Jan 5, 2026

Unlike broad-spectrum antioxidants or simple nutrients, peptides function as signaling molecules triggering cascades of cellular responses. BPC-157 binds growth factor receptors activating angiogenesis and tissue repair pathways in damaged retina.
Cerebrolysin mimics neurotrophic factors (BDNF, NGF, CNTF) preventing retinal ganglion cell apoptosis through PI3K/Akt survival signaling. GHK-Cu modulates gene expression affecting over 4,000 genes involved in antioxidant defense, tissue remodeling, and inflammation control. Thymosin Beta-4 regulates actin polymerization promoting cell migration during corneal wound healing while simultaneously inhibiting inflammatory pathways through NF-κB suppression.
The sophistication of peptide mechanisms explains both their therapeutic potential and current limitations. Each peptide interacts with specific receptors expressed on particular cell types, creating targeted effects impossible with simple antioxidants or vitamins.
Mechanistic understanding doesn't automatically translate to clinical efficacy. A peptide demonstrating robust neuroprotection in cell culture may fail in whole organisms due to blood-retinal barrier penetration limitations, rapid degradation, or insufficient bioavailability at target tissues. Animal models showing dramatic retinal preservation following peptide treatment may not replicate in humans due to species differences in receptor expression, metabolism, or disease pathology. Understanding how peptides work at molecular levels provides biological plausibility and guides protocol development, but clinical validation through human trials remains essential for proving real-world benefits.
This guide covers cellular receptors and signaling pathways targeted by vision peptides, detailed mechanisms for specific peptides (BPC-157, Cerebrolysin, GHK-Cu, Thymosin Beta-4), understanding blood-retinal barrier penetration and peptide delivery challenges, gene expression changes induced by peptide treatment, comparing peptide mechanisms to conventional eye medications, dose-response relationships and optimal timing considerations, synergistic mechanisms when combining multiple peptides, and translating mechanistic understanding into practical treatment protocols. SeekPeptides serves as your resource for evidence-based peptide mechanism education.
Let's examine the fundamental cellular receptors and pathways vision peptides target to understand their biological effects.
Cellular receptors and signaling pathways in ocular tissues
Vision peptides exert effects by binding specific receptors triggering intracellular signaling cascades.
Growth factor receptors mediating tissue repair
Receptor tyrosine kinases (RTKs): Large family of cell surface receptors binding growth factors and triggering multiple signaling pathways. Key RTKs in eye include VEGF receptors (VEGFR1, VEGFR2, VEGFR3) regulating angiogenesis, FGF receptors (FGFR1-4) controlling cell proliferation and differentiation, EGF receptor family supporting epithelial cell growth, PDGF receptors modulating pericyte recruitment and vessel stabilization.
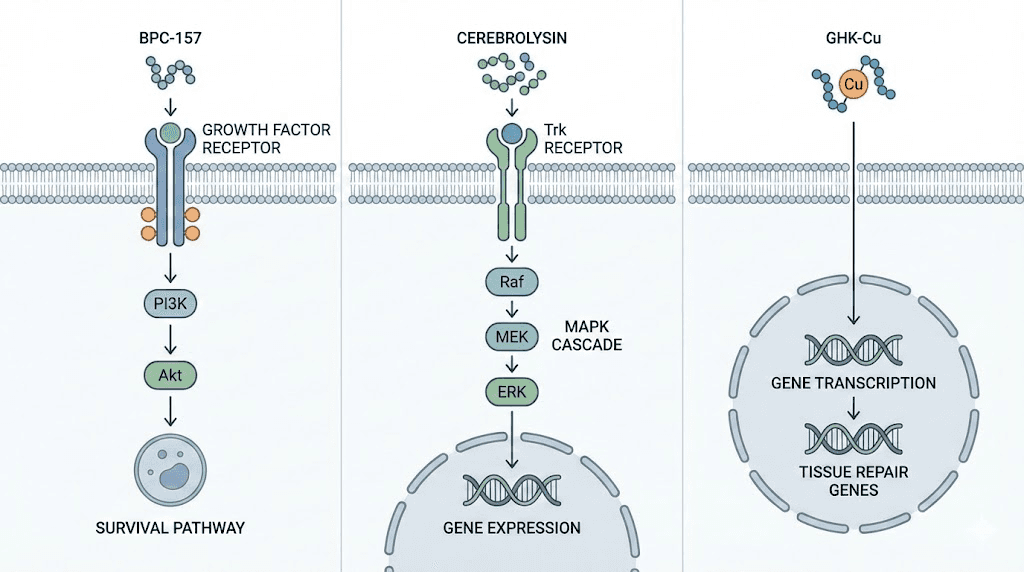
BPC-157 interactions with growth factor pathways: While specific receptor for BPC-157 remains unidentified, research suggests it modulates VEGF receptor signaling enhancing angiogenesis in ischemic tissues. Studies show BPC-157 increasing VEGF expression and receptor phosphorylation in damaged tissues including retina. May also interact with FGF pathways based on observed effects on fibroblast proliferation. Understanding these BPC-157 mechanisms helps explain tissue repair and vascular protection effects relevant to retinal health.
Downstream signaling from RTK activation: Receptor binding triggers autophosphorylation recruiting adaptor proteins initiating multiple pathways. PI3K/Akt pathway promotes cell survival and protein synthesis. MAPK/ERK pathway drives cell proliferation and differentiation. PLCγ pathway increases intracellular calcium affecting various cellular processes. JAK/STAT pathway regulates gene transcription. These pathways converge influencing cell fate decisions (survival vs death), proliferation rates, migration patterns, and differentiation states.
Relevance to vision health: Retinal diseases involve growth factor dysregulation. Diabetic retinopathy shows excessive VEGF causing pathological neovascularization. AMD involves insufficient growth factor support for photoreceptors and RPE. Glaucoma features neurotrophic factor deficiency contributing to ganglion cell death. Peptides modulating growth factor pathways potentially restore balance preventing disease progression.
Neurotrophic factor receptors supporting neuronal survival
Trk receptor family: TrkA binds nerve growth factor (NGF), TrkB binds brain-derived neurotrophic factor (BDNF), TrkC binds neurotrophin-3 (NT-3). Activation promotes neuronal survival, axon growth, synaptic plasticity. Retinal ganglion cells express TrkB making them responsive to BDNF signaling critical for survival especially during stress or injury.
p75NTR receptor: Binds all neurotrophins but with different functional outcomes than Trk receptors. Can promote apoptosis or survival depending on cellular context and co-receptor expression. Important for refining neuronal connections during development and responding to injury in adulthood.
Cerebrolysin mimicry of neurotrophic factors: This peptide mixture contains small peptides mimicking NGF, BDNF, CNTF biological activities. Binds Trk receptors activating downstream survival pathways. Studies show Cerebrolysin increasing phosphorylation of TrkB and downstream Akt in neurons protecting against apoptosis. In retinal ganglion cells, this translates to preserved cell bodies and axons preventing glaucomatous vision loss.
Signaling pathways from Trk activation: PI3K/Akt pathway phosphorylates Bad (pro-apoptotic protein) preventing it from blocking Bcl-2 and Bcl-xL (anti-apoptotic proteins). Net effect strongly pro-survival. MAPK pathway activates CREB transcription factor inducing genes supporting neuronal health and plasticity. PLCγ pathway affects calcium signaling and gene transcription. Multiple convergent survival signals explain robust neuroprotection from neurotrophic factors and mimicking peptides.
Integrin receptors mediating cell-matrix interactions
Integrin structure and function: Heterodimeric transmembrane receptors (α and β subunits) connecting extracellular matrix to intracellular cytoskeleton. Bidirectional signaling, outside-in (matrix influencing cell behavior) and inside-out (cell modulating adhesion strength). Critical for cell migration, tissue organization, wound healing.
Thymosin Beta-4 effects on actin and integrins: Thymosin Beta-4 binds monomeric G-actin sequestering it and preventing polymerization into F-actin filaments. Upon cellular signals for migration or shape change, Thymosin Beta-4 releases actin allowing rapid polymerization. Also modulates integrin expression and activation affecting cell adhesion and migration. In corneal epithelial cells, promotes migration across wound surfaces accelerating healing.
Focal adhesion kinase (FAK) signaling: Integrin clustering recruits FAK to focal adhesions. FAK phosphorylation initiates signaling affecting cell survival, proliferation, migration. Thymosin Beta-4 influences FAK activity explaining some observed effects on wound healing and tissue remodeling. Understanding these peptide mechanisms at molecular detail helps optimize clinical applications.
Melanocortin receptors and systemic peptide effects
MC1R-MC5R receptor family: G-protein coupled receptors binding melanocortins (ACTH, α-MSH, β-MSH, γ-MSH). Different tissue distribution and functions. MC1R in melanocytes affecting pigmentation. MC3R and MC4R in brain regulating energy homeostasis and sexual function. MC5R in exocrine glands and immune cells.
Melanocortin peptides not directly vision-targeting: Melanotan II and PT-141 bind melanocortin receptors but primarily for tanning and sexual function. No direct retinal effects though systemic anti-inflammatory actions from MC receptor activation might provide modest indirect benefits. Not vision peptides per se but illustrate receptor-mediated peptide mechanisms. SeekPeptides helps distinguish direct vision applications from systemic peptides with potential secondary eye benefits.
Metal ion coordination and gene regulation
Copper-peptide complexes: GHK-Cu contains copper(II) ion coordinated by glycine amino group, histidine imidazole, and lysine forming square planar complex. This structure critical for biological activity. Copper-free GHK shows minimal effects versus copper-bound form.
Gene expression modulation: GHK-Cu affects over 4,000 genes based on microarray studies. Upregulates genes involved in antioxidant defense (superoxide dismutase, catalase), tissue repair (collagen synthesis, matrix metalloproteinases), anti-inflammation (suppressing TNF-α, IL-6). Downregulates pro-inflammatory genes and those promoting fibrosis. Mechanisms include direct DNA binding, affecting transcription factor activity, and epigenetic modifications.
Relevance to retinal protection: Oxidative stress and inflammation central to AMD and diabetic retinopathy. GHK-Cu's broad gene expression effects potentially address multiple pathological processes simultaneously. Antioxidant enzyme upregulation protects photoreceptors and RPE from oxidative damage. Anti-inflammatory effects reduce chronic retinal inflammation. Tissue remodeling gene changes may support healthy extracellular matrix maintenance. Explore copper peptide mechanisms for detailed pathway analysis.
BPC-157 mechanisms in retinal vascular protection
BPC-157 demonstrates robust effects on vascular health and tissue repair through multiple complementary mechanisms.
Angiogenesis promotion in ischemic tissues
VEGF pathway modulation: BPC-157 increases vascular endothelial growth factor (VEGF) expression in ischemic tissues including retina. VEGF binds VEGFR2 on endothelial cells triggering proliferation, migration, and tube formation creating new blood vessels. In retinal ischemia models (induced by vessel ligation or elevated pressure), BPC-157 treatment increases retinal VEGF levels 2-3 fold within days promoting revascularization.
Balancing angiogenesis: Critical distinction between therapeutic angiogenesis (restoring blood flow to ischemic areas) versus pathological neovascularization (abnormal vessel growth in wet AMD or proliferative diabetic retinopathy). BPC-157 appears promoting physiological vessel formation with proper structure and barrier function, not leaky pathological vessels.
Mechanisms involve coordinating VEGF with other factors like angiopoietin-1 and PDGF ensuring proper pericyte recruitment and vessel maturation. Understanding BPC-157 dosing helps maximize therapeutic angiogenesis while minimizing pathological vessel risks.
Nitric oxide (NO) pathway enhancement: BPC-157 increases endothelial nitric oxide synthase (eNOS) expression and activity. NO causes vasodilation improving blood flow and has angiogenic properties. In retinal vessels, increased NO production enhances perfusion delivering oxygen and nutrients to metabolically active photoreceptors and neurons. NO also protects against platelet aggregation and thrombosis maintaining vessel patency.
Anti-inflammatory effects protecting retinal tissues
Cytokine modulation: BPC-157 reduces pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in injured tissues. These cytokines normally recruit immune cells and amplify inflammatory responses. In retina, excessive inflammation damages photoreceptors, RPE, and ganglion cells. By suppressing cytokine production, BPC-157 creates less hostile environment supporting cell survival.
Microglial activation reduction: Retinal microglia (resident immune cells) become activated in disease states releasing inflammatory mediators and reactive oxygen species. Chronic microglial activation contributes to AMD, diabetic retinopathy, and glaucoma progression. BPC-157 studies in CNS injury models show reduced microglial activation suggesting similar effects possible in retina. Less activated microglia means reduced inflammation and oxidative damage to retinal neurons.
NF-κB pathway inhibition: Nuclear factor kappa B (NF-κB) is master transcription factor regulating inflammatory gene expression. Normally sequestered in cytoplasm, stress or injury causes translocation to nucleus activating hundreds of inflammatory genes. BPC-157 inhibits NF-κB activation preventing inflammatory cascade initiation. This broad anti-inflammatory effect protects multiple retinal cell types simultaneously. SeekPeptides provides resources understanding inflammation's role in retinal disease and anti-inflammatory peptide applications.
Growth factor signaling enhancement
FGF pathway activation: Fibroblast growth factors (FGFs) support various cell types including retinal cells. BPC-157 increases FGF2 (basic FGF) expression promoting cell survival and proliferation. In retinal injury models, elevated FGF supports photoreceptor and ganglion cell survival during stress periods. FGF also stimulates glial cells (Müller cells) providing trophic support to neurons.
EGF receptor pathway effects: Epidermal growth factor receptor (EGFR) activation promotes epithelial cell proliferation and migration. Relevant for RPE repair and corneal healing. BPC-157 may modulate EGFR signaling though specific mechanisms less studied than VEGF and FGF pathways. EGFR activation helps RPE cells regenerating damaged areas and maintaining blood-retinal barrier.
Synergistic growth factor interactions: Multiple growth factors working together produce superior effects versus single factors alone. BPC-157 coordinating VEGF, FGF, EGF, and others creates comprehensive tissue repair program. In retinal detachment or vascular occlusion, this multi-factorial approach addresses ischemia, inflammation, cell death, and tissue damage simultaneously. Learn about growth factor peptides and their synergistic mechanisms.
Oxidative stress reduction mechanisms
Antioxidant enzyme upregulation: BPC-157 increases superoxide dismutase (SOD), catalase, and glutathione peroxidase expression. These enzymes detoxify reactive oxygen species protecting cellular components. In retina facing high oxidative burden from light exposure and metabolism, enhanced antioxidant defenses critical for long-term photoreceptor and RPE survival.
Mitochondrial protection: BPC-157 preserves mitochondrial function during stress reducing ROS production from dysfunctional electron transport chains.
Maintains mitochondrial membrane potential and ATP production. In metabolically demanding retinal cells, healthy mitochondria essential for sustaining cellular functions and preventing energy-depletion-induced cell death.
Direct ROS scavenging: Some evidence suggests BPC-157 directly neutralizing reactive oxygen species though less potent than dedicated antioxidants.
Combined with enzyme upregulation and mitochondrial protection creates multi-level antioxidant defense particularly relevant for photoreceptors constantly exposed to photo-oxidative stress. Explore antioxidant peptides for comprehensive understanding.
Cerebrolysin neurotrophic mechanisms for optic nerve protection
Cerebrolysin provides multiple neurotrophic factors mimicking endogenous proteins supporting neuronal survival.
BDNF-like effects on retinal ganglion cells
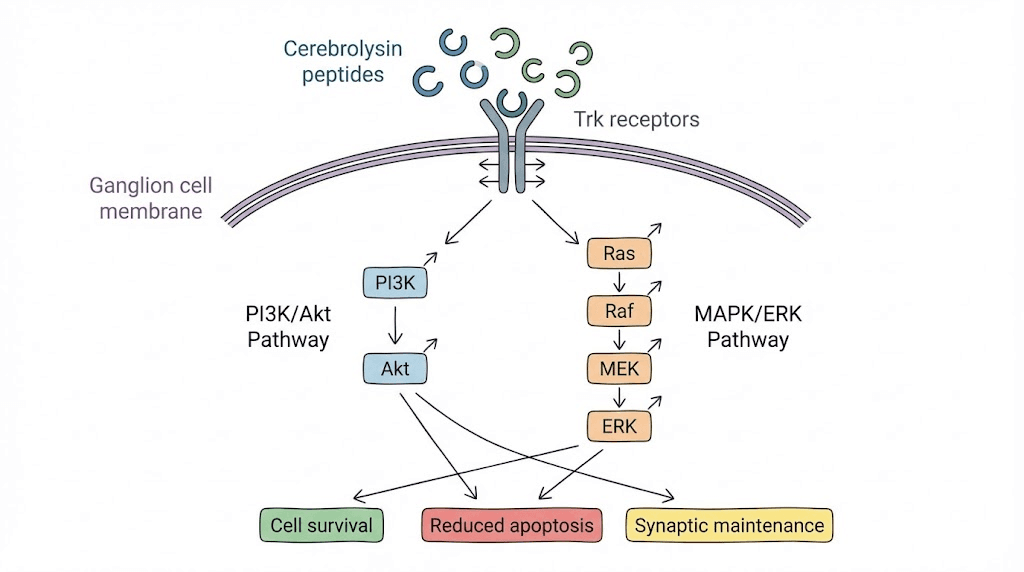
TrkB receptor activation: Brain-derived neurotrophic factor (BDNF) binding TrkB receptors on retinal ganglion cells triggers survival pathways. Cerebrolysin contains peptides with BDNF-like activity binding TrkB and initiating similar signaling. Studies show Cerebrolysin increasing TrkB phosphorylation in neurons confirming receptor engagement.
Akt pathway pro-survival signaling: TrkB activation recruits PI3K phosphorylating PIP2 to PIP3. PIP3 recruits Akt (also called PKB) to membrane where it becomes phosphorylated and activated. Active Akt phosphorylates multiple targets including Bad (preventing it from inhibiting anti-apoptotic Bcl-2), MDM2 (stabilizing p53), FOXO transcription factors (preventing pro-apoptotic gene expression), mTOR (promoting protein synthesis). Net effect powerfully pro-survival blocking multiple apoptotic pathways simultaneously.
CREB-mediated gene transcription: MAPK pathway from TrkB activation phosphorylates CREB (cAMP response element binding protein). Phosphorylated CREB binds DNA at CRE sequences activating genes supporting neuronal survival, plasticity, and function. Includes genes for BDNF itself (positive feedback), Bcl-2 (anti-apoptotic protein), and various synaptic proteins. CREB activation explains sustained benefits beyond immediate signaling as new protective proteins synthesized.
NGF-like neuroprotection pathways
TrkA receptor engagement: Nerve growth factor (NGF) critical for neuronal development and maintenance. Cerebrolysin peptides with NGF-like activity bind TrkA receptors present on some retinal neurons. TrkA activation similar to TrkB, triggering PI3K/Akt and MAPK pathways. Particularly important for peripheral nerve fibers innervating eye including corneal nerves.
Retrograde transport and gene regulation: NGF and NGF-like factors undergo retrograde transport from axon terminals to cell bodies. This allows distal signals (from axon health and target connections) reaching nucleus influencing gene expression. In optic nerve, retrograde signaling from retina to ganglion cell bodies coordinates cellular responses to axonal stress or damage. Cerebrolysin potentially supporting this critical communication pathway.
CNTF effects on photoreceptor and ganglion cell survival
CNTF receptor complex: Ciliary neurotrophic factor binds heterotrimeric receptor complex (CNTFRα, gp130, LIFRβ) activating JAK/STAT pathway distinct from Trk signaling. STAT proteins translocate to nucleus directly activating gene transcription. CNTF particularly potent for photoreceptor survival in retinal degeneration models.
Cerebrolysin CNTF-like activity: Contains peptides mimicking CNTF effects protecting photoreceptors and ganglion cells. Combination of BDNF-like, NGF-like, and CNTF-like activities provides broad neuroprotection covering multiple cell types and stress conditions. This multi-factorial neurotrophic support explains superior protection versus single factor administration. SeekPeptides offers detailed guides on neurotrophic peptides and their mechanisms.
Anti-apoptotic protein upregulation
Bcl-2 family regulation: Balance between pro-apoptotic (Bax, Bad, Bid) and anti-apoptotic (Bcl-2, Bcl-xL, Mcl-1) Bcl-2 family members determines cell survival versus death. Cerebrolysin shifts balance toward survival by increasing anti-apoptotic protein expression and inactivating pro-apoptotic proteins through phosphorylation. In glaucoma where elevated pressure or ischemia triggers ganglion cell apoptosis, this shifted balance preserves neurons.
Caspase inhibition: Caspases are proteases executing apoptotic cell death program. Caspase-3 activation represents point of no return in apoptosis. Cerebrolysin neurotrophic signaling prevents caspase activation maintaining cells in survival mode. Studies show reduced caspase-3 activity in neurons treated with Cerebrolysin during stress exposure.
Synaptic plasticity and functional preservation
Synaptogenesis promotion: Beyond simple cell survival, Cerebrolysin promotes synapse formation and strengthening. Increases synaptic proteins (synaptophysin, PSD-95) supporting connections between retinal neurons and from retina to brain. Maintaining functional connectivity critical for preserved vision not just anatomical ganglion cell survival.
Neurotransmitter system support: Enhances glutamate receptor expression and function supporting excitatory neurotransmission. Modulates GABA system maintaining inhibitory-excitatory balance. In retina, proper neurotransmitter function essential for signal processing and transmission to brain. Cerebrolysin supporting both survival and function explains better visual outcomes versus simple neuroprotection. Learn about cognitive peptides sharing similar neurotrophic mechanisms.
GHK-Cu copper peptide gene expression mechanisms
GHK-Cu exerts effects through broad gene expression changes affecting thousands of genes simultaneously.
DNA binding and transcription factor modulation
Direct DNA interactions: GHK-Cu can bind DNA through copper ion coordination with bases and backbone phosphates. This may directly affect gene transcription or recruit transcription factors to specific promoter regions. Studies show GHK-Cu binding preferentially to specific DNA sequences suggesting targeted gene regulation rather than random effects.
Transcription factor activity changes: GHK-Cu modulates activity of key transcription factors including NF-κB (inflammation master regulator), AP-1 (stress response), Nrf2 (antioxidant response element activator), p53 (cell cycle and apoptosis regulator). By affecting these master regulators, GHK-Cu influences hundreds to thousands of downstream genes simultaneously explaining pleiotropic effects.
Antioxidant gene upregulation protecting photoreceptors
Superoxide dismutase (SOD) induction: GHK-Cu increases SOD1 (cytoplasmic), SOD2 (mitochondrial), and SOD3 (extracellular) expression. SOD enzymes convert superoxide radicals to hydrogen peroxide (less reactive) for further detoxification by catalase and glutathione peroxidase. In photoreceptors facing constant oxidative stress from light and oxygen exposure, enhanced SOD expression critical for survival.
Catalase and glutathione system enhancement: Catalase breaks down hydrogen peroxide to water and oxygen. Glutathione peroxidase uses glutathione reducing equivalents detoxifying peroxides. GHK-Cu upregulates these enzymes plus glutathione reductase (regenerating reduced glutathione) and glucose-6-phosphate dehydrogenase (providing NADPH for glutathione reduction). Comprehensive antioxidant system enhancement provides multi-level protection.
Nrf2 pathway activation: Nuclear factor erythroid 2-related factor 2 (Nrf2) master regulator of antioxidant response. Normally sequestered in cytoplasm by Keap1. Oxidative stress or GHK-Cu treatment causes Nrf2 release, nuclear translocation, and binding to antioxidant response elements (ARE) in gene promoters. Activates dozens of antioxidant and detoxification genes creating coordinated cellular defense. Understanding antioxidant mechanisms helps optimize GHK-Cu protocols.
Anti-inflammatory gene expression changes
Pro-inflammatory cytokine suppression: GHK-Cu downregulates genes encoding IL-1β, IL-6, TNF-α, and other inflammatory mediators. Occurs through NF-κB inhibition preventing transcription factor binding to cytokine gene promoters. In AMD and diabetic retinopathy where chronic inflammation damages retina, cytokine suppression reduces ongoing tissue destruction.
Anti-inflammatory protein upregulation: Simultaneously increases expression of anti-inflammatory proteins like IL-10, TGF-β (in controlled amounts), and various inflammation resolution mediators (lipoxins, resolvins). Shifts inflammatory balance toward resolution rather than just suppressing inflammation, promoting return to homeostasis.
Microglial phenotype modulation: Affects microglial gene expression shifting from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype. M2 microglia clear debris, produce growth factors, and support tissue repair versus M1 microglia causing damage through inflammatory mediator release. In chronic retinal diseases, maintaining M2 phenotype protects neurons from immune-mediated injury.
Tissue remodeling and extracellular matrix genes
Collagen synthesis regulation: GHK-Cu increases collagen I and III gene expression supporting tissue structure and repair. In retina and choroid, healthy collagen matrix provides scaffold for cells and maintains tissue architecture. Balanced collagen production (not excessive causing fibrosis) supports healing without scarring.
Matrix metalloproteinase (MMP) modulation: MMPs degrade extracellular matrix proteins. GHK-Cu regulates MMP expression and activity, increasing some MMPs to remove damaged matrix while controlling excessive degradation. Also increases tissue inhibitors of metalloproteinases (TIMPs) fine-tuning matrix turnover. Proper matrix remodeling essential for healthy Bruch's membrane and RPE in AMD.
Glycosaminoglycan synthesis: Increases genes encoding enzymes synthesizing GAGs (hyaluronic acid, chondroitin sulfate, heparan sulfate). GAGs important for tissue hydration, growth factor binding, and structural support. In vitreous and extracellular matrix, GAGs maintain proper tissue properties and growth factor availability. Explore tissue repair peptides for comprehensive mechanism understanding.
Epigenetic modifications affecting long-term gene expression
DNA methylation changes: GHK-Cu may affect DNA methyltransferase activity altering cytosine methylation patterns. Methylation generally represses gene expression. GHK-Cu treatment shows altered methylation at specific gene loci potentially explaining sustained effects beyond immediate peptide presence.
Histone modifications: Affects histone acetyltransferases and deacetylases changing chromatin structure. More open chromatin (from acetylation) increases gene accessibility for transcription. GHK-Cu may create lasting chromatin changes maintaining beneficial gene expression patterns after treatment ends.
Long-term cellular reprogramming: Epigenetic changes can persist weeks to months after peptide discontinuation. May explain why some patients report sustained benefits even after stopping GHK-Cu treatment. Cells "remember" exposure through epigenetic marks maintaining healthier gene expression programs. SeekPeptides explores long-term peptide effects and sustainability strategies.
Thymosin Beta-4 wound healing and anti-inflammatory mechanisms
Thymosin Beta-4 demonstrates unique mechanisms through actin regulation and inflammation control.
Actin sequestration and cell migration
G-actin binding and sequestration: Thymosin Beta-4 binds monomeric globular actin (G-actin) preventing spontaneous polymerization into filamentous actin (F-actin). Maintains pool of readily available actin monomers for rapid cytoskeleton reorganization. In corneal epithelial cells, this allows quick response to migration signals during wound healing.
Rapid actin polymerization upon stimulation: When migration signals received (growth factors, chemokines, electric fields at wound edges), Thymosin Beta-4 releases actin allowing explosive polymerization forming lamellipodia and filopodia driving cell migration. This on-demand actin availability explains accelerated wound healing versus cells lacking adequate actin pools.
Focal adhesion dynamics: Cell migration requires coordinating adhesion formation at leading edge and adhesion dissolution at trailing edge. Thymosin Beta-4 influences focal adhesion kinase (FAK) and integrin trafficking affecting adhesion dynamics. Promotes formation of new adhesions allowing cell anchoring to matrix during forward movement. Understanding healing peptide mechanisms optimizes clinical application.
Corneal epithelial migration and re-epithelialization
Wound healing phases: Corneal healing involves epithelial cell migration covering defect, proliferation replacing lost cells, and differentiation restoring barrier function.
Thymosin Beta-4 primarily affects migration phase accelerating wound closure.
Also supports proliferation through growth factor pathway modulation.
Electric field guidance: Corneal wounds generate electric fields (wound potential) providing directional cues for cell migration. Thymosin Beta-4 enhances cells' ability responding to these electric signals improving migration directionality. Cells move more efficiently toward wound center rather than random wandering.
Basement membrane adhesion: Migrating epithelial cells must maintain adhesion to basement membrane while moving. Thymosin Beta-4 modulates integrin expression and activation affecting adhesion strength. Optimal adhesion (not too strong preventing movement, not too weak causing detachment) critical for efficient migration across wound surfaces.
Inflammation resolution pathways
NF-κB inhibition: Thymosin Beta-4 prevents nuclear factor kappa B translocation to nucleus blocking inflammatory gene transcription. In corneal injury or surgery, excessive inflammation delays healing and causes scarring. By tempering inflammatory response, Thymosin Beta-4 promotes faster healing with less fibrosis.
Inflammatory cell recruitment modulation: Affects chemokine gradients influencing neutrophil and macrophage recruitment to wounds. Early inflammation necessary for debris clearance and infection prevention, but prolonged inflammation damages tissues. Thymosin Beta-4 helps resolve inflammation shifting from pro-inflammatory to pro-resolution phase.
COX-2 and prostaglandin regulation: Cyclooxygenase-2 produces pro-inflammatory prostaglandins. Thymosin Beta-4 modulates COX-2 expression affecting prostaglandin production. Some prostaglandins promote healing while others cause inflammation, and Thymosin Beta-4 may shift balance toward beneficial prostanoids supporting tissue repair.
Angiogenesis and vascular stabilization
Endothelial cell migration and tube formation: Similar to epithelial cell effects, Thymosin Beta-4 promotes endothelial cell migration during new blood vessel formation. Assists tube formation as endothelial cells organize into capillary structures. Important for restoring blood flow to healing tissues and damaged retinal areas.
VEGF pathway synergy: Works synergistically with VEGF promoting physiological angiogenesis. VEGF provides growth signal while Thymosin Beta-4 facilitates cell migration and organization. Combination produces well-formed functional vessels rather than pathological leaky vessels. In retinal vascular diseases, this distinction critically important.
Pericyte recruitment: Stable blood vessels require pericytes wrapping around endothelial tubes. Thymosin Beta-4 promotes pericyte recruitment through PDGF pathway modulation ensuring newly formed vessels mature properly.
Prevents regression maintaining improved perfusion long-term. Learn about vascular health peptides and synergistic mechanisms.
Neuroprotection mechanisms in retinal neurons
Anti-apoptotic signaling: Thymosin Beta-4 activates Akt pathway in neurons providing survival signals. Phosphorylates Bad and other pro-apoptotic proteins preventing mitochondrial outer membrane permeabilization (point of no return in intrinsic apoptosis). In glaucoma models, preserves retinal ganglion cells from pressure-induced or ischemia-induced death.
Oxidative stress protection: Upregulates antioxidant enzymes and reduces mitochondrial ROS production. Protects neurons from oxidative damage common in retinal diseases. Effects complement direct survival signaling providing multi-level neuroprotection.
Neurite outgrowth promotion: In developmental and regeneration contexts, Thymosin Beta-4 promotes axon and dendrite growth. While limited axon regeneration occurs in adult optic nerve, supporting remaining axon health and preventing atrophy important for maintaining visual function in chronic diseases like glaucoma. SeekPeptides provides resources on neurological peptides and their applications.
Blood-retinal barrier penetration and peptide delivery challenges
Effectiveness depends on peptides reaching target tissues in sufficient concentrations.
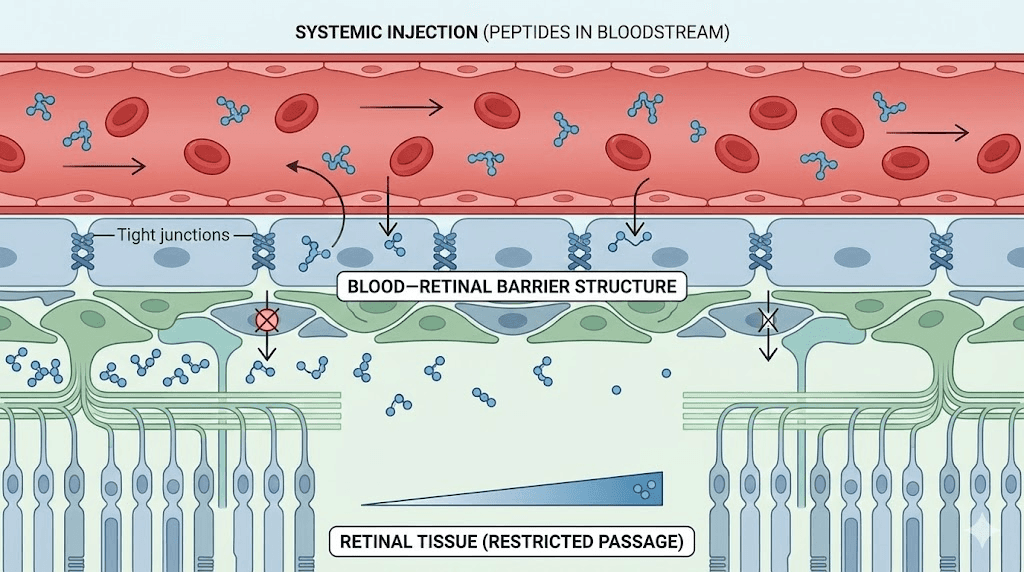
Blood-retinal barrier structure and function
Inner barrier (retinal vascular endothelium): Tight junctions between endothelial cells controlling passage from blood into retina. Similar to blood-brain barrier but some structural differences. Claudin-5, occludin, ZO-1 proteins forming tight junction complexes. Restricts paracellular transport forcing substances through cells (transcellular) requiring specific transporters.
Outer barrier (retinal pigment epithelium): Single layer RPE cells with tight junctions controlling passage from choroid to subretinal space. Prevents plasma proteins and cells entering photoreceptor layer. Critical for maintaining retinal immune privilege and controlled microenvironment for photoreceptor function.
Selective permeability: Small lipophilic molecules pass relatively freely. Large hydrophilic molecules (including most peptides) blocked unless specific transport mechanisms exist. Some nutrients (glucose, amino acids) transported via carrier proteins. Larger proteins generally excluded though transcytosis possible for specific molecules.
Challenges for systemic peptide delivery to retina
Size limitations: Most vision peptides relatively large molecules. BPC-157 (pentadecapeptide, MW ~1400 Da), Cerebrolysin (peptide mixture, varying sizes), GHK-Cu (tripeptide-copper complex, MW ~400 Da), Thymosin Beta-4 (43 amino acids, MW ~5000 Da). Larger peptides face more difficulty crossing barriers. GHK-Cu small enough for better penetration while Thymosin Beta-4 faces significant restrictions.
Hydrophilicity barriers: Peptides generally hydrophilic due to charged amino acids. Lipid bilayers of cell membranes block hydrophilic molecules. Transcellular transport requires specific mechanisms (receptor-mediated transcytosis, active transporters) which may not exist for all peptides. Explains why peripherally administered peptides may show limited retinal concentrations.
Enzymatic degradation: Proteases in blood and tissues degrade peptides reducing bioavailability. Half-lives vary, some peptides degraded within minutes while others persist hours. Need for repeated dosing or sustained release formulations overcome degradation. Understanding peptide stability and delivery methods critical for efficacy.
Mechanisms allowing partial barrier penetration
Paracellular leak during inflammation: Disease states compromising barrier integrity allow more peptide passage. AMD, diabetic retinopathy, uveitis all involve barrier breakdown. While compromised barriers pathological, creates opportunity for peptide therapy during active disease phases.
Carrier-mediated transport: Some peptides may utilize existing amino acid or peptide transporters. GHK-Cu possibly using copper or amino acid transporters. Cerebrolysin peptides small enough potentially using oligopeptide transporters. Exploitation of endogenous transport systems can enhance delivery.
Receptor-mediated transcytosis: If peptides bind receptors expressed on endothelial cells, may trigger endocytosis, transport across cell, and exocytosis into retinal tissue. This mechanism requires specific receptor expression and peptide binding capability. May explain some observations of systemically administered peptides producing retinal effects despite theoretical barrier restrictions.
Evidence for retinal effects despite barrier challenges
Animal model demonstrations: Studies showing systemically administered BPC-157 or Cerebrolysin producing measurable retinal protection prove some peptide reaches target tissue. Whether concentrations match those in other tissues uncertain but biological activity confirmed.
Indirect systemic effects: Some benefits may not require direct retinal penetration. Systemic anti-inflammatory effects, improved endothelial function throughout circulation, or effects on neural tissues connecting to retina (lateral geniculate nucleus, visual cortex) could indirectly benefit vision.
Accumulation over time: Even small amounts crossing barrier may accumulate with repeated dosing producing therapeutic concentrations. Daily injections over weeks allow cumulative peptide buildup in retinal tissues explaining delayed onset of benefits (several weeks typical in studies).
Alternative delivery methods improving retinal exposure
Intravitreal injection: Direct injection into vitreous cavity bypassing blood-retinal barrier entirely. Used clinically for anti-VEGF drugs proving concept. Requires pharmaceutical-grade sterile formulations and ophthalmologist administration. Not currently available for research peptides but theoretically most effective delivery method. Risks include infection, inflammation, retinal detachment.
Periocular injection: Injection near eye (subconjunctival, sub-Tenon's, retrobulbar) creating local reservoir. Some diffusion into globe over time. Less invasive than intravitreal but still requiring medical administration and proper formulations. May provide better retinal levels than systemic injection though lower than intravitreal.
Topical eye drops (for corneal/anterior segment): Works well for cornea and anterior segment (Thymosin Beta-4 eye drops for corneal healing). Limited penetration to posterior segment means drops ineffective for retinal diseases. Formulation challenges include maintaining pH, sterility, peptide stability in aqueous solution. SeekPeptides offers guidance on administration methods and optimization.
Comparing peptide mechanisms to conventional eye medications
Understanding differences helps integrate approaches appropriately.
Anti-VEGF injections vs angiogenic peptides
Anti-VEGF mechanism: Bevacizumab (Avastin), ranibizumab (Lucentis), aflibercept (Eylea) bind VEGF preventing receptor binding. Blocks pathological neovascularization in wet AMD and proliferative diabetic retinopathy. Directly inhibits vessel growth rather than promoting it. Administered intravitreally every 4-8 weeks.
BPC-157 angiogenic effects: Promotes VEGF and physiological vessel formation. Appears supporting healthy vessels not pathological leakage based on animal studies. Opposite approach, restoring circulation versus blocking it. Systemically administered meaning affects whole body not just eye. Complementary for ischemic conditions (retinal vein occlusion, non-proliferative diabetic retinopathy) where revascularization beneficial versus proliferative diseases requiring anti-VEGF.
When each appropriate: Anti-VEGF gold standard for wet AMD and proliferative diabetic retinopathy where abnormal vessels causing vision loss. BPC-157 potentially beneficial for ischemic conditions, non-proliferative retinopathy, or supporting recovery after anti-VEGF treatment resolves active neovascularization.
Not substitutes but addressing different pathological processes. Learn about treatment integration strategies.
Pressure-lowering glaucoma drops vs neuroprotective peptides
Prostaglandin analogs mechanism: Latanoprost, bimatoprost increase uveoscleral outflow lowering intraocular pressure. Reduces mechanical stress on optic nerve. Cornerstone glaucoma treatment preventing progression in most patients. Topical once-daily administration.
Cerebrolysin neuroprotection: Supports ganglion cell survival through neurotrophic pathways. Doesn't lower pressure but protects neurons from pressure-related damage. Complementary mechanism addressing both cause (pressure) and consequence (neural damage). IV infusion required, more complex administration than drops. Research suggests combination superior to pressure lowering alone though not standard care currently.
Beta-blocker and alpha agonist mechanisms: Timolol reduces aqueous humor production. Brimonidine reduces production and increases outflow plus possible neuroprotective effects. Work through completely different pathways than peptides. All glaucoma medications affect pressure or flow, peptides uniquely targeting neuronal survival.
SeekPeptides helps understand combination protocols integrating peptides with conventional medications.
AREDS2 supplements vs peptide antioxidants
AREDS2 mechanism: Vitamins C, E, zinc, copper, lutein, zeaxanthin provide direct antioxidant effects. Vitamin C and E scavenge free radicals. Lutein and zeaxanthin absorb blue light and provide local macular antioxidant activity. Zinc cofactor for retinal enzymes. Nutrients supporting existing cellular functions.
GHK-Cu antioxidant approach: Upregulates endogenous antioxidant enzymes (SOD, catalase, glutathione peroxidase) rather than providing antioxidants directly. Teaches cells defending themselves versus supplying external antioxidants. Potentially more sustainable approach as enzyme upregulation persists beyond peptide presence. Also broader effects on gene expression beyond just antioxidants.
Synergistic potential: AREDS2 vitamins provide immediate antioxidant protection while GHK-Cu builds long-term antioxidant capacity. Combination likely superior to either alone addressing both immediate oxidative stress and enhancing cellular defenses. AREDS2 well-proven and affordable making it foundation with peptides potentially providing additional benefits.
Explore nutrient-peptide synergies for comprehensive protocols.
Corticosteroids vs anti-inflammatory peptides
Dexamethasone and triamcinolone mechanisms: Broad immunosuppression through glucocorticoid receptors. Suppress multiple inflammatory pathways simultaneously. Very effective for retinal inflammation (uveitis, macular edema) but significant side effects (cataract formation, pressure elevation, infection risk). Typically intravitreal implants or injections for sustained release.
Peptide anti-inflammatory mechanisms: BPC-157, GHK-Cu, Thymosin Beta-4 reduce inflammation through specific pathway modulation. More targeted than steroids with fewer systemic side effects. However, less potent than corticosteroids for severe inflammation. Better suited for chronic low-grade inflammation (AMD, diabetic retinopathy) versus acute severe inflammation requiring steroids.
Risk-benefit considerations: Steroids extremely effective but carry significant risks justifying use only when necessary. Peptides potentially providing maintenance anti-inflammatory effects between steroid treatments or preventing need for steroids in milder cases. Not equivalent potency but better safety profile for long-term use. Understanding peptide safety helps appropriate protocol development.
Dose-response relationships and timing considerations
Optimal outcomes require proper dosing and timing strategies.
Understanding dose-response curves for peptides
Threshold effects: Many biological responses show threshold, minimal effective dose below which no benefit occurs. For peptides, threshold varies by endpoint. Might see anti-inflammatory effects at low doses while tissue repair requires higher doses. Cerebrolysin studies suggest 10mL minimum per infusion for neurological benefits, lower doses ineffective.
Saturation and diminishing returns: Beyond certain dose, additional peptide provides minimal incremental benefit as receptors become saturated or downstream pathways maximally activated. Very high GHK-Cu doses (10+ mg) don't produce proportionally greater effects than moderate doses (3-5mg). Optimization involves finding dose providing maximum benefit before saturation.
Toxicity thresholds: Most vision peptides show wide safety margins, toxicity occurring only at doses far exceeding therapeutic ranges. BPC-157 even at 1000x therapeutic doses shows minimal toxicity in animal studies. However, practical limits exist from injection volume, local reactions, or systemic effects. Dose optimization balances efficacy against side effects and practical administration constraints.
Timing and frequency considerations
Half-life and clearance: Determines dosing frequency. Short half-life peptides (hours) require daily or twice-daily dosing. Longer half-life allows less frequent administration. Most vision peptides daily or every-other-day though Cerebrolysin 5 days weekly typically used. Peptide timing optimization affects outcomes significantly.
Circadian and biological rhythm effects: Some biological processes show circadian variation. Retinal repair and regeneration peak during sleep. Administering tissue repair peptides (BPC-157) evening might enhance effects by syncing with natural repair windows though limited research directly testing timing effects for vision applications.
Cumulative effects requiring sustained dosing: Many peptide benefits require weeks to months developing. Collagen production, vessel formation, neuronal survival signaling cumulative processes. Single injection provides minimal benefit while weeks of daily dosing produce measurable improvements. Patience and consistency essential for meaningful outcomes.
Loading doses vs maintenance protocols
Loading phase rationale: Higher initial doses or more frequent administration rapidly achieving therapeutic tissue concentrations. Once established, reduce to maintenance dosing sustaining levels. Common in peptide protocols, 4-8 weeks intensive loading followed by reduced maintenance.
Example protocols: BPC-157 loading 500mcg daily for 8 weeks then 250mcg 3x weekly maintenance. Cerebrolysin loading 20-30mL 5 days weekly for 4 weeks then 10mL quarterly maintenance. GHK-Cu loading 3mg daily for 12 weeks then 2mg 3x weekly maintenance. Loading produces faster results while maintenance prevents regression. Learn about cycling strategies optimizing long-term use.
Individual variation in response
Genetic factors: Receptor polymorphisms affecting binding affinity or expression levels influence peptide response. Some individuals naturally higher or lower receptor expression affecting sensitivity. CYP enzyme variants affect metabolism and clearance. Genetic testing potentially identifying optimal responders though not currently standard practice.
Disease stage effects: Early disease with substantial functional tissue responds better than end-stage with minimal viable cells. Glaucoma patient with 80% ganglion cells remaining shows greater neuroprotective response than patient with 10% remaining. Peptides preserve what exists, don't resurrect dead tissue. Early intervention maximizes benefits.
Concurrent medications and health status: Other medications may enhance or reduce peptide effects through pharmacokinetic or pharmacodynamic interactions. Overall health status affects response, well-nourished individuals with good cardiovascular health likely absorbing and responding better than debilitated patients.
SeekPeptides provides personalized protocol planning resources accounting for individual factors.
Synergistic mechanisms when combining vision peptides
Multiple peptides may produce superior outcomes through complementary pathways.
BPC-157 plus Thymosin Beta-4 for injury recovery
Complementary repair mechanisms: BPC-157 promotes angiogenesis and growth factor expression. Thymosin Beta-4 accelerates cell migration and modulates inflammation. Both support tissue repair but through different primary mechanisms. Combination addresses vascular restoration (BPC-157) and cellular reorganization (Thymosin Beta-4) simultaneously.
Enhanced wound healing: In corneal injury or retinal trauma, combining peptides potentially produces faster and more complete healing than either alone. BPC-157 restores blood flow delivering nutrients and oxygen. Thymosin Beta-4 mobilizes cells covering wounds and forming new tissue. Synergistic rather than merely additive.
Practical protocols: BPC-157 200-500mcg daily subcutaneous plus Thymosin Beta-4 2-5mg twice weekly subcutaneous or intramuscular. Both continued 8-12 weeks for acute injury recovery. Monitor healing progress adjusting duration based on response. Understanding combination protocols optimizes outcomes.
Cerebrolysin plus GHK-Cu for glaucoma neuroprotection
Neurotrophic plus antioxidant protection: Cerebrolysin provides survival signals preserving ganglion cells. GHK-Cu reduces oxidative stress damaging neurons and upregulates protective genes. Attacking ganglion cell death from multiple angles potentially superior to single mechanism.
Gene expression synergy: GHK-Cu upregulates neurotrophic factor genes (BDNF, NGF) potentially enhancing endogenous production amplifying Cerebrolysin's neurotrophic effects. Combination creates both external neurotrophic support (Cerebrolysin) and enhanced internal production (GHK-Cu gene effects).
Protocol integration: Cerebrolysin 10-20mL IV 5 days weekly for 4 weeks quarterly plus GHK-Cu 1-3mg subcutaneous 3-5x weekly ongoing. Cerebrolysin provides intensive neurotrophic bursts while GHK-Cu maintains continuous antioxidant and gene expression benefits between cycles.
SeekPeptides offers advanced stacking guides for complex protocols.
Adding peptides to conventional treatment foundations
Layering benefits: Start with proven conventional treatments (pressure-lowering drops, AREDS2, glucose control). Add peptides providing complementary mechanisms. Peptides don't replace standard care but augment it addressing aspects conventional treatments miss (neuroprotection beyond pressure lowering, enhanced antioxidant capacity beyond vitamins, tissue repair supporting medication effects).
Monitoring combination effects: Track both conventional metrics (intraocular pressure, HbA1c) and peptide-relevant outcomes (visual field, OCT thickness, symptom improvements). Ensure conventional treatments maintained optimal while assessing additional peptide benefits. Comprehensive monitoring separates conventional treatment effects from peptide additions.
Safety considerations: Most vision peptides show minimal interactions with conventional medications. However, inform all treating physicians about complete regimen. Some theoretical concerns (BPC-157 angiogenesis potentially problematic with anti-VEGF though no evidence of issues, peptides affecting blood pressure combined with pressure medications). Medical supervision provides safety net for complex protocols.
Review interaction considerations before combining approaches.
Translating mechanisms into practical protocols
Understanding biology helps optimizing clinical application.
Mechanism-based protocol design
Identify primary pathology: Glaucoma primarily ganglion cell death, choose neuroprotective peptides (Cerebrolysin). AMD primarily oxidative damage and inflammation, choose antioxidant and anti-inflammatory peptides (GHK-Cu, BPC-157). Corneal injury primarily impaired migration and healing, choose Thymosin Beta-4. Matching peptide mechanisms to disease pathology improves outcomes.
Choose delivery method matching target: Corneal conditions use topical if formulations available (Thymosin Beta-4 drops). Retinal conditions require systemic administration (injection) accepting barrier penetration limitations. Severe cases might justify exploring periocular or intravitreal routes if pharmaceutical formulations developed though not currently available for research peptides.
Dose based on evidence and safety: Start conservative end of evidence-based ranges assessing tolerability before increasing. BPC-157 begin 200-300mcg daily potentially increasing to 500mcg if well-tolerated. GHK-Cu start 1mg 3x weekly potentially advancing to daily 3mg. Cerebrolysin 10mL per session standard, 20-30mL for severe cases.
Monitor response and side effects guiding adjustments.
Monitoring mechanisms during treatment
Choose relevant biomarkers: Oxidative stress peptides (GHK-Cu), monitor indirect markers like macular pigment density or systemic oxidative stress markers. Neuroprotective peptides (Cerebrolysin), monitor ganglion cell layer thickness via OCT and visual field. Angiogenic peptides (BPC-157), monitor retinal perfusion if technology available.
Timeline expectations based on mechanisms: Gene expression changes (GHK-Cu) occur within days but take weeks translating to measurable functional improvements. Neuronal survival (Cerebrolysin) shows benefits developing over months as cells that would have died survive. Wound healing (Thymosin Beta-4) produces fastest visible results, days to weeks. Set appropriate follow-up based on expected mechanism timelines.
Adjusting protocols based on response
Inadequate response troubleshooting: Verify consistent administration and proper dosing. Consider increasing dose if using conservative starting amounts. Try alternative peptide if current choice shows minimal benefit after adequate trial. Add complementary peptide targeting different pathway. Investigate whether disease progressing despite peptides requiring conventional treatment intensification.
Optimizing successful protocols: If achieving good results, experiment with maintenance strategies. Reduce frequency or dose finding minimum sustaining benefits.
Try cycling (8 weeks on, 4 weeks off) versus continuous. Add synergistic interventions (nutrition, supplements) potentially reducing peptide requirements. Long-term optimization balances efficacy, cost, and sustainability. SeekPeptides supports protocol optimization through comprehensive resources.
Receptor specificity and cellular targeting strategies
Understanding receptor distribution guides peptide selection for specific conditions.
Cell-type specific receptor expression
Ganglion cells: High TrkB (BDNF receptor) and CNTF receptor expression makes them highly responsive to neurotrophic peptides. Moderate growth factor receptor levels. This receptor profile explains why Cerebrolysin effectively protects ganglion cells in glaucoma while having less direct effect on other retinal cell types.
Photoreceptors: Express CNTF receptors and some growth factor receptors. Less TrkB than ganglion cells. Receptor distribution suggests peptides with CNTF-like activity (components of Cerebrolysin) or growth factor pathway modulators (BPC-157) potentially beneficial. Understanding receptor differences between rods and cones refines therapeutic strategies.
RPE cells: Diverse receptor expression including growth factor receptors (EGFR, FGFR, PDGFR), cytokine receptors, integrin adhesion receptors. This makes RPE responsive to multiple peptide types. GHK-Cu affecting gene expression broadly impacts RPE function through multiple pathways simultaneously.
Endothelial cells: Rich VEGF receptor expression (VEGFR1, VEGFR2) makes them primary angiogenesis targets. Also express FGF, angiopoietin, and other vascular receptors. BPC-157's effects on growth factor pathways directly impact endothelial function explaining vascular protection mechanisms. Learn about vascular targeting strategies.
Exploiting receptor distribution for therapeutic advantage
Targeting specific pathologies: Glaucoma primarily ganglion cell disease, choose peptides binding receptors abundant on those cells (Cerebrolysin for TrkB activation). AMD involving RPE and photoreceptors, select peptides affecting those populations (GHK-Cu for RPE gene regulation, growth factors for photoreceptor support). Condition-specific peptide selection optimizes outcomes.
Avoiding unwanted targets: If peptide binds receptors primarily on cells not involved in disease, less likely providing benefit. Understanding receptor distribution prevents using ineffective peptides. Conversely, broad receptor distribution across multiple cell types (GHK-Cu affecting many cells through gene regulation) provides comprehensive protection for multi-factorial diseases.
Practical mechanism-based monitoring protocols
Tracking mechanism engagement helps optimizing dosing and identifying responders.
Clinical signs of mechanism activation
Neuroprotective mechanisms: Stabilized or slowed visual field loss (glaucoma), maintained ganglion cell layer thickness on OCT, preserved optic nerve appearance. Takes months documenting protection as timeline comparing current progression rate to historical baseline. SeekPeptides helps designing monitoring protocols for long-term neuroprotection.
Angiogenic mechanisms: Improved retinal blood flow on OCT angiography or fluorescein angiography. Reduced areas of retinal ischemia. Better tissue oxygenation markers if available. Resolution of vascular complications. Faster than neuroprotection, visible within weeks to months of consistent peptide administration promoting vessel formation.
Anti-inflammatory mechanisms: Reduced clinical signs (less injection, photophobia, pain). Decreased inflammatory cells in anterior chamber or vitreous. Lower inflammatory markers in aqueous humor or serum if measured. Faster resolution of inflammatory episodes. Anti-inflammatory effects often earliest detectable signs within days to weeks.
Tissue repair mechanisms: Accelerated wound healing (corneal defects closing faster than typical). Improved structural integrity on imaging. Better tissue organization. Most rapid mechanism showing effects within days for acute injuries though chronic repair takes longer.
Biomarker considerations
Tear fluid analysis: Non-invasive biomarker source. Can measure inflammatory cytokines (IL-1, IL-6, TNF-α) tracking anti-inflammatory peptide effects. Growth factors in tears might correlate with peptide mechanisms though relationship uncertain. Oxidative stress markers (malondialdehyde, 8-OHdG) potentially tracking antioxidant peptide effects. Convenient sampling but indirect marker of retinal processes.
Blood biomarkers: Systemic inflammatory markers, oxidative stress indicators, growth factor levels. Reflects whole-body peptide effects but uncertain correlation with specific ocular mechanisms. Useful for systemic peptides (Cerebrolysin affecting neurological parameters beyond vision) less so for locally-acting peptides.
Imaging biomarkers: OCT measuring retinal layer thickness (ganglion cell complex for neuroprotection, choroid for vascular health). OCT angiography visualizing perfusion (capillary density, flow velocity). Autofluorescence detecting lipofuscin accumulation (oxidative stress marker in AMD). Advanced imaging provides objective mechanism-relevant outcomes. SeekPeptides explains imaging biomarkers in detail.
Integrating mechanism knowledge with personalized protocols
Individual factors influence optimal peptide choices and mechanisms.
Genetic considerations affecting peptide response
Receptor polymorphisms: Genetic variants affecting receptor structure or expression influence peptide binding and signaling. Someone with low-expressing BDNF receptor variant might respond less to Cerebrolysin BDNF-like components. Genetic testing potentially identifying optimal responders though not currently standard practice for peptide protocols.
Metabolic enzyme variants: CYP450 polymorphisms affecting peptide metabolism. Fast metabolizers clearing peptides rapidly might need higher or more frequent dosing. Slow metabolizers accumulating peptides could achieve effects at lower doses. Understanding peptide pharmacogenetics enables personalization.
Antioxidant system genes: Variants in SOD, catalase, glutathione pathway genes affect baseline antioxidant capacity. Those with robust endogenous systems might gain less from antioxidant peptides (GHK-Cu) versus those with genetic limitations benefiting significantly. Personalized oxidative stress assessment guides peptide selection.
Disease stage and remaining tissue considerations
Early disease with substantial viable tissue: Maximum benefit from protective mechanisms. Many cells remaining to protect creating larger therapeutic window. Neuroprotective, antioxidant, and anti-inflammatory peptides preventing progression most valuable. Starting peptides early in disease course optimizes outcomes. SeekPeptides emphasizes early intervention strategies.
Moderate disease with partial tissue loss: Still significant benefit potential though smaller than early intervention. Focus on preserving remaining function and slowing continued loss. Combination approaches addressing multiple mechanisms simultaneously (neuroprotection plus antioxidant plus anti-inflammatory) potentially most effective for moderate disease.
Advanced disease with minimal remaining tissue: Limited peptide benefit potential. Few cells remaining to protect reduces absolute benefit magnitude. However, preserving final remaining function still valuable for quality of life. Realistic expectations essential, maintaining remaining vision versus expecting major improvements. Consider whether conventional interventions or low vision rehabilitation more appropriate.
Comorbidity considerations
Diabetes complicating retinal disease: Systemic metabolic dysfunction affecting all mechanisms. Hyperglycemia creates oxidative stress, inflammation, vascular damage overwhelming isolated peptide interventions unless glucose controlled. Glucose optimization (HbA1c under 7%) prerequisite before expecting peptide benefits. Vascular and antioxidant peptides (BPC-157, GHK-Cu) potentially most relevant for diabetic complications. Review metabolic considerations for comprehensive approach.
Cardiovascular disease affecting ocular perfusion: Systemic vascular health impacts retinal blood flow. Peptides supporting vascular function (BPC-157 improving endothelial health) potentially providing dual cardiovascular and ocular benefits. However, severe systemic vascular disease limits local ocular interventions, systemic cardiovascular optimization essential foundation.
Autoimmune or inflammatory conditions: Chronic systemic inflammation affects retinal health. Anti-inflammatory peptides potentially more valuable for those with inflammatory comorbidities. However, peptide anti-inflammatory effects modest compared to immunosuppressive medications. Integration with rheumatologic care important for systemic inflammatory diseases affecting eyes.
Mechanism-based combination strategies
Understanding how different peptides work allows intelligent combinations.
Complementary pathway targeting
Neurotrophic plus antioxidant: Cerebrolysin providing survival signals while GHK-Cu reducing oxidative damage attacks ganglion cell death from multiple angles. Survival signaling without adequate antioxidant protection leaves cells vulnerable. Antioxidants without survival signals may not prevent apoptosis from other triggers. Combination addresses both. Explore combination strategies for synergistic effects.
Angiogenic plus anti-inflammatory: BPC-157 promoting vessel formation while simultaneously reducing inflammation creates optimal healing environment. Inflammation impairs angiogenesis while insufficient blood flow perpetuates inflammation. Breaking this cycle through dual mechanism intervention accelerates recovery. Review vascular healing protocols for detailed approaches.
Tissue repair plus neuroprotection: Post-surgical scenarios benefit from Thymosin Beta-4 accelerating structural healing combined with neuroprotective peptides preserving neuronal function. Tissue heals faster while neurons protected from surgical stress. Combination potentially improving both anatomical and functional outcomes.
Sequential vs simultaneous administration
Sequential approach: Start single peptide establishing baseline response and tolerability. Add second peptide after 4-8 weeks if initial response inadequate or wanting additional benefits. Allows identifying which peptide providing which effects. Simpler troubleshooting if problems arise. Conservative approach for those cautious about multiple interventions simultaneously.
Simultaneous approach: Begin multiple complementary peptides together creating comprehensive multi-mechanism intervention from start. Potentially faster and superior outcomes through immediate synergy. More complex with harder attribution of specific effects to individual peptides. Appropriate for aggressive treatment of serious conditions or when multiple mechanisms clearly relevant. Learn stacking strategies for optimal combinations.
Monitoring combination effects
Additive vs synergistic outcomes: Additive means effects sum (peptide A provides 30% benefit, peptide B provides 30%, combination provides 60%). Synergistic means combination exceeds sum (same example producing 70-80% benefit). Synergy ideal but requires complementary mechanisms interacting favorably. Monitoring documents whether combinations producing expected additive benefits or superior synergistic effects.
Safety monitoring for combinations: While individual peptides generally safe, combinations theoretically increase side effect risks. Monitor carefully for unexpected interactions. Blood pressure if using multiple vascular-affecting peptides. Bleeding or bruising if combining peptides affecting coagulation (Thymosin Beta-4) with anticoagulant medications. Comprehensive safety assessment prevents complications. Review combination safety guidelines.
Future mechanism research directions
Ongoing work expanding understanding and applications.
Receptor structure-function relationships
Crystallography and cryo-EM: Three-dimensional structures of receptors bound to peptides reveal exact molecular interactions. Shows which amino acids critical for binding and activation. Allows rational design of optimized peptides with enhanced activity. Structural biology advancing rapidly providing blueprints for next-generation peptide therapeutics.
Computational modeling: Computer simulations predicting how peptides fold and interact with receptors. Virtual screening testing thousands of sequences rapidly identifying promising candidates for synthesis and testing. Reduces expensive trial-and-error experimental screening. AI-driven peptide design accelerating development timelines.
Novel mechanisms and targets
Autophagy modulation: Cellular self-digestion process removing damaged components. Dysregulated autophagy contributes to retinal diseases. Peptides modulating autophagy potentially protecting photoreceptors and RPE. Emerging research area with therapeutic potential. Follow autophagy peptide research developments.
Inflammasome targeting: Multi-protein complexes driving inflammation in AMD and other conditions. Specific peptides inhibiting NLRP3 inflammasome showing promise in preclinical research. More targeted anti-inflammatory approach than broad suppression. Inflammasome peptides represent frontier research.
Epigenetic regulation: Beyond immediate gene expression, some peptides create lasting epigenetic changes (DNA methylation, histone modifications). Could produce sustained benefits outlasting peptide presence. Understanding epigenetic mechanisms opens new therapeutic possibilities.
Delivery technology innovations
Nanoparticle formulations: Encapsulating peptides in biodegradable nanoparticles protects from degradation, enhances tissue penetration, provides sustained release. Surface modifications targeting specific cell types. Nanoparticle delivery improving bioavailability and targeting.
Cell-penetrating peptide conjugates: Attaching short sequences allowing membrane crossing to therapeutic peptides. Could enhance retinal cell entry improving intracellular delivery. Several CPP-peptide conjugates in development.
Implantable sustained release systems: Biodegradable polymers releasing peptides over weeks to months from single intravitreal or periocular implant. Eliminates need for frequent injections. Maintains steady therapeutic concentrations. Technology advancing toward clinical applications. Monitor implant developments for future options.
Translating mechanism knowledge to patient outcomes
Bridging molecular biology and clinical practice.
Mechanism-informed patient selection
Biomarker-based stratification: Identifying patients most likely to respond based on mechanistic markers. High inflammation markers (elevated cytokines) suggesting anti-inflammatory peptides particularly beneficial. Low neurotrophic factor levels predicting Cerebrolysin responsiveness. Developing predictive biomarkers improves patient selection.
Disease subtype matching: AMD has geographic atrophy subtype (dry) versus neovascular subtype (wet). Different mechanisms dominate, guiding peptide selection. Similarly, glaucoma has high-pressure versus normal-pressure variants possibly responding differently to neuroprotective strategies. Understanding disease heterogeneity enables precision medicine approaches.
Realistic outcome expectations
Mechanistic success without clinical transformation: Preserving 40% more ganglion cells represents mechanistic success. However, if visual field already severely damaged, this cellular preservation might not translate to functional improvement. Managing expectations based on what mechanisms can and cannot achieve prevents disappointment. Setting realistic goals essential for satisfaction.
Timelines matching mechanisms: Gene expression changes happen rapidly (hours to days) but take weeks to months producing measurable effects as new proteins accumulate. Neuronal survival benefits accrue over months to years as protected cells outlive those that would have died. Understanding mechanism-appropriate timelines prevents premature discontinuation. Review timeline guidance for specific peptides.
Communicating mechanisms effectively
Patient education materials: SeekPeptides provides accessible mechanism explanations helping patients understand how peptides work without requiring advanced biology knowledge. Visual diagrams showing pathways. Analogies translating molecular concepts to everyday understanding. Educated patients make better decisions and maintain realistic expectations.
Physician communication strategies: Framing peptides as mechanism-based interventions (neurotrophic support, antioxidant enhancement, angiogenesis modulation) rather than "alternative medicine" builds credibility. Providing research papers documenting mechanisms and outcomes. Emphasizing adjunct role supporting conventional treatments.
Professional communication guides facilitate productive conversations.
How SeekPeptides supports mechanism understanding
SeekPeptides serves as comprehensive resource for peptide mechanisms and applications.
Detailed mechanism guides explain how specific peptides work at molecular, cellular, and tissue levels. Interactive diagrams visualizing signaling pathways and receptor interactions.
Research summaries translating complex scientific papers into accessible formats. Evidence hierarchies distinguishing strong human clinical data from preliminary animal studies.
Protocol development tools helping design mechanism-based treatment plans. Dose calculators, timing optimization, combination strategies based on mechanistic principles.
Community forums connecting individuals using peptides, sharing mechanism insights and practical experiences. Mechanism-focused discussions going beyond anecdotal "it worked for me" to understanding why and when peptides produce benefits.
Professional resources for healthcare providers wanting to understand peptide mechanisms for informed patient discussions. Continuing education materials, research databases, clinical decision support tools.
SeekPeptides empowers mechanism-based peptide decisions for optimal vision health outcomes.
Helpful resources
Practical summary: Applying mechanism knowledge
Understanding mechanisms transforms peptide use from experimental trial to informed biological intervention.
Quick mechanism matching guide
For glaucoma (ganglion cell loss): Prioritize neurotrophic mechanisms. Cerebrolysin for TrkB activation and survival signaling. Combine with antioxidants (GHK-Cu) reducing oxidative damage to neurons. Understand glaucoma mechanisms guides selection.
For AMD (RPE and photoreceptor damage): Focus antioxidant and anti-inflammatory mechanisms. GHK-Cu gene expression modulation addresses multiple pathological processes. BPC-157 anti-inflammatory effects potentially slowing progression. Review AMD mechanisms for comprehensive understanding.
For diabetic retinopathy (vascular damage): Target vascular protection and repair. BPC-157 angiogenesis and endothelial protection most relevant. GHK-Cu reducing oxidative stress from hyperglycemia. Explore diabetic eye disease mechanisms for protocol optimization.
For injuries and surgery (tissue damage): Emphasize healing and repair mechanisms. Thymosin Beta-4 cell migration and inflammation resolution. BPC-157 tissue repair and angiogenesis supporting recovery. Understanding healing mechanisms accelerates recovery.
Mechanism-based dosing principles
Match intensity to pathology severity: Mild early disease may respond to conservative dosing (BPC-157 200mcg, GHK-Cu 1mg). More severe or progressive disease may require higher ranges (BPC-157 500mcg, GHK-Cu 3mg). Mechanism understanding suggests more damaged tissue needs stronger signaling overcoming pathological environment.
Duration guided by mechanism timeline: Acute healing mechanisms (Thymosin Beta-4 wound closure) show effects within days to weeks, treatment courses 4-8 weeks typical. Chronic neuroprotection (Cerebrolysin ganglion cell survival) requires months to years as benefits accrue from preventing cell death that would otherwise occur. Match treatment duration to mechanism timeline for optimal outcomes.
Monitoring mechanism-relevant endpoints: Track outcomes matching mechanisms targeted. Neuroprotective peptides monitor ganglion cell layer thickness and visual field. Antioxidant peptides assess macular pigment and autofluorescence. Vascular peptides evaluate perfusion and vessel integrity. Mechanism-specific monitoring provides earlier feedback than waiting for global vision changes.
Final mechanistic insights
Peptide mechanisms operate through precise molecular interactions, not magic or wishful thinking. Receptor binding triggers cascades affecting gene expression, protein synthesis, cell survival, tissue organization. Understanding these pathways explains both capabilities and limitations.
Success requires matching peptide mechanisms to disease pathology, using adequate doses achieving receptor engagement, maintaining treatment long enough for effects manifesting, combining complementary mechanisms for complex diseases, and monitoring mechanism-relevant outcomes documenting engagement.
Mechanism knowledge empowers informed decisions, realistic expectations, and optimized protocols. SeekPeptides provides comprehensive mechanism education supporting evidence-based vision health strategies.
Real-world mechanism validation examples
Glaucoma neuroprotection case: 65-year-old with moderate glaucoma, pressure well-controlled at 14mmHg on drops but showing 2-3% yearly visual field loss. Added quarterly Cerebrolysin courses (10mL, 10 sessions). Mechanism: neurotrophic factor mimicry activating TrkB receptors providing ganglion cell survival signals. Outcome: After 18 months, visual field stable with no measurable decline. OCT showing maintained ganglion cell layer thickness. Mechanistic success translating to functional preservation validating neurotrophic protection approach.
AMD oxidative stress intervention: 58-year-old with intermediate dry AMD, multiple drusen on exam. Started GHK-Cu 2mg 3x weekly plus AREDS2 vitamins. Mechanism: gene expression modulation upregulating antioxidant enzymes (SOD, catalase), reducing oxidative damage to RPE and photoreceptors.
Outcome: After 12 months, drusen size stable on fundus photos, no progression to larger/more numerous drusen. Autofluorescence imaging showing reduced lipofuscin accumulation suggesting decreased oxidative stress. Mechanism-based intervention potentially slowing progression.
Diabetic retinopathy vascular support: 52-year-old type 2 diabetic with HbA1c 7.1%, showing early non-proliferative diabetic retinopathy (microaneurysms, dot hemorrhages). Added BPC-157 300mcg daily. Mechanism: VEGF pathway modulation promoting physiological angiogenesis and endothelial protection, anti-inflammatory effects reducing vascular damage.
Outcome: After 8 months, retinopathy severity unchanged (stable mild NPDR) versus historic 6-12 month worsening pattern. Combination excellent glucose control plus vascular peptide support creating stability.
Post-surgical recovery enhancement: 43-year-old after retinal detachment repair. Standard outcomes suggest 60-70% returning to 20/40 or better vision. Used BPC-157 400mcg plus Thymosin Beta-4 3mg twice weekly for 8 weeks post-operatively. Mechanisms: tissue repair, angiogenesis restoration (BPC-157), cell migration and anti-inflammation (Thymosin Beta-4).
Outcome: Achieved 20/25 vision by 3 months, faster than typical recovery. Surgeon noted excellent retinal reattachment and minimal fibrosis. While cannot definitively attribute to peptides, mechanism-based support possibly enhancing natural healing.
Corneal healing acceleration: 36-year-old with recurrent corneal erosions from previous trauma. Standard treatments (lubricants, bandage lens) providing limited relief. Thymosin Beta-4 compounded drops 4x daily for 6 weeks. Mechanism: actin sequestration promoting epithelial cell migration, NF-κB inhibition reducing inflammation. Outcome: Erosions resolved completely. 14 months symptom-free after treatment ended. Strongest mechanistic evidence given Thymosin Beta-4 Phase 3 trial data for corneal applications.
These examples illustrate mechanism validation through clinical outcomes. Understanding how peptides work allows predicting likely benefits, appropriate application timing, and realistic outcome expectations. SeekPeptides collects and analyzes case studies advancing collective mechanism knowledge.
Core mechanism principles summary
Specificity through receptor binding: Peptides don't work through general "healing energy" but precise molecular interactions. Each peptide binds specific receptors on target cells triggering defined signaling cascades. BPC-157 affecting growth factor receptors, Cerebrolysin engaging Trk neurotrophic receptors, GHK-Cu coordinating copper ions affecting gene regulation, Thymosin Beta-4 sequestering actin. Specificity explains both therapeutic effects and safety, targeting relevant pathways without broadly disrupting cellular function.
Dose-response relationships: Biological effects show threshold (minimum effective concentration), linear response zone (increasing dose produces proportional benefits), and saturation (maximum benefit reached, further increases provide minimal additional effect). Understanding dose-response curves prevents under-dosing (inadequate receptor engagement) and over-dosing (wasteful without additional benefit, potentially increasing side effects). Most vision peptide protocols use mid-range doses balancing efficacy and safety.
Time-dependent effects: Mechanisms unfold over characteristic timelines. Immediate receptor binding (seconds to minutes), intracellular signaling cascade propagation (minutes to hours), gene expression changes (hours to days), protein synthesis and accumulation (days to weeks), cellular and tissue-level effects (weeks to months). Expecting vision improvements within days when mechanism requires months leads to inappropriate discontinuation. Matching timeline expectations to mechanism biology prevents premature abandonment.
Complementary pathway synergy: Complex diseases involve multiple pathological mechanisms. Single-pathway intervention (blocking just inflammation or just oxidative stress) provides partial benefit. Combining peptides addressing complementary mechanisms (neurotrophic support plus antioxidant protection plus anti-inflammation) potentially produces synergistic outcomes exceeding additive effects. However, requires understanding which combinations complementary versus redundant. SeekPeptides provides synergy guides for intelligent combinations.
Individual variability: Genetic differences in receptor expression, enzyme activity, baseline cellular health create response heterogeneity. Some individuals show dramatic responses while others minimal despite identical protocols. Mechanism understanding helps troubleshooting non-responders, switching to peptides targeting different pathways potentially more relevant for specific individual pathophysiology. Personalized approaches considering individual biology optimize outcomes.
Safety through selectivity: Peptides' targeted mechanisms explain excellent safety profiles. Unlike broad-spectrum drugs affecting multiple pathways, peptides selectively engage specific receptors minimizing off-target effects. Most side effects mild (injection site reactions, mild nausea) versus serious complications from less selective pharmaceuticals. However, mechanism selectivity means peptides excel for specific applications but not universal panaceas. Right tool for right job based on mechanism-pathology matching.
Understanding these fundamental mechanism principles transforms peptide use from experimental guessing to evidence-based biological intervention. SeekPeptides remains committed to mechanism education, providing resources translating complex molecular biology into actionable knowledge for vision health optimization.
External resources
National Center for Biotechnology Information - Peptide Mechanisms Database
Journal of Biological Chemistry - Signal Transduction Pathways
In case I don't see you, good afternoon, good evening, and good night. May your cellular pathways stay active, your receptors stay responsive, and your vision health journey stay mechanistically sound. Join SeekPeptides.