Jan 5, 2026

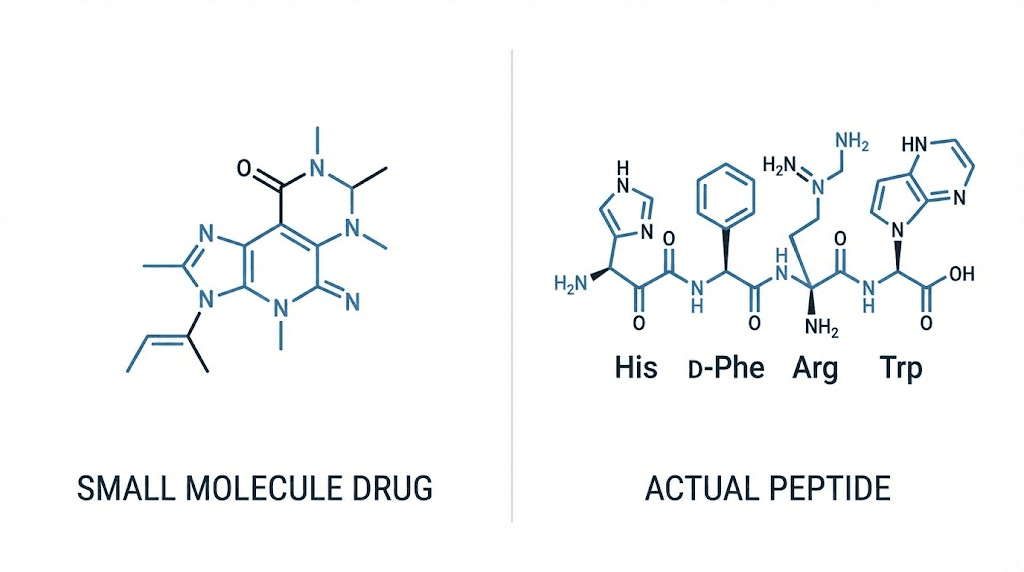
Sildenafil peptide terminology creates immediate confusion requiring clarification. Sildenafil (marketed as Viagra) is not actually a peptide, it's a small molecule phosphodiesterase-5 (PDE5) inhibitor with molecular formula C₂₂H₃₀N₆O₄S. The compound contains no peptide bonds (amino acid chains) making "sildenafil peptide" technically inaccurate.
However, the term appears frequently in research chemical markets referring to sildenafil citrate sold for laboratory research rather than human consumption. The nomenclature likely emerged from vendors grouping sildenafil alongside actual research peptides like PT-141 (bremelanotide) which does treat erectile dysfunction through peptide mechanisms.
The research chemical market operates in regulatory gray areas across jurisdictions. Sildenafil remains prescription medication in most countries, FDA-approved for erectile dysfunction and pulmonary arterial hypertension. However, chemical suppliers sell sildenafil citrate powder "for research purposes only" with explicit labeling stating "not for human consumption."
This creates legal ambiguity where purchasing sildenafil for legitimate research (academic studies, pharmaceutical development, analytical standards) remains legal while purchasing for personal use technically violates prescription requirements despite vendors not verifying end-use.
Availability varies dramatically by jurisdiction and vendor category. Academic researchers access pharmaceutical-grade sildenafil through licensed suppliers (Sigma-Aldrich, TCI Chemicals, Cayman Chemical) with institutional accounts and appropriate regulatory documentation. Independent researchers, supplement manufacturers, and individuals face more restricted options, research chemical vendors with variable quality control, international suppliers operating outside domestic regulations, and underground markets selling counterfeit or contaminated products.
Price ranges span $50-$500 per gram depending on purity, quantity, and source legitimacy with correlation between price and actual quality highly unreliable.
This guide covers regulatory status of sildenafil across major jurisdictions defining legal boundaries, legitimate research applications justifying sildenafil procurement, verified vendor categories and quality verification methods, comparing sildenafil to actual peptides addressing erectile dysfunction like PT-141, understanding why sildenafil gets grouped with research peptides despite chemical classification differences, procurement strategies for different researcher categories (academic, industry, independent), and critical safety considerations around quality verification and contamination risks in unregulated markets.
Let's clarify the fundamental chemical classification establishing whether sildenafil qualifies as peptide and why terminology confusion persists.
Chemical classification: Why sildenafil isn't actually a peptide
Understanding molecular structure clarifies nomenclature confusion and explains market categorization.
Defining peptides vs small molecule drugs
Peptides: Chains of amino acids connected by peptide bonds (CO-NH linkages between amino acid residues). Short peptides contain 2-50 amino acids (dipeptides, tripeptides, oligopeptides). Longer chains become polypeptides (50-100 amino acids) then proteins (100+ amino acids). Peptides synthesized biologically through ribosomal translation or chemically through solid-phase peptide synthesis.
Small molecule drugs: Organic compounds typically under 900 Daltons molecular weight. Synthesized through traditional organic chemistry methods. Not built from amino acid chains, instead assembled from various chemical building blocks through multi-step synthesis. Most pharmaceutical drugs fall into this category including aspirin, ibuprofen, statins, and PDE5 inhibitors.
Sildenafil structure: C₂₂H₃₀N₆O₄S with molecular weight 474.58 Da. Contains piperazine ring, pyrazolopyrimidinone core, and sulfonamide group. No peptide bonds present—entirely small molecule architecture synthesized through organic chemistry not peptide coupling reactions.
Why sildenafil gets grouped with "peptides" in research markets
Market categorization convenience: Research chemical vendors often create "peptides and research chemicals" categories lumping together non-traditional supplements regardless of chemical classification. Sildenafil, SARMs (selective androgen receptor modulators), nootropics, and actual peptides all grouped together as "research compounds" based on regulatory status not molecular structure.
Similar legal gray areas: Both sildenafil and research peptides occupy ambiguous regulatory positions—legal for research, prescription-required or prohibited for human use. Vendors selling one compound often sell others in similar legal categories creating association.
Overlapping applications: PT-141 peptide (bremelanotide), Melanotan II, and sildenafil all address sexual dysfunction creating functional grouping despite different mechanisms. Users researching erectile dysfunction treatments encounter both creating market association.
Vendor marketing: Calling sildenafil a "peptide" may reduce scrutiny versus accurately labeling as pharmaceutical drug analog. Regulatory agencies focus more on prescription drug analogs than "peptide research chemicals" making misleading nomenclature strategically beneficial for gray-market vendors.
Customer search patterns: People searching "research peptides for ED" encounter sildenafil listings creating impression it belongs in peptide category. Vendors optimize for these search patterns perpetuating misclassification.
Actual peptides addressing erectile dysfunction
PT-141 (bremelanotide): True peptide drug (Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH) containing 7 amino acids in cyclic structure. Works through melanocortin receptors in brain triggering sexual arousal centrally rather than peripherally like sildenafil. FDA-approved 2019 for hypoactive sexual desire disorder in women. Available through research chemical suppliers "for research only" alongside prescription access.
Melanotan II: Cyclic heptapeptide analog of α-melanocyte stimulating hormone. Binds melanocortin receptors producing tanning effects plus sexual enhancement side effects. Never achieved FDA approval but available through research peptide vendors. Actual peptide unlike sildenafil.
Kisspeptin: Research-stage peptide showing promise for hypogonadal hypogonadism and sexual dysfunction. Multiple isoforms (Kisspeptin-10, -13, -54) currently in clinical trials. Legitimate peptide research compound.
These represent actual peptides targeting sexual function through mechanisms completely different from sildenafil's PDE5 inhibition. Compare our PT-141 usage guide for peptide-based approaches.
Regulatory status of sildenafil across major jurisdictions
Understanding legal boundaries prevents procurement violations and clarifies available options.
United States regulatory framework
FDA classification: Sildenafil citrate approved as prescription drug under brand names Viagra (erectile dysfunction, 25mg-100mg tablets) and Revatio (pulmonary arterial hypertension, 20mg tablets). Schedule classification: Not controlled substance under DEA scheduling but requires prescription for legal human use.
Research exemption: Chemical suppliers can sell sildenafil for legitimate research purposes to qualified institutions. "For research use only" labeling allows sales to laboratories, academic institutions, pharmaceutical companies conducting studies. However, supplier must document research use and customer must have legitimate research application.
Personal possession and use: Possessing sildenafil without valid prescription technically violates prescription drug regulations. However, enforcement primarily targets distribution and trafficking not personal possession. Customs may seize international shipments but rarely prosecutes individuals for small quantities. Using sildenafil obtained through research chemical vendors constitutes off-label use without medical supervision creating liability.
Vendor requirements: Legitimate research chemical suppliers should verify institutional affiliation or research credentials before sales. Many gray-market vendors skip verification accepting anyone's money with "research only" disclaimer providing legal cover. This creates accessibility but regulatory risk for buyers.
Practical enforcement: FDA and DEA focus on large-scale distribution, counterfeit operations, and safety threats not individual researchers ordering grams for study. Academic institutions using sildenafil face scrutiny ensuring proper protocols and documentation. Independent researchers or individuals ordering for personal use operate in enforcement gray area, technically illegal but rarely prosecuted unless flagrant.
European Union regulatory status
EMA classification: Sildenafil approved prescription medicine across EU member states. Available legally only through pharmacies with valid prescription from licensed physician.
Research chemical regulations: Novel Food Regulation and pharmaceutical regulations govern sildenafil sales. Legitimate laboratory suppliers require documentation of research purpose. Gray-market vendors operating from certain jurisdictions (Eastern Europe, Asia) ship to EU customers despite questionable legality.
Country-specific variations: Germany strictly enforces prescription requirements with customs actively screening sildenafil shipments. Netherlands more permissive with research chemical access. UK post-Brexit maintains prescription requirements but enforcement varies.
Intra-EU shipping: Research chemical vendors in permissive member states ship to customers across EU single market. Legal ambiguity whether this violates pharmaceutical regulations in destination countries.
Other major jurisdictions
Canada: Prescription required through Health Canada regulations. Research exemption exists for qualified institutions. Personal importation for research technically allowed in small quantities (3-month supply guideline) though sildenafil's prescription status creates complications.
Australia: Therapeutic Goods Administration (TGA) classifies sildenafil as prescription-only medicine (Schedule 4). Stricter importation controls than US/Canada with higher seizure rates for research chemical shipments. Limited research exemptions requiring extensive documentation.
United Kingdom: Post-Brexit maintains prescription requirements. Medicines and Healthcare products Regulatory Agency (MHRA) regulates sildenafil as POM (prescription-only medicine). Research chemical imports face scrutiny though some shipments arrive successfully.
Asia (variable): India manufactures generic sildenafil with looser domestic controls. China produces raw sildenafil powder for export (pharmaceutical manufacturing use). Japan strictly controls with prescription requirements. Regulatory frameworks vary dramatically across region.
Jurisdiction | Legal Status | Research Availability | Enforcement Level | Import Risk |
|---|---|---|---|---|
United States | Prescription required | Available to institutions | Moderate (targets distribution) | Moderate (customs screens) |
European Union | Prescription required | Variable by country | Moderate-High | Moderate-High |
Canada | Prescription required | Limited exemptions | Moderate | Moderate |
Australia | Prescription required (Schedule 4) | Strict documentation | High | High |
United Kingdom | Prescription required | Limited post-Brexit | Moderate | Moderate |
Legitimate research applications for sildenafil
Understanding valid research uses clarifies legal procurement justifications and vendor expectations.
Academic and pharmaceutical research
Pharmacological mechanism studies: Investigating PDE5 inhibitor effects on cellular signaling pathways. How cyclic GMP accumulation affects smooth muscle relaxation. Comparing different PDE5 inhibitors (sildenafil, tadalafil, vardenafil) for structure-activity relationships.
Clinical trial comparisons: Testing sildenafil versus novel erectile dysfunction treatments. Comparing PDE5 inhibitors to peptide-based approaches like PT-141. Evaluating combination therapies.
Alternative indication exploration: Pulmonary hypertension research (already approved indication requiring continued study). Cardiovascular effects and blood pressure modulation. Potential cognitive benefits through cerebral blood flow enhancement. Female sexual dysfunction investigations. High-altitude performance and mountain sickness prevention.
Drug development: Using sildenafil as comparator in developing next-generation PDE5 inhibitors. Structure-activity relationship studies informing new drug design. Formulation development for novel delivery methods.
Analytical method development: Creating HPLC, mass spectrometry, or other analytical methods for detecting sildenafil in biological samples. Forensic applications identifying counterfeit products. Quality control methodology for pharmaceutical manufacturing.
Industry research and development
Supplement manufacturers: Testing sildenafil contamination in male enhancement supplements. Quality control ensuring products don't contain undeclared pharmaceutical ingredients.
Contract research organizations: Conducting studies for pharmaceutical companies. Analytical testing services requiring sildenafil reference standards.
Medical device companies: Researching devices for erectile dysfunction requiring sildenafil as comparator treatment. Combination approaches integrating devices with pharmaceuticals.
Nutraceutical research: Studying herbal products claimed to enhance sexual function. Comparing natural products to pharmaceutical sildenafil efficacy.
Independent and citizen science research
Quantified self experimentation: Amateur researchers tracking performance metrics, cardiovascular responses, cognitive effects. Data-driven self-experimentation requiring controlled substances.
Biohacking communities: Exploring nootropic effects, exercise performance enhancement, health optimization. Often operates in legal gray areas but genuine research intent present.
Quality testing: Independent verification of research chemical vendor product quality. Providing community service identifying contaminated or fake products. Using analytical chemistry skills ensuring safe sourcing.
Formulation development: Creating novel delivery methods, combination supplements, or pharmaceutical alternatives. Garage pharmacy operations developing products for potential commercialization.
Important caveat: Independent research faces ethical and legal challenges. Lacks institutional review board (IRB) oversight protecting human subjects. Self-experimentation carries medical risks without physician supervision. Procurement without institutional credentials potentially violates regulations.
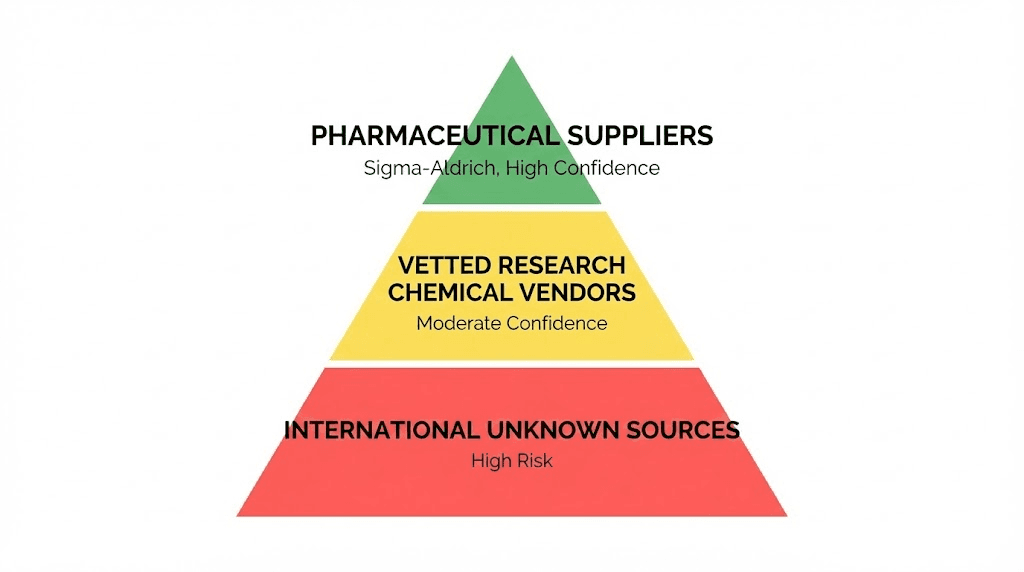
Verified vendors and procurement strategies
Sourcing legitimate sildenafil for research requires careful vendor evaluation and verification.
Pharmaceutical-grade laboratory suppliers (highest quality)
Major vendors:
Sigma-Aldrich / MilliporeSigma: Industry standard for research chemicals. Sildenafil citrate available in various purities (≥98%, ≥99%) and quantities (100mg to 1g+). Certificate of Analysis (COA) included showing HPLC verification. Pricing $150-$400 per gram depending on purity and quantity.
TCI Chemicals (Tokyo Chemical Industry): Japanese supplier with global distribution. High-purity sildenafil for pharmaceutical research. Similar pricing and quality to Sigma-Aldrich.
Cayman Chemical: Specializes in biochemicals and pharmacological research tools. Sildenafil and analogs available. Strong quality documentation.
MedChemExpress (MCE): Research chemical supplier focusing on pharmaceutical compounds. Good quality control at slightly lower prices than Sigma-Aldrich.
Advantages: Pharmaceutical-grade purity (typically ≥98%, often ≥99%), comprehensive analytical documentation (NMR, HPLC, mass spec), consistent batch quality, institutional credibility, customer support for technical questions, reliable shipping.
Disadvantages: Requires institutional accounts for most suppliers, higher pricing ($200-$500/gram vs $50-$200 from gray market), may require purchase orders and institutional documentation, minimum order quantities sometimes enforced.
Procurement process: Create institutional account if affiliated with university/research organization/company, provide business documentation (tax ID, research credentials), place order with institutional purchase order, receive product with comprehensive COA, verify against specifications.
Research chemical vendors (moderate quality, variable reliability)
Evaluation criteria for gray-market suppliers:
Certificate of Analysis verification: Request COA showing HPLC purity testing. Verify COA comes from independent lab not vendor's "in-house" testing potentially fabricated. Check batch numbers on product match COA documentation. Look for testing date within past year (old COA may not represent current batch).
Business verification: Check business registration and longevity (3+ years operating suggests more legitimate operation). Physical address (not just P.O. box) and working phone number. Professional website with clear product descriptions and policies. Responsive customer service answering technical questions.
Community reputation: Search Reddit (r/ResearchChemicals, r/Nootropics), Longecity forums, and other communities for vendor reviews. Look for pattern of satisfied customers over time not just recent shills. Check for lab testing threads where community members independently verified products. Note any scam reports or contamination warnings.
Payment security: Reputable vendors accept credit cards (provides buyer protection) or PayPal. Cryptocurrency-only raises concerns about customer recourse. Secure payment processing through established processors (Stripe, PayPal).
Shipping and packaging: Proper storage preventing degradation (sealed, desiccated, away from light/heat). Discreet packaging reducing customs scrutiny. Tracking provided for monitoring.
Pricing realism: If significantly cheaper than competitors ($30/gram vs typical $100-$200), likely low purity or counterfeit. Extreme bulk discounts (50%+ off) raise suspicions. Prices too low to maintain business suggests exit scam planning.
International suppliers (highest risk, variable quality)
Chinese manufacturers: Produce bulk sildenafil powder for pharmaceutical industry. Some sell smaller quantities to individuals. Quality ranges from pharmaceutical-grade (≥99%) to heavily contaminated (<50% actual sildenafil). Price extremely variable ($50-$500/gram). Require extensive vetting—request samples, conduct independent testing before bulk purchase. Language barriers complicate communication. Shipping times 2-4 weeks. Customs seizure risk moderate to high depending on destination.
Indian pharmaceutical suppliers: Generic drug manufacturers sometimes selling raw materials. Quality generally better than Chinese sources but still variable. Pricing mid-range $100-$300/gram. Easier English communication than Chinese sources. Shipping typically successful though occasional seizures.
Eastern European vendors: Operating from jurisdictions with looser regulations. Quality variable. Pricing competitive $75-$200/gram. EU shipping generally successful, international varies. May or may not provide legitimate COA documentation.
Verification imperative: Never trust international vendor claims without independent testing. Send samples to analytical labs ($75-$150 for HPLC testing) before consuming or using in research. Community testing groups where members pool funds for batch verification.
Quality verification and contamination risks
Ensuring product authenticity and purity critical for research validity and safety.
Independent testing methods
HPLC (High-Performance Liquid Chromatography): Gold standard for purity verification. Separates compound components measuring sildenafil percentage. Results show ≥98% or ≥99% for pharmaceutical-grade. Lower percentages indicate impurities, fillers, or contaminants.
Mass spectrometry: Confirms molecular identity. Distinguishes sildenafil from analogs (tadalafil, vardenafil) or completely different compounds. Essential for detecting substitutions where vendor ships wrong chemical entirely.
NMR (Nuclear Magnetic Resonance) spectroscopy: Provides detailed structural information. Confirms compound identity and detects structural isomers. Expensive but comprehensive.
Testing services:
Colmaric Analyticals: Peptide and pharmaceutical testing service. HPLC testing $75-$125 per sample. Mass spec available.
Janoshik: European testing service popular in research chemical communities. HPLC and mass spec. Reasonable pricing and turnaround.
Energy Control: Harm reduction testing service. Anonymous testing for contamination and purity.
University analytical facilities: Some universities offer testing services to public for fee. Access through chemistry department inquiries.
Sample submission: Send 10-50mg sample (tiny amount) with payment. Specify testing type desired. Receive results in 1-2 weeks showing purity percentage and identity confirmation.
Common contamination and adulteration patterns
Filler dilution: Sildenafil mixed with inert powder (lactose, cellulose) reducing potency. HPLC showing 50-70% purity indicates 30-50% filler. Reduces efficacy but generally low toxicity if filler itself safe.
Analog substitution: Cheaper PDE5 inhibitor (homosildenafil, thiosildenafil) substituted for sildenafil. Similar effects but unlicensed compounds lacking safety data. Mass spectrometry detects substitutions.
Complete counterfeits: No active ingredient present. HPLC showing <5% sildenafil or absence entirely. Pure fraud.
Dangerous contaminants: Heavy metals from poor manufacturing. Bacterial contamination from unsterile production. Solvent residues from incomplete purification. Random pharmaceutical adulterants. These create serious health risks beyond reduced efficacy.
Detection timeline: Test immediately upon receiving research chemical batch before use. Don't trust vendor COA, verify independently. Budget $100-$150 per vendor/batch for testing peace of mind.
Red flags indicating likely counterfeits
Physical appearance issues: Discolored powder (should be white to off-white), clumping or caking (poor storage), unusual odor (should be nearly odorless), inconsistent texture between batches from same vendor.
Vendor warning signs: Refuses to provide COA or provides obviously fake documentation, dramatically cheaper than all competitors, new vendor without track record, poor English communication suggesting overseas operation without quality control, payment exclusively through untraceable methods.
Effects discrepancies: If researching effects: no response at expected doses, unexpected side effects, inconsistent potency between batches, delayed or abnormal response timeline.
Compare our comprehensive vendor vetting guide applicable to research chemical sourcing.
Comparing sildenafil to peptide-based erectile dysfunction treatments
Understanding mechanistic differences clarifies when each approach appropriate.
Sildenafil mechanism and effects profile
PDE5 inhibition pathway: Sildenafil blocks phosphodiesterase-5 enzyme preventing cyclic GMP breakdown in smooth muscle cells. Accumulated cyclic GMP maintains relaxation of arterial walls allowing blood flow. In penis, this creates and sustains erection. Works peripherally at vascular level.
Onset and duration: Oral sildenafil absorbed in 30-60 minutes. Peak plasma concentration 1 hour. Effects last 4-6 hours. Requires sexual stimulation activating NO/cGMP pathway, doesn't create automatic erection.
Efficacy profile: 60-80% of men with erectile dysfunction respond to sildenafil with improved erections sufficient for intercourse. Effectiveness declines with severe vascular disease or neurological damage. Works reliably for psychogenic and mild-to-moderate organic erectile dysfunction.
Side effect profile: Headache (16% incidence), flushing (10%), dyspepsia (7%), nasal congestion (4%), visual disturbances (3%, blue tinge due to PDE6 cross-reactivity). Contraindicated with nitrates (severe hypotension risk). Generally well-tolerated.
PT-141 (bremelanotide) peptide mechanism
Central melanocortin pathway: PT-141 peptide activates melanocortin receptors (MC1R, MC3R, MC4R) in hypothalamus and other brain regions. Increases sexual desire and arousal centrally. Doesn't require sexual stimulation, creates spontaneous desire and physiological arousal.
Administration: Subcutaneous injection or nasal spray formulations. Injection typical dose 1.75mg. Nasal spray FDA-approved formulation for women (Vyleesi).
Onset and duration: Effects begin 30-45 minutes post-injection. Peak 2-3 hours. Duration 6-12 hours including residual effects. Prolonged arousal period versus sildenafil's shorter window.
Efficacy profile: 60-70% response rate for hypoactive sexual desire disorder. Works for both men and women. Particularly effective for psychological/desire-based dysfunction versus purely vascular. FDA-approved 2019 for premenopausal women with low libido.
Side effect profile: Nausea (40% incidence, often transient), flushing, injection site reactions, headache. Increases blood pressure moderately. Less cardiovascular risk than sildenafil but nausea more prominent.
When to choose each approach
Sildenafil appropriate for: Vascular-origin erectile dysfunction (most common type), desire present but erection insufficient, predictable timing (know when intercourse planned), avoiding injections (oral administration preferred), established safety profile desired (50+ years clinical use).
PT-141 appropriate for: Desire/libido deficits more than erectile mechanics, spontaneous arousal desired rather than planned, female sexual dysfunction (limited sildenafil efficacy in women), psychological factors primary (anxiety, performance pressure), comfortable with injection or nasal administration, exploring peptide-based approaches beyond traditional pharmaceuticals.
Combination potential: Some researchers/practitioners combine approaches—low-dose sildenafil for vascular support, PT-141 for central arousal. Synergistic effects addressing both peripheral and central pathways. However, combination increases side effect risks and requires careful monitoring.
Factor | Sildenafil | PT-141 Peptide |
|---|---|---|
Mechanism | Peripheral PDE5 inhibition | Central melanocortin activation |
Administration | Oral tablet | Injection or nasal spray |
Onset | 30-60 minutes | 30-45 minutes |
Duration | 4-6 hours | 6-12 hours |
Requires arousal | Yes (enhances natural response) | No (creates spontaneous desire) |
Primary use | Male erectile dysfunction | Low libido (both sexes) |
Side effects | Headache, flushing, visual (mild) | Nausea (more prominent) |
FDA status | Approved since 1998 | Approved 2019 (women) |
Procurement strategies for different researcher categories
Approach varies based on institutional affiliation and research legitimacy.
Academic researchers with institutional backing
Advantages: Access to pharmaceutical-grade suppliers requiring institutional accounts, purchasing through institution eliminates personal liability, IRB approval for human studies provides ethical framework, funding often covers research chemical costs, institutional chemical safety protocols protect researchers.
Procurement process: Submit research proposal to IRB if human subjects involved, obtain departmental approval for chemical purchase, create institutional account with Sigma-Aldrich or equivalent, place order using institutional funds/purchase order, receive product through institutional receiving department, store in approved chemical storage following safety protocols, document use in research records.
Cost considerations: Pharmaceutical-grade sildenafil $200-$500/gram seems expensive but covered by grants/institutional budgets. Quality assurance justifies premium over gray-market alternatives. Legitimate research shouldn't cut corners on chemical purity.
Regulatory compliance: Academic institutions maintain DEA licenses and chemical use protocols. Researchers working under institutional umbrella protected by compliance frameworks. Following institutional procedures prevents personal legal exposure.
Industry researchers (pharmaceutical, supplement, CRO)
Procurement advantages: Similar institutional purchasing as academics. Industry budgets typically larger allowing pharmaceutical-grade chemical procurement.
Quality critical for commercial product development and regulatory submissions.
Vendor relationships: Established relationships with major suppliers. Volume purchasing discounts for larger companies. Access to custom synthesis services for sildenafil analogs or derivatives.
Quality documentation requirements: Industry research generates data for regulatory submissions requiring pharmaceutical-grade chemicals with full documentation. COA and batch records essential for FDA/EMA applications. No cost-cutting through gray-market vendors acceptable.
Analytical capabilities: Most pharmaceutical companies have in-house analytical labs verifying incoming chemicals. HPLC, mass spec, NMR readily available. Independent quality verification even when purchasing from reputable suppliers.
Independent researchers without institutional affiliation
Challenges: Legitimate research interest but lacking institutional credentials for pharmaceutical supplier accounts. Higher personal liability for regulatory violations. Limited funding requiring cost-consciousness. No IRB oversight for human studies creating ethical concerns.
Sourcing options: Research chemical vendors accepting individual purchases (no institutional verification), international suppliers selling to individuals, community group buys splitting larger purchases, analytical chemistry colleagues potentially accessing institutional suppliers.
Quality verification imperative: Independent testing through commercial labs essential. Cannot trust vendor COA, verify independently. Budget 10-20% of chemical cost for testing. Community testing initiatives sharing results benefit entire independent research community.
Legal considerations: Operating in regulatory gray areas. Technically violating prescription requirements if using sildenafil personally. Research justification provides some protection but not absolute. Consult lawyer familiar with pharmaceutical regulations before procurement.
Ethical frameworks: Without IRB oversight, independent researchers must create personal ethical standards. Never experiment on others without consent and medical supervision. Self-experimentation carries personal risk, informed consent means understanding consequences. Document carefully maintaining research standards despite lacking institutional structure.
Biohackers and quantified self experimenters
Reality check: Most "research" in this category constitutes personal use with measurement. Calling it research doesn't transform personal experimentation into legitimate science. However, rigorous self-experimentation with proper controls, measurement, and documentation provides value.
Procurement realities: Gray-market research chemical vendors primary source. Quality verification through community testing groups. Risk tolerance higher than academic/industry researchers, willing to accept some quality uncertainty for access and cost.
Safety first approach: Even without institutional oversight, maintain safety standards. Independent lab testing before use. Starting with minimal doses verifying tolerability. Monitoring cardiovascular parameters if using sildenafil (blood pressure, heart rate). Medical consultation for concerning symptoms.
Data collection: Rigorous tracking distinguishes serious self-experimentation from casual use. Standardized assessments (IIEF questionnaire for erectile function), physiological measurements (blood pressure, heart rate variability), subjective ratings on consistent scales. Publishing data (blogs, forums, preprint servers) contributes to community knowledge.
Community contribution: Sharing testing results warns others about contaminated batches. Publishing protocols helps others design better experiments. Transparency about negative results prevents publication bias. Biohacking community advances through open sharing.
Compare our getting started guide for research approaches.
Critical safety considerations
Quality uncertainty in research chemical markets creates serious health risks.
Cardiovascular risks and contraindications
Mechanism-based risks: Sildenafil lowers blood pressure through vasodilation. Combined with nitrates (nitroglycerin, isosorbide) causes severe potentially fatal hypotension. Contraindicated in anyone taking nitrates for angina or heart conditions.
Pre-existing cardiovascular disease: Consultation with cardiologist essential before sildenafil use for those with heart disease, previous heart attack, stroke history, uncontrolled hypertension, or significant cardiovascular risk factors.
Drug interactions: Alpha-blockers for prostate (tamsulosin, doxazosin) interact causing excessive blood pressure drops. CYP3A4 inhibitors (ketoconazole, ritonavir, grapefruit juice) increase sildenafil levels creating higher side effect risk. Multiple medication users should verify interactions.
Monitoring requirements: Blood pressure monitoring especially first few uses. Awareness of warning signs (chest pain, severe headache, vision changes, priapism >4 hours). Emergency action plan if concerning symptoms develop.
Contamination and adulteration dangers
Unknown purity risks: Gray-market sildenafil may contain 10-90% actual compound with remainder being fillers, contaminants, or wrong chemicals entirely. Unpredictable dosing creates safety issues, thinking you're taking 50mg but actually taking 5mg (ineffective) or 150mg (excess side effects).
Toxic contaminants: Heavy metals (lead, arsenic) from poor manufacturing. Solvent residues (dichloromethane, acetone) from incomplete purification. Bacterial endotoxins from unsterile production. Unknown pharmaceutical adulterants. These create immediate poisoning risks or long-term health damage.
Analog dangers: Unlicensed sildenafil analogs (homosildenafil, thiosildenafil, acetildenafil) sold as sildenafil lack safety data. Potentially different pharmacokinetics, drug interactions, or toxicity profiles versus approved sildenafil.
Harm reduction: Independent lab testing mandatory before use. Start with quarter of expected dose testing tolerability. Monitor carefully for unusual effects. Never trust vendor claims—verify independently.
Legal and regulatory exposure
Personal use violations: Using sildenafil without prescription violates pharmaceutical regulations in most jurisdictions. While enforcement typically targets distribution, personal use can theoretically face prosecution.
Import seizures: Customs may seize international sildenafil shipments. Loss of product plus potential inquiry letters or legal action depending on quantity and jurisdiction.
Professional consequences: Medical professionals, pharmacists, or others in regulated industries face licensure risks if caught using prescription drugs without valid prescription. Academic researchers violating institutional protocols risk employment consequences.
Documentation importance: Maintaining research logs, protocols, and data provides evidence of legitimate research intent if questioned. "I bought sildenafil to research effects" more defensible with actual documented research than naked claim without supporting evidence.
When to abandon gray-market procurement
Prescription access available: If able to obtain legitimate prescription through physician (for erectile dysfunction or pulmonary hypertension), that eliminates procurement risks and ensures pharmaceutical-grade quality.
Institutional access obtained: Researchers gaining academic or industry positions should use institutional suppliers rather than maintaining gray-market sources. Quality and legality make pharmaceutical-grade sources preferable when available.
Quality concerns outweigh benefits: If testing reveals repeated contamination, extremely low purity, or wrong compounds from vendors, risk exceeds potential research value. Better to halt research than use dangerous chemicals.
Legal risk intolerance: Some individuals/situations cannot tolerate legal ambiguity. Professional licensure, security clearances, or risk-averse personalities should avoid gray markets regardless of quality verification measures.
How SeekPeptides supports research chemical decisions
SeekPeptides provides comprehensive resources for understanding both peptides and research chemicals.
Learn about actual research peptides versus small molecule pharmaceuticals like sildenafil.
Compare sildenafil to PT-141 peptide for addressing sexual dysfunction through different mechanisms.
Understand research chemical safety and quality verification methods.
SeekPeptides empowers informed research decisions.
Helpful resources
Related guides worth reading
Sexual health peptides:
Research and safety:
Vendor and quality guides:
Getting started:
Research dosing protocols and pharmacokinetics
Understanding sildenafil pharmacology essential for legitimate research applications.
Standard dosing regimens in clinical use
Erectile dysfunction approved dosing: 25mg, 50mg, or 100mg tablets taken approximately 1 hour before sexual activity. Maximum once daily. Starting dose typically 50mg with titration up or down based on response and tolerability. Elderly patients and those with hepatic/renal impairment start at 25mg.
Pulmonary arterial hypertension dosing: 20mg three times daily (60mg total daily). Lower per-dose amount but higher total daily exposure. Continuous treatment rather than on-demand use.
Pharmacokinetics: Rapidly absorbed with peak plasma concentration (Cmax) at 30-120 minutes post-dose. Absolute bioavailability approximately 40% due to first-pass hepatic metabolism. Terminal half-life 3-5 hours. Metabolized primarily by CYP3A4 and CYP2C9 hepatic enzymes producing active metabolite N-desmethylsildenafil with ~50% parent compound potency.
Food effects: High-fat meals delay absorption and reduce Cmax by 29% though total bioavailability (AUC) unchanged. Research protocols should standardize food intake timing or use fasted state for consistency.
Research protocol design considerations
Dose-response studies: Investigating effects at sub-therapeutic (5-10mg), therapeutic (25-100mg), and supra-therapeutic (150-200mg) doses. Establishing dose-response curves for various outcomes (erectile function, cardiovascular parameters, cognitive effects, exercise performance).
Timing optimization: Comparing effects at 30min, 60min, 90min, and 120min post-dose. Identifying optimal timing for peak effects versus onset speed versus duration considerations.
Chronic dosing: Daily administration (typically 25-50mg) versus on-demand use. Investigating tolerance development, sustained effects, or tachyphylaxis over weeks to months.
Combination research: Sildenafil plus testosterone replacement, sildenafil plus peptide therapies, sildenafil plus lifestyle interventions. Synergistic or additive effects exploration.
Bioavailability enhancement: Alternative delivery methods (sublingual, intranasal, transdermal) attempting to bypass first-pass metabolism. Formulation research improving absorption or targeting.
Analytical methodology for research
Plasma/serum sildenafil quantification: HPLC-MS/MS most common method. Limit of quantification typically 1-5 ng/mL. Sample preparation involves protein precipitation or solid-phase extraction. Essential for pharmacokinetic studies measuring absorption, distribution, metabolism.
Tissue distribution studies: Measuring sildenafil concentrations in various tissues (corpus cavernosum most relevant for erectile function research, lungs for pulmonary hypertension, brain for cognitive studies). Requires LC-MS/MS with tissue homogenization and extraction.
Metabolite profiling: Identifying and quantifying N-desmethylsildenafil and other metabolites. Understanding metabolic pathways and active metabolite contribution to overall effects.
In vitro assays: PDE5 enzyme inhibition assays measuring IC50 (concentration producing 50% inhibition). Comparing sildenafil potency to other PDE inhibitors. Selectivity testing against other PDE isoforms (PDE6 cross-reactivity causes visual side effects).
Cellular assays: Cyclic GMP accumulation in smooth muscle cells. NO-stimulated relaxation of vascular rings. Calcium mobilization assays in relevant cell types.
Animal model considerations
Rodent erectile dysfunction models: Cavernous nerve injury, diabetes-induced ED, aging-related dysfunction. Sildenafil effects on intracavernosal pressure, histology, molecular markers. Translational research informing human applications.
Cardiovascular research: Hypertension models, heart failure, pulmonary hypertension. Hemodynamic measurements, cardiac output, vascular remodeling. Potential therapeutic applications beyond approved indications.
Cognitive function models: Aging, Alzheimer's disease models, vascular dementia. Cerebral blood flow measurements, cognitive task performance, neuropathology assessment. Exploring sildenafil's brain effects.
Dosing conversions: Human equivalent doses calculated through allometric scaling or FDA guidance. Typical mouse dose might be 5-10mg/kg translating to ~0.4-0.8mg/kg human equivalent (roughly 25-50mg for 70kg person). Rat doses similar calculations.
International procurement and shipping realities
Cross-border sildenafil acquisition creates additional complexities beyond domestic purchases.
Customs considerations by region
United States: Customs and Border Protection (CBP) screens packages for prescription drugs. Sildenafil shipments identified through X-ray, drug-sniffing dogs, or random inspection. Small quantities (10-30 day supply) sometimes pass, larger amounts face higher seizure risk. Seizure results in notice letter explaining violation, no criminal prosecution for personal amounts. Professional or commercial quantities trigger DEA investigation.
European Union: Variable enforcement by member state. Germany strictly screens pharmaceutical imports. Netherlands more permissive. Intra-EU shipments (within single market) face minimal screening. Extra-EU imports inspected by customs of entry country. Research purposes claims may require supporting documentation.
Australia: Therapeutic Goods Administration works with customs strictly controlling pharmaceutical imports. High seizure rates even for small quantities. Personal importation scheme exists but requires prescription for Schedule 4 drugs like sildenafil. Research importation requires licenses and extensive documentation.
Canada: Canada Border Services Agency screens pharmaceutical imports. Personal quantities (90-day supply) sometimes permitted under personal importation guidelines though prescription drugs technically require prescription. Commercial quantities face serious enforcement.
Asia: Variable widely. Singapore extremely strict with harsh penalties for drug importation. Hong Kong more permissive. India and China as source countries not destination concerns. Japan strict requiring extensive documentation.
Shipping methods and success rates
Standard postal service: Cheaper but lower success rate. More scrutiny at customs. Tracking limited making seizure notification delayed. Typical success rate 60-80% depending on origin/destination countries.
Express courier (DHL, FedEx, UPS): Higher costs but faster clearance and better tracking. Professional courier screening sometimes catches items postal service misses. Success rates similar 60-80% but faster delivery when successful.
Stealth shipping: Vendors using creative packaging hiding products. Effectiveness variable and declining as customs adapt. May improve success to 75-85% but not guaranteed.
Reshipping services: Package sent to intermediary country with looser customs then forwarded to final destination. Adds complexity and cost. May improve success for residents of strict countries (Australia) receiving from permissive intermediaries (UK, Netherlands).
Success rate factors: Source country (China/India higher risk), destination country (Australia/Singapore higher interception), package size (under 100g better than kilos), packaging discretion, declared value (avoid underdeclaring too obviously), frequency (multiple shipments to same address raise flags).
Seized shipment handling
Notification: Love letter or seizure notice explaining package contained prohibited item and was confiscated. No criminal charges for small personal amounts. Explanation of appeals process (rarely successful for prescription drugs).
Appeals: Can contest seizure claiming prescription exists or research purposes. Requires documentation supporting claim. Low success rate without legitimate credentials. Most people don't appeal accepting loss.
Reshipment: Some vendors offer reshipping if initial package seized. May require additional payment or insurance. Not all vendors honor this. Verify reshipment policy before ordering from international sources.
Account consequences: Multiple seizures to same address may trigger more intensive screening of future packages. Avoid repeated orders from same vendor if seizures occurring.
Legal exposure: Single small quantity seizure rarely prosecuted. Repeated large shipments suggest distribution intent potentially triggering investigation. Extremely large amounts (kilograms) face serious legal consequences.
Alternative approaches for legitimate researchers
When direct procurement proves difficult, alternatives exist maintaining research integrity.
Pharmaceutical sample programs
Some physicians and healthcare facilities receive pharmaceutical samples from sales representatives. Researchers with healthcare connections might access expired or surplus samples for analytical method development, comparator studies, or educational purposes. Samples not for human use but suitable for chemical analysis, assay development, or reference standards.
Collaborative research agreements
Academic researchers can establish collaborations with pharmaceutical companies holding sildenafil inventory. Contract research agreements allow compound access in exchange for research results benefiting sponsor. Industry partnerships provide pharmaceutical-grade materials with proper documentation.
Generic pharmaceutical sources
Countries with generic sildenafil manufacturing (India, South Africa, Eastern Europe) produce legitimate pharmaceutical products sold domestically.
Generic tablets can be purchased through international pharmacies (online or in-person while traveling) with prescription or sometimes over-counter in permissive jurisdictions.
Extracting and purifying sildenafil from tablets provides research material though lower purity than bulk powder.
Compounding pharmacy connections
Compounding pharmacies formulate custom medications including sildenafil. Researchers with relationships to compounding pharmacies might access rejected batches, expired inventory, or custom-prepared research formulations. Requires licensed pharmacy cooperation and documentation.
Academic chemical repositories
Some universities maintain chemical inventory sharing programs. Researchers at one institution can request chemicals from others with excess inventory. Sildenafil ordered for past research but no longer needed might be available through these networks.
Case studies: Legitimate sildenafil research applications
Real-world examples illustrate valid research justifications.
High-altitude medicine research
Mountain climbers and high-altitude workers face pulmonary hypertension from low oxygen environments. Researchers study sildenafil preventing altitude sickness and improving performance at elevation.
Study design: Double-blind placebo-controlled trial, climbers receiving 50mg sildenafil or placebo three times daily during ascent, measurements include pulmonary artery pressure (via echocardiography), exercise performance (6-minute walk test), oxygen saturation, subjective symptoms (headache, nausea, fatigue).
Results: Sildenafil reduces pulmonary artery pressure at high altitude, improves exercise capacity and oxygen saturation, reduces acute mountain sickness incidence. Led to off-label use by climbers despite limited FDA indication.
Research procurement: Academic institutions conducting these studies purchase pharmaceutical-grade sildenafil from Sigma-Aldrich or similar suppliers. IRB approval, grant funding, proper storage and accountability documentation.
Cognitive enhancement investigation
University neuroscience departments investigate sildenafil's effects on cerebral blood flow and cognitive function. PDE5 present in hippocampus and other brain regions suggests potential cognitive effects beyond vascular actions.
Research questions: Does sildenafil improve memory consolidation? Enhance cognitive performance in healthy volunteers? Provide neuroprotection in aging or disease models? Augment neuroplasticity?
Study methodology: Healthy volunteers receive 50-100mg sildenafil or placebo in crossover design, cognitive testing battery (memory tasks, attention tasks, executive function), fMRI measuring brain activation and blood flow, biomarkers in blood/saliva.
Findings: Mixed results—some studies show modest memory improvements, others no significant cognitive enhancement. Cerebral blood flow increases confirmed. Ongoing research area with potential therapeutic applications for dementia.
Procurement pathway: Institutional chemical suppliers with IRB approval and institutional oversight. No gray-market vendors for legitimate published research.
Female sexual dysfunction trials
Controversial application given sildenafil's limited efficacy in women versus men. However, research continues investigating potential benefits for female arousal and satisfaction.
Mechanisms hypothesized: Clitoral and vaginal blood flow enhancement, similar vascular effects as in men. Potential central effects on sexual processing though mechanism unclear.
Clinical trials: Multiple studies testing 50-100mg sildenafil in women with sexual dysfunction. Outcomes measured include arousal, lubrication, satisfaction, orgasm quality. Subjective questionnaires and objective physiological measures.
Results: Inconsistent—some studies show modest benefits, others no advantage versus placebo. FDA did not approve for female sexual dysfunction. PT-141 peptide showing more promise through central mechanism.
Research significance: Even negative studies contribute knowledge about sex differences in drug response and sexual physiology. Justifies continued research despite lack of approved indication.
Exercise performance and sports medicine
Sildenafil's vasodilation and potential mitochondrial effects suggested ergogenic (performance-enhancing) potential prompting sports medicine research.
Study designs: Athletes receive sildenafil before exercise testing. Measures include VO2 max, time to exhaustion, power output, lactate accumulation, recovery metrics.
Findings: No consistent performance enhancement in healthy individuals at sea level. Some benefits at altitude (reduced hypoxia impact). Led to WADA monitoring though not currently banned due to lack of clear advantage.
Procurement for sports research: University sports science departments, professional sports teams research divisions. Pharmaceutical-grade sildenafil with proper oversight and athlete consent.
Long-term implications and future directions
Sildenafil research continues expanding beyond original erectile dysfunction indication.
Emerging therapeutic applications
Neurodegenerative diseases: Investigating sildenafil for Alzheimer's disease, Parkinson's disease, vascular dementia. PDE5 inhibition potentially improving cerebral blood flow, reducing neuroinflammation, enhancing neuroplasticity.
Cancer treatment adjuvant: Some research suggests sildenafil might enhance chemotherapy efficacy, reduce drug resistance, or provide direct anti-tumor effects. Preliminary stage requiring extensive validation.
Wound healing: Local application or systemic administration potentially improving angiogenesis and tissue repair. Research in diabetic ulcers, surgical wounds, burn injuries.
Heart failure: Beyond pulmonary hypertension into broader cardiac applications. Right ventricular dysfunction, diastolic heart failure, cardiac remodeling post-infarction.
Neuropathic pain: PDE5 inhibitors showing analgesic properties in some chronic pain conditions. Mechanism unclear but justifies investigation.
Novel formulations and delivery methods
Transdermal patches: Avoiding first-pass metabolism, providing sustained release, reducing peak-related side effects. Research formulation challenges and bioavailability optimization.
Sublingual/buccal formulations: Faster onset than oral, partial first-pass avoidance. Pharmaceutical development creating commercial products.
Intranasal delivery: Rapid onset similar to injection without invasiveness. Formulation stability and absorption consistency challenges.
Topical genital application: Direct application to erectile tissue. Limited systemic absorption reducing side effects. Effectiveness questions due to absorption limitations.
Sustained-release systems: Once-daily or longer formulations for chronic conditions. Polymer-based or implantable delivery systems.
Personalized medicine approaches
Genetic screening: CYP3A4 and CYP2C9 polymorphisms affecting sildenafil metabolism. Genotype-guided dosing optimizing efficacy and minimizing side effects.
Biomarker prediction: Identifying patients most likely to respond based on PDE5 expression, cardiovascular status, or other markers.
Combination therapy optimization: Personalized combinations based on individual erectile dysfunction etiology, comorbidities, and preferences.
Ethical considerations in sildenafil research
Research involving sildenafil raises important ethical questions requiring thoughtful navigation.
Human subjects research ethics
Informed consent requirements: Participants must understand sildenafil's effects, side effects, contraindications, and risks. Disclosure that sildenafil is FDA-approved prescription medication being used for research purposes. Explanation of study purpose, procedures, alternatives, and voluntary participation. Documentation through signed consent forms with adequate time for questions.
Vulnerable populations: Special protections for elderly, those with cardiovascular disease, individuals with cognitive impairment. Enhanced monitoring and exclusion criteria preventing undue risk. Careful benefit-risk assessment weighted toward subject welfare.
Placebo use considerations: Sexual dysfunction significantly impacts quality of life. Placebo-controlled trials withhold potentially effective treatment. Crossover designs allowing all participants eventually receiving active treatment address this concern. Time-limited placebo exposure balances scientific rigor with participant welfare.
Privacy and confidentiality: Sexual health research carries stigma. Extra precautions protecting participant identity. Secure data storage, coded identifiers, limited access to identifying information. Publication without revealing individual details.
IRB oversight: Institutional Review Boards evaluate research protocols ensuring ethical standards. Required for academic research, absent in independent/biohacker experiments creating ethical vacuum.
Self-experimentation ethical frameworks
Informed self-consent: Even without formal IRB, self-experimenters should document informed decision-making. Understanding risks, contraindications, potential adverse events. Written protocols and risk assessments creating accountability.
Medical screening: Cardiovascular assessment (blood pressure, ECG, stress test if indicated) before sildenafil research. Excluding contraindications (nitrate use, severe heart disease, recent stroke). Establishing baseline health ensuring safe experimentation.
Start-low-go-slow philosophy: Beginning with minimal doses (10-25mg) assessing tolerability before advancing to standard or higher doses. Gradual titration allowing identification of adverse effects before serious harm.
Medical backup: Establishing relationship with physician aware of research activities. Emergency action plan if serious adverse events occur. Not replacing medical supervision but having medical resource available.
Data integrity: Honest documentation including negative results and adverse effects. Not cherry-picking data showing desired outcomes. Contributing truthfully to community knowledge.
Harm prevention: Sharing contamination findings warning others about dangerous vendors. Publishing testing results benefiting community. Transparency about failures and risks not just successes.
Institutional accountability and oversight
Chemical inventory control: Universities and industry tracking sildenafil procurement, storage, use, and disposal. Preventing diversion to unauthorized uses or theft. Regular audits ensuring compliance.
Protocol documentation: Written research plans specifying hypothesis, methods, analyses, safety monitoring. Deviations documented with justification. Creating audit trail from procurement through publication.
Adverse event reporting: Institutional policies requiring immediate reporting of serious adverse events. Investigation determining if research should continue or requires modification. Regulatory reporting if threshold criteria met.
Publication ethics: Complete and honest reporting of methods, results, conflicts of interest. Not omitting unfavorable findings. Proper attribution and authorship. Data availability for verification.
Conflict of interest management: Researchers with financial interests in outcomes face enhanced scrutiny. Disclosure requirements, independent oversight, separation of roles minimizing bias.
Detailed procurement workflows for different scenarios
Step-by-step processes for various researcher categories.
Academic researcher workflow
Step 1 - Protocol development: Write detailed research protocol specifying objectives, methods, sildenafil dosing, safety monitoring, data analysis. Include literature review justifying sildenafil use and sample size calculations.
Step 2 - IRB submission: Submit protocol to Institutional Review Board for human subjects research or IACUC for animal research. Address reviewer questions, make requested modifications, obtain approval before any sildenafil procurement.
Step 3 - Funding acquisition: Grant applications to NIH, NSF, foundations, or industry sponsors. Budget including sildenafil costs ($200-$500/gram from pharmaceutical suppliers). Award notification and account setup.
Step 4 - Vendor account creation: Contact Sigma-Aldrich, TCI, or Cayman Chemical sales representative. Provide institutional documentation (tax ID, research credentials, institutional affiliation). Create account with institutional billing and shipping addresses.
Step 5 - Purchase order: Submit purchase order through institutional procurement system. Specify catalog number, quantity (typically 1-5g for most studies), purity grade (≥98% minimum). Obtain departmental and financial approvals.
Step 6 - Receipt and verification: Chemical arrives at institutional receiving department. Sign for package, transport to laboratory. Review COA against order specifications. Store in locked chemical storage at appropriate conditions.
Step 7 - Inventory documentation: Log sildenafil into institutional chemical inventory system. Record receipt date, quantity, storage location, responsible person. Update inventory as sildenafil used or disposed.
Step 8 - Research execution: Follow approved protocol using documented sildenafil quantities. Record usage dates, amounts, subjects/animals, observations. Maintain research notebook with complete documentation.
Step 9 - Disposal: Unused sildenafil disposed through institutional hazardous waste system. Never flush or trash. Complete disposal documentation for audit trail.
Step 10 - Publication: Report findings in peer-reviewed publications with complete methods section. Disclose funding sources, acknowledge institutional support, make data available per journal requirements.
Timeline: 3-12 months from protocol development to sildenafil receipt depending on IRB review complexity, funding acquisition, and procurement processing. Additional months to years for research completion and publication.
Independent researcher workflow
Step 1 - Research question development: Define specific testable hypothesis. Sildenafil addresses research question better than alternatives. Clear objectives and measurement plan.
Step 2 - Vendor identification: Research chemical vendor surveys checking community reputation (Reddit, Longecity), reviewing COA samples, verifying business legitimacy (domain age, contact information), comparing pricing ($100-$250/gram typical for reputable vendors).
Step 3 - Initial contact: Email vendor requesting current COA for sildenafil citrate. Assess response quality, professionalism, technical knowledge. Request references or lab testing results if available.
Step 4 - Small test order: Place minimal order (500mg-1g) testing vendor reliability and product quality before larger purchase. Use credit card for buyer protection rather than cryptocurrency if possible.
Step 5 - Receipt and documentation: Photograph packaging upon arrival. Check batch number against any provided COA. Document condition, appearance, any discrepancies.
Step 6 - Independent testing: Send 10-50mg sample to analytical lab (Colmaric, Janoshik, Energy Control). Request HPLC purity and mass spec identity confirmation. Budget $100-$150 for testing.
Step 7 - Results evaluation: If purity ≥95% and identity confirmed, vendor acceptable for larger orders. If purity <90% or wrong compound, warn community and find alternative vendor.
Step 8 - Bulk order (if needed): Purchase larger quantity from verified vendor if research requires. Maintain documentation of testing results justifying bulk purchase.
Step 9 - Research execution: Follow planned protocol with careful documentation. Start low dosages assessing tolerance. Monitor carefully for adverse effects. Record all observations objectively.
Step 10 - Results sharing: Publish findings via blog, forum post, or preprint server. Share testing results benefiting community. Honest reporting including limitations and negative findings.
Legal considerations: Document research intent with written protocols, notes, data. Maintain evidence of legitimate research vs personal use if questioned. Understand legal risks operating in gray areas.
Timeline: 2-8 weeks from vendor identification to verified sildenafil receipt (testing adds 1-2 weeks). Research execution variable based on study design.
Industry researcher workflow
Advantages: Established vendor relationships, procurement departments handling logistics, quality control labs verifying incoming materials, larger budgets allowing pharmaceutical-grade purchases without financial strain, legal department guidance on regulatory compliance.
Process: Similar to academic but through corporate channels. R&D manager approval, procurement department ordering through existing Sigma-Aldrich or Cayman contracts, receiving inspection and QC testing, inventory tracking through corporate systems, use in development or testing protocols, comprehensive documentation for regulatory filings.
Timeline: Often faster than academic (days to weeks) due to established processes and purchasing infrastructure.
Biohacker/quantified-self workflow
Reality: Most similar to independent researcher but potentially lower standards for documentation and quality verification. Higher risk tolerance accepting contamination possibilities in exchange for access and cost savings.
Best practices: Still follow testing protocols, community verification, starting low dosages, careful monitoring. Treat seriously despite informal context. Share findings and warnings contributing to community knowledge.
Risk acceptance: Acknowledge operating in legal and safety gray zones. Make informed decisions understanding consequences. Not recommended but recognizing it occurs regardless.
Final thoughts on sildenafil peptide research availability
Sildenafil availability for research purposes exists through pharmaceutical suppliers, research chemical vendors, and international sources creating tiered access depending on researcher credentials and resources.
Academic and industry researchers access pharmaceutical-grade materials through established institutional channels with proper oversight and documentation. Independent researchers navigate gray markets requiring extra verification vigilance and legal risk awareness.
The "sildenafil peptide" terminology reflects market categorization convenience rather than accurate chemical classification, sildenafil is small molecule drug not peptide despite frequent grouping with research peptides like PT-141. Understanding this distinction clarifies chemical properties, regulatory status, and appropriate research applications.
Procurement success requires realistic assessment of research legitimacy (institutional backing vs independent experimentation), understanding jurisdiction-specific regulations (prescription requirements, import controls, enforcement patterns), implementing quality verification (independent testing through analytical labs), and maintaining ethical standards (informed consent for human research, careful documentation, honest reporting).
Future sildenafil research expands beyond erectile dysfunction into neurodegenerative diseases, cardiovascular applications, high-altitude medicine, and personalized therapy optimization requiring continued access for legitimate scientific investigation while preventing diversion to inappropriate uses.
Common procurement challenges and solutions
Practical troubleshooting for researchers facing sourcing obstacles.
Challenge: Institutional supplier requires business account
Problem: Sigma-Aldrich, TCI, and similar pharmaceutical suppliers require institutional accounts. Independent researchers or small companies struggle obtaining accounts.
Solutions: Establish academic collaboration—partner with university researcher providing institutional credentials. Small companies provide business registration documents, tax ID, and research overview creating corporate account. Join professional research organizations potentially offering group purchasing. Consider research chemical vendors not requiring institutional verification though accepting quality trade-offs.
Challenge: International shipping seized by customs
Problem: Sildenafil shipment intercepted at border with seizure notice. Product lost, potential legal concerns.
Solutions: Accept loss and try domestic vendor eliminating international shipping. Use reshipment services routing through permissive intermediary countries. Reduce order sizes, multiple small shipments face less scrutiny than single large order. Verify vendor ships properly declared or underdeclared optimally. Some researchers accept 30-40% seizure rate budgeting accordingly.
Challenge: Product testing reveals low purity or wrong compound
Problem: Independent lab testing shows purchased sildenafil is 40% pure, heavily contaminated, or wrong chemical entirely.
Solutions: Warn community immediately about vendor selling fakes. Request refund documenting testing results (success rare but attempt). Find alternative vendor with better reputation. Join community testing initiatives sharing costs and results. Consider pharmaceutical-grade suppliers despite higher cost if gray market consistently unreliable.
Challenge: Research budget insufficient for pharmaceutical-grade sildenafil
Problem: $200-$500/gram exceeds available funding. Research legitimate but resource-constrained.
Solutions: Reduce required quantity, redesign study requiring less material. Apply for small grants or pilot study funding. Collaborate with better-funded researcher sharing resources. Use generic pharmaceutical tablets extracting sildenafil (lower purity but verified pharmaceutical source). Delay research until funding available rather than compromising quality.
Challenge: Legal concerns about personal use vs research distinction
Problem: Purchasing sildenafil for "research" but actually using personally without prescription. Uncertain about legal exposure.
Solutions: Obtain legitimate prescription through physician consultation, removes legal ambiguity entirely. Document genuine research activities (protocols, data collection, notes) supporting research claim if questioned. Accept legal risk making informed decision, or abandon procurement if risk intolerance. Consult attorney familiar with pharmaceutical law before proceeding.
Challenge: Vendor requires minimum order quantities exceeding need
Problem: Vendor minimum order 5g-10g but research requires only 500mg-1g. Financial and practical excess.
Solutions: Partner with other researchers sharing bulk order and costs. Purchase minimum then properly store excess for future research. Verify smaller quantity vendors exist (research chemical suppliers often sell 100mg-1g amounts). Accept premium pricing for smaller quantities from flexible vendors.
Comparative analysis: Sildenafil vs actual research peptides
Understanding differences beyond nomenclature confusion.
Procurement comparison matrix
Factor | Sildenafil | PT-141 Peptide | Other Research Peptides |
|---|---|---|---|
Chemical class | Small molecule PDE5 inhibitor | Heptapeptide (7 amino acids) | Variable (2-50+ amino acids) |
Synthesis | Multi-step organic synthesis | Solid-phase peptide synthesis | Solid-phase peptide synthesis |
FDA status | Approved (prescription required) | Approved 2019 (Vyleesi brand) | Most unapproved, research only |
Legal status | Prescription drug, gray market research | Research chemical, some Rx | Primarily research chemicals |
Vendor availability | Pharmaceutical + research chemical | Primarily research chemical | Research chemical vendors |
Pricing (1g) | $50-$500 depending on source/purity | $200-$800 typical | $50-$1000+ highly variable |
Quality verification | HPLC, mass spec standard | HPLC, mass spec, sometimes sequencing | HPLC, mass spec essential |
Storage | Room temperature, dry, dark | Refrigerated preferred, freezer long-term | Usually refrigerated or frozen |
Mechanism | PDE5 enzyme inhibition | Melanocortin receptor agonist | Highly variable by peptide |
Administration | Oral primarily | Subcutaneous injection, nasal spray | Injection, nasal, oral, topical |
Research application differences
Sildenafil research focuses: Cardiovascular pharmacology, erectile dysfunction mechanisms, drug interactions, formulation development, off-label applications (altitude, cognition). Well-established compound with decades of data.
Peptide research focuses: Novel mechanisms being elucidated, structure-activity relationships, delivery method optimization, emerging therapeutic applications. Many peptides early research stages lacking sildenafil's extensive background.
Complexity differences: Sildenafil chemically stable, straightforward handling. Peptides more labile requiring careful storage, susceptible to degradation, may need special formulations. Sildenafil simpler for beginning researchers.
Analytical differences: Sildenafil analysis well-standardized with published methods. Peptide analysis requires more sophisticated techniques, sequence verification, structure confirmation.
Why conflation persists in markets
Vendors group together based on regulatory status (research chemicals, gray market) rather than chemistry. Customers searching sexual health research find both creating association.
Marketing strategies benefit from ambiguity. Community discussions blend topics reinforcing confusion.
Researchers should maintain clear understanding, calling sildenafil a peptide perpetuates misinformation regardless of market categorization convenience.
Quality vendor checklist for sildenafil procurement
Systematic evaluation prevents problematic purchases.
Essential verification steps
✓ COA availability: Vendor provides Certificate of Analysis upon request showing HPLC purity ≥95%, batch number, testing date within past year, independent lab name (not vendor's internal testing).
✓ Business verification: Company registered with verifiable address and contact information. Domain registered 2+ years ago. Professional website with clear policies. Responsive customer service answering technical questions.
✓ Community reputation: Positive reviews on Reddit (r/ResearchChemicals, r/Nootropics), Longecity forums, or other independent platforms. Pattern of satisfied customers over months to years. No recent scam warnings or major contamination reports.
✓ Pricing realism: Within market range ($100-$250/gram for research chemical vendors, $200-$500 pharmaceutical suppliers). Suspiciously low pricing (<$75/gram) suggests poor quality. Extreme bulk discounts (50%+ off) raise exit scam concerns.
✓ Payment security: Accepts credit cards (buyer protection) or PayPal. Secure payment processing through recognized processors. Not exclusively cryptocurrency-only (reduces recourse options).
✓ Shipping reliability: Provides tracking information. Reasonable shipping times (1-2 weeks domestic, 2-4 weeks international). Packaging prevents degradation (sealed, desiccated, away from heat/light).
✓ Return/refund policy: Clear policy for quality issues or contamination. Legitimate vendors stand behind products. No return policy suggests vendor knows products won't satisfy.
Red flags requiring vendor avoidance
✗ Refuses COA provision: Won't provide testing documentation or provides obviously fake/generic COA without batch-specific information.
✗ No business documentation: Can't verify company registration, only P.O. box or no address, contact information doesn't work.
✗ Terrible communication: No response to inquiries, poor English suggesting overseas operation without quality control, evasive about sourcing or testing.
✗ Extreme discounts: Constant 50-70% off sales, pricing far below competitors ($30-$50/gram when market is $100-$200).
✗ Recent establishment: Vendor operating under 6 months without track record. Could be legitimate startup or setup for exit scam.
✗ Scam reports: Community warnings about non-delivery, fake products, credit card fraud, no customer service after purchase.
✗ Amateur presentation: Poorly designed website, spelling errors, stock photos only, no product information or details.
Documentation and record-keeping for sildenafil research
Proper documentation protects researchers and validates findings.
Research protocol documentation
Written protocols before procurement: Document hypothesis, specific aims, methodology, sildenafil dosing regimen, outcome measurements, data analysis plan, safety monitoring procedures. Creates evidence of research intent vs personal use if questioned legally. Provides roadmap ensuring systematic execution vs haphazard experimentation.
Protocol elements: Background and significance (why sildenafil appropriate for research question), specific aims (testable hypotheses), experimental design (subjects/animals, sample size, controls, randomization), sildenafil administration (dose, frequency, duration, route), measurements and endpoints (primary outcomes, secondary outcomes, timing), data analysis (statistical methods, significance thresholds), safety considerations (exclusion criteria, adverse event monitoring, stopping rules).
Version control: Date protocols, maintain version history if modifications made, document deviations from original plan with justifications.
Laboratory notebook practices
Daily documentation: Record sildenafil usage (date, time, amount, batch number, subject/animal ID), observations (immediate effects, delayed responses, adverse events), measurements (vital signs, performance metrics, laboratory values), deviations from protocol (missed doses, protocol violations, unexpected events).
Notebook standards: Bound notebooks with numbered pages preventing removal, permanent ink (not pencil), entries dated and signed, no blank spaces (line through unused areas), corrections single line through error with initials and date, contemporaneous recording (same day, not retrospective reconstruction).
Digital alternatives: Electronic lab notebooks (ELNs) with timestamp verification, backup protocols preventing data loss, secure storage protecting confidentiality, version tracking maintaining audit trail.
Chemical inventory records
Procurement tracking: Record sildenafil purchase date, vendor, quantity, batch number, COA reference, cost, receiving person. Creates accountability preventing diversion or misuse.
Storage documentation: Location (building, room, cabinet, shelf), temperature/humidity conditions, access restrictions, inventory checks confirming expected quantities present.
Usage logging: Each sildenafil use records date, amount withdrawn, purpose (study ID or protocol), remaining balance, person responsible. Regular reconciliation ensures accounting accuracy.
Disposal documentation: Unused sildenafil disposal method, date, quantity, person responsible, disposal contractor if hazardous waste service used. Complete lifecycle tracking from procurement to disposal.
Data management and security
Raw data preservation: Original measurements, observations, images, recordings maintained without alteration. Analyses performed on copies preserving originals for verification.
Data security: Encrypted storage if human subject data, password protection, limited access (only research team members), backup systems preventing loss (cloud storage, external drives, institutional servers).
Participant confidentiality: Coded identifiers replacing names, limited access to linking key, secure storage of consent forms separately from data, de-identified data sharing for publications or collaborations.
Data retention: Maintain research records minimum 3-7 years post-publication (institutional policies vary), longer for clinical trials or regulated research, secure destruction after retention period.
Publication and sharing practices
Complete methods reporting: Sildenafil source (vendor, catalog number, batch number), purity verification (testing method, results), dosing details (amount, frequency, route, timing), preparation methods if formulated, storage conditions affecting stability.
Results transparency: Report all outcomes (not just statistically significant findings), include confidence intervals and effect sizes (not just p-values), acknowledge limitations, disclose null findings preventing publication bias.
Data availability: Share underlying data through repositories (Open Science Framework, Dryad, institutional repositories), provide upon request to qualified researchers, protect participant confidentiality while maximizing transparency.
Conflict of interest disclosure: Financial interests in outcomes, industry funding, vendor relationships, consulting arrangements. Transparency builds trust and allows readers assessing potential bias.
Long-term research planning with sildenafil
Sustainable research programs require strategic thinking beyond individual experiments.
Building research trajectory
Pilot studies first: Initial small-scale research (5-20 subjects/animals) testing feasibility, refining methods, generating preliminary data. Modest sildenafil requirements (1-5g) minimizing costs while establishing proof-of-concept.
Grant applications: Preliminary data from pilots supports grant applications to NIH, NSF, foundations, or industry sponsors. Budgets include adequate sildenafil quantities (10-100g for multi-year projects), analytical testing, personnel, equipment.
Progressive complexity: Early studies establish basic effects, later research investigates mechanisms, translational research moves toward applications. Each phase building on previous requiring increasing sophistication and resources.
Publication strategy: Pilot results published in specialized journals, main findings in higher-impact venues, reviews and perspectives establishing expertise. Building citation record and reputation enabling future funding.
Collaboration and networking
Academic partnerships: Solo researchers collaborate with university investigators accessing institutional resources (IRB, chemical suppliers, analytical facilities, statistical consulting). Co-authorship and resource sharing benefit both parties.
Industry connections: Pharmaceutical company collaborations providing funding and materials in exchange for research results. Contract research organizations offering services.
Professional societies: American Urological Association, International Society for Sexual Medicine, American College of Cardiology depending on sildenafil application. Networking through conferences, committees, publications.
Community engagement: Sharing progress through blogs, forums, preprints. Soliciting feedback improving research quality. Contributing to collective knowledge even without formal publication.
Regulatory navigation
Staying informed: Following FDA guidance updates, DEA scheduling changes, state/local regulations affecting sildenafil procurement or research. Subscribing to regulatory newsletters, consulting legal experts periodically.
Preemptive compliance: Over-documenting rather than under-documenting. Maintaining pristine records demonstrating research legitimacy. Consulting IRB even if not strictly required showing good faith.
International considerations: Research spanning countries navigates multiple regulatory frameworks. Understanding import/export controls, equivalency of approvals, local requirements. Legal consultation essential for multi-national research.
Adapting to changing landscape
Vendor reliability evolution: Today's reputable vendor may deteriorate or exit scam tomorrow. Maintaining multiple qualified sources, regular quality testing even from established vendors, community monitoring for early warnings.
Regulatory tightening: Research chemical access may restrict over time. Procurement strategies requiring flexibility, shifting to pharmaceutical suppliers if gray market closes, exploring legal alternatives (PT-141 peptide vs sildenafil), modifying research focus if sildenafil access too difficult.
Technology advancement: Novel delivery methods, formulations, analogs potentially superseding sildenafil. Staying current with literature, attending conferences, networking with field leaders identifies emerging opportunities.
Resource allocation: Balancing sildenafil research against other projects. If procurement becomes too difficult or expensive, shifting focus to more accessible research areas while maintaining expertise for future opportunities.
External resources
In case I don't see you, good afternoon, good evening, and good night. May your research stay legitimate, your chemicals stay pure, and your procurement stay legal, join SeekPeptides.