Jan 24, 2026
A new weight loss peptide is making headlines. It reduces food intake by 50%. It melts fat without touching muscle. And it does all this without the nausea, constipation, and muscle wasting that plague current GLP-1 drugs like semaglutide.
This is not another incremental improvement. This is BRP, the BRINP2-related peptide that Stanford researchers discovered using artificial intelligence in March 2025. Published in Nature, one of the most prestigious scientific journals on the planet, this 12-amino-acid molecule represents a fundamentally new approach to weight loss through peptide therapy. It works through an entirely different pathway than Ozempic. It targets the brain directly. And early animal studies suggest it may solve the problems that have made GLP-1 agonists both revolutionary and controversial.
For researchers following the peptide regulation landscape, this discovery arrives at a pivotal moment. The weight loss drug market is exploding. Ozempic and Wegovy have become household names. Yet for all their effectiveness, these drugs come with significant drawbacks that limit their long-term use for many patients. BRP peptide offers something different. Something that works in the hypothalamus rather than the gut. Something that preserves muscle while burning fat. Something worth understanding deeply.
This guide covers everything researchers need to know about BRP peptide. From the science of its discovery to its mechanism of action. From the dosage protocols used in studies to how it compares with established fat-burning peptides. From availability to future clinical development. Whether you are a researcher tracking emerging compounds or someone exploring alternatives to GLP-1 drugs, the information here will help you understand why BRP matters and what comes next.
What is BRP peptide?
BRP stands for BRINP2-related peptide, a naturally occurring molecule derived from the BRINP2 protein in the human body. At just 12 amino acids long, it represents one of the smallest bioactive peptides ever identified for metabolic weight regulation.
The discovery came from Stanford Medicine, where researchers led by Dr. Katrin Svensson and Dr. Laetitia Coassolo developed an AI-powered drug discovery platform called Peptide Predictor. This system analyzed over 2,600 previously uncharacterized human peptide fragments to identify molecules with potential metabolic activity. Among the 100 candidates they tested, BRP stood out dramatically, increasing neuronal cell activity tenfold compared to controls. That is more than triple the activation seen with GLP-1 peptide.
The full amino acid sequence is THRILRRLFNLC with C-terminal amidation. That amidation is critical. Studies confirmed that non-amidated BRP shows zero biological activity. Researchers also identified leucine at position 8 as essential for function. Substituting any other amino acid at that position completely eliminates the peptide's effects.
What makes BRP particularly interesting is its origin. It comes from the cleavage of a larger protein called BRINP2 (BMP/retinoic acid inducible neural specific 2). Enzymes called prohormone convertases, specifically PCSK1, cut BRINP2 at recognition sites flanking the BRP sequence to release this small but powerful molecule. This means BRP is not a synthetic creation. It exists naturally in human biology, suggesting the body already has systems designed to respond to it.
The role of AI in discovering BRP
Traditional drug discovery might have missed BRP entirely. The peptide came from a computational approach that systematically mapped how prohormone convertases process larger proteins into smaller bioactive fragments. This is not random screening. It is targeted prediction based on understanding enzyme cleavage patterns.
The Peptide Predictor algorithm identified 2,683 unique peptides from 373 proteins that might be biologically active. Researchers focused on sequences likely to act in the brain and screened 100 candidates for their ability to activate laboratory-grown neuronal cells. GLP-1 was included as a positive control, producing a threefold increase in cellular activity. BRP produced a tenfold increase.
This discovery method represents a new paradigm for peptide research. Rather than testing compounds one by one, researchers can use computational tools to predict which molecules are worth investigating. The success with BRP validates this approach and suggests many more therapeutic peptides may remain undiscovered in the human proteome.
How BRP peptide works: mechanism of action
Understanding how BRP works requires first understanding why current weight loss drugs have the effects they do. GLP-1 receptor agonists like semaglutide and liraglutide target receptors found throughout the body, not just in the brain. These receptors exist in the gut, pancreas, and other tissues. That widespread distribution explains both their effectiveness and their side effects.
Ozempic slows food movement through the digestive tract. It affects insulin secretion from the pancreas. It creates systemic changes that ripple through multiple organ systems. The nausea, vomiting, and constipation that many users experience come directly from these widespread effects.
BRP takes a different path entirely.
Hypothalamic targeting
BRP appears to act specifically in the hypothalamus, the brain region controlling appetite and metabolism. Rather than affecting the gut or pancreas, it targets the neural circuits that regulate hunger at their source. This is a hypothalamic-centric model of weight regulation, fundamentally distinct from the peripheral approach of GLP-1 drugs.
Research shows BRP activates several hypothalamic regions including the dorsomedial hypothalamic nucleus, preoptic hypothalamic nucleus, tuberal nucleus, and arcuate nucleus. These are the command centers for appetite. By working here rather than in the digestive system, BRP may avoid the gastrointestinal disruption that characterizes current medications.
The CREB-FOS signaling pathway
At the cellular level, BRP triggers a specific signaling cascade. It activates the cAMP-PKA-CREB-FOS pathway in neurons. This is consistent with G-protein coupled receptor (GPCR) activation, though researchers have not yet identified the specific receptor that BRP binds.
The pathway works like this. BRP binds to an unknown receptor on neuronal cells. This triggers cyclic AMP (cAMP) production inside the cell. cAMP activates protein kinase A (PKA). PKA then phosphorylates CREB at serine 133. Phosphorylated CREB enters the nucleus and activates Fos expression. The result is changed neuronal activity that suppresses appetite.
Importantly, this mechanism operates independently of leptin, GLP-1 receptor, and melanocortin 4 receptor (MC4R), the three major known pathways for appetite regulation. BRP represents something genuinely new in how peptides influence metabolism.
POMC neuron activation
The research team found that BRP directly activates pro-opiomelanocortin (POMC) neurons in the arcuate nucleus. POMC neurons are key regulators of appetite and energy expenditure. When activated, they suppress hunger and increase metabolic rate. They also influence brown adipose tissue, the type of fat that burns calories to generate heat.
This POMC activation may explain why BRP reduces fat mass specifically. POMC neurons connect to thermogenic pathways. By stimulating these circuits, BRP may increase fat burning while simultaneously decreasing food intake. The result is weight loss that comes from fat rather than from muscle or other lean tissue.
BRP peptide research and study results
The March 2025 Nature publication provides the most comprehensive data on BRP effects. While the peptide has not been tested in humans yet, the animal studies are remarkably consistent across multiple models and demonstrate effects that match or exceed those of established weight loss peptides.
In vitro studies on neuronal cells
Before testing in animals, researchers examined BRP effects on laboratory-grown neuronal cells. They measured activation levels using established markers of cellular activity.
GLP-1 increased neuronal activity threefold compared to control cells.
BRP increased neuronal activity tenfold compared to controls.
That is more than triple the effect. It suggested from the very first experiments that BRP was something special.
Researchers also tested FOS expression, a marker of sustained neuronal activation. BRP produced greater than tenfold increases in FOS expression in both neuronal cells and beta-cell lines. GLP-1 produced only three to tenfold increases in the same cell types. This consistent superiority across multiple measures indicated BRP had genuine potential for therapeutic development.
Acute feeding studies in mice and minipigs
The next step was testing whether BRP actually reduced food intake in living animals. Researchers used two models: lean mice and minipigs. Minipigs are particularly valuable for metabolism research because their eating patterns and digestive systems more closely resemble human physiology than rodents do.
A single intramuscular injection of BRP before feeding reduced food intake by up to 50% over the following hour in both mice and minipigs. This effect was dose-dependent. At 5 mg/kg, food intake decreased significantly. At 20 mg/kg, feeding was nearly completely suppressed for up to three hours.
The consistency across species is encouraging. When a compound works similarly in both rodents and larger mammals, it increases confidence that effects might translate to humans. This cross-species validation is often missing from early peptide research.
Chronic weight loss studies
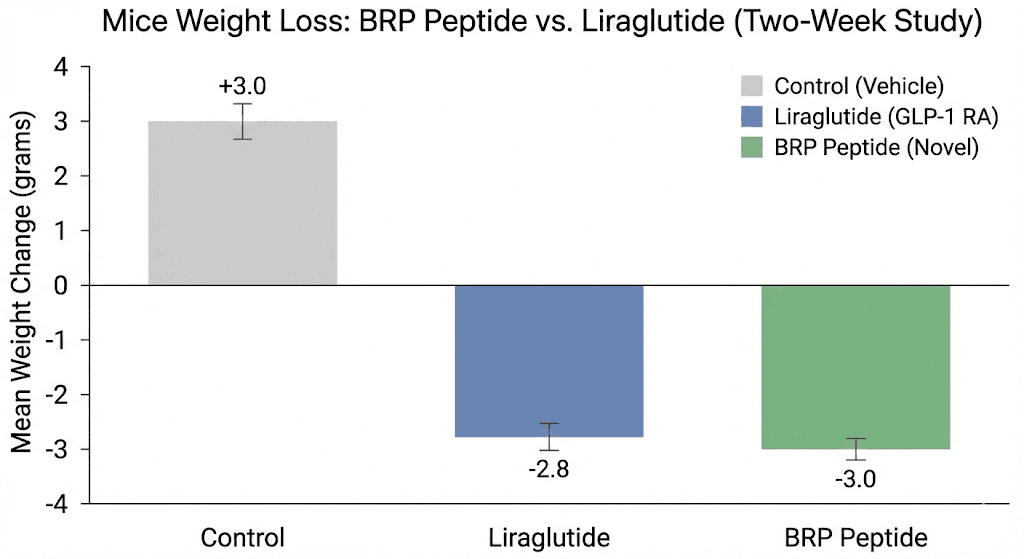
Single dose studies show acute appetite suppression. But lasting weight management requires sustained effects over time. The Stanford team conducted 14-day treatment studies in obese mice to evaluate chronic BRP administration.
Obese mice receiving daily BRP injections lost an average of 3-4 grams over two weeks. Control animals gained approximately 3 grams during the same period. That represents a roughly 6-7 gram difference in body weight between groups.
More importantly, the weight loss came almost entirely from fat. BRP selectively reduced fat mass without affecting lean body mass. This is a critical distinction from some current treatments. GLP-1 drugs can cause loss of muscle and bone mass accounting for up to 20% of total weight lost. BRP avoided this problem in the animal studies.
The treated mice also showed improved glucose tolerance and insulin sensitivity. These metabolic benefits occurred without BRP directly affecting pancreatic function. The improvements appeared secondary to weight loss rather than from direct drug effects on insulin-producing cells.
Comparison to liraglutide
Researchers directly compared BRP to liraglutide, another GLP-1 receptor agonist. At equivalent doses (100 mcg/kg), BRP achieved comparable fat mass reduction to liraglutide. Both compounds reduced subcutaneous white adipose tissue, brown adipose tissue mass, and liver weight.
The difference appeared in side effects. Liraglutide-treated animals showed expected gastrointestinal effects. BRP-treated animals did not. This head-to-head comparison strengthens the case that BRP offers similar efficacy with improved tolerability.
BRP peptide dosage information from studies
Researchers used specific dosage protocols in their animal studies. While these cannot directly translate to human use until clinical trials establish proper dosing, understanding the study parameters helps researchers evaluate the compound and plan future investigations.
Dosages used in mouse studies
The acute feeding studies tested multiple dose levels to establish a dose-response relationship. At 5 mg/kg body weight, BRP significantly reduced food intake compared to vehicle controls. At 20 mg/kg, feeding was nearly completely suppressed for up to three hours post-injection.
For the chronic 14-day weight loss studies, researchers administered daily injections. The specific dose used achieved consistent fat mass reduction over the treatment period. Mice received intramuscular injections before their normal feeding periods.
The research also identified that 100 mcg/kg was sufficient for metabolic comparison studies against liraglutide. At this dose, BRP matched the GLP-1 agonist for fat mass reduction while avoiding gastrointestinal effects.
Dosages used in minipig studies
Minipigs received intramuscular BRP injections prior to feeding. The peptide reduced food intake by 50% within one hour of administration. This rapid onset of action suggests good bioavailability following intramuscular delivery.
The cross-species consistency at roughly similar dose ranges (adjusted for body weight) provides preliminary evidence for dosing scalability. However, human doses will require careful determination through Phase 1 clinical trials.
Critical structural requirements
Beyond dosage, the studies revealed critical structural requirements for BRP activity. The C-terminal amidation is essential. Non-amidated BRP showed no activity in vitro. This modification is common in bioactive peptides and often increases stability and receptor binding.
Leucine at position 8 (the eighth amino acid in the sequence) is also critical. Researchers tested amino acid substitutions at this position. Every substitution completely eliminated BRP activity. Any synthesis or research involving BRP must preserve this residue exactly.
BRP peptide vs semaglutide: complete comparison
The obvious question for anyone familiar with GLP-1 alternatives is how BRP compares to established drugs like Ozempic. Here is a detailed comparison based on available research.
Mechanism of action differences
Semaglutide is a GLP-1 receptor agonist. It binds to GLP-1 receptors found throughout the body, including in the brain, gut, and pancreas. This widespread receptor distribution causes effects in multiple organ systems.
BRP acts through an unknown receptor in the hypothalamus. It does not bind GLP-1 receptors. It does not interact with leptin or MC4R pathways. It represents a genuinely novel mechanism for appetite suppression.
The practical difference: semaglutide affects insulin secretion, slows gastric emptying, and changes gut motility. BRP appears to work centrally in the brain without peripheral effects on digestion or pancreatic function.
Weight loss efficacy
Semaglutide has been extensively studied in humans. Clinical trials show average weight loss of approximately 12-15% of body weight over 68 weeks. Some patients lose more. Others respond less well. But the overall efficacy is well-established.
BRP has only been studied in animals. Mouse studies showed significant fat mass reduction over 14 days. The effect was comparable to liraglutide (another GLP-1 drug) at equivalent doses. Whether BRP will match semaglutide efficacy in humans remains unknown until clinical trials complete.
Side effect profile comparison
This is where BRP shows its greatest potential advantage.
Semaglutide commonly causes nausea, vomiting, diarrhea, and constipation. Up to 40% of patients experience gastrointestinal symptoms, particularly during dose escalation. Many patients cannot tolerate the drug at effective doses. Weight loss from semaglutide also includes muscle and bone loss, accounting for up to 20% of total weight lost in some studies.
BRP-treated animals showed no differences in anxiety-like behavior, water intake, locomotor activity, or fecal production compared to controls. No nausea or food aversion behaviors were observed. Most critically, weight loss came almost entirely from fat with no significant lean mass reduction.
If these findings translate to humans, BRP could offer similar weight loss without the tolerability problems that limit GLP-1 drugs for many patients.
Comparison table
Factor | Semaglutide (Ozempic) | BRP Peptide |
|---|---|---|
Mechanism | GLP-1 receptor agonist | Unknown receptor, CREB-FOS pathway |
Target location | Brain, gut, pancreas | Hypothalamus specifically |
GI side effects | Common (40%+ of patients) | Not observed in animals |
Muscle loss | Up to 20% of weight lost | Not observed, fat-specific |
Effect on blood sugar | Lowers glucose directly | Improves glucose via weight loss |
Human trial data | Extensive (FDA approved) | None yet (preclinical) |
Administration | Weekly injection | Unknown (daily in studies) |
Availability | Prescription (approved drug) | Research use only |
Benefits of BRP peptide for weight loss
Based on the published research, BRP offers several potential advantages over existing weight loss compounds. Understanding these benefits helps researchers and clinicians evaluate where BRP might fit in the evolving landscape of metabolic peptide therapies.
Fat-specific weight reduction
Perhaps the most significant finding is that BRP selectively reduces fat mass while preserving lean tissue. In the obese mouse studies, weight loss came almost entirely from adipose tissue. Muscle mass remained stable throughout treatment.
This matters because muscle loss during dieting or drug-induced weight loss has serious health consequences. Muscle is metabolically active tissue. Losing it reduces basal metabolic rate, making weight regain more likely. It also increases frailty risk, particularly in older adults. BRP's apparent muscle-sparing effect could represent a major clinical advantage.
No gastrointestinal side effects observed
GLP-1 drug side effects are not minor inconveniences. Severe nausea can affect quality of life. Gastroparesis (delayed stomach emptying) has been reported with long-term use. Some patients simply cannot tolerate these medications at doses needed for significant weight loss.
Animal studies with BRP showed no changes in fecal production, an indicator of gut function. No nausea or aversion behaviors were observed. If this translates to humans, BRP could make sustained weight loss treatment accessible to patients who cannot tolerate current options.
Improved metabolic markers
Beyond weight loss, BRP-treated animals showed improved glucose and insulin tolerance. These metabolic improvements occurred without direct effects on pancreatic beta cells. The improvements appeared secondary to fat mass reduction.
This is an important distinction. Some weight loss drugs work partly by affecting insulin secretion directly. This can complicate their use in patients with certain conditions. BRP appears to improve metabolic health through weight loss itself, potentially making it suitable for a broader patient population.
No anxiety or behavioral effects
Some appetite suppressants, particularly stimulant-based compounds, cause anxiety, agitation, or other behavioral changes. The Stanford researchers specifically tested for these effects and found none. BRP-treated animals showed normal locomotor activity, water intake, and behavior in anxiety tests.
For a compound acting in the brain, this clean behavioral profile is reassuring. It suggests BRP specifically targets appetite circuits without broader neurological effects.
Natural origin and presence in human biology
BRP is not a synthetic compound. It is derived from BRINP2, a protein that exists naturally in the human body. The cleavage sites that release BRP from BRINP2 suggest the body produces this peptide as part of normal physiology.
This natural origin does not guarantee safety in pharmacological use. Many natural compounds are harmful at high doses. But it does suggest the body has existing systems to process and respond to BRP, potentially reducing the risk of completely unexpected effects.
Potential applications of BRP peptide
While BRP remains in preclinical development, its mechanism and effects suggest several potential therapeutic applications beyond simple weight loss.
Obesity treatment
The most obvious application is as an anti-obesity drug. The efficacy shown in animal studies, combined with the improved side effect profile compared to GLP-1 drugs, positions BRP as a potential first-line or alternative treatment for obesity.
For patients who cannot tolerate semaglutide or similar drugs, BRP could provide an option. For patients concerned about muscle loss during treatment, BRP's fat-specific effects could be particularly valuable. The weight loss and muscle preservation combination is rare in current medications.
Metabolic syndrome management
BRP improved glucose and insulin tolerance in treated mice independent of direct pancreatic effects. This suggests potential utility for metabolic syndrome, the cluster of conditions including obesity, insulin resistance, and dyslipidemia that increases cardiovascular risk.
By addressing obesity while simultaneously improving metabolic markers, BRP could offer integrated treatment for metabolic syndrome. Current management often requires multiple drugs. A single agent addressing multiple components would simplify treatment.
Adjunct to lifestyle modification
Weight loss medications work best combined with diet and exercise. BRP's appetite-suppressing effects could help patients adhere to caloric restriction while its fat-specific weight loss preserves the muscle gained through exercise.
This combination could be particularly valuable for athletic or active patients who need to lose fat while maintaining or building muscle. Current GLP-1 drugs are suboptimal for this population because of muscle loss concerns.
Research tool for appetite neuroscience
Beyond therapy, BRP offers value as a research tool. Its novel mechanism, distinct from GLP-1, leptin, and MC4R pathways, provides a new probe for studying appetite regulation in the hypothalamus.
Identifying the BRP receptor will advance understanding of central appetite control. This knowledge could lead to additional drug targets and deeper understanding of obesity pathophysiology. Even if BRP itself never reaches clinical use, the research it enables has value.
Where to find BRP peptide for research
BRP peptide is currently available only for research purposes. It has not been approved by any regulatory agency for human use. The compound is being commercialized by Merrifield Therapeutics, the company co-founded by senior researcher Dr. Katrin Svensson, which plans to conduct clinical trials.
Research suppliers
Several peptide research suppliers have begun offering BRP for laboratory investigation. These include:
Phoenix Pharmaceuticals offers BRINP2-related peptide / BRP Free Acid (Human) for research applications. Their product is the free acid form for laboratory use.
NovoPro Labs provides BRP/BRINP2-related peptide at greater than 98% purity. The peptide is delivered in lyophilized form and should be stored frozen at or below -20C. They note pricing starting at $3.20 per amino acid for custom synthesis.
Cayman Chemical lists BRINP2-related Peptide (BRP) as an anorexigenic peptide available for research.
AdipoGen Life Sciences offers BRP under product code AG-CP3-0048 for research purposes.
Important considerations for research use
Researchers should note several critical factors when sourcing BRP:
The C-terminal amidation is essential for activity. Non-amidated forms will not produce biological effects. Confirm with suppliers that the product is correctly amidated.
The sequence THRILRRLFNLC must be exact. Particularly leucine at position 8 is critical for function. Any substitution eliminates activity. Verify sequence and purity with analytical data.
Proper peptide storage is essential. Lyophilized peptide should be kept frozen. Reconstituted peptide should follow standard reconstituted peptide handling protocols.
BRP is not approved for human use. Research use only. Any human studies must follow appropriate regulatory and ethical guidelines with proper institutional approval.
The future of BRP peptide: clinical development
The path from promising animal studies to approved medication is long and uncertain. Many compounds that work in mice fail in humans. However, BRP has several factors working in its favor as it moves toward clinical development.
Merrifield Therapeutics and clinical trials
Dr. Katrin Svensson co-founded Merrifield Therapeutics specifically to advance BRP toward clinical use. The company plans to launch human clinical trials in the near future. Phase 1 studies will establish safety, tolerability, and preliminary dosing in healthy volunteers.
The researchers hold patents on BRP peptides for metabolic disorders, providing intellectual property protection for commercial development. This patent position should attract investment and support continued development.
Ongoing research priorities
Several questions need answering before BRP can advance to late-stage clinical trials:
Receptor identification. The specific receptor that BRP binds remains unknown. Identifying it would enable better understanding of the mechanism, potential off-target effects, and opportunities for optimization.
Duration of action. Current studies used daily injections. Researchers are investigating how to extend BRP's effects to allow less frequent dosing. Weekly or monthly administration would be more practical for chronic use.
Human pharmacokinetics. How BRP is absorbed, distributed, metabolized, and eliminated in humans is unknown. These parameters will determine dosing schedules and delivery methods.
Long-term safety. The 14-day mouse studies showed no problems. But chronic use for obesity treatment means years of exposure. Long-term animal studies and eventually human trials will need to establish safety over extended periods.
Timeline expectations
Drug development typically takes 10-15 years from discovery to approval. BRP was first published in March 2025. If Phase 1 trials begin soon, Phase 2 studies might occur in 2027-2028. Phase 3 pivotal trials, if successful, might complete by 2030-2032. FDA approval, if everything goes well, might come in the early 2030s.
This is optimistic. Many drugs fail in development. But for researchers tracking the peptide development landscape, BRP represents one of the most promising new compounds in the obesity space.
How BRP fits into the broader peptide landscape
BRP does not exist in isolation. It joins a growing array of peptides being studied for metabolic regulation. Understanding where it fits helps researchers evaluate its potential and identify research opportunities.
Relationship to GLP-1 agonists
GLP-1 agonists like semaglutide, liraglutide, and tirzepatide dominate the current weight loss peptide market. They work. But they have limitations that BRP might address.
BRP is not competing to replace GLP-1 drugs. It offers an alternative mechanism that might work better for certain patients or complement GLP-1 therapy. Combination approaches using drugs with different mechanisms often outperform single agents. BRP plus a low-dose GLP-1 agonist might provide better weight loss with fewer side effects than either alone.
Comparison to other metabolic peptides
Several other peptides target metabolism through various mechanisms:
AOD-9604 is a fragment of human growth hormone studied for fat loss. Unlike BRP, it works through lipolytic (fat-burning) pathways rather than appetite suppression.
MOTS-c is a mitochondrial peptide that improves metabolic function and exercise capacity. It addresses metabolism differently than BRP, potentially making them complementary.
SS-31 targets mitochondrial function and has shown benefits for metabolic health. Again, the mechanism differs from BRP.
Ipamorelin and other growth hormone secretagogues affect body composition through GH release. They work through entirely different pathways than BRP.
BRP occupies a unique niche as a centrally-acting appetite suppressant with a novel mechanism. This uniqueness could make it valuable either as a standalone treatment or in combination with other approaches.
The evolving weight loss peptide market
The market for weight loss drugs is projected to reach $100 billion by 2030. GLP-1 agonists currently dominate, but their side effects and supply constraints create opportunity for alternatives. BRP arrives at an ideal moment.
Researchers and clinicians are actively seeking options for patients who cannot tolerate GLP-1 drugs. They want compounds that preserve muscle during weight loss. They need drugs that work through different mechanisms for non-responders. BRP potentially addresses all these needs.
For those involved in peptide research and development, BRP represents both a promising compound and validation of AI-driven drug discovery approaches. Its success would accelerate the search for additional peptide therapeutics.
BRP peptide safety and side effects
Safety data for BRP comes entirely from animal studies. No human safety information exists yet. However, the animal data provides initial indications of the compound's tolerability profile.
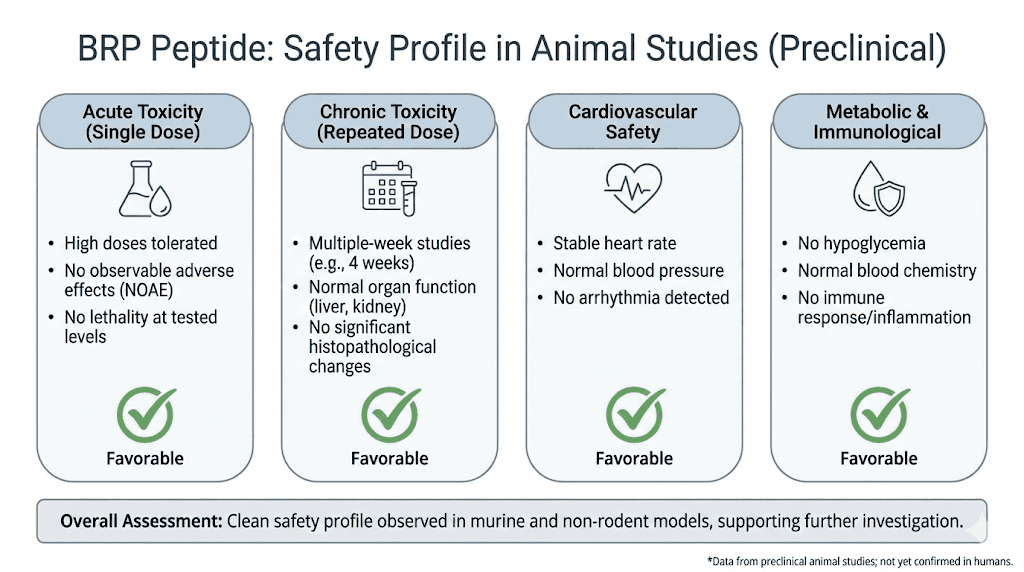
Observed safety profile in animal studies
The Stanford researchers specifically evaluated behavioral and physiological markers of safety in treated animals:
No anxiety-like behavior was observed. Animals performed normally on standard anxiety tests.
Locomotor activity was unchanged. Treated animals moved and behaved normally.
Water intake remained stable. This suggests no dehydration effects.
Fecal production was normal. This indicates preserved gut function without the constipation seen with GLP-1 drugs.
No sucrose preference changes occurred. This behavioral measure suggests no effects on reward or pleasure systems.
No nausea or food aversion behaviors were observed.
Weight loss came specifically from fat tissue. Lean mass was preserved.
What remains unknown
Animal studies cannot predict all human responses. Several safety questions will only be answered through clinical trials:
Cardiovascular effects. Weight loss drugs have had cardiovascular concerns historically. Long-term cardiac safety needs evaluation.
Cancer risk. Chronic use of any agent requires cancer surveillance in trials.
Reproductive effects. Many peptides affect fertility or pregnancy. BRP's effects here are unknown.
Drug interactions. How BRP interacts with common medications needs study.
Rare adverse events. Side effects occurring in 1 in 1000 or fewer patients only appear in large trials.
Comparison to GLP-1 drug safety profile
For context, GLP-1 drugs have known safety considerations including:
Gastrointestinal events (nausea, vomiting, diarrhea, constipation) in 30-50% of patients.
Pancreatitis risk, though the actual increase may be small.
Thyroid C-cell tumor concerns in rodents (black box warning).
Muscle and bone mass loss with weight reduction.
Gastroparesis with prolonged use in some patients.
BRP animal studies showed none of these concerns, but human data will be essential for proper safety assessment. Researchers should follow standard peptide safety protocols when working with BRP in laboratory settings.
Frequently asked questions
What does BRP stand for in peptides?
BRP stands for BRINP2-related peptide. It is a 12-amino acid peptide derived from the BRINP2 protein through enzymatic cleavage. The name comes from the parent protein, BMP/retinoic acid inducible neural specific 2 (BRINP2).
Is BRP peptide better than Ozempic?
BRP has only been tested in animals, while Ozempic (semaglutide) has extensive human trial data. Animal studies suggest BRP may offer similar weight loss with fewer side effects and better muscle preservation. However, human trials are needed to confirm these advantages. Currently, Ozempic is proven in humans while BRP is experimental.
When will BRP peptide be available for patients?
Merrifield Therapeutics plans to begin human clinical trials soon. If development proceeds smoothly, BRP might reach the market in the early 2030s. Drug development typically takes 10-15 years from discovery. BRP was first published in March 2025.
How does BRP peptide cause weight loss?
BRP activates neurons in the hypothalamus through the CREB-FOS signaling pathway. This suppresses appetite at the brain level without affecting the gut or pancreas. In studies, it reduced food intake by up to 50% and caused fat-specific weight loss while preserving muscle mass.
Can you buy BRP peptide?
BRP is available from research chemical suppliers for laboratory use only. It is not approved for human consumption. Companies including Phoenix Pharmaceuticals, NovoPro Labs, Cayman Chemical, and AdipoGen offer BRP for research purposes. Always verify C-terminal amidation and sequence accuracy when sourcing.
Is BRP peptide natural or synthetic?
BRP is naturally occurring. It is produced in the human body when enzymes cleave the BRINP2 protein. However, for research and therapeutic development, BRP is synthesized in laboratories to ensure purity and consistency. The synthetic form replicates the natural peptide sequence exactly.
Does BRP peptide affect muscle mass?
Unlike GLP-1 drugs which can cause muscle loss accounting for up to 20% of weight lost, BRP appears to spare lean tissue. In mouse studies, virtually all weight loss came from fat mass with muscle mass preserved. This is one of BRP's most significant potential advantages.
What are the side effects of BRP peptide?
In animal studies, BRP showed no significant side effects. Animals maintained normal behavior, activity, water intake, and bowel function. No nausea or food aversion occurred. Human side effects are unknown until clinical trials complete.
For researchers serious about understanding emerging peptide therapeutics, SeekPeptides provides comprehensive guides to both established and experimental compounds. The platform offers evidence-based protocols, dosing calculators, and access to a community of researchers navigating the rapidly evolving peptide landscape. Whether evaluating new discoveries like BRP or optimizing protocols for established compounds, SeekPeptides members get the depth of information needed for informed research decisions.



