Jan 13, 2026
The terminology surrounding HMG often creates confusion, as it technically describes a hormone preparation rather than a peptide, though its gonadotropin components function through peptide hormone mechanisms and the compound frequently appears in peptide-related discussions regarding fertility support, testosterone optimization, and post-cycle therapy protocols. Understanding HMG requires distinguishing it from synthetic gonadotropins, recombinant FSH preparations, and other fertility medications while recognizing its specific applications in both clinical fertility treatment and research contexts involving hormonal optimization.
The dual FSH and LH activity provides comprehensive gonadal stimulation that single-hormone preparations cannot replicate, making HMG valuable for applications requiring complete hypothalamic-pituitary-gonadal axis support rather than targeted single-pathway intervention.
This comprehensive guide examines HMG mechanisms, clinical applications, dosing protocols, comparison with alternatives, combination strategies, safety considerations, and practical implementation for those researching gonadotropin-based approaches to fertility and testosterone support. SeekPeptides provides resources for understanding peptide and hormone optimization approaches tailored to individual goals.
Understanding HMG composition and origin
HMG derives from a biological source rather than synthetic manufacturing, giving it a distinct profile compared to recombinant gonadotropin preparations. The extraction process from postmenopausal urine yields a mixture containing FSH and LH in roughly equal proportions, along with trace amounts of other urinary proteins. This natural origin creates both advantages and considerations for those using HMG.
The FSH component stimulates Sertoli cells in the testes, supporting spermatogenesis and creating optimal conditions for sperm development. The LH component acts on Leydig cells, directly stimulating testosterone production through the same pathway activated by the body's endogenous LH. This dual action distinguishes HMG from preparations containing only one gonadotropin.
Postmenopausal women produce elevated gonadotropin levels because the feedback loop from ovarian hormones no longer suppresses pituitary output.
This natural elevation makes postmenopausal urine an efficient source for gonadotropin extraction.
The resulting HMG preparation contains both hormones in bioactive forms capable of binding their respective receptors.
FSH and LH mechanisms in males
FSH action on Sertoli cells: Follicle-stimulating hormone binds receptors on Sertoli cells within the seminiferous tubules, triggering signaling cascades that support spermatogenesis. Sertoli cells respond by producing factors essential for sperm cell development and maturation. Without adequate FSH stimulation, sperm production diminishes even when testosterone levels remain sufficient.
The peptide hormone mechanisms underlying FSH action involve receptor activation, intracellular signaling through cAMP pathways, and gene expression changes promoting Sertoli cell function. This molecular cascade takes time to produce measurable effects on sperm parameters, typically requiring weeks to months of consistent stimulation.
LH action on Leydig cells: Luteinizing hormone binds receptors on testicular Leydig cells, initiating steroidogenic enzyme cascades that convert cholesterol to testosterone. This represents the same pathway through which natural LH stimulates testosterone production, making exogenous LH activity from HMG functionally equivalent to enhanced endogenous LH signaling.
The testosterone produced from LH stimulation enters circulation where it exerts systemic effects while also acting locally within the testes to support spermatogenesis. This intratesticular testosterone proves essential for optimal sperm production, explaining why external testosterone administration, which suppresses intratesticular levels, impairs fertility despite maintaining circulating testosterone.
HMG versus recombinant gonadotropins
Recombinant FSH preparations like follitropin alpha provide pure FSH without LH activity, manufactured through genetic engineering rather than extraction from biological sources. These preparations offer consistency and purity advantages but lack the LH component present in HMG. For applications requiring both FSH and LH, recombinant preparations require combining separate products.
HMG provides convenience through combined FSH and LH in single preparation, though the exact ratio may vary slightly between batches due to biological source variability. Pharmaceutical grade HMG undergoes quality control ensuring consistent potency, but the natural origin means complete standardization remains challenging compared to recombinant products.
Cost considerations often favor HMG over recombinant preparations, particularly when both FSH and LH activity are desired. The peptide cost calculator can help compare expenses across different protocol approaches, though gonadotropin costs typically exceed most research peptides.
Clinical applications of HMG
HMG sees primary clinical use in fertility medicine, where its dual gonadotropin activity supports both male and female reproductive function. Understanding these established medical applications provides context for research applications in hormonal optimization.
Male fertility treatment
Men with hypogonadotropic hypogonadism, a condition where the pituitary fails to produce adequate FSH and LH, benefit from HMG therapy that replaces the missing gonadotropin signals. The combined FSH and LH activity stimulates both spermatogenesis and testosterone production, addressing the complete spectrum of gonadal dysfunction rather than just one aspect.
Clinical protocols typically involve: HMG administered 2-3 times weekly at doses of 75-150 IU, often combined with human chorionic gonadotropin which provides additional LH-like activity. Treatment continues for several months, as spermatogenesis requires approximately 74 days for complete sperm development cycles. Fertility improvements often take 6-12 months to manifest in improved semen parameters.
The testosterone and peptide optimization literature often references HMG in contexts of maintaining or restoring fertility alongside testosterone optimization goals. Men seeking to preserve reproductive capacity while addressing testosterone concerns find HMG relevant to their research.
Post-cycle therapy applications
Researchers investigating recovery from hypothalamic-pituitary-gonadal axis suppression often include HMG in recovery protocols. Following periods of exogenous hormone use that suppresses natural production, the axis requires stimulation to resume normal function. HMG provides this stimulation through direct gonadotropin activity.
Recovery protocol considerations: HMG during recovery periods stimulates testicular activity that may have diminished during suppression. The FSH component helps maintain Sertoli cell function and spermatogenic capacity, while LH activity stimulates Leydig cell testosterone production. This dual support addresses both aspects of testicular function affected by axis suppression.
Combining HMG with other recovery agents creates comprehensive protocols addressing multiple recovery mechanisms. The cycle planning guide covers considerations for structuring protocols involving gonadotropin support.
Testosterone support research
Beyond fertility applications, HMG research explores testosterone optimization through enhanced gonadotropin signaling. Men with suboptimal testosterone production from inadequate LH stimulation rather than testicular failure may respond to HMG's LH activity with improved testosterone levels.
This application overlaps with but differs from testosterone peptide approaches using growth hormone secretagogues or other indirect mechanisms. HMG works through direct gonadotropin action rather than the upstream or supportive mechanisms of peptide-based testosterone optimization.
Research contexts examining HMG for testosterone support typically use lower doses than fertility protocols, as testosterone production requires primarily LH activity while spermatogenesis demands both FSH and higher overall gonadotropin stimulation. Protocols focusing on testosterone may emphasize the LH activity while accepting the accompanying FSH as benign rather than targeting it specifically.
HMG dosing protocols
Dosing HMG requires understanding International Units measurement, injection frequency requirements, and protocol duration based on specific goals. Unlike many peptides dosed in micrograms, HMG uses IU measurement reflecting biological activity.
Understanding IU measurement
International Units for HMG reflect the biological potency of FSH and LH activity rather than simple mass measurement. A typical HMG vial contains 75 IU, meaning it contains FSH and LH activity equivalent to standardized reference preparations. This biological standardization ensures consistent effects despite the natural origin variability.
The IU system differs from the mass-based dosing used for most peptide dosing, requiring adjustment in thinking about dose calculations. The peptide calculator focuses on mass-based compounds, while HMG dosing relies on the IU designation on product labeling.
Fertility protocol dosing
Clinical fertility protocols typically use 75-150 IU HMG administered intramuscularly or subcutaneously every other day or three times weekly. Higher doses may be used in cases of more severe hypogonadism or poor initial response. Treatment duration extends months rather than weeks, reflecting the lengthy spermatogenic cycle.
Standard fertility protocol structure:
Initial phase: 75 IU HMG 3x weekly plus 1000-2000 IU HCG 2x weekly
Duration: 3-6 months minimum, often 12+ months for fertility restoration
Monitoring: Semen analysis every 3 months, testosterone and hormone levels monthly initially
Adjustment: Increase to 150 IU if inadequate response after 3 months
The combination with HCG provides additional LH-like activity supporting testosterone production while HMG's FSH component specifically targets spermatogenesis. This combination approach appears throughout clinical fertility literature.
Post-cycle therapy dosing
Recovery protocols typically use shorter durations with moderate dosing aimed at jumpstarting suppressed gonadal function. Unlike fertility protocols requiring months of treatment, recovery applications may use HMG for 2-4 weeks during the critical early recovery period.
Recovery protocol example:
Week 1-2: 75 IU HMG every other day (4 doses weekly)
Week 3-4: 75 IU HMG every 3 days (2-3 doses weekly)
Combination: Often paired with HCG, SERMs, or other recovery agents
Transition: Move to maintenance or cessation based on recovery markers
The HCG peptide protocols often overlap with HMG use, as both provide gonadotropin activity supporting testicular function during recovery periods.
Testosterone optimization dosing
Research into HMG for testosterone support typically uses lower doses than fertility protocols, focusing on maintaining adequate gonadotropin signaling rather than maximizing spermatogenesis. Doses of 75 IU 2-3 times weekly often appear in such protocols.
Testosterone support protocol:
Dosing: 75 IU HMG twice weekly
Duration: 8-12 weeks with monitoring
Assessment: Testosterone levels at baseline, 4 weeks, and 8 weeks
Adjustment: Based on response and side effect profile
Lower doses reduce the cost burden of HMG use while potentially providing sufficient LH activity for testosterone support. The accompanying FSH activity may support overall testicular health even when spermatogenesis is not the primary goal.
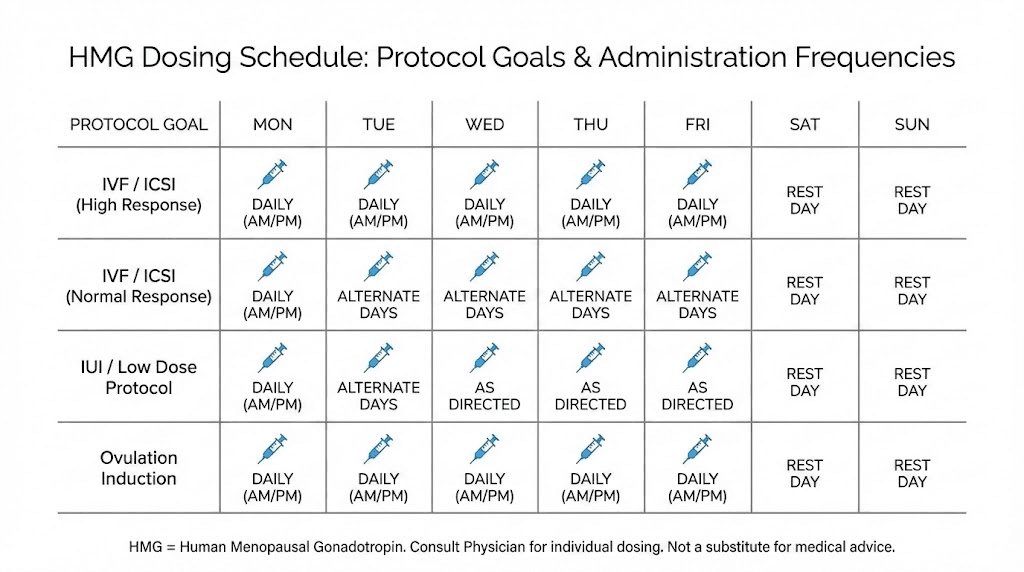
HMG versus HCG comparison
Human chorionic gonadotropin represents the most common alternative to HMG for gonadotropin therapy, with important differences in mechanism and application. Understanding this comparison helps select appropriate compounds for specific research goals.
Mechanism differences
HCG provides exclusively LH-like activity, binding LH receptors on Leydig cells to stimulate testosterone production. It lacks FSH activity entirely, meaning it cannot directly support Sertoli cell function or spermatogenesis through the FSH pathway. HCG works well for testosterone maintenance but incompletely for fertility restoration.
HMG provides both FSH and LH activity, offering comprehensive gonadal stimulation. The FSH component makes HMG more complete for fertility applications while the LH activity matches HCG's testosterone-supporting effects. For applications requiring only testosterone support, the FSH component in HMG may be unnecessary.
The peptides for men guide covers various approaches to male hormone optimization, including gonadotropin-based strategies using HMG, HCG, or combinations.
Application selection
When HMG may be preferred:
Fertility restoration requiring spermatogenesis support
Complete HPG axis recovery after prolonged suppression
Situations where Sertoli cell function needs direct stimulation
Research requiring both FSH and LH activity in single preparation
When HCG may be preferred:
Testosterone maintenance without fertility focus
Cost-sensitive protocols (HCG typically less expensive)
Situations where FSH activity is unnecessary or unwanted
Simpler protocols requiring only LH-like activity
Many protocols combine both, using HCG for consistent LH activity with periodic HMG to provide FSH support. This combination approach balances cost efficiency with comprehensive gonadal stimulation.
Practical considerations
HCG's longer history and wider availability make it more accessible than HMG in many contexts. Pharmaceutical HMG may require fertility clinic prescription while HCG availability extends more broadly. Research settings may find HCG easier to obtain consistently.
Cost differences typically favor HCG, particularly for long-term protocols. HMG's higher cost reflects the more complex extraction process and dual-hormone content. Budget constraints may push protocols toward HCG-dominant approaches with occasional HMG supplementation.
The peptide vendor evaluation principles apply when sourcing gonadotropins, with emphasis on pharmaceutical grade products given the importance of accurate dosing and purity for these hormonal compounds.
Combining HMG with other compounds
HMG often appears in combination protocols rather than standalone use, with various compounds complementing its gonadotropin activity. Strategic combinations address multiple mechanisms for comprehensive hormonal optimization.
HMG and HCG combinations
The most common HMG combination pairs it with HCG to provide enhanced LH-like activity alongside HMG's balanced FSH/LH profile. HCG's longer half-life provides sustained LH receptor activation between HMG doses, while HMG ensures FSH activity reaches target tissues.
Typical combination approach:
HCG: 500-1000 IU twice weekly (continuous LH support)
HMG: 75 IU twice weekly (FSH and additional LH)
Duration: Based on protocol goals (recovery, fertility, optimization)
This combination provides robust gonadotropin stimulation exceeding either compound alone. The redundant LH activity from both compounds ensures maximal Leydig cell stimulation while HMG adds the FSH component HCG lacks.
HMG with SERMs
Selective estrogen receptor modulators like clomiphene and tamoxifen block estrogen feedback at the hypothalamus and pituitary, increasing endogenous gonadotropin release. Combining SERMs with exogenous gonadotropins like HMG provides both direct gonadotropin activity and enhanced natural production.
SERM combination rationale: SERMs increase natural FSH and LH production by blocking negative feedback, while HMG provides direct gonadotropin supplementation. The combination may produce greater total gonadotropin activity than either approach alone, potentially accelerating recovery or optimization.
The enclomiphene option provides a more selective SERM approach that some researchers prefer when combining with gonadotropin therapy.
HMG with growth hormone peptides
Growth hormone secretagogue peptides like ipamorelin and CJC-1295 provide complementary benefits alongside HMG's gonadotropin activity. While HMG directly stimulates testicular function, GH peptides support overall hormonal environment, recovery capacity, and body composition.
Comprehensive optimization stack:
HMG: 75 IU twice weekly for gonadotropin support
Ipamorelin/CJC-1295: Daily dosing for GH optimization
BPC-157: Optional for systemic support and recovery
This combination addresses hormonal optimization through multiple pathways. The peptide stacking guide covers principles for combining compounds with complementary mechanisms.
HMG with testosterone (TRT contexts)
Men using testosterone replacement therapy often add HMG to maintain testicular function and fertility potential despite exogenous testosterone suppressing natural production. The HMG provides direct gonadotropin stimulation keeping testes active when the natural HPG axis is suppressed.
TRT combination approach:
Testosterone: Base TRT dosing as prescribed
HMG: 75 IU 2-3x weekly to maintain testicular activity
Alternative: Some use HCG alone, others prefer HMG for FSH component
This application specifically addresses the fertility and testicular atrophy concerns many men have with TRT. The FSH component in HMG provides protection for spermatogenesis that HCG alone may not fully deliver.
Administration and handling
HMG requires proper reconstitution, storage, and injection technique for effective use. The handling requirements parallel those of other injectable peptides and hormones.
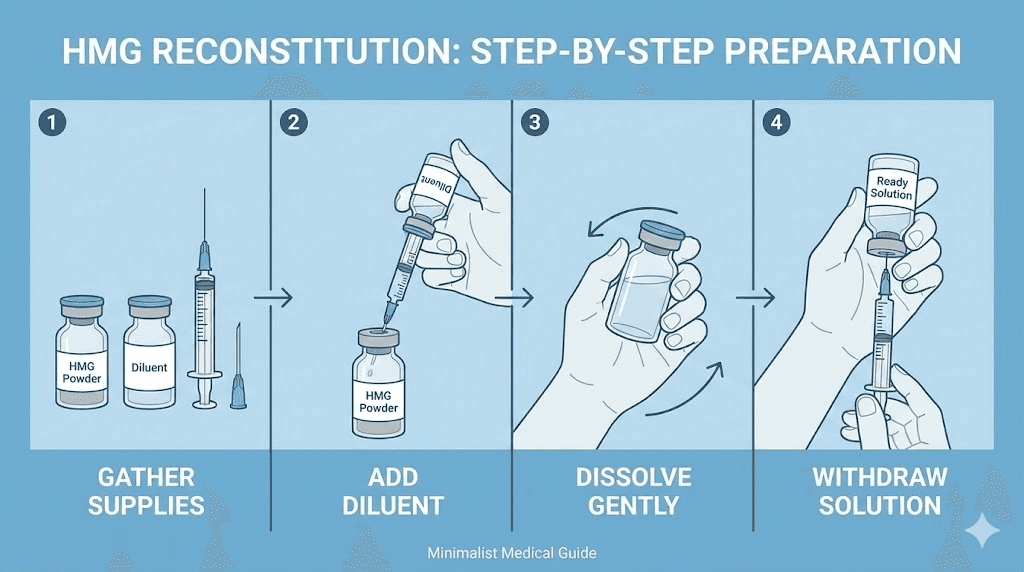
Reconstitution process
Pharmaceutical HMG typically arrives as lyophilized powder requiring reconstitution with provided diluent or bacteriostatic water. The reconstitution process follows standard principles applicable to peptide preparation.
Reconstitution steps:
1. Allow vial to reach room temperature
2. Add diluent slowly, directing flow down vial side
3. Gently swirl to dissolve, never shake
4. Verify complete dissolution before use
5. Store reconstituted solution in refrigerator
The peptide reconstitution guide covers detailed technique applicable to HMG preparation. The reconstitution calculator helps with volume calculations, though HMG often uses specific diluent volumes matching product labeling.
Storage requirements
Unreconstituted HMG typically requires refrigeration, with stability extending months when properly stored. Reconstituted HMG has shorter stability, generally requiring use within 1-2 weeks when refrigerated. Following product-specific storage guidelines ensures potency throughout the use period.
Storage principles:
Unreconstituted: Refrigerate at 36-46°F, protect from light
Reconstituted: Refrigerate, use within labeled timeframe (often 14 days)
Never freeze: Both forms damaged by freezing
Travel: May require cooled transport for longer trips
The peptide storage guide provides comprehensive storage information applicable to gonadotropin preparations.
Injection technique
HMG administration uses either intramuscular or subcutaneous injection depending on product formulation and prescribing guidance. Subcutaneous injection offers convenience and comfort for self-administration, while intramuscular may provide slightly faster absorption.
Subcutaneous injection sites:
Abdomen (1-2 inches from navel)
Upper thigh
Upper arm
Rotate sites to prevent tissue irritation
The injection technique guide covers proper subcutaneous administration applicable to HMG. Standard insulin syringes with 29-31 gauge needles work well for subcutaneous HMG administration.
Monitoring and response assessment
Effective HMG use requires monitoring to assess response and guide protocol adjustments. Blood work and clinical assessment help optimize outcomes while identifying any concerning developments.
Hormone panel testing
Baseline testing before beginning HMG establishes reference points for measuring response. Key markers include testosterone, FSH, LH, estradiol, and for fertility applications, semen analysis. These baseline values guide protocol design and provide comparison points for progress assessment.
Essential monitoring panel:
Testosterone (total and free): Primary outcome for testosterone goals
FSH and LH: Shows endogenous production alongside HMG supplementation
Estradiol: Monitor for estrogen elevation from testosterone aromatization
Semen analysis: Essential for fertility-focused protocols
Complete blood count: General health monitoring
Testing frequency depends on protocol duration and goals. Short recovery protocols may need only baseline and endpoint testing, while extended fertility protocols benefit from monthly or quarterly monitoring. The peptide research resources include guidance on monitoring hormone optimization protocols.
Response timeline expectations
Testosterone response to HMG's LH activity may appear within weeks, as Leydig cell stimulation produces relatively rapid effects on testosterone synthesis. Measurable testosterone increases often appear on blood work within 2-4 weeks of beginning HMG.
Fertility response takes much longer due to the spermatogenic cycle duration. New sperm development requires approximately 74 days, meaning semen analysis improvements may not appear for 3+ months after beginning HMG. Full fertility restoration often requires 6-12 months of consistent treatment.
Expected timeline:
Week 2-4: Potential testosterone increase visible on testing
Month 2-3: Testicular volume may increase
Month 3-6: First semen analysis improvements possible
Month 6-12: Maximum fertility response in most responders
The peptide response timeline provides context for understanding how different compounds produce effects over varying timeframes.
Dose adjustment criteria
Response assessment guides dose modifications for optimal outcomes. Inadequate response may warrant dose increases, while excessive response or side effects may require reduction. Monitoring provides objective data for these adjustments.
Adjustment considerations:
Inadequate testosterone response after 4-6 weeks: Consider dose increase or adding HCG
Estradiol elevation: May need aromatase inhibitor or dose reduction
Minimal spermatogenesis improvement after 6 months: Increase dose or extend duration
Side effects: Reduce dose or frequency
Working with healthcare providers experienced in fertility medicine helps optimize HMG protocols based on individual response patterns.
Side effects and safety considerations
HMG use carries potential side effects and safety considerations requiring attention. Understanding these risks enables informed decision-making and appropriate monitoring.
Common side effects
Injection site reactions including redness, swelling, and discomfort occur with some frequency, though proper technique minimizes these issues. Rotating injection sites and using appropriate needle sizes reduces local reactions.
Typical side effects:
Injection site reactions: Mild and temporary in most cases
Headache: Occasionally reported, usually mild
Mood changes: Hormonal fluctuation may affect mood
Breast tenderness: From elevated estrogen or hormone changes
Testicular discomfort: Particularly during initial stimulation phase
The peptide safety guide covers general principles for managing side effects from injectable compounds.
Estrogen-related concerns
Increased testosterone production from HMG may lead to elevated estrogen through aromatization. Men sensitive to estrogen or those with baseline elevation may experience symptoms including water retention, gynecomastia, and mood effects.
Managing estrogen:
Monitor estradiol levels with hormone testing
Consider aromatase inhibitor if estrogen exceeds optimal range
Adjust HMG dose if estrogen issues persist
Body composition improvement reduces aromatase activity
The balance between adequate testosterone and controlled estrogen requires individual optimization. Some men need additional estrogen management while others tolerate HMG-induced testosterone increases without issues.
Overstimulation syndrome considerations
While ovarian hyperstimulation syndrome represents a serious concern in female fertility treatment, male overstimulation risks differ. Excessive gonadotropin stimulation in males may cause testicular discomfort or, rarely, more significant issues. Conservative dosing and monitoring minimize these risks.
The lower doses typically used in male applications compared to female fertility protocols reduce overstimulation risk.
However, attention to testicular response remains important, particularly during initial treatment when sensitivity may be highest.
Quality and authenticity concerns
Gonadotropin preparations including HMG represent high-value targets for counterfeiting. Ensuring pharmaceutical grade product from reliable sources protects against receiving inactive or contaminated material. The vendor evaluation principles apply with particular importance for hormonal compounds.
Authenticity verification:
Pharmaceutical packaging with proper labeling and lot numbers
Verifiable source with prescription or legitimate research supply channels
Proper storage during shipping and at source
Consistent effects matching expected response to genuine HMG
HMG in fertility restoration protocols
Fertility restoration represents HMG's primary clinical application, with established protocols addressing various causes of male infertility. Understanding these applications provides context for research into hormonal optimization.
Hypogonadotropic hypogonadism treatment
Men with pituitary or hypothalamic dysfunction producing inadequate gonadotropins represent ideal HMG candidates. These individuals have functional testes capable of responding to gonadotropin stimulation but lack the signals to activate them. HMG provides the missing signals, enabling normal testicular function.
Treatment approach:
Confirm diagnosis with low testosterone plus low or normal FSH/LH
Begin HMG 75-150 IU three times weekly
Add HCG 1000-2000 IU twice weekly for additional LH activity
Monitor testosterone monthly initially, semen analysis quarterly
Continue 12-24 months for fertility restoration
Success rates vary based on underlying cause and treatment duration. Men with acquired hypogonadotropic hypogonadism often respond better than those with congenital forms, though both may achieve fertility with prolonged treatment.
Post-steroid fertility recovery
Anabolic steroid use suppresses the HPG axis, causing secondary hypogonadism similar in presentation to other forms of hypogonadotropic hypogonadism. The suppression may persist for extended periods after discontinuation, particularly following prolonged or high-dose use.
HMG provides direct gonadotropin stimulation potentially accelerating recovery compared to waiting for natural axis restoration. The FSH component specifically addresses spermatogenesis recovery while LH activity supports testosterone production.
Recovery protocol principles:
Begin HMG after clearing exogenous hormones from system
Combine with HCG and potentially SERMs for comprehensive recovery
Monitor testosterone and semen parameters throughout recovery
Continue until parameters normalize or plateau
Transition to maintenance or cessation based on sustained recovery
The cycle planning considerations include post-cycle recovery as a critical phase where HMG may play a role.
Idiopathic infertility support
Some men experience infertility without identifiable cause, with normal hormone levels but impaired semen parameters. HMG may benefit some of these cases by providing supraphysiological gonadotropin stimulation potentially enhancing spermatogenesis beyond baseline levels.
Response in idiopathic infertility proves less predictable than in clear hypogonadotropic cases, as the underlying problem may not involve gonadotropin insufficiency. Trial treatment periods help identify responders who may benefit from continued HMG therapy.
Comparing HMG to other gonadotropin preparations
Multiple gonadotropin preparations exist for fertility and hormonal applications, each with distinct characteristics. Understanding the landscape helps select optimal compounds for specific applications.
Highly purified HMG products
Modern HMG preparations undergo extensive purification removing most non-gonadotropin proteins present in earlier products. These highly purified preparations reduce allergic reaction risk and may provide more consistent activity. Brand-name highly purified HMG products represent current pharmaceutical standard.
The purification improvements address earlier concerns about antibody formation and injection reactions from the protein content of less-refined preparations. Most contemporary HMG products meet high purity standards suitable for clinical and research use.
Recombinant FSH products
Recombinant FSH preparations provide pure follicle-stimulating hormone manufactured through genetic engineering rather than biological extraction. These products offer complete consistency without batch variation and contain only FSH without LH activity. Examples include follitropin alfa and follitropin beta.
Recombinant FSH characteristics:
Pure FSH without LH contamination or activity
Consistent batch-to-batch potency
No biological source variability
Requires adding separate LH source if LH activity desired
Generally higher cost than HMG
For applications requiring only FSH, recombinant products provide targeted activity. For applications needing both gonadotropins, HMG or combination approaches may prove more practical.
Recombinant LH products
Recombinant LH exists but sees limited use compared to HCG which provides equivalent LH-receptor activity at lower cost. The availability of inexpensive HCG reduces demand for recombinant LH in most applications.
Specific situations where pure LH activity without HCG's longer half-life might be preferred remain limited.
For most practical purposes, HCG adequately addresses LH-activity needs while HMG or recombinant FSH addresses FSH requirements.
Selection framework
Goal-based selection:
Fertility with both FSH and LH needs: HMG (convenient) or recombinant FSH + HCG (precise)
Testosterone support primarily: HCG alone often sufficient
Spermatogenesis with existing LH support: May add recombinant FSH to HCG
Complete axis recovery: HMG provides both components in single product
Cost, availability, and protocol complexity all influence selection. The protocol design principles emphasize matching compound selection to specific goals.
Practical protocol examples
Concrete protocol examples illustrate how HMG integrates into various applications. These examples provide starting frameworks requiring individual adjustment based on response and goals.
Fertility restoration protocol
For men with documented hypogonadotropic hypogonadism seeking fertility:
Phase 1: Testosterone normalization (months 1-3)
HCG 1500 IU Monday, Wednesday, Friday
Goal: Normalize testosterone before adding HMG
Monitoring: Testosterone levels monthly
Phase 2: Adding FSH support (months 3+)
HCG 1500 IU twice weekly
HMG 75 IU three times weekly
Goal: Stimulate spermatogenesis
Monitoring: Semen analysis every 3 months
Phase 3: Intensification if needed (month 6+)
Increase HMG to 150 IU if poor semen response
Continue minimum 12 months total
Consider specialist consultation if no response by 12 months
Post-cycle recovery protocol
For researchers investigating axis recovery after suppression:
Week 1-2: Aggressive stimulation
HMG 75 IU every other day
HCG 1000 IU every other day (alternating with HMG)
Optional SERM: Clomiphene 25-50mg daily or enclomiphene 12.5-25mg daily
Week 3-4: Moderate stimulation
HMG 75 IU twice weekly
HCG 500 IU twice weekly
Continue SERM if using
Week 5-8: Transition
Taper HMG to once weekly then discontinue
HCG may continue or taper based on testosterone levels
SERM may continue for additional weeks
Testing testosterone and symptoms guides transition timing. Some individuals recover quickly while others need extended support.
TRT fertility preservation protocol
For men on testosterone replacement seeking to maintain fertility potential:
Ongoing protocol:
Continue prescribed TRT
Add HMG 75 IU twice weekly
Alternative: HCG 500 IU twice weekly plus HMG 75 IU once weekly
Pre-conception intensification:
When actively trying to conceive, consider pausing TRT temporarily
Increase HMG to 75 IU three times weekly
Add HCG 1000 IU twice weekly
Monitor semen parameters monthly
This approach maintains testicular activity during TRT while allowing intensification when conception is the immediate goal.
Lifestyle factors supporting HMG effectiveness
Maximizing HMG response involves attention to lifestyle factors influencing gonadal function and hormone optimization. These factors complement pharmacological intervention.
Nutrition for fertility and testosterone
Adequate nutrition supports both testosterone production and spermatogenesis. Key nutritional factors include sufficient calories, adequate fat intake for hormone synthesis, and specific micronutrients supporting reproductive function.
Key nutrients:
Zinc: Essential for testosterone synthesis and sperm production
Selenium: Supports sperm motility and morphology
Vitamin D: Associated with testosterone levels and fertility
Omega-3 fatty acids: Support hormone production and sperm membrane health
Antioxidants: Protect sperm from oxidative damage
Testing for deficiencies and correcting as needed provides foundational support for HMG protocols. The male optimization guide covers nutritional factors relevant to hormone and fertility optimization.
Exercise and training considerations
Moderate exercise supports testosterone production and overall reproductive health. However, excessive training may impair hormonal function, particularly when combined with inadequate nutrition or recovery. Finding appropriate training balance optimizes HMG response.
Training principles:
Resistance training: Supports testosterone through exercise-induced hormone release
Avoid overtraining: Excessive exercise suppresses reproductive hormones
Allow recovery: Rest days and sleep support hormonal restoration
Temperature management: Avoid excessive testicular heat from prolonged cycling or hot environments
The muscle growth peptides may complement HMG protocols for those also pursuing training adaptations.
Stress and sleep optimization
Chronic stress suppresses reproductive hormones through cortisol elevation and direct hypothalamic effects. Managing stress supports natural gonadotropin release and may enhance response to exogenous gonadotropins like HMG.
Sleep quality directly impacts hormone production, with testosterone and growth hormone peaking during deep sleep phases. Optimizing sleep supports the hormonal environment HMG seeks to enhance. The sleep peptides may complement gonadotropin protocols for those with sleep-related hormonal impairment.
Frequently asked questions
What is the difference between HMG and HCG?
HMG contains both FSH and LH activity, providing comprehensive gonadal stimulation supporting both spermatogenesis and testosterone production. HCG provides only LH-like activity, stimulating testosterone but not directly supporting spermatogenesis through FSH pathways. For fertility applications, HMG offers more complete stimulation.
How long does HMG take to work for fertility?
Testosterone improvements may appear within 2-4 weeks, but fertility restoration takes much longer due to the spermatogenic cycle. Expect 3-6 months minimum before seeing improvements in semen analysis, with full fertility restoration often requiring 6-12 months of consistent treatment.
Can HMG be used for testosterone support without fertility goals?
Yes, the LH component in HMG stimulates testosterone production. However, HCG alone may be more cost-effective if fertility is not a concern, as the FSH component becomes less relevant. Some researchers prefer HMG for comprehensive testicular support even without fertility focus.
Is HMG a peptide?
Technically, HMG contains glycoprotein hormones (FSH and LH) rather than simple peptides. However, these hormones function through peptide hormone mechanisms and frequently appear in peptide-related discussions about hormonal optimization. The distinction matters more for classification than for practical application.
What are the main side effects of HMG?
Common side effects include injection site reactions, headache, and mood changes. Estrogen elevation from increased testosterone may cause water retention or breast tenderness in some users. Serious side effects are uncommon with appropriate dosing and monitoring.
How should HMG be stored?
Store unreconstituted HMG in the refrigerator. After reconstitution, continue refrigerated storage and use within the timeframe specified on product labeling, typically 1-2 weeks. Never freeze HMG as it damages the hormone proteins.
How SeekPeptides supports hormonal optimization research
SeekPeptides provides comprehensive resources for understanding hormonal optimization approaches including gonadotropin-based protocols. The platform offers educational content and tools supporting informed research decisions.
The peptide calculator and reconstitution calculator support preparation of various compounds used alongside gonadotropins.
The stacking calculator helps design combination protocols integrating multiple compounds.
Educational guides covering testosterone optimization, stacking strategies, and cycle planning provide context for gonadotropin protocols. The platform supports researchers navigating complex hormonal optimization decisions.
SeekPeptides remains committed to evidence-based education for safe and effective peptide and hormone optimization research.
Helpful resources
Related guides worth reading
Hormone optimization:
In case I don't see you, good afternoon, good evening, and good night. May your gonadotropins stay balanced, your fertility stay protected, and your hormonal health stay optimized. Join SeekPeptides