weight loss intervention, peptide-assisted or not, helps preserve muscle mass. This becomes even more important when weight loss is rapid, as can occur with GLP-1 agonists. The combination of muscle-building peptides with weight loss compounds represents one research approach to this challenge, though evidence for such stacking remains largely anecdotal.
Cardiovascular exercise supports the metabolic adaptations that bioactive peptides promote. Peptides that enhance mitochondrial function, like MOTS-c and SS-31, may produce synergistic effects with endurance training, as exercise itself stimulates mitochondrial biogenesis. The combination could theoretically produce greater metabolic improvements than either intervention alone.
The relationship between peptides and athletic performance extends beyond weight loss. Many of the same compounds that influence body composition also affect energy levels, recovery, and exercise capacity. Understanding these connections helps in designing protocols that support overall health rather than just the number on the scale.
Sleep and stress considerations
Sleep deprivation disrupts virtually every metabolic process, including those that bioactive peptides target. Poor sleep increases hunger hormones, reduces satiety hormones, impairs insulin sensitivity, and creates an overall metabolic environment that favors weight gain. No peptide can fully overcome the effects of chronic sleep deprivation.
Stress similarly impacts metabolic health through cortisol elevation and behavioral changes. Chronic stress promotes visceral fat accumulation specifically, the type of fat most associated with metabolic disease. Some peptides may help with stress and anxiety, but addressing the underlying sources of stress remains important for long-term weight management success.
Safety considerations and side effects
Every bioactive compound has potential side effects. Understanding the safety profile of different peptide categories helps in making informed decisions and recognizing problems early if they occur.
GLP-1 agonist side effects
Gastrointestinal side effects are nearly universal with GLP-1 therapy, at least during the titration phase. Nausea is the most common complaint, followed by constipation, diarrhea, and reduced appetite (which is technically the desired effect). These symptoms typically improve over time but may persist in some users.
More concerning but less common side effects include:
Pancreatitis, inflammation of the pancreas that causes severe abdominal pain
Gallbladder problems, as rapid weight loss can precipitate gallstone formation
Retinopathy progression in diabetic patients
Potential thyroid tumor risk (based on rodent studies, though human relevance is unclear)
The side effects of different peptides vary significantly based on their mechanisms. Growth hormone releasing peptides, for instance, produce different side effect profiles than GLP-1 agonists. Understanding these differences helps in anticipating what to expect.
Research peptide safety considerations
Peptides that lack FDA approval present additional safety concerns beyond their pharmacological effects. Purity is not guaranteed with research chemical suppliers. Contamination is possible. Dosing recommendations are based on limited data. Long-term effects in humans are unknown.
The legal status of peptides varies by jurisdiction and by specific compound. Understanding these regulations is important for anyone considering research peptides. Some compounds are perfectly legal to purchase for research purposes. Others may have restrictions. Medical professionals rarely prescribe or supervise the use of non-approved peptides.
Contraindications and interactions
Certain medical conditions may make some bioactive peptides inappropriate. GLP-1 agonists should generally be avoided in people with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. They may not be suitable for people with a history of pancreatitis. Pregnancy and breastfeeding are contraindications for most pharmaceutical peptides.
Drug interactions exist, particularly for compounds that affect gut motility. GLP-1 agonists slow gastric emptying, which can change the absorption of oral medications. People taking medications with narrow therapeutic windows may need dosing adjustments. Always discuss new compounds with a healthcare provider who knows your complete medical history.
Comparing different bioactive peptide approaches
With so many options available, comparing different approaches helps in selecting the most appropriate strategy for specific goals and circumstances.
Comparison table: bioactive peptides for weight loss
Peptide Category | Primary Mechanism | Typical Results | Accessibility | Key Considerations |
|---|---|---|---|---|
GLP-1 agonists (semaglutide, tirzepatide) | Appetite suppression via gut-brain axis | 15-22% body weight loss | Prescription required | GI side effects common; muscle preservation needs attention |
Growth hormone peptides (tesamorelin, ipamorelin, CJC-1295) | Enhanced growth hormone signaling | Modest fat loss with lean tissue preservation | Prescription (tesamorelin) or research chemical | Less dramatic weight loss; better body composition effects |
Fat metabolism peptides (AOD-9604, 5-amino-1MQ) | Direct lipolysis enhancement or NNMT inhibition | Variable; less clinical data available | Research chemical | Limited human research; regulatory gray area |
Mitochondrial peptides (MOTS-c, SS-31) | Mitochondrial optimization and energy expenditure | Variable; primarily research applications | Research chemical or clinical trials | Emerging field; mechanisms well-understood but human data limited |
Food-derived peptides (whey, collagen, marine) | Satiety enhancement and metabolic support | Modest; supportive rather than transformative | Over-the-counter supplements | Safe and accessible but effects are subtle |
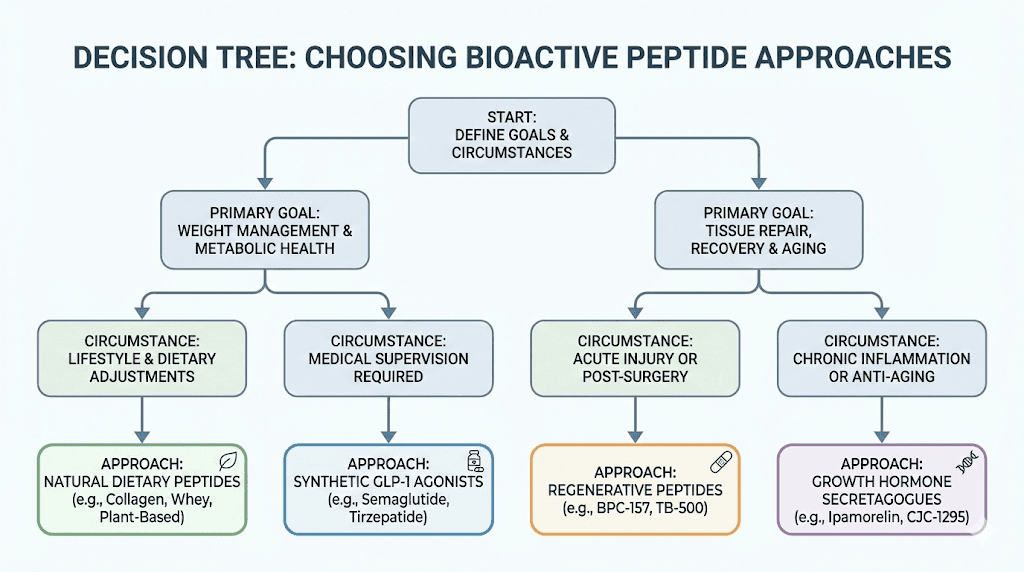
Choosing the right approach
For most people struggling with significant obesity, the evidence clearly supports GLP-1 agonists as the most effective pharmaceutical intervention currently available. The weight loss achieved rivals surgical outcomes without the risks of surgery. The main barriers are access, cost, and tolerability of side effects.
For those seeking more modest body composition improvements, or who cannot access or tolerate GLP-1 medications, other approaches may be appropriate. Growth hormone releasing peptides can support fat loss while preserving muscle, though effects are less dramatic. Food-derived peptides can provide marginal benefits with minimal risk.
The combination of multiple approaches, both pharmaceutical and lifestyle-based, often produces better results than any single intervention. A comprehensive approach might include optimized nutrition with adequate protein and food-derived peptides, regular exercise including resistance training, good sleep hygiene, stress management, and pharmaceutical peptides if appropriate and accessible.
The future of bioactive precision peptides
The field of bioactive peptides for weight management is evolving rapidly. Several developments on the horizon could change the landscape significantly.
Next-generation GLP-1 therapies
Pharmaceutical companies are developing oral formulations of GLP-1 agonists that could eliminate the need for injections. Rybelsus, an oral form of semaglutide, already exists for diabetes, though it is not approved for weight loss at the higher doses used for obesity treatment. Higher-dose oral formulations and novel delivery mechanisms are in development.
Combination therapies that target multiple pathways simultaneously represent another frontier. Beyond the GLP-1/GIP dual agonism seen with tirzepatide, researchers are exploring triple agonists that also activate glucagon receptors, potentially producing even greater metabolic benefits. Some experimental compounds combine GLP-1 activity with other beneficial peptide functions in single molecules.
AI-driven peptide discovery
Artificial intelligence is accelerating the discovery of novel bioactive peptides. Machine learning models can now predict peptide properties from their amino acid sequences, identify promising candidates from vast libraries of potential compounds, and optimize peptide design for specific therapeutic goals. This technology identified BRP, the naturally occurring peptide that may rival GLP-1 agonists while potentially avoiding their side effects.
The implications extend beyond weight loss. AI-driven discovery could identify peptides optimized for specific metabolic outcomes, whether maximizing fat loss, preserving muscle, improving insulin sensitivity, or enhancing mitochondrial function. Personalized peptide therapy, where compounds are selected or even designed based on individual metabolic profiles, may become possible.
Improved delivery methods
Peptide stability and delivery remain challenges for therapeutic applications. Most peptides require injection because they would be digested if taken orally. Research into protective formulations, nanoparticle delivery systems, and alternative administration routes like nasal sprays or transdermal patches could make peptide therapy more accessible and convenient.
The development of nasal spray peptides has already progressed significantly for some compounds. If these delivery methods can be extended to metabolic peptides, the practical barriers to their use would decrease substantially.
Frequently asked questions
Are bioactive precision peptides safe for long-term use?
The safety profile varies dramatically depending on the specific peptide. FDA-approved GLP-1 agonists like semaglutide have been studied in large clinical trials extending over two years, with safety data supporting their long-term use under medical supervision. Food-derived peptides from sources like whey and marine collagen have long safety records through their presence in the human diet. Research peptides that lack FDA approval have less established safety profiles, and their long-term effects in humans remain unknown.
How quickly do bioactive peptides produce weight loss results?
Results vary by compound and individual. GLP-1 agonists typically produce noticeable appetite changes within the first week or two, with measurable weight loss evident within the first month. Most of the weight loss occurs within the first year of treatment, with results stabilizing thereafter. Food-derived peptides produce much subtler effects that may require weeks or months to become apparent, if they produce noticeable results at all. Research peptides fall somewhere in between, with reported timelines varying widely.
Can I use bioactive peptides without exercising?
Bioactive peptides that work through appetite suppression, like GLP-1 agonists, can produce significant weight loss even without exercise. However, the quality of weight loss, meaning the proportion of fat versus muscle lost, improves considerably with resistance training. Exercise also provides metabolic benefits that complement peptide effects. For optimal outcomes, combining peptide therapy with appropriate exercise is strongly recommended.
Do I need a prescription for bioactive peptides?
It depends on the specific peptide. Semaglutide (Wegovy, Ozempic) and tirzepatide (Mounjaro, Zepbound) require prescriptions and are available only through healthcare providers. Tesamorelin is also a prescription medication. Food-derived peptide supplements like whey protein and marine collagen are available over the counter. Research peptides like MOTS-c, 5-amino-1MQ, and AOD-9604 occupy a gray area where they can be purchased without prescription but are not approved for human use and not legally sold for human consumption.
What happens when I stop taking bioactive peptides?
For pharmaceutical peptides like GLP-1 agonists, discontinuation typically leads to weight regain. Studies show that patients who stop semaglutide regain approximately two-thirds of their lost weight within one year. This reflects the fact that these medications treat the underlying biology of obesity but do not cure it. Food-derived peptides have no withdrawal effects, as they are simply nutritional compounds. The long-term effects of discontinuing research peptides are not well characterized.
Can bioactive peptides help with stubborn belly fat specifically?
Some peptides do appear to target visceral fat preferentially. Tesamorelin was FDA-approved specifically for reducing visceral fat in HIV-positive patients. GLP-1 agonists produce significant reductions in visceral fat along with overall weight loss. AOD-9604 and HGH Fragment 176-191 have been studied for their effects on abdominal fat specifically. However, no peptide can target subcutaneous belly fat exclusively while leaving fat elsewhere untouched, as spot reduction remains physiologically impossible.
Are bioactive peptides the same as weight loss drugs like Ozempic?
Ozempic is the brand name for semaglutide when used for diabetes, while Wegovy is the brand name for the same compound at higher doses for obesity. Both are GLP-1 receptor agonists, which are indeed bioactive peptides. So some bioactive peptides are weight loss drugs, but the category is much broader. It includes food-derived compounds, research chemicals not approved as drugs, and pharmaceutical agents used for purposes other than weight loss. The term encompasses any peptide with biological activity related to metabolism and body composition.
External resources
MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation
Bioactive peptides from food proteins as potential anti-obesity agents
Gut Hormones and Appetite Control: A Focus on PYY and GLP-1
For researchers serious about understanding how bioactive peptides can transform body composition, SeekPeptides offers the most comprehensive resource available ever.



