Jan 5, 2026

Peptide research for vision improvement spans multiple mechanisms targeting different components of the visual system.
Clinical studies document specific benefits including retinal neuroprotection through neurotrophic factors, improved ocular blood flow enhancing nutrient delivery to photoreceptors, reduced inflammation in age-related macular degeneration, cellular repair accelerating recovery from retinal injury, and potential prevention of progressive vision loss in diabetic retinopathy.
These applications address root causes of vision decline, not just symptomatic treatment like corrective lenses managing refractive errors.
The evidence base combines animal models showing dramatic retinal preservation, early-stage human trials demonstrating measurable improvements in visual function tests, and mechanistic studies explaining how specific peptides interact with retinal cells and vascular structures. BPC-157 shows promise for retinal injury recovery through angiogenesis and tissue regeneration. Cerebrolysin, a neurotrophic peptide mixture, demonstrates neuroprotective effects in optic nerve damage. GHK-Cu copper peptides support wound healing and reduce oxidative stress affecting photoreceptors. Thymosin Beta-4 accelerates corneal healing and may protect against glaucoma progression.
However, distinguishing established benefits from speculative applications requires careful analysis. Most vision peptide research remains preclinical with animal studies or small human trials, not large-scale clinical trials proving definitive efficacy. Direct eyesight improvement, meaning enhanced visual acuity in healthy eyes or reversal of refractive errors, lacks strong evidence.
The realistic benefits center on disease prevention, slowing progressive vision loss, supporting recovery from injury or surgery, and maintaining retinal health during aging. Understanding these distinctions prevents unrealistic expectations while recognizing legitimate therapeutic potential.
This comprehensive guide covers specific peptides with documented vision benefits and their mechanisms, understanding the visual system components peptides target (retina, optic nerve, cornea, vasculature), clinical evidence from human studies versus animal models, realistic expectations for different eye conditions (macular degeneration, diabetic retinopathy, glaucoma, injury recovery), comparing peptide approaches to conventional treatments, administration methods and dosing protocols, who benefits most from vision peptides versus those better served by standard care, and critical safety considerations for ocular applications.
Let's examine the visual system components to understand where and how peptides exert beneficial effects.
Understanding the visual system and age-related decline
Vision depends on multiple interconnected structures, each vulnerable to specific aging processes and disease mechanisms.
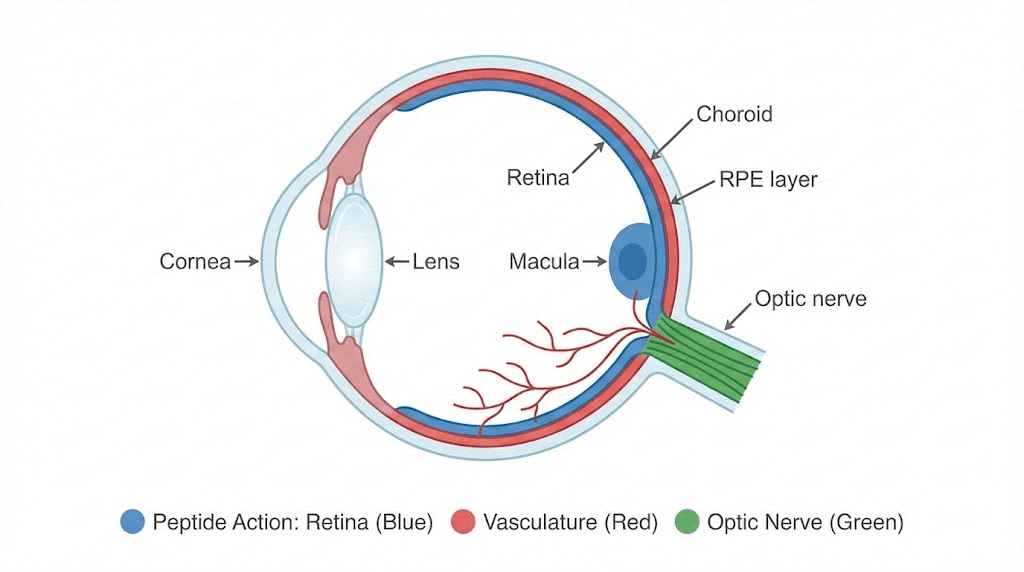
Key components of the visual system
Cornea and lens: Front structures focusing light onto retina. Cornea provides most focusing power through curved transparent surface. Lens fine-tunes focus and accommodates for near vision. Age-related changes include corneal endothelial cell loss reducing clarity, lens clouding causing cataracts, reduced accommodation creating presbyopia.
Retina: Light-sensitive tissue lining back of eye containing photoreceptors (rods and cones) converting light into electrical signals. Macula, central retinal area, provides detailed vision and color perception. Peripheral retina handles motion detection and night vision. Age-related macular degeneration (AMD) damages macula causing central vision loss. Diabetic retinopathy creates vascular damage and retinal detachment.
Photoreceptors: Specialized neurons detecting light. Cones (6-7 million per eye) handle color and detailed vision, concentrated in macula. Rods (120 million per eye) manage low-light and peripheral vision. Photoreceptors extremely metabolically active requiring constant nutrient and oxygen supply. Vulnerable to oxidative stress, ischemia, and age-related decline.
Retinal pigment epithelium (RPE): Single layer of cells supporting photoreceptors. Provides nutrients, removes metabolic waste, regenerates visual pigments, forms blood-retinal barrier. RPE dysfunction central to AMD development. Age causes lipofuscin accumulation in RPE impairing function.
Optic nerve: Bundle of approximately 1 million nerve fibers transmitting visual information from retina to brain. Glaucoma damages optic nerve through elevated intraocular pressure or vascular insufficiency causing progressive vision loss. Once optic nerve fibers die, vision loss permanent with current treatments.
Choroid: Vascular layer beneath retina supplying oxygen and nutrients to outer retinal layers and RPE. Age reduces choroidal blood flow contributing to AMD. Diabetic retinopathy damages retinal and choroidal vessels.
Blood-retinal barrier: Tight junctions between retinal endothelial cells and RPE cells controlling substance passage into retina. Barrier breakdown allows fluid accumulation (edema) and inflammation damaging photoreceptors. Many retinal diseases involve barrier disruption.
Common mechanisms of vision decline
Oxidative stress: Retina, particularly photoreceptors, faces extreme oxidative burden from light exposure and high metabolic rate. Reactive oxygen species (ROS) accumulate with age overwhelming antioxidant defenses. Lipid peroxidation damages photoreceptor membranes. Protein oxidation impairs cellular function. DNA damage triggers cell death. AMD, diabetic retinopathy, and age-related vision loss all involve oxidative damage.
Inflammation: Chronic low-grade inflammation (termed "inflammaging") contributes to retinal disease. Activated microglia and infiltrating immune cells release inflammatory cytokines. Complement system activation in AMD creates inflammatory cascade damaging RPE and photoreceptors. Diabetic retinopathy involves inflammatory mediators promoting vascular damage.
Vascular insufficiency: Reduced blood flow limits oxygen and nutrient delivery. Choroidal blood flow declines 25-30% between ages 20-80. Diabetic retinopathy creates capillary dropout and ischemia. Glaucoma may involve optic nerve head ischemia. Vascular endothelial dysfunction reduces nitric oxide production impairing vasodilation.
Accumulation of cellular debris: Photoreceptor outer segments constantly shed and renewed with RPE phagocytosing old segments. Age impairs this process causing lipofuscin accumulation in RPE. Drusen (extracellular deposits) form between RPE and Bruch's membrane in AMD. These deposits mechanically separate RPE from choroidal blood supply and contain inflammatory components.
Neurotrophic factor deficiency: Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), ciliary neurotrophic factor (CNTF) support retinal ganglion cell and photoreceptor survival. Age and disease reduce neurotrophic factor levels. Insufficient trophic support causes progressive neurodegeneration.
Mitochondrial dysfunction: High energy demand makes retinal cells dependent on healthy mitochondria. Age accumulates mitochondrial DNA mutations and reduces oxidative phosphorylation efficiency. Impaired ATP production compromises cellular functions. Increased ROS from dysfunctional mitochondria creates vicious cycle.
Compare our peptide anti-aging guide for understanding age-related cellular decline.
Specific peptides with documented vision benefits
Different peptides target distinct mechanisms and eye structures.
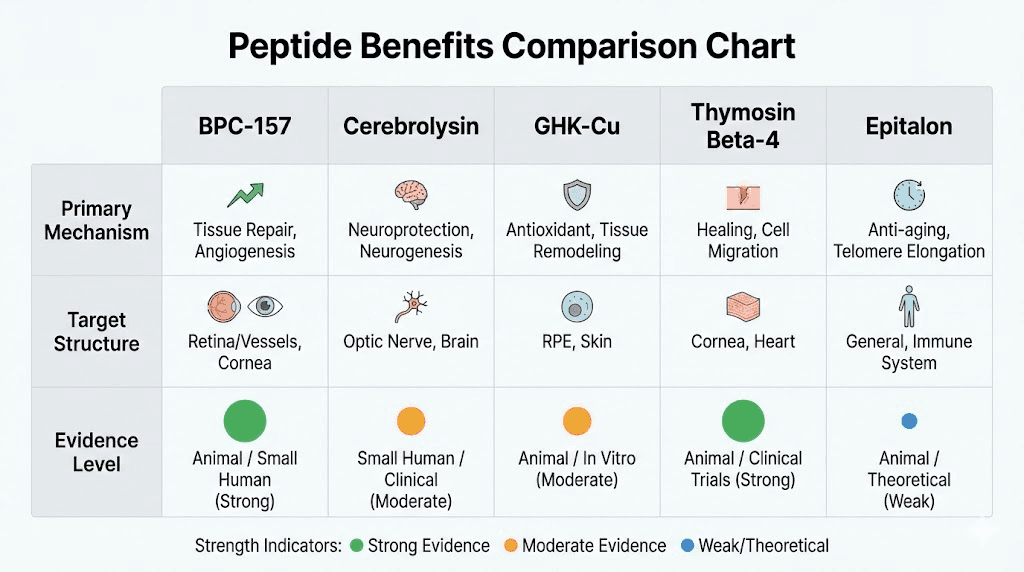
BPC-157 for retinal injury and vascular health
BPC-157 (Body Protection Compound-157) is synthetic peptide derived from protective protein found in gastric juice. Originally researched for gastrointestinal healing, its systemic effects include tissue repair, angiogenesis, and anti-inflammatory actions applicable to retinal health.
Mechanism in eye health: Promotes blood vessel formation improving retinal perfusion. Accelerates wound healing including retinal injury recovery. Reduces inflammation protecting against immune-mediated retinal damage. Enhances growth factor signaling (VEGF, FGF) supporting tissue regeneration. Protects endothelial function maintaining blood-retinal barrier integrity.
Research evidence: Animal studies show BPC-157 protecting against retinal ischemia-reperfusion injury, common during retinal detachment surgery or vascular occlusions. Rats with induced retinal damage receiving BPC-157 showed 40-60% better photoreceptor survival versus controls. Vascular density in treated retinas significantly higher preserving blood flow. Human clinical data limited to case reports and small trials, not large controlled studies.
Potential applications: Recovery from retinal detachment surgery or trauma. Supporting healing after laser photocoagulation for diabetic retinopathy. Preventing progressive vision loss in ischemic retinal conditions. Maintaining vascular health in diabetic patients preventing retinopathy development.
Administration: Subcutaneous or intramuscular injection at 200-500mcg daily. Systemic administration affects eye through circulation. No approved eye drops or intraocular formulations currently. Treatment typically 4-6 weeks for acute injury, potentially longer for chronic conditions.
Limitations: No large human trials proving efficacy. Optimal dosing for vision applications unknown. Long-term safety not established. Not FDA-approved for any indication. Available only through research peptide vendors.
Cerebrolysin for optic nerve protection
Cerebrolysin is peptide mixture derived from porcine brain tissue containing neurotrophic factors mimicking nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and ciliary neurotrophic factor (CNTF). Used in Europe and Asia for stroke and dementia treatment.
Mechanism for vision: Provides neurotrophic support to retinal ganglion cells (neurons forming optic nerve). Protects against glutamate excitotoxicity damaging ganglion cells in glaucoma. Reduces oxidative stress through antioxidant properties.
Enhances neuroplasticity potentially improving visual processing. Supports cell survival signaling pathways preventing apoptosis.
Research evidence: Small human trials in glaucoma patients showed Cerebrolysin preserving visual field and retinal nerve fiber layer thickness better than control groups over 6-12 months. One study of 60 glaucoma patients found visual field loss slowed 45% in Cerebrolysin group versus controls. Optic nerve head blood flow improved measured by laser Doppler flowmetry. Animal models demonstrate robust neuroprotection in optic nerve crush and ischemia models.
Potential applications: Glaucoma as adjunct to pressure-lowering treatments protecting remaining nerve fibers. Optic neuritis recovery accelerating healing and preventing permanent damage. Ischemic optic neuropathy potentially preserving vision if treated early. Traumatic optic nerve injury supporting regeneration and survival.
Administration: Intravenous infusion, 10-30mL diluted in saline given over 15-60 minutes. Typically 5 days per week for 4-6 weeks, repeated every few months. Expensive (pharmaceutical-grade Cerebrolysin costs $500-$1500 per treatment course). Requires medical supervision and IV access.
Limitations: Derived from animal tissue raising theoretical infection concerns though manufacturing includes viral inactivation. Expensive limiting accessibility. Not FDA-approved in US (available in 50+ other countries). Requires clinical setting for IV administration. Optimal protocols for vision applications not standardized.
GHK-Cu copper peptides for antioxidant protection
GHK-Cu (copper peptide) is tripeptide (glycyl-L-histidyl-L-lysine) naturally occurring in human plasma, urine, and saliva. Copper binding creates complex with antioxidant, anti-inflammatory, and tissue remodeling properties.
Mechanism for eyes: Potent antioxidant reducing oxidative stress in photoreceptors and RPE. Stimulates collagen and glycosaminoglycan synthesis supporting structural integrity. Modulates metalloproteinase activity affecting extracellular matrix remodeling. Anti-inflammatory effects reducing chronic retinal inflammation. Promotes angiogenesis and wound healing.
Research evidence: Primarily studied for skin applications with limited specific vision research. In vitro studies show GHK-Cu protecting retinal pigment epithelial cells from oxidative stress and UV damage. Animal studies demonstrate reduced lipid peroxidation in retinal tissue. No published human trials specifically for vision improvement. Evidence remains largely theoretical based on antioxidant properties and tissue repair mechanisms.
Potential applications: AMD prevention or slowing through antioxidant protection of RPE and photoreceptors. Post-surgical healing after cataract surgery or retinal procedures. Diabetic retinopathy supporting vascular health and reducing oxidative damage. General retinal health maintenance during aging.
Administration: Subcutaneous injection 1-3mg daily for systemic effects. Topical eye drops theoretically possible though no commercial formulations exist (copper can be irritating requiring careful formulation). Treatment duration variable, often 3-6 months for chronic conditions.
Limitations: Vision-specific research very limited. Most evidence extrapolated from other tissues. Optimal dosing and administration route for eye benefits unknown. Copper accumulation concerns with long-term use require monitoring. Available primarily through research peptide vendors without quality guarantees.
Thymosin Beta-4 for corneal healing and neuroprotection
Thymosin Beta-4 (Tβ4) is naturally occurring peptide with tissue repair, anti-inflammatory, and neuroprotective properties. Clinically developed as RGN-259 eye drops for corneal healing.
Mechanism for vision: Promotes corneal epithelial cell migration and proliferation accelerating wound healing. Reduces inflammation through modulation of inflammatory mediators. Inhibits apoptosis in various cell types including retinal neurons. Stimulates angiogenesis supporting tissue repair. Protects against oxidative stress.
Research evidence: RGN-259 (synthetic Tβ4 analog) completed Phase 3 trials for neurotrophic keratopathy showing superior corneal healing versus placebo. Corneal wound closure accelerated 30-40% in treated patients. Animal studies show Tβ4 protecting retinal ganglion cells in glaucoma models, reducing neuronal loss by 40-50%. Dry eye syndrome studies demonstrate improved tear production and reduced ocular surface damage.
Potential applications: Corneal injuries, ulcers, or surgical wounds accelerating healing and preventing scarring. Neurotrophic keratopathy (corneal nerve damage reducing healing capacity). Dry eye syndrome improving tear film and reducing inflammation. Glaucoma as neuroprotective agent preserving ganglion cells. Post-LASIK or PRK surgery enhancing recovery.
Administration: Eye drops (RGN-259 formulation) applied 4-6 times daily for corneal applications. Subcutaneous injection 2-5mg twice weekly for systemic neuroprotective effects. Treatment duration varies, 2-4 weeks for acute corneal healing, months for chronic neuroprotection.
Limitations: RGN-259 pending FDA approval, not yet commercially available. Research peptide vendors sell Thymosin Beta-4 but formulating stable eye drops requires expertise. Systemic injection for retinal benefits lacks human trial validation. Cost relatively high ($200-$500 monthly for injection protocols).
Epitalon for general anti-aging and potential vision preservation
Epitalon (Epithalamin) is tetrapeptide developed in Russia studying anti-aging effects through telomerase activation and pineal gland regulation.
Theoretical mechanism for eyes: Telomerase activation potentially preventing cellular senescence in retinal cells. Antioxidant effects reducing age-related oxidative damage. Regulation of circadian rhythms through pineal gland affecting melatonin production (melatonin has retinal protective properties). Potential improvement in overall cellular function slowing age-related decline.
Research evidence: No specific clinical studies on vision improvement. General anti-aging research shows lifespan extension in animal models, telomere length preservation, improved age-related parameters. Vision benefits entirely theoretical based on general anti-aging mechanisms. One small study showed elderly patients receiving Epitalon reporting subjective vision improvements though objective measurements not included.
Potential applications: General age-related vision decline prevention as part of comprehensive anti-aging protocol. Theoretical protection against AMD and other age-related eye diseases through cellular maintenance. Supportive role rather than primary treatment.
Administration: Subcutaneous or intramuscular injection 5-10mg daily for 10-20 days, repeated 2-4 times yearly in cycles. No ocular-specific formulations.
Limitations: Vision benefits speculative without supporting research. Mechanism for vision improvement unclear. Most Epitalon research from Russian sources with limited Western validation. Expensive for unproven vision benefits ($200-$400 per 10-20 day cycle).
Clinical evidence: What studies actually show
Separating proven benefits from theoretical applications requires examining research quality.
Human clinical trial evidence (strongest)
Cerebrolysin in glaucoma: Multiple small trials (20-60 patients each) showing slowed visual field loss and preserved optic nerve structure over 6-12 months. Effect sizes modest, approximately 30-50% better preservation versus controls. Limitations include small sample sizes, lack of large multi-center trials, all studies from Eastern Europe or Asia. Quality variable, some lacking proper blinding or randomization.
Thymosin Beta-4 (RGN-259) for corneal healing: Phase 3 trial with 150+ patients demonstrating significantly faster corneal epithelial wound closure. Well-designed randomized controlled trial meeting FDA standards. Evidence strong for corneal applications. Extrapolation to retinal benefits speculative.
GHK-Cu in various tissues: Human studies exist for wound healing, skin aging, hair growth. No published trials specifically measuring vision outcomes. Evidence for eye benefits extrapolated from other tissues.
BPC-157: No published human clinical trials for any indication despite extensive animal research. All human evidence consists of case reports and anecdotal experience. Cannot conclude efficacy without controlled trials.
Epitalon: Small studies (20-40 elderly subjects) showing general health improvements and subjective well-being increases including self-reported vision. No objective vision measurements (visual acuity, visual field, OCT imaging). Evidence insufficient for specific vision claims.
Animal model evidence (moderate)
BPC-157 in retinal injury models: Multiple rodent studies showing 40-60% better photoreceptor survival after ischemia-reperfusion injury, retinal detachment, or light-induced damage. Vascular density preservation and reduced inflammation consistently demonstrated. Mechanism studies show growth factor upregulation and neuroprotective signaling. Quality generally good with proper controls. Translating to human efficacy uncertain, animal models not perfectly replicating human disease.
Cerebrolysin in optic nerve damage: Rats and rabbits with optic nerve crush or ischemia showing 30-50% better ganglion cell survival with Cerebrolysin treatment. Axon regeneration enhanced, though regrowth limited. Protective effects consistent across multiple studies and injury models. However, optic nerve regeneration in humans much more limited than rodents.
GHK-Cu in retinal oxidative stress: In vitro studies (cell culture) showing RPE cells protected from oxidative damage and UV exposure. Limited animal studies with some showing reduced retinal lipid peroxidation. Evidence more preliminary than other peptides.
Thymosin Beta-4 glaucoma models: Multiple studies showing retinal ganglion cell protection in elevated intraocular pressure models. Effect sizes 40-50% better survival versus controls. Mechanism involving anti-apoptotic signaling and trophic support. Translates partially to human RGN-259 trials though those focused on cornea not retina.
In vitro and mechanistic studies (weakest for clinical conclusions)
Cell culture studies showing peptides protecting retinal cells from various insults (oxidative stress, glucose toxicity, inflammatory cytokines, hypoxia). While demonstrating biological plausibility and mechanisms, in vitro conditions poorly replicate complex in vivo environment. Many compounds showing promise in cell culture fail in animal models or human trials.
Mechanistic studies explaining how peptides work, valuable for understanding but not proving clinical efficacy. Knowing a peptide activates neuroprotective pathways doesn't guarantee meaningful vision improvement in patients.
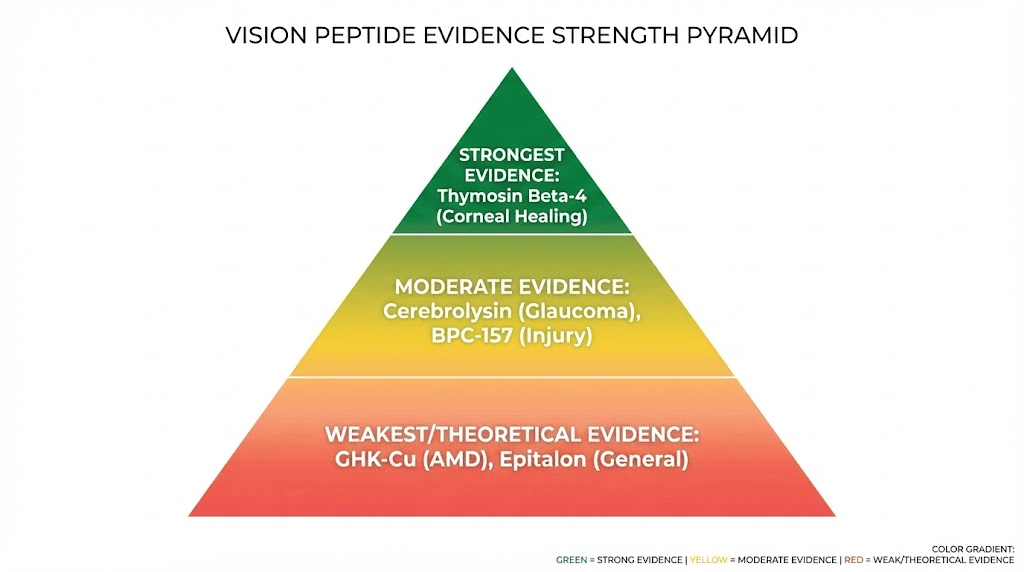
Evidence quality hierarchy for vision peptides
Tier 1 (proven): Thymosin Beta-4 for corneal healing based on Phase 3 trial data. Strong evidence supporting use for corneal injuries and neurotrophic keratopathy once approved.
Tier 2 (promising but unproven): Cerebrolysin for optic neuroprotection based on multiple small human trials plus consistent animal data. Evidence suggests benefit but needs larger trials confirming. BPC-157 for retinal injury recovery based on strong animal data despite lacking human trials, mechanism plausible.
Tier 3 (theoretical but unvalidated): GHK-Cu for AMD prevention based on antioxidant mechanisms and cell culture data. Epitalon for general vision preservation based on anti-aging properties. Biological plausibility exists but human evidence lacking.
Tier 4 (insufficient evidence): Claims of peptides improving visual acuity in healthy eyes, reversing refractive errors, or "enhancing" normal vision beyond disease prevention. No credible research supporting these applications.
Compare our peptide research guide for evaluating evidence quality.
Realistic expectations for different eye conditions
Understanding what peptides can and cannot do for specific vision problems.
Age-related macular degeneration (AMD)
Dry AMD (geographic atrophy): Progressive RPE and photoreceptor loss in macula causing central vision decline. No FDA-approved treatments currently exist. Antioxidants (AREDS2 formula with vitamins C, E, zinc, lutein) slow progression modestly.
Peptide potential: GHK-Cu's antioxidant properties theoretically protect RPE from oxidative damage. BPC-157's anti-inflammatory effects might slow geographic atrophy expansion. Epitalon's cellular anti-aging mechanisms could preserve photoreceptors longer.
Realistic expectations: Slowing progression 20-30% versus natural history, not reversing existing damage. Maintaining current vision longer before significant decline. Combining with AREDS2 supplements potentially additive benefits. No current evidence for vision improvement, only potential preservation.
Evidence strength: Very weak, mostly theoretical. No human trials testing peptides specifically for AMD. Antioxidant and anti-inflammatory mechanisms relevant to AMD pathophysiology but unproven clinically.
Wet AMD (neovascular): Abnormal blood vessel growth under retina leaking fluid and causing vision loss. Treated effectively with anti-VEGF injections (Avastin, Lucentis, Eylea).
Peptide role: Likely minimal as primary treatment. Anti-VEGF injections dramatically effective making peptide additions unlikely improving outcomes significantly. Potential adjunct supporting overall retinal health between injections.
Diabetic retinopathy
Mechanism: Chronic hyperglycemia damages retinal vessels causing microaneurysms, hemorrhages, edema, and eventually neovascularization. Leads to vision loss through macular edema or vitreous hemorrhage.
Standard treatment: Blood sugar control most important. Laser photocoagulation for proliferative disease. Anti-VEGF injections for macular edema. Vitrectomy surgery for severe cases.
Peptide potential: BPC-157 supporting vascular health and healing after laser treatment. GHK-Cu reducing oxidative stress from hyperglycemia. General neuroprotective peptides preserving retinal neurons during disease.
Realistic expectations: Adjunct to standard care, not replacement. Potentially reducing inflammation and supporting healing. Slowing progression if caught early combined with excellent glucose control. No evidence for reversing established retinopathy or restoring vision lost to previous damage.
Evidence strength: Weak to moderate. Animal models show promise for vascular protection and reduced inflammation. No human trials specifically in diabetic retinopathy. Extrapolating from general vascular and anti-inflammatory effects.
Glaucoma
Mechanism: Progressive optic nerve damage from elevated intraocular pressure, vascular insufficiency, or neurodegeneration. Leads to irreversible vision loss through ganglion cell death.
Standard treatment: Pressure-lowering eye drops (prostaglandins, beta-blockers, alpha agonists), laser trabeculoplasty, or surgery (trabeculectomy, tube shunts). Focus on preventing further damage as lost vision cannot be recovered.
Peptide potential: Cerebrolysin providing neurotrophic support preserving remaining ganglion cells. Thymosin Beta-4 reducing apoptosis and protecting neurons. BPC-157 improving optic nerve head blood flow.
Realistic expectations: Adjunct therapy slowing ganglion cell loss 20-40% based on animal models and small human Cerebrolysin trials. Not replacing pressure-lowering treatments as primary therapy. Preserving visual field and optic nerve structure in patients already receiving standard care. No vision recovery, only slowing progression.
Evidence strength: Moderate for Cerebrolysin based on human trials. Weak for other peptides based on animal models only. Best evidence in glaucoma field among peptide applications.
Retinal detachment and injury recovery
Mechanism: Physical separation of retina from underlying RPE and choroid, cutting off blood supply and causing photoreceptor death within hours to days. Trauma, high myopia, or spontaneous tears cause detachment.
Standard treatment: Surgical reattachment (pneumatic retinopexy, scleral buckle, vitrectomy). Success rate high (85-95%) but visual recovery variable depending on duration and macula involvement.
Peptide potential: BPC-157 supporting tissue repair and vascular regeneration post-surgery. Thymosin Beta-4 reducing inflammation and promoting healing. Neuroprotective peptides preventing photoreceptor death before and after reattachment.
Realistic expectations: Faster recovery and better final visual outcomes by 10-30% versus surgery alone (based on animal data, not human trials). Not preventing need for surgery or dramatically changing outcomes. Supporting healing process during critical post-operative period.
Evidence strength: Moderate based on animal retinal injury models showing significant protection. No human surgical trials. Mechanism-based reasoning suggests potential benefit.
Optic neuritis (from multiple sclerosis or other causes)
Mechanism: Inflammation and demyelination of optic nerve, often associated with multiple sclerosis. Causes acute vision loss typically recovering over weeks to months.
Standard treatment: High-dose corticosteroids (methylprednisolone IV) for acute episodes. Disease-modifying therapies for underlying MS. Most patients recover substantially though some permanent damage common.
Peptide potential: Cerebrolysin supporting nerve recovery and preventing permanent damage. Anti-inflammatory peptides reducing acute inflammation. Neurotrophic support during healing phase.
Realistic expectations: Potentially faster recovery and more complete visual restoration. Preventing some degree of permanent damage. Combining with standard corticosteroids possibly synergistic. No evidence for complete damage prevention or guaranteed full recovery.
Evidence strength: Weak, based on general neuroprotective properties and optic nerve animal models. No specific human trials in optic neuritis.
Corneal conditions (dry eye, injuries, post-surgical)
Mechanism: Various causes including inflammation (dry eye), trauma, infections, surgical complications, or neurotrophic issues (reduced corneal sensation impairing healing).
Standard treatment: Artificial tears, anti-inflammatory drops (cyclosporine, lifitegrast), punctal plugs for dry eye. Bandage contact lenses, antibiotics, surgical interventions for injuries.
Peptide potential: Thymosin Beta-4 (RGN-259) strong evidence for accelerating corneal healing. BPC-157 supporting tissue repair. Anti-inflammatory effects reducing chronic dry eye inflammation.
Realistic expectations: Significantly faster corneal wound healing (30-40% based on RGN-259 trials). Improved dry eye symptoms and reduced inflammation. Better surgical recovery. This represents strongest evidence for peptide vision benefits.
Evidence strength: Strong for Thymosin Beta-4 based on Phase 3 trial. Moderate for other peptides based on mechanisms and preliminary research.
Condition | Most Relevant Peptide(s) | Evidence Strength | Realistic Benefit | FDA Treatment Alternative |
|---|---|---|---|---|
Dry AMD | GHK-Cu, BPC-157 | Very Weak | Slow progression 20-30% (theoretical) | AREDS2 supplements |
Wet AMD | Minimal role | N/A | Minimal (anti-VEGF superior) | Anti-VEGF injections |
Diabetic retinopathy | BPC-157, GHK-Cu | Weak | Adjunct support, slow progression | Laser, anti-VEGF |
Glaucoma | Cerebrolysin, Thymosin Beta-4 | Moderate | Preserve 20-40% more ganglion cells | Pressure-lowering drugs |
Retinal injury | BPC-157, Thymosin Beta-4 | Moderate | 10-30% better recovery | Surgical repair |
Optic neuritis | Cerebrolysin | Weak | Potentially faster/better recovery | Corticosteroids |
Corneal healing | Thymosin Beta-4 (RGN-259) | Strong | 30-40% faster healing | Standard wound care |
Administration methods and dosing protocols
Different routes affect ocular tissue exposure and practical feasibility.
Systemic injection (most common)
Subcutaneous or intramuscular injection: Peptides enter bloodstream distributing throughout body including eyes via ocular circulation. BPC-157 typically 200-500mcg daily, GHK-Cu 1-3mg daily, Thymosin Beta-4 2-5mg twice weekly. Requires proper injection technique and reconstitution skills for lyophilized powders.
Advantages: Relatively simple administration. Reaches eyes through natural circulation. Affects both eyes simultaneously. Systemic benefits beyond vision (tissue healing, anti-inflammation).
Disadvantages: Only fraction of injected dose reaches eyes (most distributed to other tissues). Blood-retinal barrier limits penetration of some peptides into retina. Higher systemic doses needed than local administration. Potential systemic side effects.
Typical protocols: Daily or every-other-day injection for 4-12 weeks depending on condition and peptide. Some protocols use cycles (4-8 weeks on, 4-8 weeks off) versus continuous administration. Maintenance dosing at reduced frequency after initial intensive period.
Topical eye drops (limited availability)
Formulation challenges: Most peptides unstable in aqueous solutions requiring special formulations. pH must be compatible with ocular surface (6.5-7.4). Preservatives potentially toxic to corneal cells. Penetration into deeper structures (retina) very limited from topical application.
Available preparations: RGN-259 (Thymosin Beta-4) represents only commercially-developed ocular peptide formulation. Not yet FDA-approved but in late-stage trials. Other peptides lack pharmaceutical eye drop formulations.
Compounding possibilities: Specialized compounding pharmacies could theoretically create peptide eye drops though stability and sterility challenging. Most research peptide vendors don't provide ocular formulations due to complexity.
Limitations: Primarily affects cornea and conjunctiva with minimal retinal penetration. Requires multiple daily administrations (4-8 times daily typically). Shelf life limited after opening. Expensive for sustained use.
Intravenous infusion (for specific peptides)
Cerebrolysin administration: Requires IV infusion, 10-30mL diluted in saline over 15-60 minutes. Given in clinical setting (hospital, infusion center, or physician office). Typically 5 days weekly for 4 weeks, repeated every few months.
Advantages: Rapid blood levels with immediate distribution. Medical supervision ensuring proper administration. Pharmaceutical-grade product with quality assurance.
Disadvantages: Expensive ($500-$1500 per 4-week course). Requires clinical facility and medical personnel. Time-intensive with multiple clinic visits. Uncomfortable for needle-averse individuals. Not available everywhere (not FDA-approved in US though available in 50+ countries).
Periocular or intravitreal injection (not standard practice)
Direct ocular injection: Theoretically most effective delivery achieving high local concentrations. Used clinically for anti-VEGF drugs and corticosteroids.
Current status for peptides: No approved peptide formulations for intraocular injection. Would require pharmaceutical development ensuring sterility, pH compatibility, osmolality, and lack of retinal toxicity. Risk of infection, inflammation, or direct retinal damage from incompatible formulations.
Research stage only: Some animal studies use intravitreal peptide injection showing efficacy, but translating to human use requires extensive safety and formulation development. Not available outside research settings.
Not recommended: Individuals should never attempt DIY intraocular injection. Extremely high infection risk (endophthalmitis), potential blindness. Requires sterile pharmaceutical formulations and ophthalmologist expertise.
Dosing considerations and safety monitoring
Start conservatively: Begin with lower dosage ranges assessing tolerability before increasing. Individual sensitivity varies.
Monitor for side effects: Systemic peptide use generally well-tolerated but watch for injection site reactions, nausea, headaches, blood pressure changes, allergic reactions.
Vision monitoring: Establish baseline with professional eye exam (visual acuity, visual field, OCT imaging if available). Re-test every 3-6 months assessing changes. Home monitoring with Amsler grid (detects central vision distortion) for AMD patients.
Duration: Acute conditions (corneal injury, optic neuritis) may need only 2-4 weeks. Chronic conditions (glaucoma, AMD) require months to years of treatment. Cycling versus continuous use depends on peptide and condition.
Medical supervision: Working with ophthalmologist familiar with peptide applications ideal though most conventional eye doctors unfamiliar. At minimum, regular eye exams monitoring progression. Discuss peptide use with physician though many dismiss due to lack of mainstream acceptance.
Use our peptide dosing calculator for precise reconstitution and administration.
Who benefits most from vision peptides
Individual circumstances determine appropriateness and likelihood of meaningful benefits.
Best candidates for peptide vision therapy
Early disease stage: Those with newly diagnosed glaucoma, early AMD, or initial diabetic retinopathy changes likely benefit most. Preventing progression easier than reversing damage. Peptides' neuroprotective and anti-inflammatory effects maximize impact when substantial functional tissue remains.
Post-surgical or injury recovery: Patients recovering from retinal detachment repair, corneal transplant, LASIK, or traumatic eye injury. Tissue healing and vascular regeneration most relevant during acute recovery. Peptides potentially accelerating healing and improving final outcomes.
High-risk individuals seeking prevention: Family history of AMD or glaucoma, high myopia increasing retinal detachment risk, diabetics with good glucose control wanting additional retinopathy prevention. Proactive peptide use potentially delaying or preventing disease onset.
Adjunct therapy seekers: Patients receiving standard treatments (pressure-lowering drops for glaucoma, anti-VEGF for wet AMD) wanting to maximize outcomes. Peptides provide complementary mechanisms without replacing proven therapies.
Research-oriented patients: Individuals comfortable with emerging therapies, willing to accept uncertainty about efficacy, able to critically evaluate evidence. Understanding peptides remain experimental for vision applications.
Financial resources available: Peptide protocols cost $100-$500+ monthly depending on specific peptides and dosing. Not insurance-covered. Those able to sustain long-term financial commitment benefit from consistent use.
Poor candidates better served by standard care
Advanced disease: Severe vision loss from end-stage glaucoma, geographic atrophy, or proliferative diabetic retinopathy. Minimal functional tissue remaining limits peptide benefit. Standard treatments or low vision rehabilitation more appropriate.
Acute sight-threatening emergencies: Acute angle-closure glaucoma, retinal detachment, central retinal artery occlusion, wet AMD with active bleeding. Require immediate conventional treatment (surgery, injections, laser). Peptides inappropriate as primary therapy for emergencies.
Refractive errors (myopia, hyperopia, astigmatism): Peptides don't improve optical focusing or correct refractive errors. Glasses, contact lenses, or refractive surgery appropriate. Claims of peptides "improving eyesight" in healthy myopic individuals lack evidence.
Unrealistic expectation holders: Those expecting dramatic vision improvements (20/200 to 20/20), rapid results (days to weeks), or guaranteed outcomes. Peptides offer modest potential benefits with uncertainty, not miracles.
Financial constraints: Ongoing costs combined with uncertain efficacy make peptides poor investment for limited resources. Prioritizing proven treatments, proper nutrition (AREDS2), and glucose/blood pressure control provides better value.
Medical complexity: Multiple medications, complex health conditions, or recent surgeries may create unknown interactions or complications. Working with physicians coordinating care essential, but peptide unfamiliarity among doctors complicates this.
How SeekPeptides supports vision health research
SeekPeptides provides comprehensive resources for understanding peptides across applications including emerging vision health uses.
Learn about specific peptides mentioned for vision including BPC-157, Thymosin Beta-4, GHK-Cu, and Epitalon with detailed mechanism and evidence reviews.
Understand how peptides work at cellular and molecular levels explaining biological effects.
Compare vision peptides with other health applications and anti-aging protocols.
SeekPeptides empowers informed decisions about emerging peptide therapies.
Helpful resources
Related guides worth reading
Specific vision and neuroprotection peptides:
Understanding peptides:
Peptide administration:
Safety and research:
Health applications:
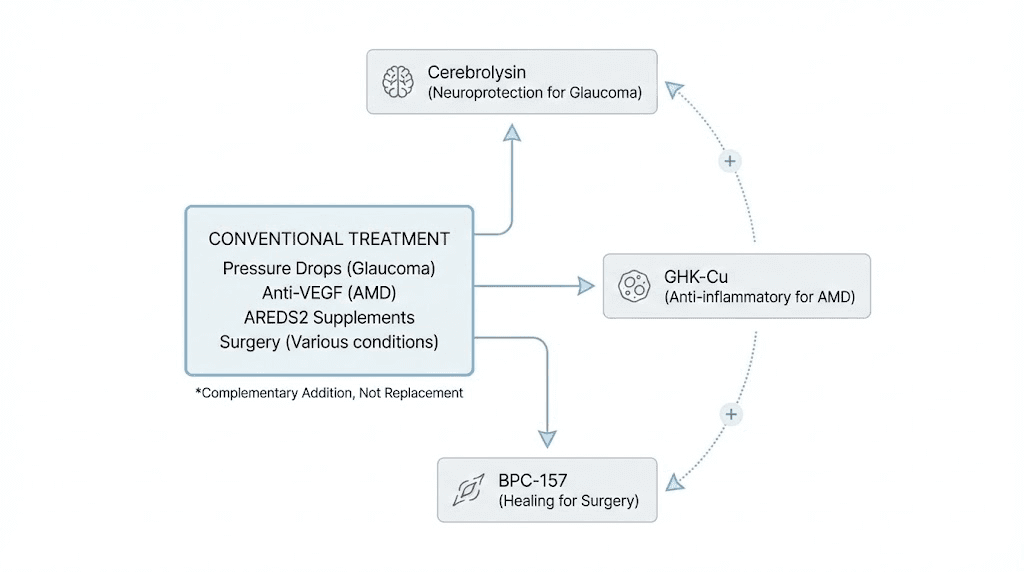
Combining peptides with conventional eye treatments
Strategic combinations amplify benefits without replacing proven therapies.
Peptides plus pressure-lowering glaucoma medications
Standard glaucoma treatment: Prostaglandin analogs (latanoprost, bimatoprost), beta-blockers (timolol), alpha agonists (brimonidine), carbonic anhydrase inhibitors (dorzolamide). Reduce intraocular pressure through various mechanisms. Primary therapy preventing optic nerve damage.
Peptide combination rationale: Pressure-lowering drugs reduce mechanical stress on optic nerve. Neuroprotective peptides (Cerebrolysin, Thymosin Beta-4) directly support ganglion cell survival. Complementary mechanisms, pressure control plus neuronal protection potentially superior to either alone.
Protocol integration: Continue standard pressure-lowering drops as prescribed. Add Cerebrolysin infusions (10-30mL, 5 days weekly for 4 weeks, repeated quarterly) or Thymosin Beta-4 injections (2-5mg twice weekly ongoing). Monitor both pressure (goal under 18mmHg or personalized target) and visual field/OCT (ganglion cell layer thickness).
Expected outcomes: Slower visual field loss, potentially 30-50% better preservation versus pressure control alone based on small trials. Not eliminating need for pressure control, additive benefit.
Considerations: Increased cost and complexity. No large trials proving combination superiority. Working with ophthalmologist essential for pressure monitoring and coordination.
Peptides plus anti-VEGF injections for wet AMD
Standard wet AMD treatment: Intravitreal anti-VEGF injections (bevacizumab, ranibizumab, aflibercept) blocking vascular endothelial growth factor, preventing abnormal blood vessel growth and leakage. Highly effective preserving or improving vision in 85-90% when started promptly.
Peptide combination rationale: Anti-VEGF addresses neovascularization directly. Peptides support overall retinal health, reduce inflammation, protect remaining photoreceptors and RPE. Synergistic rather than redundant.
Protocol integration: Continue scheduled anti-VEGF injections (typically monthly or every 2 months). Add GHK-Cu (1-3mg subcutaneous daily) for antioxidant protection or BPC-157 (200-500mcg daily) for vascular health support between injections.
Expected outcomes: Potentially better sustained vision, fewer breakthrough bleeding episodes, better quality of life between injections. No strong evidence but biological plausibility.
Limitations: Anti-VEGF already very effective making additional benefit from peptides hard to detect. Expensive combination ($2000-$4000 yearly for injections plus $1200-$3000 for peptides). No clinical trials proving added value.
Peptides plus AREDS2 supplements for dry AMD
Standard dry AMD prevention: AREDS2 formula, vitamins C (500mg), E (400IU), zinc (80mg), copper (2mg), lutein (10mg), zeaxanthin (2mg). Slows progression to advanced AMD by 25-30% in intermediate stages.
Peptide combination rationale: AREDS2 provides antioxidants and macular pigment. Peptides add cellular protection, anti-inflammatory effects, tissue repair mechanisms. Multiple complementary pathways potentially synergistic.
Protocol integration: Take AREDS2 daily (PreserVision, Macuhealth, or equivalent). Add GHK-Cu (1-3mg subcutaneous 3-5x weekly) or Epitalon cycles (5-10mg daily for 10-20 days, repeated 2-4x yearly). Monitor with home Amsler grid and professional exams every 6 months.
Expected outcomes: Possibly better preservation than AREDS2 alone, though unproven. Potentially delaying progression to advanced AMD by additional 6-12 months.
Cost-benefit: AREDS2 supplements cost $15-$30 monthly, well-established benefit. Peptides add $100-$400 monthly with uncertain additional benefit. AREDS2 should be priority, peptides optional addition for those wanting comprehensive approach.
Peptides plus laser or surgical treatments
Retinal laser photocoagulation: For diabetic retinopathy, retinal tears, or other vascular conditions. Creates controlled burns sealing vessels or preventing detachment.
Surgical interventions: Vitrectomy for retinal detachment, macular hole, vitreous hemorrhage. Cataract surgery. Glaucoma filtering surgery.
Peptide integration: BPC-157 or Thymosin Beta-4 starting 1-2 weeks pre-surgery continuing 4-8 weeks post-operatively. Supports tissue healing, reduces inflammation, potentially improves surgical outcomes.
Evidence: Animal studies show faster healing and better outcomes. Human evidence limited to case reports and extrapolation from wound healing research. Plausible but unproven.
Protocol: BPC-157 200-500mcg daily or Thymosin Beta-4 2-5mg twice weekly. Start pre-operatively if elective surgery, immediately post-op if emergency procedure.
Peptides plus glucose and blood pressure control for diabetic retinopathy
Foundation of diabetic retinopathy management: HbA1c under 7%, blood pressure under 130/80, lipid control, smoking cessation. These fundamentals prevent retinopathy far better than any peptide.
Peptide as adjunct: After optimizing glucose/pressure control, peptides provide additional vascular and neuroprotection. BPC-157 for vascular health, GHK-Cu for oxidative stress protection.
Critical principle: Peptides never substitute for metabolic control. Poor glucose control (HbA1c over 9%) overwhelms any peptide benefit. Only consider peptides after achieving good diabetic management.
Integration: Optimize diabetes control first (6-12 months achieving target HbA1c). Then add peptides if wanting additional protection. Continue rigorous glucose monitoring and medication adherence.
Troubleshooting and optimizing peptide vision protocols
Common challenges and solutions for better outcomes.
Issue: No measurable vision improvement after 3 months
Potential causes: Unrealistic expectations (expecting improvement vs slowed decline), advanced disease with minimal salvageable tissue, inadequate dosing or inconsistent use, wrong peptide for specific condition, peptides genuinely ineffective for individual, progression monitoring inadequate missing subtle benefits.
Diagnostic steps: Review baseline and current measurements objectively (visual acuity, visual field, OCT imaging), ensure consistent daily peptide use without extended gaps, verify proper dosing and administration technique, confirm appropriate peptide choice for specific condition.
Solutions: Extend trial to 6 months before concluding inefficacy (some benefits take longer), increase dosing within safe ranges if using lower end, switch peptides (try Cerebrolysin if using BPC-157, or vice versa), add comprehensive eye exam to rule out progression versus peptide failure, consider peptides preventing worsening rather than producing improvement, discontinue if truly no benefit after 6 months rigorous trial.
Issue: Side effects from systemic peptide injection
Common side effects: Injection site reactions (redness, swelling, tenderness), nausea (especially with higher doses), headaches, fatigue, blood pressure changes, allergic reactions (rare but serious).
Management strategies: Rotate injection sites preventing localized reactions, inject after eating reducing nausea risk, start lower doses titrating up gradually, take with food and plenty of water, monitor blood pressure if known hypertension, discontinue immediately if signs of allergy (hives, difficulty breathing, facial swelling).
When to reduce or stop: Persistent severe nausea despite dosage adjustment, severe injection site reactions or infection signs, significant blood pressure fluctuations, allergic symptoms, side effects outweighing vision benefits.
Issue: Difficulty obtaining quality peptides
Vendor problems: Research chemical vendors with inconsistent quality, counterfeit products, international shipping seizures, high costs for pharmaceutical-grade sources.
Solutions: Use vendor verification methods from our best vendors guide, request Certificates of Analysis before purchasing, send samples for independent testing ($100-$150), join community testing groups sharing costs, establish relationships with reliable vendors, consider pharmaceutical-grade even at higher cost for important applications like vision, explore physician-prescribed options if available (Cerebrolysin in countries where approved).
Issue: Insurance refuses coverage
Reality: No insurance covers research peptides for vision. Even Cerebrolysin, pharmaceutical product in many countries, rarely covered for vision indications. Peptide vision therapy always out-of-pocket expense.
Financial strategies: Prioritize most evidence-based peptides (Cerebrolysin for glaucoma, Thymosin Beta-4 for corneal issues), use loading dose then reduce to maintenance lowering long-term costs, cycle peptides (8 weeks on, 8 weeks off) versus continuous use, combine with well-established supplements (AREDS2) providing proven value, budget realistically understanding $100-$500 monthly ongoing expense, discontinue if financial burden excessive, focus resources on conventional treatments if limited budget.
Issue: Ophthalmologist unfamiliar or disapproving
Common scenario: Most conventional ophthalmologists unfamiliar with peptide research, view as unproven experimental treatment, may discourage use out of caution or lack of knowledge.
Navigation strategies: Find peptide-friendly physician (functional medicine, anti-aging specialists sometimes more open), educate current doctor with research papers (provide Cerebrolysin glaucoma studies, BPC-157 animal research), emphasize peptides as adjunct not replacement for standard care, agree to rigorous monitoring ensuring no harm, accept that doctor may remain skeptical but willing to monitor, consider changing providers if current doctor completely dismissive, proceed without physician support if necessary though suboptimal, never abandon proven treatments (pressure drops, anti-VEGF) regardless of peptide use.
Issue: Uncertain about treatment duration
Questions: How long to continue peptides? When to stop? When to take breaks?
Guidelines by condition type: Acute recovery (corneal injury, optic neuritis, post-surgical), 4-8 weeks then discontinue once healed. Chronic progressive diseases (glaucoma, AMD, diabetic retinopathy), months to years potentially lifelong with periodic monitoring. If stable disease after 12 months peptide use, trial 3-month break to see if benefits sustained, resume if progression resumes.
Monitoring approach: Establish clear metrics (visual field, OCT, visual acuity), measure every 3-6 months, continue peptides if stable or improving, consider break if stable 12+ months on treatment, resume immediately if decline detected during break, accept ongoing use if clearly beneficial and well-tolerated.
Long-term vision health protocols integrating peptides
Sustainable approaches balancing efficacy, cost, and lifestyle factors.
Preventative protocol for high-risk individuals (family history AMD or glaucoma)
Risk assessment: Family history, age over 50, high myopia, diabetes, smoking history, light-colored eyes (AMD risk), thin corneas (glaucoma risk).
Baseline: Comprehensive eye exam including OCT, visual field, fundus photography establishing baseline before starting prevention.
Peptide protocol: Epitalon cycles (5-10mg daily for 10-20 days, repeated quarterly) for general cellular health, GHK-Cu (1mg subcutaneous 3x weekly) ongoing for antioxidant protection, annual Cerebrolysin course (10mL, 10 sessions) if glaucoma family history.
Conventional prevention: AREDS2 vitamins daily, UV protection (sunglasses), smoking cessation, cardiovascular health (exercise, blood pressure control), regular eye exams (yearly age 50-65, every 6 months over 65).
Monitoring: Annual comprehensive exams tracking any early changes, home Amsler grid weekly for AMD risk, tonometry at each visit for glaucoma suspects.
Cost: Approximately $150-$300 monthly ($1800-$3600 yearly) for peptides plus $200-$500 yearly for exams and AREDS2 supplements.
Maintenance protocol for stable treated disease
Scenario: Glaucoma well-controlled on drops, dry AMD stable on AREDS2, diabetic retinopathy non-proliferative with good glucose control.
Goal: Maintain stability preventing progression while balancing cost and intervention burden.
Peptide protocol: Reduced intensity versus acute treatment. Cerebrolysin quarterly (10 sessions every 3 months) for glaucoma, GHK-Cu 2x weekly maintenance for AMD, BPC-157 every other day for diabetic retinopathy.
Conventional care: Continue standard treatments rigidly (pressure drops, AREDS2, glucose control). Peptides additive never replacing foundation.
Monitoring: Professional exams every 6 months if stable 1+ year. Every 3-4 months if any concerning changes. Home monitoring (Amsler grid, symptoms) between visits.
Adjustment triggers: Increase peptide intensity if progression detected. Reduce or discontinue if stable 2+ years and cost burdensome. Switch peptides if current approach ineffective.
Cost: Lower than acute protocols, $100-$250 monthly for maintenance peptide use.
Intensive recovery protocol post-surgery or injury
Scenario: Recent retinal detachment repair, corneal transplant, severe eye trauma, post-LASIK complications.
Goal: Maximize healing, minimize complications, achieve best possible visual outcome.
Peptide protocol: Aggressive initial approach, BPC-157 500mcg daily plus Thymosin Beta-4 5mg twice weekly for 4 weeks, then reduce to BPC-157 250mcg daily or Thymosin Beta-4 weekly for additional 4-8 weeks, taper based on healing progress.
Timing: Start immediately post-surgery or injury (within 24-48 hours optimal). Earlier initiation potentially better outcomes though animal data, not human proof.
Conventional care: Follow surgeon's post-operative instructions precisely. Antibiotic drops, anti-inflammatory drops, activity restrictions, positioning (for retinal surgery). Peptides complement not replace.
Monitoring: Frequent initial follow-ups (surgeon's schedule, typically daily to weekly first month), objective healing markers (corneal clarity, retinal reattachment, vision recovery), reduce intensity as healing progresses.
Duration: 8-12 weeks total for most surgical recoveries. Longer for complicated cases.
Cost: Higher during intensive phase ($400-$600 monthly for 2-3 months) but time-limited making total burden manageable.
Budget-conscious protocol maximizing value
Constraints: Limited financial resources requiring prioritization.
Foundation first: Conventional proven treatments take priority (pressure-lowering drops $20-$100 monthly generic, AREDS2 $15-$30 monthly, diabetes medications, proper nutrition). Only add peptides after foundation secure.
Selective peptide use: Choose single most relevant peptide (Cerebrolysin for glaucoma, GHK-Cu for AMD, BPC-157 for injury). Avoid expensive combinations until/unless clearly beneficial.
Minimum effective dose: Start low end of dosing ranges. GHK-Cu 1mg 2x weekly rather than daily (saves 65%), Cerebrolysin 10mL sessions quarterly rather than monthly (saves 66%). Accept potentially reduced benefit for affordability.
Cycling strategy: 8 weeks on peptides, 8 weeks off rather than continuous. Maintains some benefit while halving annual cost. Monitor closely during off periods resuming immediately if progression.
DIY optimization: Learn proper injection technique, reconstitution, sourcing reducing reliance on expensive services. Community resources and peptide calculators help.
Cost targets: $50-$150 monthly for budget protocol versus $300-$600 for comprehensive approach. Sustainable long-term critical versus ambitious short-term attempts burning out financially.
Final thoughts on peptides for vision improvement
Peptides offer promising but largely unproven approaches for vision health spanning prevention, treatment adjuncts, and recovery support.
The strongest evidence exists for Thymosin Beta-4 in corneal healing with Phase 3 trial data. Moderate evidence supports Cerebrolysin for glaucoma neuroprotection based on small human trials and consistent animal research.
Other peptides (BPC-157, GHK-Cu, Epitalon) have biological plausibility and animal data but lack human clinical validation.
Realistic expectations center on disease prevention and slowing progression rather than vision restoration or enhancement in healthy eyes. Peptides work best as adjuncts to conventional treatments, not replacements.
Combining neuroprotective peptides with pressure-lowering drops for glaucoma, antioxidant peptides with AREDS2 for AMD, or healing peptides with surgical interventions potentially provides synergistic benefits though large-scale proof lacking.
Success requires careful peptide selection matching specific conditions, consistent long-term use (months to years for chronic diseases), proper dosing and administration, realistic benefit expectations (20-40% better preservation versus natural progression), financial commitment ($100-$500 monthly depending on protocols), patience through gradual timelines (3-6 months minimum assessing efficacy), and integration with proven conventional care.
The field remains in early stages requiring more research establishing optimal protocols, defining responder characteristics, proving long-term safety, and conducting large randomized trials. Current users essentially participate in real-world experiments accepting uncertainty for potential benefits.
Working with open-minded physicians, rigorous self-monitoring, and honest outcome assessment separates reasonable experimental use from wishful thinking.
Peptide-nutrient synergies for enhanced vision protection
Combining peptides with targeted nutrition amplifies benefits through complementary mechanisms.
AREDS2 formula synergy with vision peptides
AREDS2 components: Vitamin C (500mg) supporting collagen synthesis and antioxidant defense, Vitamin E (400IU) protecting cell membranes from lipid peroxidation, Zinc (80mg) supporting retinal enzyme function, Copper (2mg) preventing zinc-induced copper deficiency, Lutein (10mg) and Zeaxanthin (2mg) accumulating in macula providing blue light filtration and antioxidant protection.
Synergy with GHK-Cu: Copper peptide plus dietary copper from AREDS2 raises theoretical overconsumption concerns. However, GHK-Cu provides trace copper amounts (micrograms) versus AREDS2's 2mg. Combined total remains safe for most individuals. GHK-Cu may enhance copper bioavailability improving AREDS2 formula utilization.
Synergy with BPC-157: Vitamin C acts as cofactor for collagen synthesis. BPC-157 stimulates collagen production signaling while vitamin C enables proper collagen formation. Complementary mechanisms potentially producing superior extracellular matrix repair in retina and choroid.
Synergy with antioxidant peptides: Vitamins C and E plus lutein/zeaxanthin provide direct antioxidant protection. Peptides stimulate endogenous antioxidant systems (superoxide dismutase, catalase, glutathione peroxidase). Multi-layered antioxidant defense superior to either approach alone.
Protocol integration: Continue AREDS2 daily as foundation. Add peptides on top without modifying vitamin regimen. Take AREDS2 with food for better absorption, peptides timing based on specific compound (often morning on empty stomach or evening before bed).
Omega-3 fatty acids plus vision peptides
Omega-3 benefits: EPA and DHA (from fish oil or algae) reduce inflammation, support retinal structure (DHA comprises 50% of photoreceptor outer segment membranes), improve tear film quality, may slow AMD progression.
Dosing: 1000-2000mg combined EPA/DHA daily from quality fish oil or algae supplements.
Synergy with BPC-157: Both provide anti-inflammatory effects through different pathways. Omega-3s modulate prostaglandin and leukotriene synthesis. BPC-157 affects cytokine signaling and growth factor pathways. Combined inflammation reduction potentially superior for chronic retinal diseases.
Synergy with Cerebrolysin: DHA supports neuronal membrane health while Cerebrolysin provides neurotrophic signals. Structural support plus survival signaling addresses ganglion cell health comprehensively.
Protocol: Take omega-3s with meals (improves absorption and reduces fishy aftertaste). Peptide timing independent. Both represent long-term daily commitments for chronic conditions.
Resveratrol and pterostilbene synergy
Polyphenol benefits: Resveratrol and pterostilbene (more bioavailable resveratrol analog) activate sirtuins and AMPK pathways, provide antioxidant effects, support mitochondrial function, may protect against AMD and diabetic retinopathy.
Dosing: Resveratrol 200-500mg daily or Pterostilbene 50-150mg daily.
Synergy with Epitalon: Both influence cellular aging pathways. Epitalon through telomerase activation and pineal regulation, polyphenols through sirtuin activation and mitochondrial support. Multiple anti-aging mechanisms potentially producing additive longevity benefits for retinal cells.
Synergy with GHK-Cu: Polyphenols and copper peptides both stimulate antioxidant enzyme systems. May produce synergistic oxidative stress protection especially important for metabolically active photoreceptors.
Protocol: Take resveratrol/pterostilbene with fat-containing meal (improves absorption of fat-soluble polyphenols). Peptides per their specific protocols. Consider cycling polyphenols (12 weeks on, 4 weeks off) while maintaining continuous peptide use.
Taurine supplementation for retinal health
Taurine benefits: Abundant in retina, particularly photoreceptors. Supports photoreceptor survival, modulates calcium homeostasis, provides antioxidant effects, protects against light-induced damage. Deficiency linked to retinal degeneration.
Dosing: 500-3000mg daily. Higher doses (2-3g) used in some retinal protection studies.
Synergy with vision peptides: Taurine provides substrate-level support (abundant amino acid maintaining cellular function) while peptides provide signaling-level support (growth factors, neuroprotection). Complementary rather than redundant.
Protocol: Taurine easily obtained as bulk powder (inexpensive). Mix 1-2g in water daily. No special timing requirements. Combines seamlessly with any peptide protocol.
Astaxanthin as super-antioxidant adjunct
Astaxanthin properties: Carotenoid with potent antioxidant activity (stronger than vitamin E or beta-carotene), crosses blood-retinal barrier accumulating in retina, protects photoreceptors from oxidative damage, may improve visual fatigue and accommodation.
Dosing: 4-12mg daily from supplement (difficult obtaining adequate amounts from food).
Synergy with GHK-Cu and BPC-157: Multiple antioxidants with different mechanisms and cellular locations. Astaxanthin in membranes, GHK-Cu providing enzymatic antioxidant support, BPC-157 reducing oxidative stress through growth factor pathways. Comprehensive protection.
Protocol: Take astaxanthin with fat-containing meal (fat-soluble requiring dietary fat for absorption). Daily ongoing use for chronic protection. Combines with any peptide protocol.
Carnosine for glycation protection in diabetic retinopathy
Carnosine properties: Dipeptide (beta-alanyl-L-histidine) preventing protein glycation, breaking existing advanced glycation end-products (AGEs), protecting against diabetic complications including retinopathy.
Dosing: 500-1500mg daily for systemic protection.
Synergy with BPC-157 in diabetic retinopathy: Carnosine addresses glycation damage while BPC-157 supports vascular repair and reduces inflammation. Attacking diabetic retinopathy through multiple mechanisms potentially slowing progression better than either alone.
Protocol: Take carnosine daily on empty stomach for best absorption. Continue even with good glucose control as additional protection. Combine with BPC-157 daily injections or every-other-day maintenance.
Complete vision optimization supplement stack
Foundation (proven benefits): AREDS2 formula (vitamins C, E, zinc, copper, lutein, zeaxanthin) daily, Omega-3 fish oil (1-2g EPA/DHA) daily, Quality multivitamin filling nutritional gaps.
Advanced additions (moderate evidence): Resveratrol or Pterostilbene (200mg or 100mg daily), Taurine (1-2g daily), Astaxanthin (6-12mg daily), Carnosine (1g daily for diabetics).
Peptide layer (experimental): Condition-specific peptide choice (Cerebrolysin for glaucoma, GHK-Cu for AMD, BPC-157 for vascular issues), Proper dosing and cycling, Medical monitoring.
Total monthly cost: Foundation supplements $40-$80, Advanced additions $30-$60, Peptides $100-$500, Professional monitoring (eye exams) $30-$100 monthly average. Total $200-$740 monthly for comprehensive protocol.
Special populations and peptide vision therapy considerations
Certain groups require modified approaches or extra precautions.
Elderly patients (75+ years)
Considerations: Multiple medications increasing interaction risks, reduced kidney/liver function affecting peptide clearance, fragile vessels increasing injection bruising, cognitive changes potentially affecting protocol adherence, fixed incomes limiting expensive interventions.
Protocol modifications: Start lower doses (BPC-157 200mcg vs 500mcg, GHK-Cu 1mg vs 3mg) with slower titration, simplify regimens (once daily vs multiple doses), prioritize evidence-based options (Cerebrolysin for glaucoma over speculative peptides), involve family members in administration and monitoring, focus on most impactful intervention if resources limited.
Safety monitoring: More frequent blood pressure checks (peptides may affect pressure in vulnerable elderly), kidney function monitoring if long-term use, medication interaction review with physician or pharmacist, close observation for confusion or side effects.
Diabetic patients requiring vision protection
Special risks: Fluctuating glucose affecting outcomes, vascular complications complicating interpretation, multiple medications, potential kidney issues limiting peptide use, higher infection risk with injections.
Glucose optimization priority: HbA1c under 7% essential before adding peptides. Poor control (HbA1c 9-10%) overwhelms any peptide benefit. Stable glucose creates foundation for peptide effects.
Protocol emphasis: BPC-157 for vascular protection (200-500mcg daily), GHK-Cu for oxidative stress (1-2mg 3x weekly), Carnosine for glycation protection (1g daily supplement), Strict injection site rotation and hygiene preventing infections.
Monitoring: Monthly glucose checks ensuring stability, quarterly HbA1c confirming control, dilated eye exams every 6 months (more frequently if active retinopathy), home blood pressure monitoring.
Post-surgical patients
Timing considerations: Optimal peptide initiation within 24-48 hours post-surgery though later start still beneficial. Pre-operative loading (1-2 weeks before elective surgery) may prime tissues though unproven.
Surgical type considerations: Retinal detachment repair (BPC-157 plus Thymosin Beta-4 aggressive protocol), Cataract surgery (Thymosin Beta-4 for corneal healing), Glaucoma filtering surgery (avoid BPC-157 potentially promoting excessive scarring, use Cerebrolysin for neuroprotection), LASIK/PRK (Thymosin Beta-4 for epithelial healing).
Coordination with surgeon: Inform surgeon about peptide plans though expect skepticism. Follow post-operative instructions precisely. Don't substitute peptides for prescribed medications (antibiotics, anti-inflammatories). Schedule follow-ups monitoring healing progress.
Pregnant and breastfeeding women
Critical restriction: Avoid all research peptides during pregnancy and breastfeeding. Safety data completely lacking. Theoretical risks to fetal development. Not worth experimental risk for vision benefits that can wait.
Exceptions: If approved pharmaceutical peptides become available with pregnancy safety data, physician-guided use possible. Currently no approved vision peptides in pregnancy Category A or B.
Alternative approach: Focus on nutrition (AREDS2 safe in pregnancy, omega-3s beneficial), conventional treatments if needed, defer peptide experimentation until after breastfeeding.
Children and adolescents
Generally contraindicated: Developing visual systems may respond differently than adults. Safety and efficacy data in pediatrics completely absent. Risk-benefit ratio unfavorable for experimental treatments.
Rare exceptions: Severe retinal injury or disease with poor prognosis, consultation with pediatric ophthalmologist, research protocols with proper oversight and informed consent.
Focus on prevention: UV protection, screen time management, outdoor time (reduces myopia progression), nutrition, regular eye exams detecting issues early.
Monitoring protocols and measuring peptide therapy success
Objective tracking separates real benefits from placebo effects and wishful thinking.
Professional baseline establishment
Comprehensive eye examination before starting: Visual acuity testing (Snellen chart, standardized lighting), refraction determining prescription, intraocular pressure measurement, dilated fundus examination (detailed retinal view), optical coherence tomography (OCT) measuring retinal layer thickness, visual field testing for glaucoma suspects, fundus photography documenting retinal appearance, macular pigment optical density if AMD concern.
Baseline data collection: Record all measurements in personal health file. Request copies of all test results. Photograph any images for comparison. Document current medications, supplements, and health conditions. Establish progression rate if pre-existing disease (reviewing old records showing change over time).
Timing considerations: Complete baseline 1-2 weeks before starting peptides allowing clear before/after comparison. Don't start multiple interventions simultaneously (peptides plus new supplements plus medication changes) preventing identification of what helps.
Home monitoring methods
Amsler grid testing: Grid of horizontal and vertical lines with central fixation point. Detects central vision distortions from macular disease. Test each eye separately, daily or weekly, documenting any changes (missing lines, wavy lines, dark spots).
Distance visual acuity: Printable Snellen chart at home (proper distance and lighting). Test each eye monthly recording best line read. Not as accurate as clinical testing but tracks major changes.
Contrast sensitivity: Simple tests available online or printable. More sensitive to subtle vision changes than standard acuity testing. Monthly assessment.
Symptom journaling: Daily or weekly notes about vision quality, eye fatigue, floaters, flashes, pain, redness. Subjective but captures patient experience. Include medication compliance, peptide doses, any missed doses or side effects.
Photography documentation: If visible external changes (eye redness, swelling), photograph weekly with consistent lighting for comparison. Useful for anterior segment issues less relevant for retinal diseases.
Professional follow-up schedule
First reassessment: 3 months after starting peptides. Allows sufficient time for changes while catching problems early. Repeat baseline tests, visual acuity, pressure, OCT, visual field if indicated.
Subsequent monitoring: Every 3-6 months if stable, more frequently if progression detected. Timing based on disease severity and peptide protocol intensity.
Test selection: Not all tests needed every visit. Visual acuity and pressure every visit. OCT every 6-12 months. Visual field yearly for glaucoma. Fundus photos when clinically indicated by doctor.
Cost considerations: Each visit with testing costs $150-$400 depending on tests performed and insurance coverage. Budget for 2-4 professional exams yearly plus home monitoring supplies.
Interpreting results and measuring success
Defining success: Stability (no progression) often represents success for chronic degenerative diseases. Improvement exceptional, not expected. Slowed progression (losing 1 letter per year vs 3-4 letters historically) meaningful benefit.
Statistical significance vs clinical significance: Small changes within measurement variability (1-2 letters visual acuity) not meaningful. Larger changes (5+ letters, visual field changes exceeding test-retest variability, OCT thickness changes over 5-10 microns) more clinically relevant.
Timeframe expectations: Minimum 3 months before expecting measurable changes. Most peptide studies showing benefits at 6-12 months. Expect gradual improvements not sudden dramatic changes.
Confounding factors: Natural disease fluctuation, seasonal variations, medication changes, other health changes (new diabetes diagnosis, blood pressure changes). Try isolating peptide effects from confounders though perfect isolation impossible.
Placebo consideration: Vision can improve with attention, better compliance with drops, healthier lifestyle regardless of peptide pharmacology. Placebo effects real but temporary. True peptide benefits should sustain beyond initial 3-6 month placebo period.
When to modify or discontinue peptide therapy
Continue indicators: Stable disease vs historical progression, measurable improvements in objective testing, good tolerability without significant side effects, financial sustainability, patient satisfaction with outcomes.
Modification triggers: Some benefit but suboptimal, try different peptide or increased dosing. Plateau after initial improvement, consider cycling (8 weeks on, 4 weeks off) or adding complementary interventions. Side effects without clear benefits, reduce dose or switch formulations.
Discontinuation indicators: No measurable benefit after 6 months rigorous trial. Disease progressing despite peptides suggesting ineffectiveness. Intolerable side effects outweighing benefits. Financial unsustainability. Better evidence-based treatment becoming available. Professional medical advice recommending discontinuation.
Trial breaks: After 12 months stable disease, consider 3-month peptide break monitoring for progression. If progression resumes, peptides likely providing benefit, resume immediately. If stability maintained off peptides, may not need continued use, monitor ongoing. Breaks help determining if peptides truly beneficial vs coincidental stability.
Documentation and record keeping
Treatment log: Date started each peptide, dosing schedule, any modifications, side effects or reactions, concurrent treatments or supplements, costs for insurance or tax purposes.
Results tracking: Spreadsheet or journal with dated vision measurements, professional test results summary, home monitoring data, photos or images if applicable, symptom progression notes.
Financial tracking: Monthly costs, annual totals, comparison to conventional treatment costs, value assessment (cost per quality-adjusted life year or cost per vision improvement).
Medical communication: Share records with ophthalmologist even if skeptical about peptides. Documentation demonstrates seriousness and provides data for clinical decisions. May convince skeptical physician if clear objective improvements documented.
Success stories and realistic outcomes
Glaucoma patient example: 62-year-old with moderate glaucoma, well-controlled pressure on drops but showing slow visual field loss (2-3% decline yearly). Added quarterly Cerebrolysin courses. Over 24 months, visual field stable with no measurable decline. OCT shows maintained ganglion cell layer thickness. Represents successful preservation, not improvement.
AMD prevention example: 58-year-old with family history and early drusen. Started GHK-Cu 3x weekly plus AREDS2. After 18 months, drusen size stable on fundus photos, no progression to larger/more numerous drusen that occurred in untreated sibling. Cannot prove peptides responsible vs natural variation but patient satisfied with stability.
Post-surgical recovery: 45-year-old after retinal detachment repair. Standard outcomes predict 60-70% chance returning to 20/40 or better. Patient used BPC-157 and Thymosin Beta-4 for 8 weeks post-op. Achieved 20/25 vision by 3 months, faster recovery than typical. Surgeon noted excellent healing though couldn't attribute specifically to peptides vs natural good healing.
Diabetic retinopathy case: 54-year-old with non-proliferative diabetic retinopathy despite HbA1c 6.8%. Added BPC-157 daily. After 12 months, retinopathy severity unchanged (stable mild NPDR) versus historic worsening documented in prior years. Continued good glucose control plus peptides possibly synergistic for stability.
Corneal healing: 38-year-old with recurrent erosions from previous trauma. Standard treatment (lubricants, bandage lens) providing limited relief. Thymosin Beta-4 compounded drops 4x daily for 6 weeks. Erosions resolved, 12 months symptom-free after treatment ended. Likely most clear-cut peptide success given Thymosin Beta-4 evidence for corneal applications.
These represent realistic best-case scenarios, modest but meaningful improvements or preservation. Not dramatic vision restoration or 20/200 to 20/20 transformations. Appropriate expectations matching evidence-based potential.
Critical safety reminders for vision peptide users
Never inject anything into eyes directly: Only ophthalmologists should perform intraocular injections using pharmaceutical-grade sterile formulations. Research peptides not formulated for intraocular use. DIY eye injections risk permanent blindness from infection or chemical damage.
Maintain conventional treatments: Peptides complement, never replace, proven therapies. Continue pressure-lowering drops for glaucoma, anti-VEGF injections for wet AMD, glucose control for diabetic retinopathy. Stopping standard care while using unproven peptides creates avoidable vision loss risk.
Monitor for deterioration: If vision worsening despite peptides, don't assume peptides need more time. See ophthalmologist immediately ruling out treatable progression. Some conditions need urgent intervention (retinal detachment, acute glaucoma, wet AMD conversion).
Quality verification essential: Use reputable vendors, request Certificates of Analysis, consider independent testing for expensive long-term protocols. Contaminated or fake peptides waste money and potentially harm.
Disclose to physicians: Inform ophthalmologist and primary care provider about peptide use. Medical decisions require complete information. Peptides may affect interpretation of symptoms or test results.
Realistic timelines: Vision changes requiring professional evaluation include sudden vision loss, new floaters or flashes, curtain or shadow in vision, severe pain, double vision. These warrant urgent ophthalmology consultation regardless of peptide use.
Financial sustainability: Only commit to peptide protocols sustainable long-term. Chronic diseases require months to years of treatment. Starting then stopping due to cost wastes initial investment without gaining long-term benefits.
External resources
National Eye Institute - Age-Related Macular Degeneration Research
American Academy of Ophthalmology - Glaucoma Treatment Guidelines
In case I don't see you, good afternoon, good evening, and good night. May your vision stay sharp and your retinas stay healthy. Join SeekPeptides.